Abstract
Background and objective
The differential diagnosis for exercise-associated breathlessness is broad, however, when a young athletic individual presents with respiratory symptoms, they are most often prescribed inhaler therapy for presumed exercise-induced asthma (EIA). The purpose of this study was therefore to use a novel sound-based approach to assessment to evaluate the prevalence of exertional respiratory symptoms and characterise abnormal breathing sounds in a large cohort of recreationally active individuals.
Methods
Cross-sectional field-based evaluation of individuals completing Parkrun.
Phase 1
Prerace, clinical assessment and baseline spirometry were conducted. At peak exercise and immediately postrace, breathing was monitored continuously using a smartphone. Recordings were analysed retrospectively and coded for signs of the predominant respiratory noise.
Phase 2
A subpopulation that reported symptoms with at least one audible sign of respiratory dysfunction was randomly selected and invited to attend the laboratory on a separate occasion to undergo objective clinical workup to confirm or refute EIA.
Results
Forty-eight participants (22.6%) had at least one audible sign of respiratory dysfunction; inspiratory stridor (9.9%), expiratory wheeze (3.3%), combined stridor+wheeze (3.3%), cough (6.1%). Over one-third of the cohort (38.2%) were classified as symptomatic. Ten individuals attended a follow-up appointment, however, only one had objective evidence of EIA.
Conclusions
The most common audible sign, detected in approximately 1 in 10 individuals, was inspiratory stridor, a characteristic feature of upper airway closure occurring during exercise. Further work is now required to further validate the precision and feasibility of this diagnostic approach in cohorts reporting exertional breathing difficulty.
Keywords: asthma, exercise, respiratory measurement
Key messages.
What is the key question?
To use a novel sound-based approach to assessment to evaluate and characterise abnormal breathing sounds in a large cohort of recreationally active individuals.
What is the bottom line?
The most common audible sign, detected in approximately 1 in 10 individuals, was inspiratory stridor, a characteristic feature of upper airway closure occurring during exercise.
Why read on?
This study demonstrates the potential value of field-based smartphone audio recordings as a simple screening modality or adjunctive tool to aid the assessing clinician in an office-based setting to guide clinical workup and inform subsequent diagnostic referral.
Introduction
The differential diagnosis for exercise-associated breathlessness with wheeze is broad,1 however, when a young athletic individual reports this problem, they are most often prescribed inhaler therapy for a presumed diagnosis of exercise-induced asthma (EIA).2 This is despite a substantial body of evidence indicating a poor association between the presence of exertional respiratory symptoms and objective evidence of EIA.3 4
The limited predictive value of symptoms, in this context, is likely explained by the presence of conditions that can act to mimic EIA. In this respect, closure of the laryngeal inlet (upper airway) during exercise—a condition termed exercise-induced laryngeal obstruction (EILO) is recognised to precipitate breathlessness and wheeze.5 6 EILO is a common condition, affecting between 5% and 7% in adolescents,7 with a higher prevalence in athletes and military personnel (15%–35%),8 9 but can also exist as a comorbid phenomenon in approximately one in four patients with asthma.10 11
A key difference between EILO and EIA is the nature and quality of the respiratory sound that develops during exercise. Specifically, EILO is typically associated with an inspiratory phase stridor, that is present during exercise, whereas in contrast, EIA can be associated with expiratory wheeze, often maximal in the postexercise period. Thus, a unique but mandatory requirement, in the assessment of patients who report exertional breathlessness, is the ability to evaluate respiratory sounds during exercise (ie, not simply in the clinic room).
The gold-standard method to confirm a diagnosis of EILO is the continuous laryngoscopy during exercise test (CLE)12, a technique that involves flexible nasendoscopy, to allow visualisation of the laryngeal structures during laboratory-based exercise. Although the feasibility of this approach is well established, CLE is currently only available at specialist centres across Europe and requires expensive comprehensive setup and expertise, and thus deemed impractical for field-based assessment. Alternative detection methods used in this setting have included evaluating the origin of respiratory sounds by auscultating the neck and chest, during exercise. Previously, a high prevalence of inspiratory stridor has been reported when employing sound-based stethoscope assessment during exercise challenge testing in athletes screened for EIA.13
The purpose of this study was therefore to use a novel sound-based approach to assess and evaluate the prevalence of exertional respiratory symptoms and characterise abnormal breathing sounds in a large cohort of recreationally active individuals. Due to the typical features associated with upper and lower airway obstruction during exercise (ie, high-pitched stridor or ‘whistle’ on inspiration and expiratory wheeze, respectively), we hypothesised that self-recorded audio using smartphones could offer utility as a screening modality to guide clinical workup and inform subsequent diagnostic referral.
Methods
Study population
Two hundred and twenty recreational runners (men: n=100) currently meeting the American College of Sports Medicine physical activity recommendations were enrolled into the study.14 All were non-smokers and free from respiratory, cardiovascular, metabolic and psychiatric disease and any other significant medical condition except asthma.
Patient and public involvement
Patients were not involved in the design of this study.
Experimental design
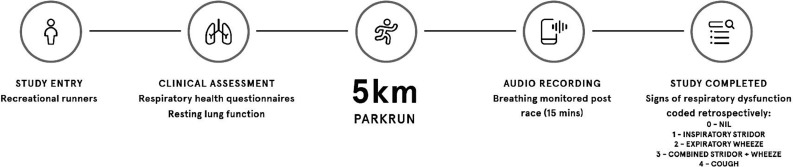
The study was conducted as a cross-sectional field-based evaluation of individuals completing Parkrun across Northern England between 2017 and 2019. Phase 1. Prerace, preparticipation health screening, clinical assessment and baseline spirometry were conducted prior to completing a 5 km time-trial run. At peak exercise and immediately postrace, breathing was self-recorded by the participant and analysed, retrospectively (figure 1). Phase 2. A subpopulation that reported symptoms and at least one audible sign of respiratory dysfunction was randomly selected and invited to attend the laboratory on a separate occasion to undergo objective clinical workup to confirm or refute EIA.
Figure 1.
Schematic detailing experimental design.
Phase 1
Clinical assessment and baseline lung function
Respiratory symptoms were assessed via completion of the Allergy Questionnaire for Athletes (AQUA) (AQUA score: 0–30) and Dyspnoea-12 (D-12 score:1–36). The AQUA has previously been validated to evaluate allergic and respiratory symptoms in athletes15 16—whereas D-12 quantifies the physical and affective components of breathlessness.17 A combined positive AQUA score ≥5 and D-12 score ≥1 was used to confirm symptomatic status. Lung function was assessed by maximal forced flow-volume spirometry with established reference values employed in accordance with international guidelines.18
Audio-recordings and sound analysis
Breathing sounds during exercise were self-recorded by the participant using an in-built audio-recording application on a smartphone. At the start of the race, participants launched the application in preparation to begin recording at peak exercise (ie, approaching and crossing the finish line) and immediately postrace for 15 min or until resting tidal breathing had resumed. To optimise signal-to-noise ratio (ie, minimise distortion and background noise), participants were instructed to hold the smartphone approximately 10–15 cm away from their mouth. Audio-recordings were analysed retrospectively by two independent researchers (JS and OJP) and coded for signs of the predominant respiratory noise: 0=nil; 1=inspiratory stridor (defined as a monophonic high-pitched ‘whistle’ on inspiration); 2=expiratory wheeze (defined as a polyphonic ‘whistle or rattle’ on expiration); 3=combined stridor+wheeze; 4=cough.
Phase 2
Fractional exhaled nitric oxide and eucapnic voluntary hyperpnoea
Airway inflammation was assessed via fractional exhaled nitric oxide (FeNO) using a handheld measuring device (NIOX VERO; Aerocrine AB, Stockholm, Sweden).19 Eucapnic voluntary hyperpnoea (EVH) was conducted as previously described.20 In brief, participants breathed a dry compressed gas mixture (21% O2, 5% CO2, balance N2) at a target ventilation rate equivalent to 85% (baseline FEV1×30) of their predicted maximal voluntary ventilation (MVV) for 6 min. Spirometry was performed in triplicate at baseline and in duplicate at 3, 5, 7, 10 and 15 mins post-EVH. Values within 5% were considered acceptable. The highest recorded value at each time point was used for analysis. A positive diagnosis for EIA was defined by a fall in forced expiratory volume in one second (FEV1)≥15% at one time point.21
Statistical analysis
Data were stratified with cross tabulation and reported descriptively according to the prevalence of respiratory symptoms and audible code (ie, sign of respiratory dysfunction). Independent t-tests (continuous variables) and χ2 tests (categorical variables) were employed to evaluate between-group differences. The relationship between variables was evaluated using Spearman’s correlation coefficient. Data are reported as mean±SD (normal distribution) or median and IQR (non-normal distribution). Data were analysed using SPSS Statistics V.24 statistical software package (SPSS, V.21, Chicago, Illinois, USA). p<0.05 was considered statistically significant.
Results
Clinical characteristics and baseline lung function
Two-hundred and twenty recreational runners consented to participate in the study. Eight individuals failed to provide a postrace audio recording and thus 212 participants (men: n=95) were included in the analysis. The majority of the cohort (86%) had normal resting lung function (FEV1>80% predicted and FEV1/FVC ratio >70%). Forty-one (19.3%) (men: n=19) reported a prior physician-based asthma diagnosis. Three individuals (7.3%) with asthma had evidence of expiratory airflow limitation (FEV1/FVC ratio <70%). Participants with asthma had a lower FEV1/FVC ratio (p=0.003), however, no difference was observed in any other pulmonary function parameters (p>0.05) (table 1).
Table 1.
Clinical characteristics and baseline lung function
| Variables | Mean (±SD) | Median (IQR) | |||
| Age (year) | 36 | ± | 13 | 35 | (25) |
| Height (cm) | 172.1 | ± | 9.2 | 172.0 | (13.3) |
| Weight (kg) | 71.6 | ± | 12.8 | 69.0 | (16.3) |
| BMI (kg·m-2) | 24 | ± | 3 | 24 | (4) |
| FEV1 (L) | 3.72 | ± | 0.85 | 3.7 | (1.2) |
| FEV1 %predicted | 97.8 | ± | 15.5 | 97.2 | (23.5) |
| FVC (L) | 4.06 | ± | 1.07 | 4.5 | (1.5) |
| FVC %predicted | 117.8 | ± | 17.2 | 118.2 | (24.3) |
| FEV1/FVC (%) | 82.6 | ± | 7.6 | 83.0 | (9) |
| D-12 score | 3 | ± | 5 | 2 | (5) |
| AQUA score | 8 | ± | 8 | 6 | (13) |
AQUA, Allergy Questionnaire for Athletes; BMI, body mass index; D-12, Dyspnoea-12; Forced expiratory volume in one second, FEV1; Forced vital capacity, FVC.
Phase 1
Exertional respiratory symptoms
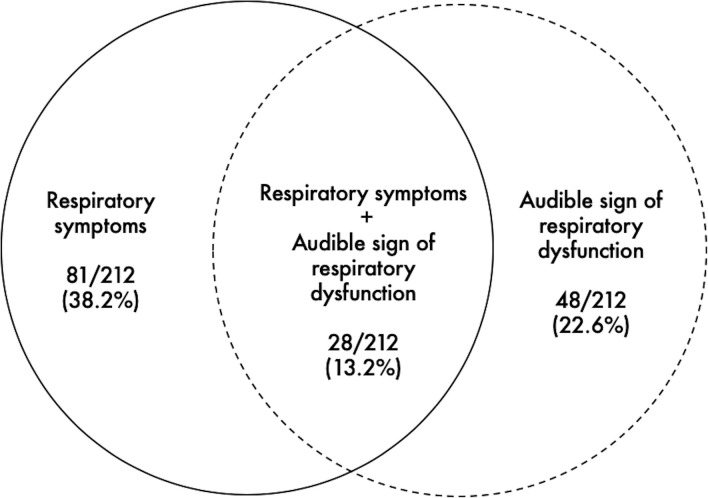
One hundred and eighteen participants (55.6%) provided a positive AQUA questionnaire (AQUA score range: 0–30) and 121 (57.1%) reported heightened perceived breathlessness (D-12 score range: 0–26). Eighty-one (38.2%) were thus classified as being symptomatic (ie, positive to both AQUA and D-12) (figure 2). Of these, 54 were women (66.7%) and 27 were men (33.3%) (p=0.008). A higher proportion of individuals with asthma reported exertional breathing difficulty in comparison to their healthy counterparts (p<0.001).
Figure 2.
Prevalence of exertional respiratory symptoms and audible signs of respiratory dysfunction.
Audible signs of respiratory dysfunction
Forty-eight participants (22.6%) had at least one audible sign of respiratory dysfunction; inspiratory stridor (9.9%), expiratory wheeze (3.3%), combined stridor+wheeze (3.3%), cough (6.1%) (ie, inspiratory stridor was the most common audible sign) (see online supplemental audio file 1), with no difference apparent between men and women (p=0.62). Fifteen individuals with a prior asthma diagnosis (36.6%) also had an audible sign; inspiratory stridor (14.6%), expiratory wheeze (7.3%), combined stridor+wheeze (7.3%), cough (7.3%). The proportion of individuals with an audible sign was higher in those with asthma in comparison to healthy individuals (p=0.02).
bmjresp-2020-000618supp001.mp3 (2MB, mp3)
Exertional respiratory symptoms+audible sign
Twenty-eight participants (13.2%) had evidence of both symptoms and an audible sign: inspiratory stridor (4.2%); expiratory wheeze (1.9%); combined stridor+wheeze (2.8%); cough (4.2%). Of these, 22 were women (78.6%) and 6 (21.4%) were men (p=0.008). The proportion of individuals with both symptoms and an audible sign was higher in those with an asthma diagnosis when compared with healthy individuals (p=0.002). A weak association was observed between audible signs and symptomatic status (r=0.2, p=0.001). Importantly, 20 asymptomatic individuals (9.4%) had an audible sign: inspiratory stridor (5.7%); expiratory wheeze (1.4%); combined stridor+wheeze (0.5%); cough (1.9%)—whereas 33 (15.6%) symptomatic individuals did not present with an audible sign.
Phase 2
EIA screening outcome
Ten symptomatic individuals with audible respiratory dysfunction (3 inspiratory stridor, 3 combined stridor+wheeze and 4 cough) agreed to attend a follow-up appointment to complete FeNO and EVH. Of these, three had elevated airway inflammation (ie, FeNO ≥25 ppb) but normal baseline lung function. All met the accepted minimal target ventilation for a valid test EVH (ie, minute ventilation ≥60% MVV). Only one individual (combined stridor+wheeze) had objective evidence of EIA (−25% reduction in FEV1) (table 2).
Table 2.
Screening outcome following EVH
| Gender | Age (year) | BMI (kg·m-2) | Audible sign | FeNO (ppb) | EVH (% fall in FEV1) | EIA |
| M | 55 | 24 | Inspiratory stridor | 37* | −1.6 | Negative |
| F | 50 | 24 | Inspiratory stridor | 18 | −5.3 | Negative |
| F | 38 | 25 | Inspiratory stridor | 18 | −3.7 | Negative |
| F | 24 | 22 | Stridor+wheeze | 31* | −25.1† | Positive |
| M | 26 | 27 | Stridor+wheeze | 19 | −4.2 | Negative |
| F | 21 | 23 | Stridor+wheeze | 17 | −1.4 | Negative |
| F | 59 | 24 | Cough | 15 | −5.5 | Negative |
| F | 46 | 29 | Cough | 16 | −6.0 | Negative |
| M | 25 | 28 | Cough | 19 | −8.3 | Negative |
| F | 36 | 24 | Cough | 40* | −0.5 | Negative |
*FeNO ≥25 ppb (intermediate).
†EIA ≥15% fall in FEV1.
BMI, body mass index; EIA, exercise-induced asthma; EVH, eucapnic voluntary hyperpnoea; FeNO, fractional exhaled nitric oxide.
Discussion
In a large cohort of recreational runners, we found that a substantial proportion of individuals has an audible sign of respiratory dysfunction, during their routine weekly exercise. Importantly, the most frequently detected noise, affecting approximately 1 in 10 individuals, was inspiratory stridor. This challenges the widespread misconception that any exercise-associated ‘wheeze’ must be arising from EIA and is supported by the finding that the majority of those with a prior physician-based asthma diagnosis actually had evidence of inspiratory stridor.
Respiratory noise on exertion is thought to be generated by vibrations within the airway structures in the presence of turbulent airflow or vortices.22 In healthy individuals undertaking exercise, both the upper and lower airways dilate mildly to facilitate ventilation and decrease airway resistance.23–25 The presence of stridor, a monophonic high-pitched sound heard on inspiration, during exercise, indicates narrowing in the extrathoracic airway and most often transient closure of the larynx.26 In contrast, expiratory wheeze is commonly associated with lower airway narrowing and often detected in conditions characterised by expiratory airflow limitation (ie, intrathoracic airway obstruction).26 Despite this, the presence of any ‘abnormal’ loud or noisy breathing on exertion is most often associated and interpreted as a characteristic feature of EIA.27 The low prevalence of objectively confirmed airway hyper-responsiveness with bronchial provocation testing, in the subgroup of patients with audible respiratory dysfunction, underlines the poor predictive value of a symptom-based approach to clinical assessment.3 4 Likewise, it has been recognised for some time that resting spirometry provides limited predictive value in this setting28 and thus highlights the importance and indeed requirement to detect and evaluate exertional respiratory disorders during physical activity. Overall, therefore, our findings challenge the validity of a ‘clinic-based approach’ and typical view or practice that breathlessness and wheeze on exertion equate to asthma. Our findings also act to demonstrate the potential value of employing modern technologies to conduct field-based testing to truly understand the cause of an individual’s respiratory symptoms. From a practical perspective, audio recordings may therefore offer value as an adjunctive tool to aid the assessing clinician in an office-based setting to ensure that patients are referred for the most appropriate tests to achieve early accurate diagnosis. It is important to recognise, however, that despite their apparently ‘asymptomatic’ status, 20 individuals had an audible sign of respiratory dysfunction. We used screening questionnaires to detect symptoms and it may be that direct questioning±consultation may have provided further clinical insight. It is also plausible that individuals who have experienced exertional breathing difficulty for a sustained period become accustomed and associate the sensation of dyspnoea as a ‘normal’ or a typical response to vigorous exercise.4 Indeed, screening studies in athletic cohorts consistently reveal individuals who fail to perceive respiratory symptoms yet report improvements in exercise ventilatory function following intervention.29 In this respect, audio recordings may also have utility as a low-cost pragmatic first step to widespread screening programmes in sports teams, schools or athletic squads.
The prevalence of respiratory symptoms in the current study was comparable with the previous population-based research in athletic cohorts.30 31 Indeed, over half of the study population scored positively on the AQUA questionnaire15—with a similar number reporting a heightened D-12 score. Although the development of breathlessness and a degree of respiratory discomfort is considered a physiologically appropriate response to exercise, potentially reflecting heightened ventilatory drive due to increased work of breathing32; in some individuals, these sensations are considered excessive or inappropriate and may relate to an underlying abnormality or pathological condition.33 We used a combined positive AQUA and D-12 score that resulted in over one-third of the cohort being classified as symptomatic, and similar to previous studies, respiratory symptoms were most frequently reported in women.31 34 35 Although the underlying physiological mechanism(s) to explain the disparity in symptom perception between genders remains unclear, previous reports suggest that the prevalence of EILO is most common in adolescents and young women.36 37 In keeping with this concept, a significantly higher proportion of symptomatic women also had an audible sign of respiratory distress. Other factors that may explain or contribute to gender differences include the fact that women have lower absolute lung volumes impacting expiratory flow limitation, smaller airway diameters, differences in respiratory muscle function and a lower surface area for pulmonary gas exchange in comparison with men.38 39 The higher prevalence of symptoms in those with asthma is expected given exercise is one of the most frequently reported symptom triggers and a prominent clinical characteristic of the disease.40
Methodological considerations and future research
A key limitation of the present study is the lack of validity work demonstrating that inspiratory stridor was caused by EILO. Thus, a logical extension of this study and focus for future research should be to determine the value of our approach to sound recordings in asymptomatic and symptomatic individuals against objective test results (ie, bronchial provocation or CLE testing) to determine diagnostic sensitivity and specificity. It is important to highlight that we were unable to detect a sign of respiratory dysfunction in 33 symptomatic individuals and thus a sound-based approach to detection should not be used to preclude underlying airflow limitation. It is also worth noting that differences in smartphone brand or model may impact audio quality (ie, ability to detect ‘abnormal’ breathing sounds) and despite attempting to standardise the position of the smartphone, a degree of between-subject variability is inevitable when employing this approach. Irrespective, our findings indicate that it is possible to discern between audible signs of respiratory dysfunction in most cases by using modern smartphone audio recording technology. Indeed, the accessible and user-friendly nature of these devices and current widespread use of social fitness network applications (eg, Strava and MapMyRun, etc) within the general population highlights the potential to implement and use this approach as a simple screening modality. The development of a targeted ‘fit for purpose’ ambulatory device with increased signal-to-noise ratio to detect, analyse and interpret signs of respiratory dysfunction therefore remains an important avenue for future research.
Conclusion
In summary, a high proportion of individuals engaging in recreational sport develop respiratory symptoms and audible respiratory dysfunction during exercise. The most common audible sign, detected in approximately 1 in 10 individuals, was inspiratory stridor, a characteristic feature of upper airway closure occurring during exercise. Further work is now required to further validate the precision and feasibility of this diagnostic approach in cohorts reporting exertional breathing difficulty.
Acknowledgments
The authors wish to extend their gratitude to all Parkrun Event Directors who kindly offered assistance to support project delivery.
Footnotes
Twitter: @oliverjprice
Contributors: OJP, JHH designed the study. JS, HA, LD acquired the data. JS, OJP and ESW interpreted the data. All authors were involved in drafting and critical revision of the manuscript; and final approval of the version to be published. OJP confirms full responsibility for the content of the manuscript, including data and analysis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Parkrun Research Board and Leeds Beckett University Research Ethics Committee (ethics ID: 34844). All participants provided written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. N/A.
References
- 1.Price OJ, Walsted ES, Hull JH. Understanding the total airway response to exercise: current perspectives and future challenges. Curr Opin Physiol 2019;10:185–92 https://doi.org/ 10.1016/j.cophys.2019.05.014 [DOI] [Google Scholar]
- 2.Hall A, Thomas M, Sandhu G, et al. Exercise-Induced laryngeal obstruction: a common and overlooked cause of exertional breathlessness. Br J Gen Pract 2016;66:e683–5. 10.3399/bjgp16X687001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price OJ, Ansley L, Bikov A, et al. The role of impulse oscillometry in detecting airway dysfunction in athletes. J Asthma 2016;53:1–7. 10.3109/02770903.2015.1063647 [DOI] [PubMed] [Google Scholar]
- 4.Price OJ, Hull JH, Ansley L, et al. Exercise-induced bronchoconstriction in athletes - A qualitative assessment of symptom perception. Respir Med 2016;120:36–43. 10.1016/j.rmed.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 5.Hull JH, Backer V, Gibson PG, et al. Laryngeal dysfunction: assessment and management for the clinician. Am J Respir Crit Care Med 2016;194:1062–72. 10.1164/rccm.201606-1249CI [DOI] [PubMed] [Google Scholar]
- 6.Halvorsen T, Walsted ES, Bucca C, et al. Inducible laryngeal obstruction: an official joint European respiratory Society and European Laryngological Society statement. Eur Respir J 2017;50:1–3. 10.1183/13993003.02221-2016 [DOI] [PubMed] [Google Scholar]
- 7.Johansson H, Norlander K, Berglund L, et al. Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax 2015;70:57–63. 10.1136/thoraxjnl-2014-205738 [DOI] [PubMed] [Google Scholar]
- 8.Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc 2013;45:2030–5. 10.1249/MSS.0b013e318298b19a [DOI] [PubMed] [Google Scholar]
- 9.Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest 1999;116:1676–82. 10.1378/chest.116.6.1676 [DOI] [PubMed] [Google Scholar]
- 10.Hull JH, Walsted ES, Pavitt MJ, et al. High prevalence of laryngeal obstruction during exercise in severe asthma. Am J Respir Crit Care Med 2019;199:538–42. 10.1164/rccm.201809-1734LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low K, Lau KK, Holmes P, et al. Abnormal vocal cord function in difficult-to-treat asthma. Am J Respir Crit Care Med 2011;184:50–6. 10.1164/rccm.201010-1604OC [DOI] [PubMed] [Google Scholar]
- 12.Heimdal J-H, Roksund OD, Halvorsen T, et al. Continuous laryngoscopy exercise test: a method for visualizing laryngeal dysfunction during exercise. Laryngoscope 2006;116:52–7. 10.1097/01.mlg.0000184528.16229.ba [DOI] [PubMed] [Google Scholar]
- 13.Rundell KW, Spiering BA. Inspiratory stridor in elite athletes. Chest 2003;123:468–74. 10.1378/chest.123.2.468 [DOI] [PubMed] [Google Scholar]
- 14.Price OJ, Tsakirides C, Gray M, et al. ACSM Preparticipation health screening guidelines: a UK university cohort perspective. Med Sci Sports Exerc 2019;51:1047–54. 10.1249/MSS.0000000000001868 [DOI] [PubMed] [Google Scholar]
- 15.Bonini M, Braido F, Baiardini I, et al. Aqua: allergy questionnaire for athletes. development and validation. Med Sci Sports Exerc 2009;41:1034–41. 10.1249/MSS.0b013e318193c663 [DOI] [PubMed] [Google Scholar]
- 16.Allen H, Hull JH, Backhouse SH, et al. The allergy questionnaire for athletes provides value in ruling-out exercise-induced bronchoconstriction. Allergy 2019;74:1794–6. 10.1111/all.13778 [DOI] [PubMed] [Google Scholar]
- 17.Yorke J, Russell A-M, Swigris J, et al. Assessment of dyspnea in asthma: validation of the Dyspnea-12. J Asthma 2011;48:602–8. 10.3109/02770903.2011.585412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American thoracic Society and European respiratory Society technical statement. Am J Respir Crit Care Med 2019;200:e70–88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dweik RA, Boggs PB, Erzurum SC, et al. An official ats clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602–15. 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price OJ, Ansley L, Hull JH. Diagnosing exercise-induced bronchoconstriction with eucapnic voluntary hyperpnea: is one test enough? J Allergy Clin Immunol Pract 2015;3:243–9. 10.1016/j.jaip.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 21.Price OJ, Ansley L, Levai IK, et al. Eucapnic voluntary hyperpnea testing in asymptomatic athletes. Am J Respir Crit Care Med 2016;193:1178–80. 10.1164/rccm.201510-1967LE [DOI] [PubMed] [Google Scholar]
- 22.Sarkar M, Madabhavi I, Niranjan N, et al. Auscultation of the respiratory system. Ann Thorac Med 2015;10:158–68. 10.4103/1817-1737.160831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurbis CG, Schild JA. Laryngeal changes during exercise and exercise-induced asthma. Ann Otol Rhinol Laryngol 1991;100:34–7. 10.1177/000348949110000106 [DOI] [PubMed] [Google Scholar]
- 24.Beaty MM, Wilson JS, Smith RJ. Laryngeal motion during exercise. Laryngoscope 1999;109:136–9. 10.1097/00005537-199901000-00026 [DOI] [PubMed] [Google Scholar]
- 25.Gotshall RW. Airway response during exercise and hyperpnoea in non-asthmatic and asthmatic individuals. Sports Med 2006;36:513–27. 10.2165/00007256-200636060-00005 [DOI] [PubMed] [Google Scholar]
- 26.Mellis C. Respiratory noises: how useful are they clinically? Pediatr Clin North Am 2009;56:1–17. 10.1016/j.pcl.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 27.Hull JH, Godbout K, Boulet L-P. Exercise-associated dyspnea and stridor: thinking beyond asthma. J Allergy Clin Immunol Pract 2020;8:2202–8. 10.1016/j.jaip.2020.01.057 [DOI] [PubMed] [Google Scholar]
- 28.Bonini M, Lapucci G, Petrelli G, et al. Predictive value of allergy and pulmonary function tests for the diagnosis of asthma in elite athletes. Allergy 2007;62:1166–70. 10.1111/j.1398-9995.2007.01503.x [DOI] [PubMed] [Google Scholar]
- 29.Jackson AR, Hull JH, Hopker JG, et al. Impact of detecting and treating exercise-induced bronchoconstriction in elite footballers. ERJ Open Res 2018;4:1–3. 10.1183/23120541.00122-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robson-Ansley P, Howatson G, Tallent J, et al. Prevalence of allergy and upper respiratory tract symptoms in runners of the London marathon. Med Sci Sports Exerc 2012;44:999–1004. 10.1249/MSS.0b013e318243253d [DOI] [PubMed] [Google Scholar]
- 31.Turcotte H, Langdeau J-B, Thibault G, et al. Prevalence of respiratory symptoms in an athlete population. Respir Med 2003;97:955–63. 10.1016/S0954-6111(03)00123-9 [DOI] [PubMed] [Google Scholar]
- 32.Parshall MB, Schwartzstein RM, Adams L, et al. An official American thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012;185:435–52. 10.1164/rccm.201111-2042ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hull JH, Ansley L, Robson-Ansley P, et al. Managing respiratory problems in athletes. Clin Med 2012;12:351–6. 10.7861/clinmedicine.12-4-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langdeau J-B, Day A, Turcotte H, et al. Gender differences in the prevalence of airway hyperresponsiveness and asthma in athletes. Respir Med 2009;103:401–6. 10.1016/j.rmed.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 35.Johansson H, Norlander K, Malinovschi A. Increased prevalence of exercise-induced airway symptoms - A five-year follow-up from adolescence to young adulthood. Respir Med 2019;154:76–81. 10.1016/j.rmed.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 36.Røksund OD, Maat RC, Heimdal JH, et al. Exercise induced dyspnea in the young. larynx as the bottleneck of the airways. Respir Med 2009;103:1911–8. 10.1016/j.rmed.2009.05.024 [DOI] [PubMed] [Google Scholar]
- 37.Christensen PM, Thomsen SF, Rasmussen N, et al. Exercise-Induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Otorhinolaryngol 2011;268:1313–9. 10.1007/s00405-011-1612-0 [DOI] [PubMed] [Google Scholar]
- 38.Ekström M, Schiöler L, Grønseth R, et al. Absolute values of lung function explain the sex difference in breathlessness in the general population. Eur Respir J 2017;49:1–3. 10.1183/13993003.02047-2016 [DOI] [PubMed] [Google Scholar]
- 39.Sheel AW, Dominelli PB, Molgat-Seon Y. Revisiting dysanapsis: sex-based differences in airways and the mechanics of breathing during exercise. Exp Physiol 2016;101:213–8. 10.1113/EP085366 [DOI] [PubMed] [Google Scholar]
- 40.Price OJ, Hull JH. Asthma in elite athletes: who cares? Clinical Pulmonary Medicine 2014;21:68–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2020-000618supp001.mp3 (2MB, mp3)