Abstract
Aims
To evaluate an immunohistochemical panel differentiating endometrial stromal sarcoma (ESS) from uterine leiomyosarcoma (ULMS) and leiomyoma (LM).
Methods
94 cases (28 ESS, 41 ULMS, 25 LM) were retrieved and arrayed. 10 immunomarkers (estrogen receptor (ER), progesterone receptor (PR), CD10, smooth muscle actin, desmin, h-caldesmon, transgelin, GEM, ASC1, stathmin1) were used. A predictive model was constructed and examined by receiver operating characteristics curve analysis to determine area under the curve (AUC).
Results
The combination of ER+/PR+/CD10+/GEM−/h-caldesmon−/transgelin− can predict ESS versus ULMS with AUC predictive value of 0.872 (95% CI 0.784 to 0.961, p<0.0001). The combination of ER+/PR+/CD10+/h-caldesmon−/transgelin− can predict low grade (LG) ESS from ‘LG’ ULMS with AUC predictive value of 0.914 (95% CI 0.832 to 0.995, p<0.0001). Finally, ULMS and ESS, including the LGs, were more likely to be stathmin1+ than LM.
Conclusions
Due to the different clinical course and management, adding novel antibodies (GEM, transgelin) to the well established immunohistochemistry panel seemed to be useful in distinguishing ESS from ULMS and LG ESS from ‘LG’ ULMS. Finally, stathmin1 expression could be of value in differentiating LM from uterine sarcomas.
INTRODUCTION
Endometrial stromal sarcomas (ESSs) and uterine leiomyosarcomas (ULMSs) represent the majority of uterine mesenchymal tumours.1,2 The new 2014 WHO classified ESS into low grade (LG) ESS, high grade ESS and undifferentiated endometrial sarcoma (UES).3 LG ESSs are composed of a proliferation of cells reminiscent of endometrial stromal cells in proliferative phase. They invade the myometrium in a characteristic fashion and have a high frequency of lymphatic invasion. ESSs are low-malignant tumours with an indolent course and late recurrences. The standard treatment recommendation of ESSs is generally surgery (total hysterectomy with salpingo-oophorectomy) followed by progestin therapy in selected cases with excellent survival outcomes. The prognosis largely depends on the extent of disease at the initial diagnosis with 5-year survival rates of 90–100% for stage I-II and 60–70% for stage III-IV On the other hand, UESs, less common tumours than LG-ESSs, are malignant tumours that lack stromal differentiation. They are aggressive and most women are dead of disease at 2 years after diagnosis. The primary treatment is surgery followed by radiation therapy for local control and chemotherapy for systemic control.4-7 ULMSs are also aggressive tumours with an overall poor prognosis, with 5-year survival of 15–25%. Tumour staging seems to be the most important prognostic factor, where stage I and II tumours have a better prognosis with 5-year survival of 25–70%. The main treatment of ULMS is surgery. Adjuvant therapy including chemotherapy/radiation therapy has been used to reduce recurrences, but its clinical efficacy is uncertain. Hormonal therapy is usually not used in patients with ULMS.8-10 Because of the distinct difference in prognosis, management and treatment between ESS and ULMS, the need for an accurate diagnosis is imperative.
Immunohistochemistry (IHC) is often employed as an adjunct to morphology in uterine mesenchymal lesions, particularly in cases with equivocal features. The routine immunomarker panel used by most surgical pathologist to distinguish ESS from ULMS consists of estrogen receptor (ER), progesterone receptor (PR), desmin, smooth muscle actin (SMA), h-caldesmon and CD10.11-19 Immunoprofiles such as ER+/PR+/desmin−/ SMA−/h-caldesmon−/CD10+ usually support the diagnosis of ESS.20 Unfortunately, however, there is much overlap and both entities can be immunoreactive to the same antibodies. New immunomarkers are thus needed to face this challenging problem.21 Novel gene expression signatures differentiating ESS from ULMS, conducted by Davidson et al,22 have recently emerged. The authors found that genes that were overexpressed in ESS were: SLCA7A10/ASC1, EFNB3, CCND2, ECEL1, ITM2A, NPW, PLAG1 and GCGR. Genes that were overexpressed in ULMS were: CDKN2A, FABP3, TAGLN, JPH2, GEM, NAV2 and RAB23.22 Of all of these proteins, transgelin (TAGLN) was the only antibody shown in a small number of uterine sarcomas and soft tissue sarcomas to have promising results.23,24
The aim of this study is to evaluate antibodies that are routinely used such as ER, PR, desmin, SMA, h-caldesmon and CD10, as well as four novel antibodies, including stathmin 1, ASC1, GEM and transgelin, in series of uterine sarcomas and leiomyomas with the goal of incorporating these markers in the current IHC panel.
MATERIALS AND METHODS
Case selection
After institutional review board approval was obtained, patients with a first time diagnosis of ESS, ULMS and leiomyoma (LM) were retrieved from the archives of the Departments of Pathology at the University of Southern California and the University of Texas at Dallas from 2005 to 2014. This was a retrospective study. Only cases with available paraffin-embedded tissue were included in the study. A total of 69 hysterectomy specimens with a diagnosis of uterine sarcomas were available; 28 cases were ESS (19 LG and 9 UES) and 41 cases were ULMS (28 ‘LG’ and 13 ‘high grade’). Even though there is no clear-cut consensus on grading ULMS, a tumour was classified as ‘LG ULMS’ when there was mild cytological atypia and mitotic activity <20/high power filed (HPF) and as ‘high grade ULMS’ when there was moderate to severe atypia and mitotic activity ≥20/10HPF.10 All histological diagnoses were made on hysterectomy specimens.
Tissue microarray building
For tumour microarray construction, paraffin-embedded tissues from these 94 cases were used as described previously.25 Briefly, morphologically representative regions were carefully selected on each individual paraffin-embedded block (donor blocks) and a core tissue biopsy of 1 mm was punched and transferred to a composite paraffin-embedded block (receiver block). To account for tumour heterogeneity and tissue loss, three core biopsies were taken from different areas of each tumour. One section was stained with H&E to evaluate the presence of the tumour by light microscopy. Whole sections of 10 normal cases of endometrium/myometrium were also included.
Immunohistochemistry
For immunohistochemical (IHC) analysis, 4 μm thick sections were deparaffinised with xylene, and washed with ethanol. Sections were cooled for 20 min then incubated 10 min with 3% H2O2 to quench endogenous peroxidase activity. Blocking was performed using serum-free protein block, DakoCytomation (Carpenteria, California, USA), for 30 min. Ten antibodies were used (ER, PR, SMA, desmin, h-caldesmon, CD10, GEM, solute carrier family 7 (ASC1), transgelin, and stathmin1). The conditions of these antibodies are summarised in table 1. The evaluation of the antibodies was performed twice by two independent expert gynaecological pathologists, blinded of the original diagnosis, separated by a 1-month period. The percentage was assessed as follows: 0%, ≤10%, 11–50%, 51–75% and 76–100%; and the intensity as absent (0), weak (1+), moderate (2+) and strong (3+). Whenever there were discrepancies among the immunostaining evaluation in any given case, the higher intensity was taken as the final score. The immunostains evaluation of the first and second assessments was reviewed and when there was a discrepancy in scoring, a consensus was reached. The staining score was obtained by multiplying percentage with intensity and this score was used for our statistics analysis.
Table 1.
Conditions and titrations of the 10 antibodies
| Antibodies | Company | Dilution | Condition | Control tissue |
|---|---|---|---|---|
| ER | ABCAM (SP1) | 1:100 | PH 8.0 20 min | Breast CA |
| Stathmin1 | GENE TEX | 1:500 | PH 8.0 20 min | Breast CA |
| GEM | NOVUS | 1:50 | PH 8.0 20 min | Normal skin |
| Transgelin | GENE TEX | 1:25 | PH 8.0 20 min | Head and neck SCC |
| PR | NOVOCASTRA | 1:200 | PH 6.0 20 min | Breast |
| CD10 | LEICA (56C6) | ready to use | PH 8.0 20 min | Tonsil |
| SMA | LEICA (αSM-1) | ready to use | PH 6.0 20 min | Ovary |
| Desmin | LEICA (DE-R-11) | ready to use | PH 8.0 20 min | striated muscle |
| h-caldesmon | Cell Marque (E89) | 1:50 | PH 6.0 20 min | striated muscle |
| ASC1 | Novus | 1:100 | PH 8.0 20 min | liver |
ASC1, solute carrier family 7; CA, California; CD10, cluster of differentiation 10; ER, estrogen receptor; PR, progesterone receptor; SMA, smooth muscle actin.
Statistical analysis
The primary interest of statistical comparison was to identify useful biomarkers to distinguish ESS from leiomyosarcoma (LMS). The secondary interest was to identify useful biomarkers to distinguish LG ESS from ‘LG’ ULMS. First, composition scores for the 10 biomarkers tested were determined based on IHC results (range 0–12) as intensity (range 0–3) multiplied by per cent expression (range 0–4). Then, expression patterns of the 10 biomarkers were examined, and the cut-off for composition score was determined based on the distribution of score across the histology subtype groups. Based on the cut-off score, univariate analysis with χ2 test or Fisher’s exact test was performed for each marker and the magnitude of statistical significance was expressed with OR and 95% CI. Sensitivity (Sen), specificity (Spe), positive predictive value (PPV), negative predictive value (NPV) and accuracy were also determined. Among statistically significant biomarkers, receiver operating characteristics (ROC) curve analysis was performed based on descending order, adding each biomarker one by one, and the statistical significance was expressed with area under the curve (AUC) and 95% CI. ΔAUC change was calculated as interval AUC increment change by adding one additional biomarker. p Values less than 0.05 were considered statistically significant (all, two-tailed tests). SPSS (V.12.0, Chicago, Illinois, USA) was used for the statistical analysis.
RESULTS
The patterns of expression were as follows: nuclear for ER, PR; cytoplasmic for SMA, desmin, h-caldesmon, transgelin and GEM, and nuclear/cytoplasmic for stathmin1 and ASC1. The normal myometrium was moderately positive for ER and PR, strongly positive for desmin, SMA, h-caldesmon, ASC1, transgelin and GEM, weakly positive for stathmin1, and negative for CD10. The normal endometrium was strongly positive for ER, PR and CD10, weakly positive for transgelin, GEM, ASC1 and SMA, and negative for desmin, h-caldesmon and stathmin 1.
The cases were distributed as follows: 28 cases were ESS (19 LGs and 9 UES), 41 cases were ULMS (28 LGs and 13 high grades) and 25 cases were LM (10 regular LM, 10 cellular LM, 5 atypical LM). Expressions of each of the 10 antibodies in our series are summarised in table 2.
Table 2.
Expressions of the 10 antibodies in 94 cases of leiomyoma, ESS and ULMS
| Leiomyoma | Uterine leiomyosarcoma |
Endometrial stromal sarcoma |
p Value | |||
|---|---|---|---|---|---|---|
| Low grade | High grade | Low grade | High grade | |||
| Total | n=25 | n=28 | n=13 | n=19 | n=9 | |
| ER | % | % | % | % | % | <0.0001 |
| Score 0 | 12.0 | 71.4 | 92.3 | 21.1 | 55.6 | |
| Score 1–7 | 12.0 | 25.0 | 7.7 | 26.3 | 11.1 | |
| Score 8–12 | 76.0 | 3.6 | 0 | 52.6 | 3 (33.3) | |
| PR | <0.0001 | |||||
| Score 0 | 8.0 | 57.1 | 100 | 26.3 | 66.7 | |
| Score 1–7 | 12.0 | 28.6 | 0 | 0 | 11.1 | |
| Score 8–12 | 80.0 | 14.3 | 0 | 73.7 | 22.2 | |
| Desmin | 0.004 | |||||
| Score 0 | 4.0 | 39.3 | 38.5 | 55.6 | 37.5 | |
| Score 1–7 | 0 | 0 | 7.7 | 11.1 | 12.5 | |
| Score 8–12 | 96.0 | 60.7 | 53.8 | 33.3 | 50.0 | |
| SMA* | 0.001 | |||||
| Score 0 | 0 | 18.5 | 38.5 | 28.8 | 55.6 | |
| Score 1–7 | 4.0 | 0 | 0 | 22.2 | 11.1 | |
| Score 8–12 | 96.0 | 81.5%) | 61.5 | 50.0 | 33.3 | |
| CD10** | 0.005 | |||||
| Score 0 | 64.0 | 60.7 | 38.5 | 27.8 | 33.3 | |
| Score 1–7 | 32.0 | 17.9 | 30.8 | 11.1 | 11.1 | |
| Score 8–12 | 4.0 | 21.4 | 30.8 | 61.1 | 55.6 | |
| h-caldesmon* | 0.005 | |||||
| Score 0 | 8.0% | 25.9 | 23.1 | 55.6 | 55.6 | |
| Score 1–7 | 4.0 | 3.7 | 15.4 | 16.7 | 0 | |
| Score 8–12 | 88.0 | 70.4 | 61.5 | 27.8 | 44.4 | |
| Transgelin* | 0.036 | |||||
| Score 0 | 0 | 10.7 | 8.3 | 11.1 | 11.1 | |
| Score 1–7 | 0 | 17.9 | 8.3 | 33.3 | 0 | |
| Score 8–12 | 100 | 71.4 | 83.3 | 55.6 | 88.9 | |
| GEM*** | 0.056 | |||||
| Score 0 | 20.0 | 44.4 | 25.0 | 44.4 | 11.1 | |
| Score 1–7 | 28.0 | 25.9 | 0 | 33.3 | 33.3 | |
| Score 8–12 | 52.0 | 29.6 | 75.0 | 22.2 | 55.6 | |
| ASC1** | 0.30 | |||||
| Score 0 | 0 | 7.1 | 0 | 5.3 | 0 | |
| Score 1–7 | 20.0 | 17.9 | 0 | 5.3 | 0 | |
| Score 8–12 | 80.0 | 75.0 | 100 | 89.5 | 100 | |
| Stathmin1* | 0.001 | |||||
| Score 0 | 8.0 | 14.8 | 0 | 11.1 | 0 | |
| Score 1–7 | 52.0 | 11.1 | 0 | 22.2 | 0 | |
| Score 8–12 | 40.0 | 74.1 | 100 | 66.7 | 100 | |
Number (%) is shown. Score represents composite score based on intensity and % expression. χ2 test for p values
2 missing
1 missing
3 missing.
CD10, cluster of differentiation 10; ER, estrogen receptor; ESS, endometrial stromal sarcoma; GEM, Guanosine-5′- triphosphate (GTP)-binding protein overexpressed in skeletal muscle; and ASC1 solute carrier family 7; PR, progesterone receptor; SMA, smooth muscle actin; ULMS, uterine leiomyosarcoma.
Examples of expressions of different immunomarkers are illustrated in figures 1A-D and 2A-F. The Spe, Sen, PPV and NPV of each of the 10 immunomarkers to distinguish ESS from ULMS are illustrated in table 3. The three most specific immunomarkers to distinguish ESS from ULMS in descending order were; ER (Spe 97.6%, Sen 46.4%, PPV 92.9% and NPV 72.7%), PR (Spe 90.2%, Sen 57.1%, PPV 80% and NPV 75.5%) and CD10 (Spe 75.6%, Sen 59.3%, PPV 61.5% and NPV 73.8%). The three most sensitive immunomarkers in descending order were ASC1 (Sen 92.9%, Spe 17.5%, PPV 44.1% and NPV 77.8%), GEM (Sen 88.9%, Spe 35.9%, PPV 49% and NPV 82.4%) and h-caldesmon (Sen 70.4%, Spe 60%, PPV 54.3% and NPV 75%).
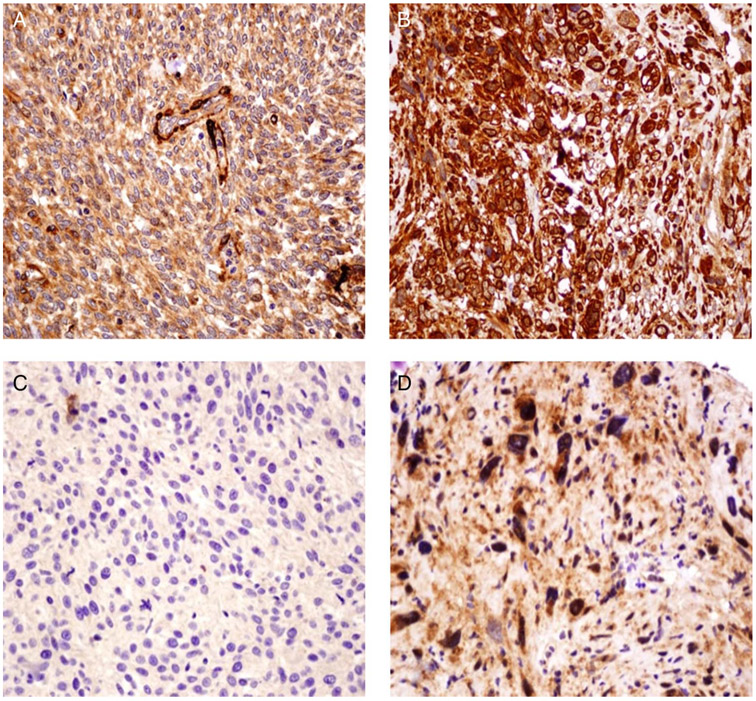
Figure 1.
(A). Negative expression of transgelin in a case of endometrial stromal sarcoma (ESS) (×40). (B) A case of uterine leiomyosarcoma (ULMS) strongly positive for transgelin. The expression was cytoplasmic in pattern. (C) high grade endometrial stromal sarcoma (HGESS) negative for Guanosine-5′- triphosphate (GTP)-binding protein overexpressed in skeletal muscle (GEM). (D) ‘HG’ leiomyosarcoma strongly positive for GEM. GEM was expressed in a cytoplasmic pattern.
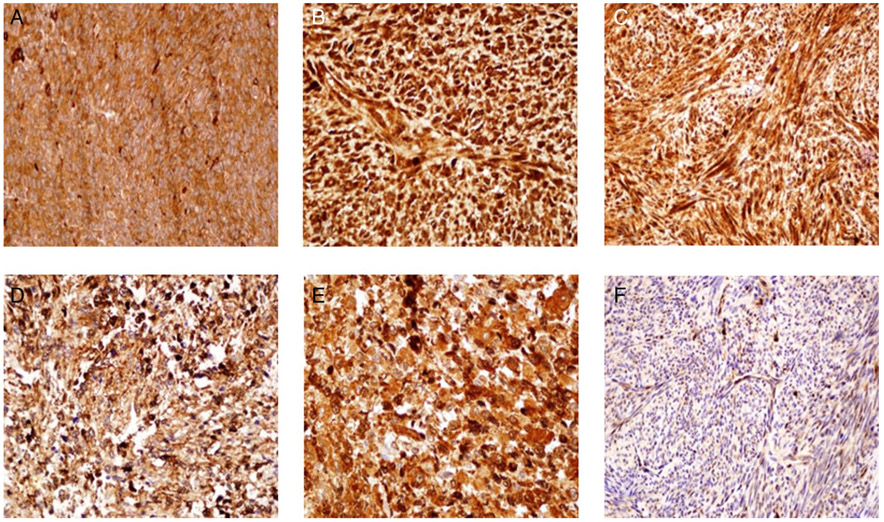
Figure 2.
Positive expression of ASC1 in (A) low grade (LG) endometrial stromal sarcoma (ESS), (B) ‘LG’ uterine leiomyosarcoma (ULMS), (C) leiomyoma (LM). ASC1 was strongly expressed in cytoplasmic and nuclear patterns. Positive expression of stathmin1 in (D) in ESS, (E) leiomyosarcoma and (F) LM. Stathmin1 was strongly expressed in cytoplasmic and nuclear patterns.
Table 3.
Comparison of biomarker expressions between all ESS and ULMS
| Score | (%) | OR (95% CI) | p Value | Sen (%) | Spe (%) | PPV (%) | NPV (%) | Acc (%) | |
|---|---|---|---|---|---|---|---|---|---|
| (%) ER | <0.0001 | ||||||||
| LMS | ≥8 | 27.30 | 1 | ||||||
| ESS | ≥8 | 92.90 | 34.7 (4.17 to 288) | 46.40 | 97.60 | 92.90 | 72.70 | 76.80 | |
| PR | <0.0001 | ||||||||
| LMS | ≥8 | 24.50 | 1 | ||||||
| ESS | ≥8 | 80.00 | 12.3 (3.45 to 44.1) | 57.10 | 90.20 | 80.00 | 75.50 | 76.80 | |
| CD10 | 0.005 | ||||||||
| LMS | ≥8 | 26.20 | 1 | ||||||
| ESS | ≥8 | 61.50 | 4.51 (1.58 to 12.9) | 59.30 | 75.60 | 61.50 | 73.80 | 69.10 | |
| GEM | 0.043 | ||||||||
| LMS | ≤8 | 17.60 | 1 | ||||||
| ESS | ≤8 | 49.00 | 4.48 (1.14 to 17.6) | 88.90 | 35.90 | 49.00 | 82.40 | 57.60 | |
| h-caldesmon | 0.024 | ||||||||
| LMS | ≤8 | 25.00 | 1 | ||||||
| ESS | ≤8 | 54.30 | 3.56 (1.26 to 10.1) | 70.40 | 60.00 | 54.30 | 75.00 | 64.20 | |
| Trangelin | 0.044 | ||||||||
| LMS | ≤8 | 28.90 | 1 | ||||||
| ESS | ≤8 | 55.20 | 3.02 (1.10 to 8.32) | 59.30 | 69.20 | 57.10 | 71.10 | 65.20 | |
| SMA | 0.045 | ||||||||
| LMS | ≤8 | 30.00 | 1 | ||||||
| ESS | ≤8 | 55.60 | 2.92 (1.06 to 8.06) | 55.60 | 70.00 | 55.60 | 70.00 | 64.20 | |
| ASC1 | 0.29 | ||||||||
| LMS | ≥8 | 22.20 | 1 | ||||||
| ESS | ≥8 | 44.10 | 2.76 (0.53 to 14.4) | 92.90 | 17.50 | 44.10 | 77.80 | 48.50 | |
| Desmin | 0.21 | ||||||||
| LMS | ≤8 | 20.30 | 1 | ||||||
| ESS | ≤8 | 47.10 | 2.04 (0.75 to 5.57) | 61.50 | 56.10 | 47.10 | 69.70 | 58.20 | |
| Stathmin1 | 0.43 | ||||||||
| LMS | ≤8 | 37.00 | 1 | ||||||
| ESS | ≤8 | 47.60 | 1.55 (0.55 to 4.41) | 37.00 | 72.50 | 47.60% | 63.00 | 58.20 |
χ2 test for p values. Score represents composition score based on intensity and % expression.
Acc, accuracy; CD10, cluster of differentiation 10; ER, estrogen receptor; ESS, endometrial stromal sarcoma; GEM, Guanosine-5′- triphosphate (GTP)-binding protein overexpressed in skeletal muscle; and ASC1, solute carrier family 7; LMS, leiomyosarcoma; NPV, negative predictive value; PPV, positive predictive value; PR, progesterone receptor; Sen, sensitivity; SMA, smooth muscle actin; Spe, specificity; ULMS, uterine leiomyosarcoma.
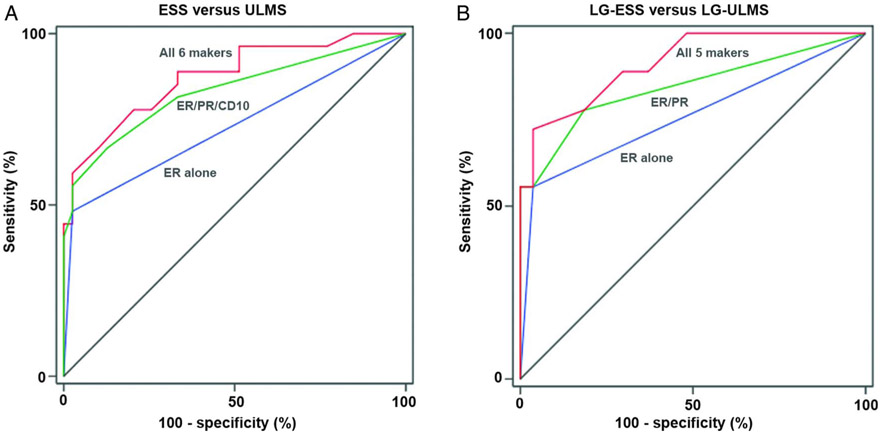
Figure 3 A shows the ROC curves with ER+/PR+/CD10+/GEM−/h-caldesmon−/transgelin− being the best combination of markers for predicting ESS from ULMS with AUC 0.872 (95% CI 0.784 to 0.961, p<0.0001). The second best combination was ER+/PR+/CD10+/GEM−/h-caldesmon− with AUC 0.846 (95% CI 0.741 to 0.950, p<0.0001) (table 4).
Figure 3.
Receiver operating characteristics curves for prediction of endometrial stromal sarcoma (ESS) and low grade (LG) ESS. (A) Comparison of ESS (n=28) and uterine leiomyosarcoma (ULMS) (n=41). All 6 markers include ER, PR, CD10, GEM, h-caldesmon and transgelin. (B) Comparison of LG ESS (n=19) and ‘LG’ ULMS (n=28). All 5 markers include ER, PR, CD10, h-caldesmon and transgelin. Abbreviations: LG-ESS, low-grade endometrial stromal sarcoma; LG-ULMS, low-grade uterine leiomyosarcoma; ER, estrogen receptor; PR, progesterone receptor; and CD10, cluster of differentiation 10.
Table 4.
Predictive model of ESS over ULMS
| Combination of immunomarker expressions |
AUC (95% CI) | ΔAUC change |
p Value |
|---|---|---|---|
| ER alone | 0.728 (0.595 to 0.861) | 0.002 | |
| ER+PR | 0.797 (0.677 to 0.917) | 0.069 | <0.0001 |
| ER+PR+CD10 | 0.831 (0.722 to 0.939) | 0.034 | <0.0001 |
| ER+PR+CD10+GEM | 0.823 (0.706 to 0.940) | −0.008 | <0.0001 |
| ER+PR+CD10+GEM+h-Caldesmon | 0.846 (0.741 to 0.950) | 0.023 | <0.0001 |
| ER+PR+CD10+GEM+h-Caldesmon+Transgelin | 0.872 (0.784 to 0.961) | 0.026 | <0.0001 |
Using the six biomarkers shown to be significant in univariate analysis in table 3, receiver operating characteristics curve analysis was performed based on the magnitude of significance (OR) for ESS over ULMS. Delta AUC change represents interval AUC increment change by adding one additional biomarker.
AUC, area under the curve; C-cal, C-caldesmon; CD10, cluster of differentiation 10; ER, estrogen receptor; ESS, endometrial stromal sarcoma; GEM, Guanosine-5′- triphosphate (GTP)-binding protein overexpressed in skeletal muscle; LMS, leiomyosarcoma; PR, progesterone receptor; ULMS, uterine leiomyosarcoma.
On the other hand, when we evaluated the Sen, Spe, PPV and NPV in the 47 LG cases only (19 ESS and 28 ULMS), it appeared that the most specific markers to distinguish LG ESS from ‘LG’ ULMS in descending order were ER (Spe 96.4%, Sen 52.6%, PPV 90.9% and NPV 75%), PR (Spe 85.7%, Sen 73.7%, PPV 77.8% and NPV 82.8%) and CD10 (Spe 78.6%, Sen 61.1%, PPV 64.7% and NPV 75.9%). The most sensitive markers in descending order were GEM (Sen 94.4%, Spe 22.2%, PPV 44.7% and NPV 85.7%), ASC1 (Sen 89.5%, Spe 25%, PPV 44.7% and NPV 77.8%) and PR (Sen 73.7%, Spe 85.7%, PPV 77.8% and NPV 82.8%) (table 5).
Table 5.
Comparison of biomarker expressions between LG ESS and ‘LG’ ULMS
| Score | (%) | OR (95% CI) | p Value | Sen (%) | Spe (%) | PPV (%) | NPV (%) | Acc (%) | |
|---|---|---|---|---|---|---|---|---|---|
| ER | <0.0001 | ||||||||
| ‘LG’ ULMS | ≥8 | 25.00 | 1 | ||||||
| LG ESS | ≥8 | 90.90 | 30.0 (3.36 to 268) | 52.60 | 96.40 | 90.90 | 75.00 | 78.70 | |
| PR | <0.0001 | ||||||||
| ‘LG’ ULMS | ≥8 | 17.20 | 1 | ||||||
| LG ESS | ≥8 | 77.80 | 16.8 (3.86 to 73.1) | 73.70 | 85.70 | 77.80 | 82.80 | 80.90 | |
| CD10 | 0.012 | ||||||||
| ‘LG’ ULMS | ≥8 | 24.10 | 1 | ||||||
| LG ESS | ≥8 | 64.70 | 5.76 (1.56 to 21.3) | 61.10 | 78.60 | 64.70 | 75.90 | 71.70 | |
| GEM | 0.22 | ||||||||
| ‘LG ULMS | ≤8 | 14.30 | 1 | ||||||
| LG ESS | ≤8 | 44.70 | 4.86 (0.53 to 44.3) | 94.40 | 22.20 | 44.70 | 85.70 | 51.10 | |
| C-caldesmon | 0.033 | ||||||||
| ‘LG’ ULMS | ≤8 | 22.70 | 1 | ||||||
| LG ESS | ≤8 | 56.50 | 4.42 (1.21 to 16.1) | 72.20 | 63.00 | 56.50 | 77.30 | 66.70 | |
| Transgelin | 0.034 | ||||||||
| ‘LG’ ULMS | ≤8 | 24.00 | 1 | ||||||
| LG ESS | ≤8 | 57.10 | 4.22 (1.20 to 14.9) | 66.70 | 67.90 | 57.10 | 76.00 | 67.40 | |
| SMA | 0.11 | ||||||||
| ‘LG’ ULMS | ≤8 | 30.00 | 1 | ||||||
| LG ESS | ≤8 | 60.00 | 3.50 (0.96 to 12.8) | 50.00 | 77.80 | 60.00 | 70.00 | 66.70 | |
| ASC1 | 0.28 | ||||||||
| ‘LG’ ULMS | ≥8 | 22.20 | 1 | ||||||
| LG ESS | ≥8 | 44.70 | 2.83 (0.52 to 15.5) | 89.50 | 25.00 | 44.70 | 77.80 | 51.10 | |
| Desmin | 0.14 | ||||||||
| ‘LG’ ULMS | ≤8 | 27.30 | 1 | ||||||
| LG ESS | ≤8 | 50.00 | 2.67 (0.78 to 9.15) | 66.70 | 57.10 | 50.00 | 72.70 | 60.90 | |
| Stathmin1 | 0.76 | ||||||||
| ‘LG’ ULMS | ≤8 | 37.00 | 1 | ||||||
| LG ESS | ≤8 | 44.40 | 1.36 (0.40 to 4.58) | 44.40 | 63.00% | 44.40 | 63.00 | 55.60 |
χ2 test for p values. Score represents composition score based on intensity and % expression.
Acc, accuracy; CD10, cluster of differentiation 10; ER, estrogen receptor; GEM, Guanosine-5′- triphosphate (GTP)-binding protein overexpressed in skeletal muscle; and ASC1, solute carrier family 7; LG ESS, low-grade endometrial stromal sarcoma; NPV, negative predictive value; PPV, positive predictive value; PR, progesterone receptor; Sen, sensitivity; SMA, smooth muscle actin; Spe, specificity; ULMS, uterine leiomyosarcoma.
Figure 3B shows the ROC curves with ER+/PR+/CD10+/h-caldesmon−/transgelin− to be the best combinations of markers to predict LG ESS from ‘LG’ ULMS with AUC 0.914 (95% CI 0.832 to 0.995, p<0.0001). The second best combination of makers would be ER+/ PR+/CD10+/ h-caldesmon− with AUC 0.903 (95% CI 0.816 to 0.991, p<0.0001) (table 6).
Table 6.
Predictive model of LG ESS over ‘LG’ ULMS
| Combination of antibodies expressions |
AUC (95% CI) | ΔAUC change |
p Value |
|---|---|---|---|
| ER alone | 0.759 (0.602 to 0.916) | 0.004 | |
| ER+PR | 0.844 (0.713 to 0.974) | 0.085 | <0.0001 |
| ER+PR+CD10 | 0.877 (0.763 to 0.990) | 0.033 | <0.0001 |
| ER+PR+CD10+h-Cal | 0.903 (0.816 to 0.991) | 0.026 | <0.0001 |
| ER+PR+CD10+h-Cal+Trang | 0.914 (0.832 to 0.995) | 0.011 | <0.0001 |
Using the five significant biomarkers shown to be significant in univariate analysis in table 5, receiver operating characteristics curve analysis was performed based on the magnitude of significance (OR) for LG ESS over ‘LG’ ULMS. Delta AUC change represents interval AUC change by adding one additional biomarker.
AUC, area under the curve; h-Cal, h-caldesmon; CD10, cluster of differentiation 10; ER, estrogen receptor; LG ESS, low-grade endometrial stromal sarcoma; PR, progesterone receptor; Trang, Transgelin; ULMS, uterine leiomyosarcoma.
Finally, we evaluated the expression of stathmin1 in all 25 cases of LM and 69 cases of ESS and ULMS. The data shows that sarcoma cases (ESS and ULMS) were more likely to express stathim1 than LM cases (ULMS 82.5%, ESS 77.8% and LM 40.0%, p=0.001). Also, stathmin 1 was valid in differentiating LM from LG ESS and ‘LG’ ULMS (n=45) (71.1% vs 40.0%, p=0.005).
DISCUSSION
Uterine sarcomas comprise less than 10% of uterine malignancies, with ULMS and ESS constituting the majority of cases.1,2 ULMS and ESS can show LG and high grade features, a factor that can play a role in histological diagnosis, but is not necessarily used for classification.6,8,9 Distinguishing ESS from ULMS, as well as LG tumours and LM from LG uterine sarcomas (ESS and ULMS), is usually straightforward, particularly on a hysterectomy specimen. But distinguishing ESS from ULMS can be very challenging on core biopsies or small excisional biopsies. Therefore, IHC must fulfil this purpose. Transgelin is an actin-binding protein of the calponin family and correlates with smooth muscle differentiation. Transgelin was found to be overexpressed in ULMS. A study was recently conducted by Robin et al.23 to determine the value of transgelin as a smooth muscle immunomarker in soft tissue tumours, including high numbers of LMS cases. The authors found that, unlike h-caldesmon and desmin, which lack Sen to distinguish LMS from other soft tissue tumours (50% and 45%, respectively), transgelin emerged as the best diagnostic marker with high Sen (83%) and high Spe (83%). However, the authors failed to mention how many of those LMS cases were from uterine origin. A very recent small study by Tawfik et al using transgelin antibody on 13 cases of ESS and 8 of uterine LMS found that transgelin was 100% sensitive and specific in distinguishing LMS from ESS.24 However in our series, transgelin seemed to have a more modest Sen and Spe of 59.3% and 69.2%, respectively. When distinguishing LG ULMS from LG ESS, transgelin proved to be 66.7% specific and 67.9% sensitive. The difference in results between our series and that above might due to our larger series of cases (69 vs 21) and the differing scoring systems used.
GEM is a Guanosine-5′- triphosphate (GTP)-binding mitogen-induced T cell protein. It is located on 8q22.1 and it is overexpressed in skeletal muscle.26 It has been suggested that GEM might be a regulatory protein that participates in receptor mediated signal transduction at the plasma membrane.27 The role of GEM in distinguishing ESS from ULMS has not yet been explored. In our series GEM proved to be a very sensitive immunomarker in distinguishing ESS from ULMS and also LG ESS from ‘LG’ ULMS (88.9% and 94.4%, respectively). However, GEM was lacking Spe in both cases.
The traditional routine immunomarker panel used by most surgical pathologists to distinguish ESS from ULMS consists of ER, PR, desmin, SMA, h-caldesmon and CD10, with the immunoprofile ER+/PR+/desmin−/ SMA−/ h-caldesmon−/ CD10+ supporting the diagnosis of ESS.20 However, in ULMS, wide ranges of ER and PR frequencies have been reported, varying from 20% to 87% for ER and 17% to 73% for PR.11-14 Even though desmin and SMA are usually expressed in ULMS, they have also been reported to be positive in 10–40% of ESS cases.28 Furthermore, positivity for CD10 ranged from 75% to 100% of ESS cases and from 0% to 60% of ULMS cases.15,19,29 Finally, even though h-caldesmon is very specific for ULMS, its Sen is only 50%.23 High grade or more undifferentiated uterine sarcomas might lose expression of some of these proteins, making use of these immunomarkers somewhat limited. In our series, we found that only a few of these individual markers were either sensitive or specific in distinguishing ESS from ULMS; these included ER (Spe 97.6%, Sen 46.4%), PR (Spe 90.2%, Sen 57.1%), CD10 (Spe 75.6%, Sen 59.3%) and h-caldesmon (Sen 70.4%, Spe 60%).
As a general rule, in making an accurate diagnosis with high predictive value no one immunomarker is sensitive and specific enough to stand on its own. Therefore, surgical pathologists normally run an IHC panel to reach a diagnosis in their challenging cases. We found that a panel consisting of ER/PR/CD10/GEM/h-caldesmon was the best predictive panel in distinguishing ESS from ULMS; tumour cells that are ER, PR, CD10 positive and GEM, trangelin negative are most likely to be ESS. The best panel with high PV for a tumour to be LG ESS rather than ‘LG’ ULMS would be ER/PR/CD10/h-caldesmon/transgelin; tumours ER, PR, CD10 positive and h-caldesmon and transgelin negative are more likely to be LG ESS than ‘LG’ LMS. This is a crucial distinction due to the differing prognosis and treatment, as LG ESSs have a better prognosis and are hormone-responsive, while ‘LG’ LMS are more aggressive, hormone-insensitive tumours with a questionable response to adjuvant therapy. ASC1 or SLCA7A10 (solute carrier family 7) is a 523 amino acid protein that has been found to be overexpressed in ESS.23 However, in our series ASC1 failed to show any PV in distinguishing uterine sarcomas.
When high-grade uterine sarcomas exhibit a high mitotic rate and severe atypia, distinguishing them from LM is straightforward. LG tumours, however, can create a major diagnostic challenge, especially on core biopsies and small samples, where making the right diagnosis has a profound effect on patient management.18 To distinguish LM from LG ESS and ‘LG’ ULMS, there is no established reliable immunomarker. The PI3k-AKT signalling pathway has been shown to play a critical role in the development of LMS and other malignancies.30,31 Stahmin1 is a candidate oncogene and seems to be a marker for the PI3 K pathway activation.32 Stathmin1 is a major regulator of the microtubule dynamics and plays a role in regulating cell division, motility and migration. In studying 25 cases of LM, of which 6 were atypical and 4 were cellular, stahmin1 seemed to be a good marker to distinguish ULMS and ESS from LMs. If a tumour expressed stahmin1, it was 36 times more likely to be malignant. All atypical and cellular LMs were negative for stahmin1. Even though our series has a limited number of atypical LMs, which create the most diagnostic challenges, these results are very promising and should be confirmed by larger studies.
The major shortcoming of our study is the lack of comparison between our results and others in the literature. The only published data are on transgelin; other antibodies are only mentioned in two very short abstracts with small numbers of cases (unpublished work). Therefore, our data is very promising and should be confirmed by others before being put into general use.
In summary, combining novel antibodies (GEM and transgelin) with the traditional markers (ER, PR, CD10, h-caldesmon) showed promising results in distinguishing ESS from ULMS. Furthermore, the novel antibody stahmin1 deserves future validation in differentiating LM from ESS and ULMS.
Take home messages.
It is important to differentiate endometrial stromal sarcoma (ESS) from uterine leiomyosarcoma (ULMS).
Adding novel antibodies (GEM, transgelin) to the established panel (estrogen receptor, progesterone receptor, CD10, smooth muscle actin, desmin, h-caldesmon) seemed to distinguish ESS from ULMS and low grade (LG) ESS from ‘LG’ ULMS which is vital due to their different clinical course and management.
Acknowledgements
The authors thank Dr Thomas Chong for his help in doing the figures.
Footnotes
Competing interests None declared.
Patient consent Obtained.
Ethics approval IRB.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Brooks SE, Zhan M, Cote T, et al. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol Oncol 2004;93:204–8. [DOI] [PubMed] [Google Scholar]
- 2.Abeler VM, Royne O, Thoresen S, et al. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009;54:355–64. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Carcangiu ML, Herrington CS, et al. Mesenchymal tumours WHO classification of tumours of female reproductive organs. 4th edn. Lyon: IARC Press, vol 6, 2014. [Google Scholar]
- 4.Koivisto-Korander R, Martinsen JI, Weiderpass E, et al. Incidence of uterine leiomyosarcoma and endometrial stromal sarcoma in Nordic countries: results from NORDCAN and NOCCA databases. Maturitas 2012;72:56–60. [DOI] [PubMed] [Google Scholar]
- 5.Somoye G, Lawton H, Havenga S. Endometrial stromal sarcoma; experience from a district hospital and literature review. Eur J Gynaecol Oncol 2009;30:664–7. [PubMed] [Google Scholar]
- 6.Xue WC, Cheung ANY. Endometrial stromal sarcoma of uterus. Best Pract Res Clin Obstet Gynaecol 2011;25:719–32. [DOI] [PubMed] [Google Scholar]
- 7.El-Khalfaoui K, du Bois A, Heitz F, et al. Current and future options in the management and treatment of uterine sarcoma. Ther Adv Med Oncol 2014;6:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amant F, Floquet A, Friedlander M, et al. Gynecologic cancer intergroup (GCIG) consensus review for endometrial stromal sarcoma. Int J Gynecol Cancer 2014;24:S67–72. [DOI] [PubMed] [Google Scholar]
- 9.Hensley ML, Barrette BA, Baumann K, et al. Gynecologic cancer intergroup (GCIG) consensus review. Uterine and ovarian leiomyosarcomas. Int J Gynecol Cancer 2014;24:S61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang WL, Soslow R, Hensley M, et al. Histopathologic prognostic factors in stage I leiomyosarcoma of the uterus: a detail analysis of 27 cases. Am J Surg Pathol 2011;35:522–9. [DOI] [PubMed] [Google Scholar]
- 11.Leitao MM, Soslow RA, Nonaka D, et al. Tissue microarray immunohistochemical expression of estrogen, progesterone, and androgen receptors in uterine leiomyomata and leiomyosarcoma. Cancer 2004;101:1455–62. [DOI] [PubMed] [Google Scholar]
- 12.Reich O, Regauer S, Urdl W, et al. Expression of estrogen and progesterone receptors in low-grade endometrial stromal sarcomas. Br J Cancer 2000;82:1030–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley TW, Borden EC, Goldblum JR. Estrogen and progesterone receptor expression in uterine and extrauterine leiomyosarcomas. An immunohistochemical study. Appl Immunohistochem Mol Morphol 2004;12:338–41. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho JC, Thomas DG, Lucas DR. Cluster analysis of immunohistochemical markers in leiomyosarcoma delineates specific anatomic and gender subgroups. Cancer 2009;115:4186–95. [DOI] [PubMed] [Google Scholar]
- 15.Chu PG, Arber DA, Weiss LM, et al. Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: An immunohistochemical comparison of 34 cases. Mod Pathol 2001;14:465–71. [DOI] [PubMed] [Google Scholar]
- 16.Agoff NS, Grieco VS, Garcia R, et al. Immunohistochemical distinction of endometrial stromal sarcoma and cellular leiomyoma. Appl Immunohist Mol Morphol 2001;9:164–9. [DOI] [PubMed] [Google Scholar]
- 17.McCluggage WG, Sumathi VP, Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and endometrial stromal neoplasms. Histopathology 2001;39:273–8. [DOI] [PubMed] [Google Scholar]
- 18.Rush DS, Tan J, Baergen RN, et al. h-Caldesmon, a novel smooth muscle-specific antibody, distinguishes between cellular leiomyoma and endometrial stromal sarcoma. Am J Surg Pathol 2001;25:253–8. [DOI] [PubMed] [Google Scholar]
- 19.Nucci MR, O’Connell JT, Huettner PC, et al. h-Caldesmon expression effectively distinguishes endometrial stromal tumors from uterine smooth muscle tumors. Am J Surg Pathol 2001;25:455–63. [DOI] [PubMed] [Google Scholar]
- 20.Mittal K, Sowslow R, McCluggage WG. Application of immunohistochemistry to gynecologic pathology. Arch Pathol Lab Med 2008;132:402–23. [DOI] [PubMed] [Google Scholar]
- 21.Oliva E, Young RH, Amin MB, et al. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: a study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol 2002;26:403–12. [DOI] [PubMed] [Google Scholar]
- 22.Davidson B, Abeler VM, Hellesylt E, et al. Gene expression signatures differentiate uterine endometrial stromal sarcoma from leiomyosarcoma. Gynecol Oncol 2013;128:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robin YM, Penel N, Perot G, et al. Transgelin is a novel marker of smooth muscle differentiation that improves diagnostic accuracy of leiomyosarcomas: a comparative immunohistochemical reappraisal of myogenic markers in 900 soft tissue tumors. Mod Pathol 2013;26:502–10. [DOI] [PubMed] [Google Scholar]
- 24.Tawfik O, Rao D, Nothnick WB, et al. Transgelin, a novel marker of smooth muscle differentiation, effectively distinguishes endometrial stromal tumors from uterine smooth muscle tumors. IJGORMR 2014;1:26–31. [PMC free article] [PubMed] [Google Scholar]
- 25.Mhawech P, Greloz V, Assaly M, Herrmann F.Immunohistochemical expression of 14-3-3 sigma protein in human urological and gynecological tumors using a multi-tumor microarray analysis. Pathol Int 2005;55:77–82. [DOI] [PubMed] [Google Scholar]
- 26.Beguin P, Nagashima K, Gonoi T, et al. Regulation of Ca(2+) channel expression at the cell surface by the small G-protein kir/Gem. Nature 2001;411:701–6. [DOI] [PubMed] [Google Scholar]
- 27.Piddini E, Schmid JA, de Martin R, et al. The Ras-like GTPase Gem is involved in cell shape remodelling and interacts with the novel kinesin-like protein KIF9. EMBO J 2001;20:4076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franquemont DW, Frierson HF Jr, Mills SE. An immunohistochemical study of normal endometrial stroma and endometrial stromal neoplasms. Evidence for smooth muscle differentiation. Am J Surg Pathol 1991;15:861–70. [DOI] [PubMed] [Google Scholar]
- 29.Ammant F, Schurmans K, Steenkiste E, et al. Immunohistochemical expression of CD10 antigen in uterine adenosarcoma. Int J Gynecol Cancer 2004;14:1118–21. [DOI] [PubMed] [Google Scholar]
- 30.Hernando E, Charytonowicz E, Dudas ME, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med 2007;13:748–53. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe A, Suzuki H, Yokobori T, et al. Stathmin1 regulates p27 expression, proliferation and drug resistance, resulting in poor clinical prognosis in cholangiocarcinoma. Cancer Sci 2014;105:690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karst AM, Levanon K, Duraisamy S, et al. Stathmin1, a marker of PI3K pathway activation and regulator of microtubule dynamics, is expressed in early pelvic serous carcinomas. Gynecol Oncol 2011;123:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]