Abstract
Objective
To validate the revised 2018 International Federation of Gynecology and Obstetrics (FIGO) staging system for cervical cancer, with a particular focus on stage IB and stage III disease.
Methods
Two retrospective cohort studies were conducted using The Surveillance, Epidemiology, and End Results Program between 1988 and 2014. The stage IB cohort consisted of node-negative FIGO stage IB1 (tumor size <2 cm), IB2 (2–3.9 cm), and IB3 (≥4 cm) cervical cancer. The stage III cohort consisted of FIGO stage IIIA, IIIB, and stage IIIC1 (any pelvic nodal metastasis) cervical cancer. Multivariable analysis was performed for cause-specific survival based on cancer stage.
Results
In the stage IB cohort (n = 8909), stage IB1 tumors were more likely to be adenocarcinoma and low-grade compared to other the groups (P < 0.001). On multivariable analysis, stage IB2 disease was independently associated with a nearly two-fold increased risk of cervical cancer mortality compared to stage IB1 disease (adjusted-hazard ratio [HR] 1.98, 95% confidence interval [CI] 1.62–2.41, P < 0.001). In the stage III cohort (n = 11,733), stage IIIC1 was independently associated with improved cause-specific survival compared to stage IIIB disease (adjusted-HR 0.79,95%CI 0.74–0.85, P < 0.001). Survival of stage IIIC1 disease significantly differed based on T = stage, (5-year rates: 74.8% for T1, 58.7% for T2, and 39.3% for T3) with a 35.3% difference in absolute survival (P < 0.001).
Conclusion
The 2018 FIGO staging system for cervical cancer is useful to distinguish survival groups; stage IB1 and stage IB2 disease have distinct characteristics and survival outcomes, while survival in stage IIIC1 varies depending on local tumor factors.
Keywords: Cervical cancer, Staging, International Federation of Gynecology and, Obstetrics, FIGO, Validation, 2018
1. Introduction
In 2018, The International Federation of Gynecology and Obstetrics (FIGO) revised the staging system for carcinoma of the uterine cervix [1]. One of the major changes from the prior 2014 FIGO staging system is that stage IB disease now includes three sub-groups rather than two (Table 1) [1,2]. In the former system, stage IB disease was defined as (i) clinically and macroscopically visible lesions limited to the uterine cervix or (ii) microscopic lesions greater in size than stage IA disease. Tumor size of 4 cm served as the cutoff for classification of sub-stage: stage IB1 (≤4 cm) and stage IB2 (>4 cm). In the revised system for stage IB disease, sub-stage increases with every 2 cm increase in tumor size: stage IB1 (<2 cm), stage IB2 disease (2–3.9 cm), and stage IB3 (≥4 cm). Effectively, what was previously stage IB1 disease is now further subdivided into two groups in the revised staging system.
Table 1.
Changes in cervical cancer staging system.
| Characteristics | 2014 FIGO system | 2018 FIGO system |
|---|---|---|
| Stage IB1 | Tumor size ≤4 cm | Tumor size <2 cm |
| Stage IB2 | Tumor size >4 cm | Tumor size 2–3.9 cm |
| Stage IB3 | n/a | Tumor size ≥4 cm |
| Stage IIIC1 | n/a | Pelvic lymph node metastasis onlya |
| Stage IIIC2 | n/a | Para-aortic lymph node metastasisa |
Either radiographic (r)or histologic (p).
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
Another major change in the current staging system is the incorporation of nodal status into stage III disease staging. Cases with lymph node metastasis are now specifically designated as stage IIIC disease, stage IIIC1 for pelvic lymph node metastasis only or stage IIIC2 for para-aortic lymph node metastasis (Table 1). This new staging system clearly reflects the importance of lymph node metastasis as a major prognostic factor in cervical cancer [3]. In both clinically early-stage disease as well as in locally-advanced stage disease, lymph node metastasis is indeed a major prognostic factor associated with decreased survival [4,5].
The revised FIGO staging system has yet to be evaluated using population-based statistics, especially with regards to the important changes made to the definitions of stage IB and stage III cervical cancer. Thus, the objective of this study was to validate the revised 2018 FIGO staging system for stage IB and stage III cervical cancer.
2. Materials and methods
2.1. Data source
This is a retrospective observational study examining The Surveillance, Epidemiology, and End Results (SEER) Program between 1988 and 2014. The SEER Program is a population-based database launched in 1973 that is supported and managed by the National Cancer Institute in the United States [6]. SEER covers approximately 28% of the US population and is publicly available and deidentified. This study was deemed exempt by the University of Southern California and Tokai University Institutional Review Boards.
2.2. Eligibility criteria
For the stage IB cohort, women with stage IB cervical cancer who underwent primary hysterectomy with available tumor size information were included. For the stage III cohort, women with stage IIIA, IIIB, and IIIC1 cervical cancer with known regional lymph node status were included. Between 1988 and 2014, information for para-aortic lymph node status was not available in the SEER database, and only pelvic lymph node results are recorded. Therefore, this variable was used as a surrogate for 2018 FIGO stage IIIC1 disease. Histology types were limited to squamous, adenocarcinoma, and adenosquamous utilizing ICD-O-3 codes [7].
Cases prior to 1988 were not included due to lack of detailed information. For the stage IB cohort, women who did not undergo hysterectomy, who received radiotherapy prior to hysterectomy, or who had lymph node metastasis were excluded. For the stage III cohort, key exclusionary criteria included unknown cancer stage, unknown lymph node status, and 2018 FIGO stages I, II, and IV disease.
2.3. Clinical information
Among cases that met the inclusion criteria, patient demographics (age, calendar year at diagnosis, race/ethnicity, marital status, and registry area), tumor characteristics (histology types, tumor differentiation grade, T-stage, N-stage, and M-stage, and tumor size), treatment type (hysterectomy type, pelvic lymphadenectomy, radiotherapy, and chemotherapy), and survival outcome (cause-specific survival) were abstracted from the database. Cause-specific survival was defined as the time interval between cervical cancer diagnosis and death from cervical cancer. Cases deemed alive at the last known follow-up were censored.
2.4. Statistical consideration
For the stage IB cohort, the primary objective of the study was to examine patient demographics and tumor characteristics associated with 2018 FIGO stage IB cervical cancer. The secondary objective was to examine cancer mortality in light of the new 2018 FIGO staging system.
For the stage III cohort, the primary objective of the study was to examine the survival of women with 2018 FIGO stage IIIC1 cervical cancer compared to stage IIIA and IIIB disease. A secondary objective was to examine survival of women with 2018 FIGO stage IIIC1 cervical cancer based on T-stage (T1, T2, and T3 diseases).
Multi-group comparisons were performed with the Mann-Whitney U test, Kruskal-Wallis H test, and chi-square test, as appropriate. For survival analysis, the Kaplan-Meier method was used to construct survival curves, and the log-rank test was used to examine the statistical difference between curves. A Cox proportional hazard regression model was used for multivariable analysis. Patient demographics, tumor characteristics, and treatment type were entered in the final model, and the magnitude of statistical significance was expressed with hazard ratios (HR) and 95% confidence intervals (CI).
The Joinpoint Regression Program (version 4.6.0.0) provided by the National Cancer Institute was also utilized to evaluate temporal trends, which were analyzed by linear segmented regression. Logtransformation was then performed to determine annual percent change (APC) of the slope with 95%CI as previously described [8]. All hypotheses were two-tailed, and a P < 0.05 was considered statistically significant. Statistical Package for Social Sciences (SPSS, version 25.0, IBM Corp, Armonk, NY, USA) was used for analyses.
3. Results
3.1. Stage IB cohort
The patient selection schema is shown in Fig. S1. Among 72,552 cases of cervical cancer between 1988 and 2014, there were 8909 women with stage IB cervical cancer who underwent primary hysterectomy and had no nodal metastasis. Using the 2018 FIGO staging system, proportion of stage IB2 disease (n = 3620, 40.6%) and stage IB1 disease (n = 3604, 40.5%) were similar whereas stage IB3 disease was the least common in this surgical cohort (n = 1685, 18.9%).
Patient demographics across the three sub-stages are shown in Table 2. White women were more likely to have early sub-stage disease whereas Black and Hispanic women, as well as single women, were more likely to have higher sub-stage disease (all, P < 0.001). Temporal trends in the proportional distribution of sub-stages are shown in Fig. S2. There was a significant increase in stage IB1 disease (APC 1.33, 95% CI 0.76–1.90) and a decrease in stage IB3 disease (APC −1.57, 95% CI −2.36 to −0.78) during the study period (both, P < 0.001). There was no difference in stage IB2 disease between 2000 and 2014 (P = 0.17).
Table 2.
Patient demographics of stage IB cervical cancer (N = 8909).
| Characteristic | Stage IB1 |
Stage IB2 |
Stage IB3 |
P-value§ | P-value† |
|---|---|---|---|---|---|
| n = 3604 (40.5%) | n = 3620 (40.6%) | n = 1685 (18.9%) | |||
| Age (years) | 44 (37–54) | 43.5 (37–53) | 44 (37–54) | 0.83 | 0.71 |
| ≥70 | 195 (5.4%) | 197 (5.4%) | 98 (5.8%) | ||
| 60–69 | 369 (10.2%) | 373 (10.3%) | 171 (10.1%) | ||
| 50–59 | 633 (17.6%) | 621 (17.2%) | 313 (18.6%) | ||
| 40–49 | 1182 (32.8%) | 1153 (31.9%) | 528 (31.3%) | ||
| <40 | 1225 (34.0%) | 1276 (35.2%) | 575 (34.1%) | ||
| Race/ethnicity | <0.001 | <0.001 | |||
| White | 2124 (58.9%) | 1979 (54.7%) | 851 (50.5%) | ||
| Black | 289 (8.0%) | 330 (9.1%) | 183 (10.9%) | ||
| Hispanic | 801 (22.2%) | 835 (23.1%) | 452 (26.8%) | ||
| Others | 390 (10.8%) | 476 (13.1%) | 199 (11.8%) | ||
| Marital status | <0.001 | 0.033 | |||
| Single | 780 (21.6%) | 872 (24.1%) | 428 (25.4%) | ||
| Married | 1995 (55.4%) | 1914 (52.9%) | 822 (48.8%) | ||
| Others | 829 (23.0%) | 834 (23.0%) | 435 (25.8%) | ||
| Registry area | 0.30 | 0.22 | |||
| West | 2240 (62.2%) | 2278 (62.9%) | 1065 (63.2%) | ||
| Central | 652 (18.1%) | 600 (16.6%) | 270 (16.0%) | ||
| East | 712 (19.8%) | 742 (20.5%) | 350 (20.8%) | ||
| Year at diagnosis | <0.001 | 0.02 | |||
| Before 2000 | 653 (18.1%) | 855 (23.6%) | 414 (24.6%) | ||
| 2000–2009 | 1931 (54.6%) | 1777 (49.1%) | 872 (51.8%) | ||
| 2010 or later | 1020 (28.3%) | 988 (27.3%) | 399 (23.7%) | ||
| Histology | <0.001 | <0.001 | |||
| Squamous | 2030 (56.3%) | 2071 (57.2%) | 1090 (64.7%) | ||
| Adenocarcinoma | 1367 (37.9%) | 1261 (34.8%) | 463 (27.5%) | ||
| Adenosquamous | 207 (5.7%) | 288 (8.0%) | 132 (7.8%) | ||
| Grade | <0.001 | <0.001 | |||
| 1 | 680 (18.9%) | 458 (12.7%) | 164 (9.7%) | ||
| 2 | 1539 (42.7%) | 1427 (39.4%) | 591 (35.1%) | ||
| 3 | 966 (26.8%) | 1446 (39.9%) | 792 (47.0%) | ||
| Unknown | 419 (11.6%) | 289 (8.0%) | 138 (8.2%) | ||
| Pelvic lymphadenectomy* | <0.001 | <0.001 | |||
| Performed | 3106 (86.3%) | 3288 (90.9%) | 1496 (88.8%) | ||
| Not performed | 495 (13.7%) | 330 (9.1%) | 186 (11.1%) | ||
| Surgery type | <0.001 | <0.001 | |||
| Total hysterectomy | 1273 (35.3%) | 1099 (30.4%) | 528 (31.3%) | ||
| Extended, mRH, RH | 2120 (58.8%) | 2283 (63.1%) | 1035 (61.4%) | ||
| Hysterectomy, NOS | 211 (5.9%) | 238 (6.6%) | 122 (7.2%) | ||
| Adjuvant radiotherapy | <0.001 | <0.001 | |||
| WPRT ± VBT | 506 (14.0%) | 1043 (28.8%) | 759 (45.0%) | ||
| Other type RT | 57 (1.5%) | 73 (2.0%) | 54 (3.2%) | ||
| Not performed | 3041 (84.4%) | 2504 (69.2%) | 872 (51.8%) | ||
| Chemotherapy | <0.001 | <0.001 | |||
| Performed | 219 (6.1%) | 496 (13.7%) | 435 (25.8%) | ||
| None/unknown | 3385 (93.9%) | 3124 (86.3%) | 1250 (74.2%) |
Number (%) or median (inter-quartile range) is shown. Significant P-values are emboldened.
trend analysis for all groups.
sensitivity analysis for stage IB1 and IB2.
excluding 8 cases unknown lymphadenectomy status.
Abbreviations: NOS, not otherwise specified; mRH, modified hysterectomy; RH, radical hysterectomy; WPRT, whole pelvic radiotherapy; VBT, vaginal brachytherapy; and RT, radiotherapy.
Tumor characteristics were examined across the groups (Table 2). Stage IB1 disease was more likely to have adenocarcinoma histology and be low-grade, whereas stage IB3 disease was more likely to have squamous histology and be high-grade (both, P < 0.001). Women with stage IB2 disease were more likely to undergo pelvic lymphadenectomy and radical hysterectomy among the groups (both, P < 0.001). Women with stage IB1 disease were the least likely to receive postoperative therapy (both, P < 0.001). When stage IB2 disease was compared to stage IB1 disease, similar results were observed (Table 2).
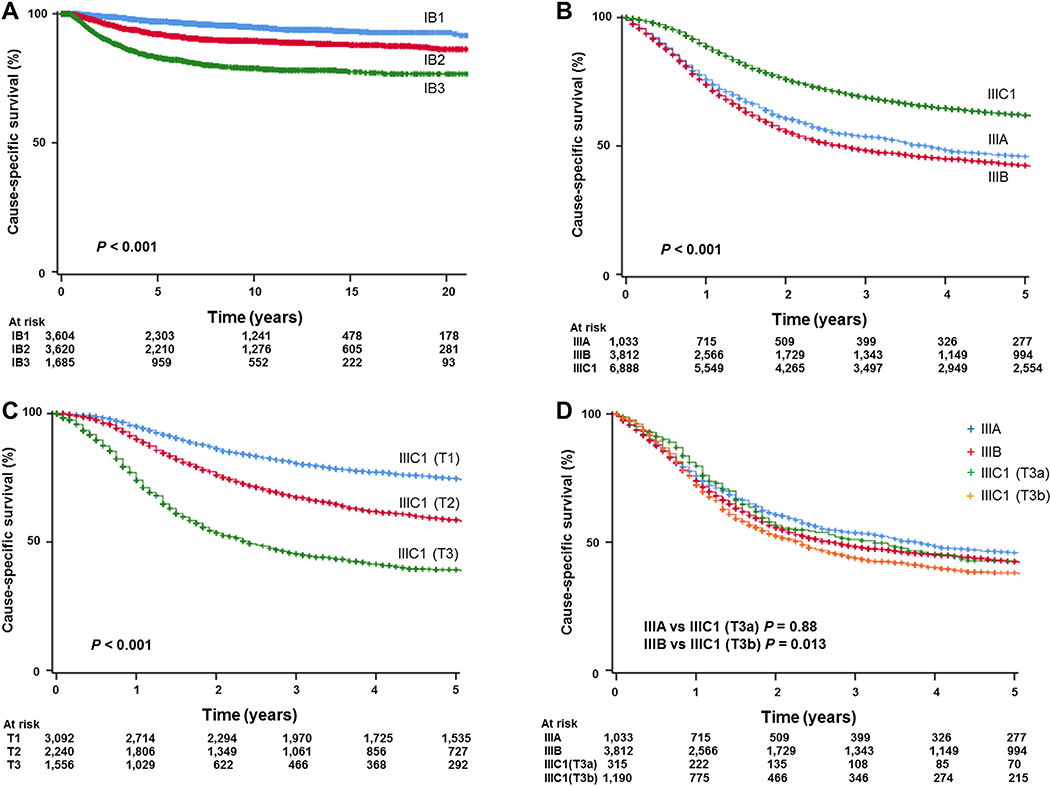
When survival outcomes were examined, there were 757 (8.5%) events classified as death from cervical cancer during the follow-up period. The median follow-up time of censored cases was 6.8 (interquartile range, 3.0–12.2) years for the whole cohort. On univariable analysis, the 2018 FIGO staging system was significantly associated with cause-specific survival for stage IB disease; 5-year survival rates were 97.0% for stage IB1 disease, 92.1% for stage IB2 disease, and 83.1% for stage IB3 disease (P < 0.001, Fig. 1A).
Fig. 1.
Kaplan-Meier curves based on 2018 FIGO cancer staging system. Log-rank test for P-values. Cause-specific survival is shown for (A) stage IB cervical cancer, (B) stage III cervical cancer, (C) stage IIIC1 cervical cancer, and (D) stage III cervical cancer based on T-stage.
On multivariable analysis, the 2018 FIGO staging system remained an independent prognostic factor for cause-specific survival (Table 3). Specifically, when compared to stage IB1 disease, stage IB2 disease was associated with a nearly two-fold increased risk of cervical cancer mortality (adjusted-HR 1.98, 95% CI 1.62–2.41, P < 0.001), and stage IB3 disease was associated with four-hold increased risk of cervical cancer mortality (adjusted-HR 4.07, 95% CI 3.33–4.97, P < 0.001). Similarly, stage IB3 disease was significantly associated with decreased cause-specific survival compared to stage IB2 disease (adjusted-HR 2.11, 95% CI 1.80–2.47, P < 0.001).
Table 3.
Multivariable analysis for cause-specific survival in stage IB cohort (N = 8909).
| No. | Survival rate (%) |
Multivariable (vs stage IB1) |
Multivariable (vs stage IB2) |
|||
|---|---|---|---|---|---|---|
| 5-yr | HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| Age (years) | ||||||
| ≥70 | 490 | 90.5 | 1.39 (1.02–1.89) | 0.04 | 1.39 (1.02–1.89) | 0.04 |
| 60–69 | 913 | 91.5 | 1.21 (0.95–1.56) | 0.13 | 1.21 (0.95–1.56) | 0.13 |
| 50–59 | 1567 | 94.3 | 0.82 (0.65–1.04) | 0.10 | 0.82 (0.65–1.04) | 0.10 |
| 40–49 | 2863 | 92.4 | 1.04 (0.87–1.24) | 0.70 | 1.04 (0.87–1.24) | 0.70 |
| <40 | 3076 | 91.8 | 1 | 1 | ||
| Race/ethnicity | ||||||
| White | 4954 | 93.1 | 1 | 1 | ||
| Black | 802 | 87.6 | 1.51 (1.21–1.89) | <0.001 | 1.51 (1.21–1.89) | <0.001 |
| Hispanic | 2088 | 93.4 | 0.95 (0.78–1.16) | 0.61 | 0.95 (0.78–1.16) | 0.61 |
| Others | 1065 | 90.7 | 1.18 (0.94–1.49) | 0.14 | 1.18 (0.94–1.49) | 0.14 |
| Marital status | ||||||
| Single | 2080 | 91.2 | 1.08 (0.90–1.29) | 0.44 | 1.08 (0.90–1.29) | 0.44 |
| Married | 4731 | 93.0 | 1 | 1 | ||
| Others | 2098 | 92.0 | 1.04 (0.86–1.24) | 0.71 | 1.04 (0.86–1.24) | 0.71 |
| Registry area | ||||||
| Western | 5583 | 92.5 | 1.05 (0.85–1.30) | 0.65 | 1.05 (0.85–1.30) | 0.65 |
| Central | 1522 | 92.4 | 1 | 1 | ||
| Eastern | 1804 | 91.9 | 0.98 (0.77–1.25) | 0.88 | 0.98 (0.77–1.25) | 0.88 |
| Year at diagnosis | ||||||
| Before 2000 | 1922 | 89.5 | 1 | 1 | ||
| 2000–2009 | 4580 | 92.2 | 0.81 (0.68–0.95) | 0.01 | 0.81 (0.68–0.95) | 0.01 |
| 2010 or later | 2407 | 93.5 | 0.90 (0.70–1.17) | 0.44 | 0.90 (0.70–1.17) | 0.44 |
| Stage | ||||||
| IB1 | 3604 | 97.0 | 1 | 0.51 (0.42–0.62) | <0.001 | |
| IB2 | 3620 | 92.1 | 1.98 (1.62–2.41) | <0.001 | 1 | |
| IB3 | 1685 | 83.1 | 4.07 (3.33–4.97) | <0.001 | 2.06 (1.76–2.41) | <0.001 |
| Histology | ||||||
| Squamous | 5191 | 91.8 | 1 | 1 | ||
| Adenocarcinoma | 3091 | 94.5 | 1.11 (0.93–1.32) | 0.27 | 1.11 (0.93–1.32) | 0.27 |
| Adenosquamous | 627 | 87.0 | 1.54 (1.22–1.94) | <0.001 | 1.54 (1.22–1.94) | <0.001 |
| Grade | ||||||
| 1 | 1302 | 96.7 | 1 | 1 | ||
| 2 | 3557 | 93.7 | 1.54 (1.14–2.08) | 0.005 | 1.54 (1.14–2.08) | 0.005 |
| 3 | 3204 | 88.6 | 2.23 (1.66–3.02) | <0.001 | 2.23 (1.66–3.02) | <0.001 |
| Unknown | 846 | 94.7 | 1.20 (0.82–1.75) | 0.36 | 1.20 (0.82–1.75) | 0.36 |
| Lymphadenectomy | ||||||
| Performed | 7890 | 92.6 | 1 | 1 | ||
| Not performed | 1019 | 90.2 | 1.71 (1.37–2.12) | <0.001 | 1.71 (1.37–2.12) | <0.001 |
| Surgery type | ||||||
| Total hysterectomy | 2900 | 92.8 | 1 | 1 | ||
| Extended, mRH, RH | 5438 | 92.4 | 1.05 (0.88–1.25) | 0.61 | 1.05 (0.88–1.25) | 0.61 |
| Hysterectomy, NOS | 571 | 89.6 | 1.55 (1.17–2.06) | 0.003 | 1.55 (1.17–2.06) | 0.003 |
Cox proportional hazard regression models for multivariable analysis. All listed covariates are entered in the final model. Significant P-values are emboldened.
including high-grade tumors.
Abbreviations: HR, hazard ratio; CI, confidence interval; n.a., not available; hyst, hysterectomy; mRH, modified hysterectomy; RH, radical hysterectomy; NOS, not otherwise specified; and 5-yr (%), 5-year proportion.
3.2. Stage III cohort
The patient selection schema is shown in Fig. S3. There were 11,733 women with stage III cervical cancer during the study period. The most common sub-stage was stage III C1 (n = 6888, 58.7%), followed by stage IIIB (n = 3812, 32.5%) and stage IIIA (n = 1033, 8.8%). Patient demographics are shown in Table 4. Women with stage IIIC1 disease were younger and were more likely to be of non-Black race, married, residents of the Western US, and more recently diagnosed compared to those with stage IIIA or IIIB disease (all, P < 0.001). Stage IIIC1 disease was also more likely to have adenocarcinoma or adenosquamous histology and to be higher grade but have smaller tumor size (all, P < 0.001). Women with stage IIIC1 disease were more likely than those with stage IIIA or IIIB disease to undergo hysterectomy or receive chemotherapy (both, P < 0.001).
Table 4.
Patient demographics of stage III cervical cancer (N = 11,733).
| Characteristic | Stage IIIA |
Stage IIIB |
Stage IIIC1 |
P-value |
|---|---|---|---|---|
| n = 1033 (8.8%) | n = 3812 (32.5%) | n = 6888 (58.7%) | ||
| Age (years) | 62 (49–74) | 55 (46–68) | 46 (38–57) | <0.001 |
| ≥70 | 352 (34.1%) | 837 (22.0%) | 544 (7.9%) | |
| 60–69 | 214 (20.7%) | 697 (18.3%) | 812 (11.8%) | |
| 50–59 | 203 (19.7%) | 997 (26.2%) | 1496 (21.7%) | |
| 40–49 | 176 (17.0%) | 850 (22.3%) | 2027 (29.4%) | |
| <40 | 88 (8.5%) | 431 (11.3%) | 2009 (29.2%) | |
| Race/ethnicity | <0.001 | |||
| White | 520 (50.3%) | 1932 (50.7%) | 3609 (52.4%) | |
| Black | 206 (19.9%) | 706 (18.5%) | 927 (13.5%) | |
| Hispanic | 183 (17.7%) | 771 (20.1%) | 1589 (23.1%) | |
| Others | 124 (12.1%) | 403 (10.6%) | 763 (11.1%) | |
| Marital status | <0.001 | |||
| Single | 243 (23.5%) | 1038 (27.2%) | 1906 (27.7%) | |
| Married | 330 (31.9%) | 1245 (32.7%) | 3103 (45.0%) | |
| Others | 460 (44.5%) | 1529 (40.1%) | 1879 (27.3%) | |
| Registry area | <0.001 | |||
| West | 521 (50.4%) | 1977 (51.9%) | 4083 (59.3%) | |
| Central | 243 (23.5%) | 808 (21.2%) | 1237 (18.0%) | |
| East | 269 (26.0%) | 1027 (26.9%) | 1568 (22.8%) | |
| Year at diagnosis | <0.001 | |||
| Before 2000 | 315 (30.5%) | 1010 (26.5%) | 1309 (19.0%) | |
| 2000–2009 | 526 (50.9%) | 2059 (54.0%) | 3357 (48.7%) | |
| 2010 or later | 192 (18.6%) | 743 (19.5%) | 2222 (32.3%) | |
| Histology | <0.001 | |||
| Squamous | 879 (85.1%) | 3357 (88.1%) | 5327 (77.3%) | |
| Adenocarcinoma | 129 (12.5%) | 360 (9.4%) | 1129 (16.4%) | |
| Adenosquamous | 25 (2.4%) | 95 (2.5%) | 432 (6.3%) | |
| Grade | <0.001 | |||
| 1 | 62 (6.0%) | 179 (4.7%) | 301 (4.4%) | |
| 2 | 320 (31.0%) | 1260 (33.1%) | 2345 (34.0%) | |
| 3* | 383 (37.1%) | 1325 (34.8%) | 3018 (43.8%) | |
| Unknown | 268 (25.9%) | 1048 (27.5%) | 1224 (17.8%) | |
| Tumor size (cm)† | 5.8 (4.0–7.0) | 6.0 (5.0–8.0) | 4.5 (3.0–6.0) | <0.001 |
| Hysterectomy | <0.001 | |||
| Performed | 132 (12.8%) | 165 (4.3%) | 3353 (48.7%) | |
| Not performed | 901 (87.2%) | 3647 (95.7%) | 3535 (51.3%) | |
| Radiotherapy | <0.001 | |||
| WPRT ± VBT | 831 (80.4%) | 3186 (83.6%) | 5688 (82.6%) | |
| Other type RT | 53 (5.2%) | 242 (6.4%) | 386 (5.6%) | |
| Not performed | 149 (14.4%) | 384 (10.1%) | 814 (11.8%) | |
| Chemotherapy | <0.001 | |||
| Performed | 555 (53.7%) | 2475 (64.9%) | 4951 (71.9%) | |
| None/unknown | 478 (46.3%) | 1337 (35.1%) | 1937 (28.1%) |
Number (%) or median (inter-quartile range) is shown. Univariable analysis with chi-square test or Kruskal-Wallis H test for P-values. Significant P-values are emboldened.
included high-grade tumors.
included VBT and not otherwise specified type of radiotherapy.
excluding 4158 cases of unknown tumor size.
Abbreviations: WPRT, whole pelvic radiotherapy; VBT, vaginal brachytherapy; and RT, radiotherapy.
Survival analysis was also performed (Table 5). The median follow-up of censored cases was 5.0 years (interquartile range 1.6–10.1), and there were 4922 (42.0%) deaths from cervical cancer in this cohort. On univariable analysis, the 2018 FIGO staging system was significantly associated with cause-specific survival in stage III cervical cancer (P < 0.001, Fig. 1B). Specifically, women with stage IIIC1 disease had significantly improved survival compared to those with other sub-stages (5-year survival rates: 46.0% for stage IIIA, 42.6% for stage IIIB, and 62.1% for stage IIIC1 disease, respectively). On multivariable analysis (Table 5), stage IIIC1 disease was independently associated with improved cause-specific survival compared to stage IIIA (adjusted-HR 0.88, 95% CI 0.70–0.98, P = 0.018) or stage IIIB disease (adjusted-HR 0.79, 95% CI 0.74–0.85, P < 0.001).
Table 5.
Multivariable analysis for cause-specific survival in stage III cohort (N = 11,733).
| Characteristic | Survival rate (%) |
Multivariable (vs stage IIIB) |
Multivariable (vs stage IIIA) |
|||
|---|---|---|---|---|---|---|
| No. | 5-yr | HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Age (years) | ||||||
| ≥70 | 1733 | 39.2 | 1.43 (1.29–1.59) | <0.001 | 1.43 (1.29–1.59) | <0.001 |
| 60–69 | 1723 | 51.9 | 1.08 (0.97–1.20) | 0.15 | 1.08 (0.97–1.20) | 0.15 |
| 50–59 | 2696 | 53.0 | 1.08 (0.99–1.19) | 0.08 | 1.08 (0.99–1.19) | 0.08 |
| 40–49 | 3053 | 58.3 | 0.99 (0.92–1.09) | 0.98 | 0.99 (0.92–1.09) | 0.98 |
| <40 | 2528 | 61.8 | 1 | 1 | ||
| Race/ethnicity | ||||||
| White | 6061 | 53.9 | 1 | 1 | ||
| Black | 1839 | 45.2 | 1.08 (1.01–1.17) | 0.049 | 1.08 (1.01–1.17) | 0.049 |
| Hispanic | 2543 | 60.0 | 0.83 (0.76–0.90) | <0.001 | 0.83 (0.76–0.90) | <0.001 |
| Others | 1290 | 58.8 | 0.81 (0.73–0.90) | <0.001 | 0.81 (0.73–0.90) | <0.001 |
| Marital status | ||||||
| Single | 3187 | 53.4 | 1.14 (1.06–1.23) | 0.001 | 1.14 (1.06–1.23) | 0.001 |
| Married | 4678 | 60.0 | 1 | 1 | ||
| Others | 3868 | 48.1 | 1.60 (1.08–1.24) | <0.001 | 1.60 (1.08–1.24) | <0.001 |
| Registry area | ||||||
| Western | 6581 | 56.4 | 0.98 (0.91–1.06) | 0.63 | 0.98 (0.91–1.06) | 0.63 |
| Central | 2288 | 51.1 | 1 | 1 | ||
| Eastern | 2864 | 52.4 | 0.98 (0.90–1.07) | 0.63 | 0.98 (0.90–1.07) | 0.63 |
| Year at diagnosis | ||||||
| Before 2000 | 2634 | 50.0 | 1 | 1 | ||
| 2000–2009 | 5942 | 55.7 | 0.90 (0.83–0.96) | 0.003 | 0.90 (0.83–0.96) | 0.003 |
| 2010 or later | 3157 | 56.0 | 0.85 (0.77–0.94) | 0.001 | 0.85 (0.77–0.94) | 0.001 |
| 2018 FIGO stage | ||||||
| IIIA | 1033 | 46.0 | 0.89 (0.81–0.98) | 0.02 | 1 | |
| IIIB | 3812 | 42.6 | 1 | 1.12 (1.02–1.24) | 0.022 | |
| IIIC | 6888 | 62.1 | 0.79 (0.74–0.85) | <0.001 | 0.88 (0.70–0.98) | 0.018 |
| Histology | ||||||
| Squamous | 9563 | 54.9 | 1 | 1 | ||
| Adenocarcinoma | 1618 | 50.5 | 1.49 (1.38–1.62) | <0.001 | 1.49 (1.38–1.62) | <0.001 |
| Adenosquamous | 552 | 56.5 | 1.28 (1.11–1.46) | <0.001 | 1.28 (1.11–1.46) | <0.001 |
| Grade | ||||||
| 1 | 542 | 60.8 | 1 | 1 | ||
| 2 | 3925 | 57.5 | 1.27 (1.09–1.47) | 0.002 | 1.27 (1.09–1.47) | 0.002 |
| 3 | 4726 | 53.3 | 1.49 (1.29–1.73) | <0.001 | 1.49 (1.29–1.73) | <0.001 |
| Unknown | 2540 | 49.9 | 1.26 (1.08–1.47) | 0.003 | 1.26 (1.08–1.47) | 0.003 |
| Hysterectomy | ||||||
| Performed | 3650 | 71.4 | 1 | 1 | ||
| Not performed | 8083 | 45.9 | 2.52 (2.32–2.73) | <0.001 | 2.52 (2.32–2.73) | <0.001 |
| Radiotherapy | ||||||
| Performed | 10,386 | 55.3 | 1 | 1 | ||
| Not performed | 1347 | 47.2 | 1.73 (1.58–1.89) | <0.001 | 1.73 (1.58–1.89) | <0.001 |
| Chemotherapy | ||||||
| Performed | 7981 | 56.5 | 1 | 1 | ||
| None/unknown | 3752 | 49.9 | 1.22 (1.13–1.30) | <0.001 | 1.22 (1.13–1.30) | <0.001 |
Cox proportional hazard regression models for multivariable analysis. All listed covariates are entered in the final model. Significant P-values are emboldened.
including high-grade tumors.
Abbreviations: HR, hazard ratio; CI, confidence interval; 5-yr, 5-year proportion.
Survival outcomes were then compared among cases with stage IIIC1 disease based on T-stage (Table S1). On univariable analysis, 5-year cause-specific survival rates varied significantly depending on T-stage: 74.8% for T1 stage, 58.7% for T2 stage, and 39.3% for T3 stage, with an absolute survival difference of 35.3% (P < 0.001, Fig. 1C). On multivariable analysis, T-stage remained an independent prognostic factor for cause-specific survival in women with 2018 FIGO stage IIIC1 cervical cancer (adjusted-P < 0.001). Survival of women with stage IIIC1 cervical cancer with T3b disease was significantly poorer compared to those with stage IIIB (no nodal metastasis) cervical cancer (38.1% versus 42.6%, HR 1.12, 95%C1 1.02–1.22, P = 0.013, Fig. 1D). Contrary, survival of women with stage IIIC1 cervical cancer with T3a disease was similar compared to those with stage IIIA (no nodal metastasis) cervical cancer (42.9% versus 45.9%, HR 1.01, 95%CI 0.85–1.22, P = 0.88, Fig. 1D).
4. Discussion
4.1. Stage I cohort
Key findings of this study are that (i) the revised FIGO system is useful to classify stage IB cervical cancer based on tumor size and that (ii) the new staging system for randomization clearly demonstrated distinct survival differences among the three sub-groups. This is particularly important because survival is significantly different between 2018 FIGO stage IB1 and IB2 disease, with nearly two-hold increased risk in cervical cancer mortality in stage IB2 disease compared to IB1 disease. This difference in cancer mortality is quite meaningful and clearly suggests that distinguishing the two groups is necessary when caring for women with what was previously classified as just stage IB1 (<4 cm) cervical cancer.
The majority of women with stage IB cervical cancer undergo surgical treatment, and tumor size is a well-known surgical-pathological factor that impacts survival [9]. A recent large-scale cohort study demonstrated that tumor size ≤2 cm was associated with significantly improved survival compared to >2 cm among 2014 FIGO stage IB1 cervical cancers [10]. A tumor size of ≤2 cm is also used as a cutoff when considering fertility-sparing trachelectomy in women with stage IB1 cervical cancer [11]. Lastly, tumor size ≥2 cm seems to be also a prognostic factor when minimally-invasive radical hysterectomy is performed for women with stage IB1 cervical cancer [12]. Taken together, the revised 2018 staging system, which stratifies sub-stage by 2 cm increments in tumors size, seems to be of value in the management of women with stage IB cervical cancer.
The new FIGO staging system has clinical utility in various aspects of the management of women with cervical cancer. First, it facilitates risk-stratification in stage IB cervical cancer. For instance, the current guideline recommendation for fertility-sparing trachelectomy is for 2018 FIGO stage IB1 disease but not stage IB2 [11]. Second, given the impact of tumor size on the outcome of minimally-invasive radical hysterectomy for early-stage cervical cancer [12], per the 2018 FIGO staging system, minimally invasive surgical approaches are associated with decreased survival in stage IB2 disease but not stage IB1 disease. This information supports and complements the results of a recent phase III randomized controlled trial that showed inferior survival with minimally-invasive surgery compared to open approaches in 2009 FIGO stage IB1 (<4 cm) cervical cancer, however, their study did not stratify the tumor size per the 2018 FIGO staging system [13].
Our analysis clearly supports the benefit and utility of the revised FIGO staging system in stage IB cervical cancer. Additional validation is warranted to further support this new system, as this study does not have detailed information for other surgical-pathological factors such as lympho-vascular space invasion, and the exact relationship to survival is not completely assessable in this study. Moreover, current criteria for high-intermediate risk cervical cancer use a tumor size of 4 cm [9]. It will be of interest to see if specifying tumor size between 2 and 4 cm adds more information or alters the definition of these risk criteria. Particular attention should also be paid to avoid confusion between the different definitions of stage IB2 disease in the current and former staging systems.
4.2. Stage III cohort
Key findings of this study are that stage III cervical cancer, per the 2018 FIGO staging system, reflects a diverse range of survival outcomes, and women with stage IIIC1 were found to have superior cervical cancer-specific survival compared to those with stage IIIA-B disease.
In other gynecologic malignancies, including endometrial cancer, location of lymph node metastases is specified in the 2009 FIGO staging system [14]. In endometrial cancer, tumors with pelvic or para-aortic lymph node metastasis are considered advanced-stage (stage IIIC) and are associated with decreased survival outcomes comparable to stage IIIA-B disease [15]. In cervical cancer, however, survival of stage IIIC1 disease was significantly better compared to stage IIIA-B disease, implying that local tumor factors, in addition to nodal status, are important determinants of survival.
Indeed, survival of women with 2018 FIGO stage IIIC1 cervical cancer varies widely by T-stage, ranging 39.3% to 74.8% (Fig. 1C). These 5-year survival rates were also comparable to stage IIIA (39.7%) and IB2 (75.7%) disease [16]. This clearly implies that stage IIIC1 cervical cancer is not homogenous, and that local tumor factors remain salient prognostic factors in cervical cancer. When the impact of lymph node metastasis on survival was examined in stage T3b disease, cases with nodal involvement were statistically significantly associated with decreased survival (stage IIIB versus IIIC1 (T3b), Fig. 1D). But, this association was not seen in stage T3a disease. Given that survival varies widely in stage IIIC1 disease based on the cervical tumor stage, modifications to the FIGO staging system to reflect T stage would be useful to group patients based on survival statistics.
A strength of this study is that this is likely the first population-based study to examine the validity of the revised 2018 FIGO staging system for cervical cancer. Weaknesses of this study include that this is a retrospective study, and there may be missing confounding surgical-pathological factors other than tumor size, grade, and histology type. Moreover, evidence of lymph node metastasis was not retrievable in this study, and it is unknown if documented lymph node metastasis was based on radiographic data alone versus histology-proven metastasis. This aspect is particularly important because histologic diagnosis generally has a higher sensitivity for detecting nodal metastasis than radiologic studies [17].
It is also likely that survival of stage IIIC1 disease differs based on degree of lymph node metastasis, such as macroscopic bulky lymphadenopathy versus microscopic metastasis or isolated tumor cells, however, this information was also not available in the SEER database. Lastly, information regarding the presence or absence of para-aortic lymph node metastasis was not available during the study period in this database, therefore the outcome of women with stage IIIC2 cervical cancer could not be assessed in this study. Thus, due to this limitation, it is possible that those with stage IIIC2 disease in reality were erroneously included as stage IIIC1 disease in this study.
In conclusion, the revised 2018 FIGO staging system for cervical cancer is useful to distinguish survival groups. In stage IB cervical cancer, stage IB1 and stage IB2 disease have distinct characteristics and outcomes, and risk-stratification based on this new classification is crucial in treatment algorithms. In stage III disease, stage IIIC1 reflects a heterogeneous group of tumors with a wide range of survival statistics based on local tumor factors, and physicians should be aware that stage IIIC1 cervical cancer is not a single disease entity.
Supplementary Material
HIGHLIGHTS.
FIGO revised cervical cancer staging in 2018
Revised staging was validated in a population-based tumor registry.
Stage IB1 and IB2 disease have distinct tumor characteristics and survival.
Stage IIIC1 disease has superior survival compared to stage IIIA-B disease.
Survival of stage IIIC1 disease depends on local tumor factors.
Acknowledgments
Funding support
Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
Disclosure statement
Honorarium, Chugai (K.M.); none for others.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2018.10.026.
References
- [1].Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R, Cancer of the cervix uteri, Int. J. Gynaecol. Obstet 143 (2018) 22–36. [DOI] [PubMed] [Google Scholar]
- [2].FIGO staging for carcinoma of the vulva, cervix, and corpus uteri, Int. J. Gynaecol. Obstet. 125 (2014) 97–98. [DOI] [PubMed] [Google Scholar]
- [3].Waggoner SE, Cervical cancer, Lancet 361 (2003) 2217–2225. [DOI] [PubMed] [Google Scholar]
- [4].Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr., Alberts DS, Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix, J. Clin. Oncol. 18 (2000) 1606–1613. [DOI] [PubMed] [Google Scholar]
- [5].Singh AK, Grigsby PW, Dehdashti F, Herzog TJ, BA Siegel FDG -PET lymph node staging and survival of patients with FIGO stage IIIb cervical carcinoma, Int. J. Radiat. Oncol. Biol. Phys. 56 (2003) 489–493. [DOI] [PubMed] [Google Scholar]
- [6].National Cancer Institute, The Surveillance, Epidemiology, and End Results (SEER) Program, http://seer.cancer.gov/, Accessed date: 8 June 2018.
- [7].Matsuo K, Machida H, Shoupe D, Melamed A, Muderspach LI, Roman LD, Wright JD, Ovarian conservation and overall survival in young women with early-stage cervical cancer, Obstet. Gynecol. 129 (2017) 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Matsuo K, Ross MS, Machida H, Blake EA, Roman LD, Trends of uterine carcinosarcoma in the United States, J. Gynecol. Oncol. 29 (2018), e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ, A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a gynecologic oncology group study, Gynecol. Oncol. 73 (1999) 177–183. [DOI] [PubMed] [Google Scholar]
- [10].Kato T, Takashima A, Kasamatsu T, Nakamura K, Mizusawa J, Nakanishi T, Takeshima N, Kamiura S, Onda T, Sumi T, Takano M, Nakai H, Saito T, Fujiwara K, Yokoyama M, Itamochi H, Takehara K, Yokota H, Mizunoe T, Takeda S, Sonoda K, Shiozawa T, Kawabata T, Honma S, Fukuda H, Yaegashi N, Yoshikawa H, Konishi I, Kamura T, Clinical tumor diameter and prognosis of patients with FIGO stage IB1 cervical cancer (JCOG0806-A), Gynecol. Oncol. 137 (2015) 34–39. [DOI] [PubMed] [Google Scholar]
- [11].National Comprehensive Cancer Network, Clinical Practice Guideline in Oncology . Cervical Cancer, https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf, Accessed date: 8 June 2018. [Google Scholar]
- [12].Margul DJ, Yang J, Seagle BL, Kocherginsky M, Shahabi S, Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer, 2018 Annual Meeting of American Society of Clinical Oncology, Chicago, IL, June 1–5, 2018. [Google Scholar]
- [13].Ramirez RT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, Buda A, Yan X, Robledo K, Gebski V, Coleman RL, Obermair A, Phase III Randomized Trial of Laparoscopic or Robotic Radical Hysterectomy vs. Abdominal Radical Hysterectomy in Patients with Early-Stage Cervical Cancer: LACC Trial, 49th Annual Meeting on Women’s Cancer, New Orleans, LA, March 24–27, 2018, 2018. [Google Scholar]
- [14].Pecorelli S, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium, Int. J. Gynaecol. Obstet. 105 (2009) 103–104. [DOI] [PubMed] [Google Scholar]
- [15].Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, Heintz AP, Ngan HY, Pecorelli S, Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological cancer, Int. J. Gynaecol. Obstet. 95 (2006) S105–S143. [DOI] [PubMed] [Google Scholar]
- [16].Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz A, Ngan H, Pecorelli S, Carcinoma of the cervix uteri, Int. J. Gynaecol. Obstet. 95 (2006) S43–S103. [DOI] [PubMed] [Google Scholar]
- [17].Monk BJ, Tian C, Rose PG, Lanciano R, Which clinical/pathologic factors matter in the era of chemoradiation as treatment for locally advanced cervical carcinoma? Analysis of two Gynecologic Oncology Group (GOG) trials, Gynecol. Oncol. 105 (2007) 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.