Abstract
Objective
This article aims to evaluate the diagnostic value of 68Gallium-PSMA positron emission tomography/computerized tomography (68Ga-PSMA PET/CT) for lymph node (LN) staging in patients with prostate cancer (PCa) by a meta-analysis of diagnostic tests.
Methods
We systematically retrieved articles from Web of Science, EMBASE, Cochrane Database, PubMed. The time limit is from the creation of the database until June 2019, and Stata 15 was used for calculation and statistical analyses.
Results
Sensitivity, specificity, positive and negative likelihood ratio (PLR, NLR), diagnostic odds ratio (DOR) and 95% confidence intervals (CI) be used to evaluate the diagnostic value. A total of 10 studies were included in our meta-analysis, which included 701 individuals. The results of each consolidated summary are as follows: sensitivity of 0.84 (95% CI 0.55–0.95), specificity of 0.95 (95% CI 0.87–0.98), PLR and NLR was 17.19 (95% CI 6.27, 47.17) and 0.17 (95% CI 0.05–0.56), respectively. DOR of 100 (95% CI 18–545), AUC of 0.97 (95% CI 0.95–0.98).
Conclusion
Our study demonstrates that 68Ga-PSMA PET/CT has a high overall diagnostic value for LN staging in patients with moderate and high-risk PCa. But our conclusions still require a larger sample size, multi-center prospective randomized controlled trial to verify.
Keywords: Prostate cancer, Lymph node, 68Gallium- prostate-specific membrane antigen, Meta-analysis
Introduction
Prostate cancer (PCa) is the second most common cancer and the fifth leading cause of cancer death in developed countries [1]. Although the clinical symptoms of early PCa are not obvious, pelvic lymph node (LN) and bone metastasis are also prone to occur [2]. About 15% of patients with medium and high-risk prostate cancer were found to have harbor lymph node invasion when performing pelvic lymph node dissection (PLND) during radical prostatectomy (RP) [3]. PLND is undoubtedly the most reliable way to determine whether there is LNI in PCa, but this method is both invasive and expensive [4]. And complications such as lymphocele, lymphedema are also headaches [5]. Before performing PLND, it is necessary to routinely use nomograms to predict the extent of lymph node invasion before surgery. According to these nomograms, patients with a serum prostate-specific antigen (PSA) of over 10 ng/mL, a Gleason score of over 6, or a stage T3 tumour (according to the Tumour, Nodes, and Metastases [TNM] staging system) defined by digital rectal examination (DRE), have a 5–65% risk of lymph node involvement [6–8]. According to the EAU guidelines, patients with moderate to high risk PCa who have an LN-positive estimated risk of more than 5% should undergo extend pelvic lymph node dissection (ePLND) [9]. Therefore, accurate assessment of LN staging before surgery is important for the operation and patient’s prognosis.
Presurgical computerized tomography (CT) scan or magnetic resonance imaging (MRI) is helpful for LN staging, but with limited accuracy [10]. Prostate-specific membrane antigen (PSMA) is a surface protein that is expressed in almost normal prostate tissues, and its expression level is higher in PCa tissues [11]. In recent years, PSMA has been introduced into positron emission tomography (PET) imaging [12], 68Gallium-PSMA positron emission tomography/computerized tomography (68Ga-PSMA PET/CT) has been proven to be better than ordinary imaging tests in detecting metastatic PCa [13]. Related studies have also shown that 68Ga-PSMA PET/CT is more sensitive for recurrent PCa with a low prostate-specific antigen (PSA) [14, 15].
Till now, some scholars have done some research on the accuracy of 68Ga-PSMA PET/CT to determine the lymph node staging of PCa preoperative, but it’s still unclear how its diagnostic value is. The diagnostic accuracy of 68Ga-PSMA PET/CT to determine lymph node staging of PCa preoperative remains unclear based on the current literature. The current study aims to establish the status of 68Ga-PSMA PET/CT in PCa's LN staging.
Methods
Literature search and eligibility criteria
A systematic search of the published literature using Web of Science, EMBASE, Cochrane Database, PubMed was performed. We have selected relevant literature from the creation of the database to June 2019. And used prostate cancer, Gallium, lymph node, positron emission tomography/computerized tomography, prostate-specific membrane antigen as the search terms and the search language was limited to English. We also searched for the relevant bibliography to avoid omissions.
The studies that were included in our research should meet the demands as follows: patients diagnosed with LN metastatic PCa using the gold standard pathological biopsy, studies with the diagnostic value of 68Ga-PSMA PET/CT reflected in the studies, and studies with sufficient data on true positive (TP), false positive (FP), false negative (FN), and true negative (TN). For duplicate articles, low-quality research, letters, reviews, case report, we analyze and exclude respectively. This process was assessed by two authors (PL and LJZ), independently.
Data extraction
We incorporate the following data from each article into the meta-analysis: author, publication year, study design, patient recruitment time, lymph node dissection, average patient age, preoperative PSA, patient population characteristics: high/medium risk. The PCa and health group was diagnosed as positive and negative by 68Ga-PSMA PET/CT to calculate the number of true positives, false positives, true negatives, and false negatives. Data extraction was performed by two authors (PL and LJZ) with any discrepancies resolved by a third author (WTQ).
Quality evaluation
The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool was used to evaluate the quality of included studies [13]. Key domains are assessed to determine the risk of bias and applicability. Signalling questions are included to facilitate judgements, with the risk being low if all signaling answer for a domain is 'yes', and if the answer to any question is 'no' suggesting potential bias exists. Concerns about applicability are determined as 'low', 'high', or 'unclear'.
Statistical analysis
We used Stata 15 (StataCorp LP, University City, Texas, USA) for statistical analysis. Q test and chi-square tests were used to verify the heterogeneity between the included works of literature. If I2 > 50%, the differences between the literature were considered significant [16]. We used a bivariate model to calculate the pooled sensitivity, specificity, positive and negative likelihood ratios (PLRs and NLRs), diagnostic odds ratio (DOR) and the 95% confidence interval (CI) [17]. We calculated the area under the receiver operator characteristic curve (SROC, AUC). AUC varied from 0.5 to 1. If the area was equal to 1, then a diagnostic test was extremely valuable. If the area was 0.5, then the diagnostic ability was considered as poor [18]. Deeks’s funnel plot was used to assess the publication bias, and Fagan plots showed the relationship between the prior probability, the likelihood ratio, and the posterior test probability [19]. P < 0.05 was considered statistically significant.
Results
Study selection and study characteristics
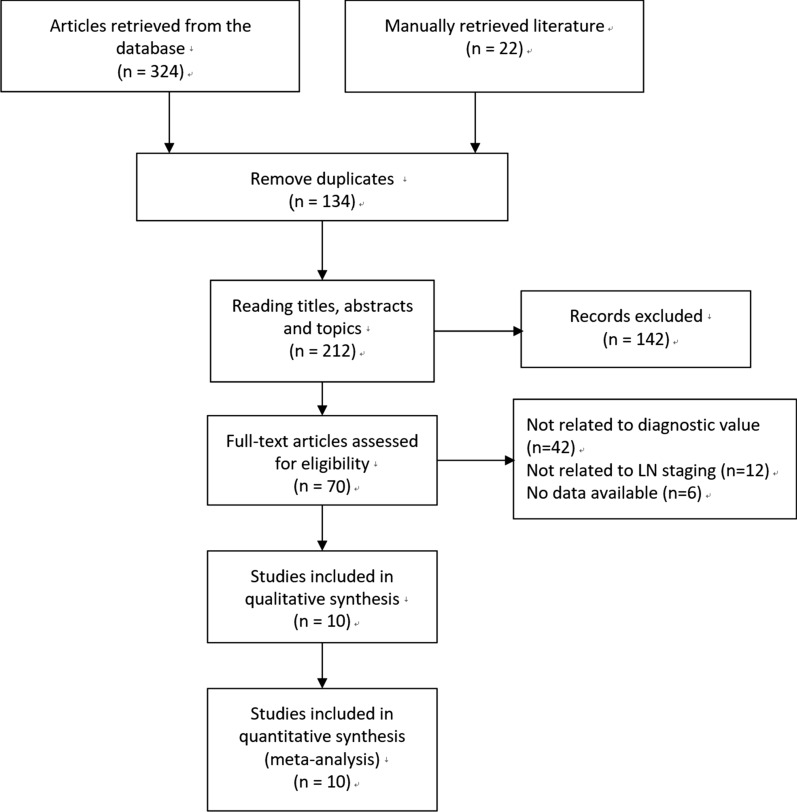
The literature search selection steps retrieval in Fig. 1. Initially, we retrieved a total of 324 articles in the selected database and manually retrieved 22 articles by referring to relevant literature citations. In these studies, we excluded 134 duplicates records. By analyzing titles, abstracts, and topics, 142 articles were also excluded. Through full-text analysis and assessment of eligibility, 42 articles that were not related to diagnostic value, 12 articles that were no related to LN staging, and 6 studies that had no access data. Finally, we included 10 studies in our meta-analysis [2, 12, 13, 20–26].
Fig. 1.
Flow diagram of studies selection process
In Table 1, we presented the basic data of the articles included in the meta-analysis of 68Ga-PSMA PET/CT for LN staging of PCa preoperative. These articles were published between 2016 and 2019. A total of 701 sample individuals were included in our analysis. Most of them are single-center retrospective studies in regions including Asia (China, India, and Turkey), Europe (Germany) and Australia. At the same time, we also display the age, PSA, patient characteristics and other information of patients included in each study.
Table 1.
Characteristics of the included studies in the meta-analysis
| References | Country | Study design | Study population | Recruiting time | Uptake time (min) | Lymphade-nectomy | Age Median (range) Median (IQRe) | PSAd median (range) Median (IQR) | Patient characteristics |
|---|---|---|---|---|---|---|---|---|---|
| Budaus [25] | Germany | Retrospective | Single center | June. 2014 to Mar. 2015 | NA | ePLNDf | 63 (44–75) | 8.8 (1.4–376) | High risk |
| Herlemann [20] | Germany | Retrospective | Single center | Jan. 2014 to Aug. 2015 | 60 | PLNDg | 70.5 (59–80)b | 56 (3.3–36.3)b | 4 intermediate risk and 16 high risk |
| Maurer [24] | Germany | Retrospective | Single center | Dec. 2012 to Nov. 2014 | 59.8 ± 17.8a | PLND | 66 (45–84) | 11.6 (0.57–244) | 42 intermediate risk and 88 high risk |
| Gupta [22] | India | Retrospective | Single center | Dec. 2014 to Dec. 2015 | Nearly 60 | ePLND | NA | NA | High risk |
| Obek [21] | Turkey | Retrospective | Single center | July. 2014 to Oct. 2015 | 45–60 | ePLND | 64 ± 6.0a | 26.5 ± 21.4a | 44 high risk and 7 very high risk |
| Van Leeuwen [26] | Australia | Prospective | Single center | Apr. to Oct. 2015 | 60 | ePLND | 65 (60–71) | 65 (55–82) | 3 intermediate risk and 27 high risk |
| Zhang [12] | China | Retrospective | Single center | Mar. to July. 2017 | 60 | PLND | 69 (55–82) | 37.25 (7.2–348) | 17 intermediate risk and 25 high risk |
| Van Leeuwen [2] | Australia | Retrospective | Single center | Feb. 2015 to Oct. 2017 | 60 | ePLND | NA | 9.4 | 140 intermediate risk and high risk |
| Yilmaz [23] | Turkey | Retrospective | Single center | May. 2016 to Apr. 2018 | Approximately 60 | rLNDc | 62.8 (49–73)b | 12 (2.4–32)b | 15 intermediate risk and 6 high risk |
| Yaxley [13] | Australia | Retrospective | Single center | July. 2014 to Sep. 2017 | 45–60 | PLND | 68 (44–80) | 7.6 (1.5–51) | 85 intermediate risk and 123 high risk |
aMean ± SD
bMean (range)
crLND, regional lymph node dissection
dPSA, prostate specific antigen
eIQR, interquartile range
fePLND, extend pelvic lymph node dissection
gPLND, pelvic lymph node dissection
Quality assessment
Based on the evaluation results of the QUADAS-2 scale, we reflected the quality evaluation results of each article in Table 2. The final score of each article was 11 or better, and 7 of them received quite high scores.
Table 2.
Summary estimated of diagnostic performance of the 68Ga-PSMA PET/CT for lymph node staging in patient with Prostate Cancer
| References | Sample size | TPa | FPb | FNc | TNd | Quality |
|---|---|---|---|---|---|---|
| Budaus [25] | 30 | 33 | 0 | 67 | 100 | 11 |
| Herlemann [20] | 34 | 91 | 33 | 9 | 67 | 10 |
| Maurer [24] | 130 | 66 | 1 | 34 | 99 | 11 |
| Gupta [22] | 12 | 100 | 20 | 0 | 80 | 10 |
| Obek [21] | 51 | 53 | 14 | 47 | 86 | 10 |
| Van Leeuwen [26] | 30 | 64 | 5 | 62 | 94 | 11 |
| Zhang [12] | 42 | 93 | 4 | 7 | 96 | 9 |
| Van Leeuwen [2] | 140 | 60 | 12 | 40 | 88 | 10 |
| Yilmaz [23] | 24 | 38 | 6 | 62 | 94 | 10 |
| Yaxley [13] | 208 | 38 | 6 | 62 | 94 | 11 |
| Pooled analysis | Inconsistency (I2) (95% CI) | 99% (98–99) | ||||
| Sample Size | 701 | |||||
| SENe (95% CI) | 0.84 (0.55, 0.95) | |||||
| SPEf (95% CI) | 0.95 (0.87, 0.98) | |||||
| PLRg (95% CI) | 17.2 (6.3, 47.2) | |||||
| NLRh (95% CI) | 0.17 (0.05, 0.56) | |||||
| DORi (95% CI) | 100 (18, 545) | |||||
| Youden Index | 0.79 | |||||
68Ga-PSMA PET/CT, 68Gallium-PSMA positron emission tomography/computerized tomography
aTP, true positive
bFP, false positive
cFN, false negative
dTN, true positive
eSEN, Sensitivity
fSPE, Specificity
gPLR, Positive Likelihood Ratio
hNLR, Negative Likelihood Ratio
iDOR, Diagnostic Odds Ratio
Pooled diagnostic values
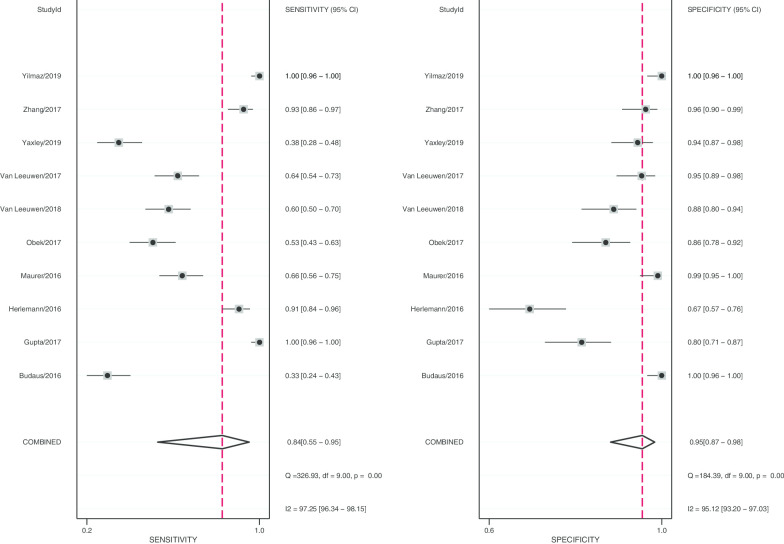
After statistical tests, the value of I2 is greater than 50%, it can be considered that the included documents have high heterogeneity. Therefore, a random effect model is used to combine sensitivity and specificity, thereby obtaining more conservative results. The diagnostic value is shown in Table 2. The pooled sensitivity and specificity were recorded as 0.84 (95% CI 0.55–0.95) and 0.95 (95% CI 0.87–0.98, Fig. 2), respectively. The Youden index was 0.79. The pooled PLR and NLR was 17.19 (95% CI 6.27–47.17) and 0.17 (95% CI 0.05–0.56), and DOR of 100 (95% CI 18–545). The overall SORC curve is presented in Fig. 3, with an AUC of 0.97 (95% CI 0.95–0.98). Figure 4 is shown in the Fagan plot. The prior probability was 20%, and the post-test probability was 81% for LR-positive and 4% for LR-negative. The diagnostic accuracy for detecting 68Ga-PSMA PET/CT for LN staging in PCa was found to be generally better.
Fig. 2.
Forest plot of pooled sensitivity and specificity of 68Ga-PSMA PET/CT for lymph node staging in patient with prostate cancer
Fig. 3.

The SORC curve of 68Ga-PSMA PET/CT for lymph node staging in patient with prostate cancer
Fig. 4.
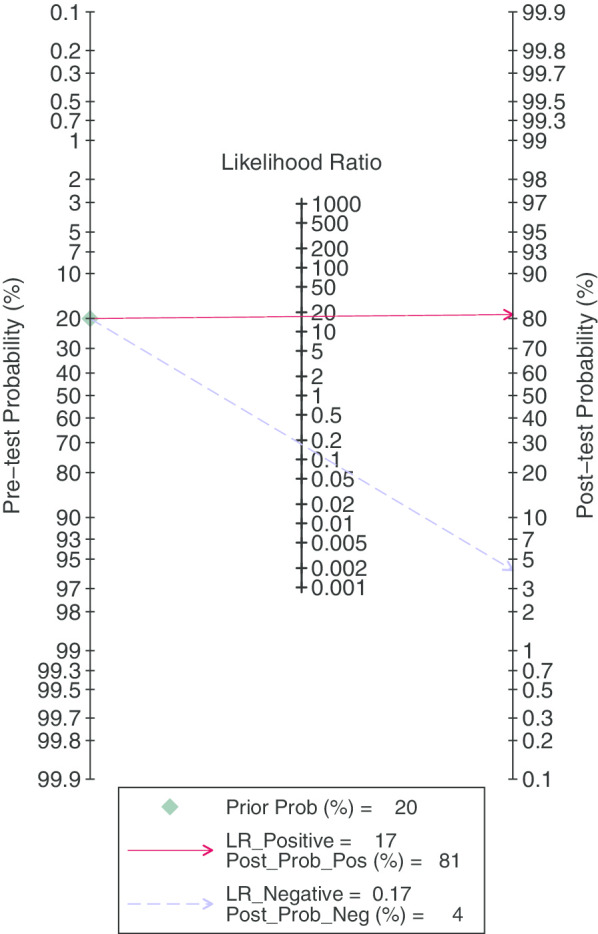
Fagan diagram evaluating the overall diagnostic value of 68Ga-PSMA PET/CT for lymph node staging in patient with prostate cancer
Publication bias
The publication bias (P = 0.02, Fig. 5) was shown in Deek’s funnel plot.
Fig. 5.
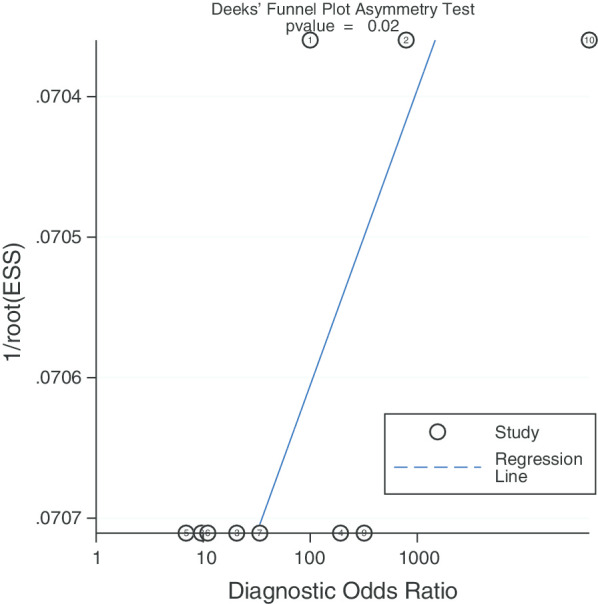
Deek’s funnel plot to evaluate the publication bias
Heterogeneity and sensitivity analysis
According to the results of the forest plot, the heterogeneity of 68Ga-PSMA PET/CT was high in both sensitivity (I2 = 97.25%) and specificity (I2 = 95.12%). Due to the small number of studies we included (n = 10) that prevented meta-regression from being implemented, a random-effects model was used to pool the data of 68Ga-PSMA PET/CT.
Meta regression and subgroup analysis
Meta-regression and subgroup analysis were performed on study design (predesign), gold standard selection and description (samemth and reftest), diagnostic test to be evaluated (index), and patient characteristics (subject). It can be seen from the forest plot that in the study we included, the five independent variables had no statistically significant effects on sensitivity and specificity (P > 0.05, Fig. 6).
Fig. 6.
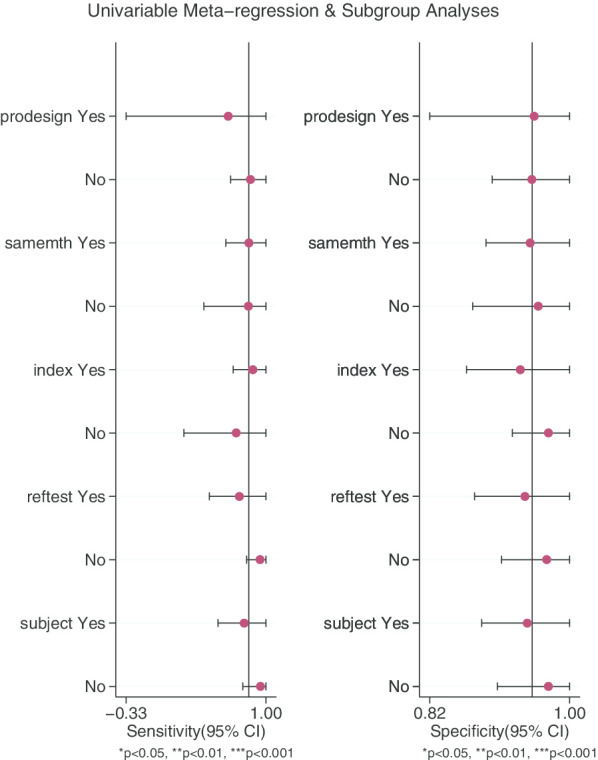
Meta regression and subgroup analysis
Discussion
Based on the currently published research, our meta-analysis fills the gap of 68Ga-PSMA PET/CT in the diagnosis of middle/high-risk prostate cancer lymph node staging to a certain extent. We found that the indicators and results of 68Ga-PSMA PET/CT show a satisfactory diagnostic efficacy.
In previous treatments, LN staging of prostate cancer patients can only be determined by performing PLND according to the situation during the operation. However, this method is not always feasible [27]. Clinically, the use of PLND is not always feasible, being restricted for the following reasons: not every patient will receive PLND, limited anatomical locations performed, and consideration regarding complications [28–30], resulting in suboptimal lymph node staging. 68Ga-PSMA PET/CT is a more accurate method to determine the LN stage of PCa patients before surgery [31], which can be incorporated into clinical practice. The common lymph node metastasis sites for prostate cancer are obturator lymph node, internal iliac lymph node, external iliac lymph node, common iliac lymph node, presacral lymph node, abdominal aortic lymph node and mediastinum, supraclavicular lymph node. Studies have shown that the most common lymph node metastasis sites for prostate cancer are external iliac lymph nodes and obturator lymph nodes, with the median percentages being 12.2% and 11.6%, respectively [32]. Based on the current evidence, the diagnostic performance of auxiliary imaging and nomograms tool for prostate cancer lymph node metastasis still has limitations, especially for lymph nodes < 5 mm. Preoperative assessment of the degree of lymph node metastasis can guide the selection of surgical procedures, which can further assist the management of patients after radical prostatectomy [33–35]. Therefore, it is particularly important to find a non-invasive and better diagnostic method.
Our meta-analysis included 10 studies from different countries and regions, involving a total of 701 patients. The pooled specificity of 0.95 (95% CI 0.87–0.98) for 68Ga-PSMA PET/CT. But pooled sensitivity was recorded as 0.84 (95% CI 0.55–0.95), which is not enough to convince us to give up the remaining 20% of the patient. The Youden index was 0.79. AUC was 0.97 (95% CI 0.95–0.98), which was in line with our initial predictions. Through these comprehensive indicators, we can think that 68Ga-PSMA PET/CT has better diagnostic efficacy in preoperative LN staging in patients with prostate cancer. In the past few decades, CT and MRI have been used to determine the LN staging before radical prostatectomy, but their accuracy rate is still low compared to the gold standard [9]. Hovels et al. Performed a meta-analysis of 24 studies to assess CT/MRI for preoperative evaluation of LN staging. In his research, for CT, pooled sensitivity and specificity was 0.42 (95% CI 0.26–0.56) and 0.82 (95% CI 0.8–0.83) respectively. For MRI, pooled sensitivity and specificity was 0.39 (95% CI 0.22–0.56) and 0.82 (95% CI 0.79–0.83) [36]. From this point of view, the performance of CT and MRI in judging LN staging is not satisfactory. If clinicians rely on CT or MRI, they will easily make the wrong decision on the patient’s condition. In our analysis, we could see that two studies have lower sensitivity, 0.38(95%: 0.28–0.48) and 0.33 (95% CI 0.24–0.43) respectively [13, 25]. The reason for this analysis was that, due to the technical level of the test, the sample size, and bias between the samples, it might have lead to different final results. The specificity value provided in one study is significantly lower [20]. We thought that the main reason is that the patients included in Herlemann’s [20] study received different PLNDs. Among them, 20 received primary PLND and 14 received secondary PLND, which may be the main reason for the lower specificity. Multi-parameter magnetic resonance (mpMR) also plays a large role in the preoperative evaluation of prostate cancer, especially in judging extraprostatic extension (EPE) of the tumor, invasion of the seminal vesicle (SVI) [23]. In a retrospective study by Van Leeuwen et al. They also compared the diagnostic accuracy of mpMR and 68Ga-PSMA PET/CT for LN metastasis in a patient with intermediate high-risk PCa. The sensitivity was 14% and 53%, respectively, and the specificity was 99% and 88%, respectively [2]. All of these indicated that 68Ga-PSMA PET/CT has better diagnostic efficacy and was expected to be popularized and used in clinical.
The higher the value of DOR, the better the diagnostic value of this diagnostic method. In our study, The DOR value was 100 (95% CI 18–545), indicating that the overall accuracy was high. Pooled PLR and NLR value was 17.19 (95% CI 6.27–47.17) and 0.17 (95% CI 0.05–0.56), respectively. This can be understood as the probability of 68Ga-PSMA PET/CT correctly judging LN metastasis is 17 times that of misjudging, and the probability of correctly judging LN non-metastasis is 0.17 times that of misjudging. At the same time, we also noticed that the publication bias shown by Deek’s funnel plot (Fig. 5) has a P-value of 0.02. It is understandable that most of the articles we included were retrospective trials, which led to the results. In addition, meta regression and subgroup analysis showed that there is no statistical difference between the above-mentioned influencing factors for the obtained sensitivity and specificity values, excluding the influence of other factors on the results. From the data results, it is worth trying to use 68Ga-PSMA PET/CT to diagnose LN staging in patients with PCa.
We perform this meta-analysis strictly according to PRISMA checklist [37]. However, there are still some limitations in our meta-analysis. First, most of the studies we included were single-center retrospective studies, and the existence of selection bias may affect our judgment. Second, the sample populations included in these articles are only Asia, Europe, and Australia, so population bias is unavoidable. Third, the sample size is too small. The current research is not registered, and there may be small deviations, but we still strictly follow the steps of systematic reviews. Due to the clinical application of 68Ga-PSMA PET/CT in the future, there is not a sufficiently large sample size to be included in our meta-analysis. Compared with PLND, although 68Ga-PSMA PET/CT has only moderate sensitivity and better specificity, it can perform relatively accurate LN staging of detected PCa patients. At this point, 68Ga-PSMA PET/CT is due to any other imaging examinations, but due to many limitations, our conclusions still require a larger sample size, multi-center prospective randomized controlled trial to verify.
Conclusion
Based on the current evidence, 68Ga-PSMA PET/CT is bound to have a strong performance in the diagnosis of medium/high-risk prostate cancer lymph node staging due to its relatively excellent diagnostic accuracy. However, in a short period of time in the future, limited by the capacity of different medical institutions and the development of regional economic levels, it will take some time to fully demonstrate its advantages in the field of prostate cancer. Further studies in 68Ga-PSMA PET/CT are needed, and efforts are warranted to improve understanding and intervention of these diagnostic deviations and effectiveness.
Acknowledgements
The authors thank Ms Xinnan Gou for providing continuous encouragement to Dr Lei Peng to pursue his career in medicine.
Authors’ contributions
Conceived and designed the experiments: YL. Analysed the data: LP, JL, CM. Contributed reagents/materials/analysis: JL, CY, DT, TW and WX. Wrote the manuscript: LP, and JL. All authors have read and approved the final manuscript.
Funding
This study was funded by National Natural Science Foundation of China, grant number: 81900617, application code: H0503, relying unit code: 63700708A0140-0268.
Availability of data and materials
All data generated and analysed during this study are included in this published article.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
There are no details on individuals reported within this manuscript, and no consent for publication was collected.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Peng, Jinze Li, Chunyang Meng have contributed equally to this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.van Leeuwen PJ, Donswijk M, Nandurkar R, Stricker P, Ho B, Heijmink S, Wit EMK, Tillier C, van Muilenkom E, Nguyen Q, et al. Gallium-68-prostate-specific membrane antigen ((68) Ga-PSMA) positron emission tomography (PET)/computed tomography (CT) predicts complete biochemical response from radical prostatectomy and lymph node dissection in intermediate- and high-risk prostate cancer. BJU Int. 2019;124(1):62–68. doi: 10.1111/bju.14506. [DOI] [PubMed] [Google Scholar]
- 3.Gandaglia G, Fossati N, Zaffuto E, Bandini M, Dell'Oglio P, Bravi CA, Fallara G, Pellegrino F, Nocera L, Karakiewicz PI, et al. Development and internal validation of a novel model to identify the candidates for extended pelvic lymph node dissection in prostate cancer. Eur Urol. 2017;72(4):632–640. doi: 10.1016/j.eururo.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 4.Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, Severens JL, Barentsz JO. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63(4):387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Fossati N, Willemse PM, Van den Broeck T, van den Bergh RCN, Yuan CY, Briers E, Bellmunt J, Bolla M, Cornford P, De Santis M, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: a systematic review. Eur Urol. 2017;72(1):84–109. doi: 10.1016/j.eururo.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Harbin AC, Eun DD. The role of extended pelvic lymphadenectomy with radical prostatectomy for high-risk prostate cancer. Urol Oncol. 2015;33(5):208–216. doi: 10.1016/j.urolonc.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Heesakkers RAM, Hövels AM, Jager GJ, van den Bosch HCM, Witjes JA, Raat HPJ, Severens JL, Adang EMM, van der Kaa CH, Fütterer JJ, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 2008;9(9):850–856. doi: 10.1016/S1470-2045(08)70203-1. [DOI] [PubMed] [Google Scholar]
- 8.Silberstein JL, Laudone VP. Pelvic Lymph Node Dissection for Prostate Cancer. In: Radical Prostatectomy, edn.. 2014. pp. 57–74.
- 9.Mottet N, van den Bergh RCN, Briers E, Bourke L, Cornford P, De Santis M, Gillessen S, Govorov A, Grummet J, Henry AM, et al. EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer 2018. In: European Association of Urology Guidelines 2018 Edition. Volume presented at the EAU Annual Congress Copenhagen 2018, edn. Arnhem, The Netherlands: European Association of Urology Guidelines Office; 2018.
- 10.Luiting HB, van Leeuwen PJ, Busstra MB, Brabander T, van der Poel HG, Donswijk ML, Vis AN, Emmett L, Stricker PD, Roobol MJ. Use of gallium-68 prostate-specific membrane antigen positron-emission tomography for detecting lymph node metastases in primary and recurrent prostate cancer and location of recurrence after radical prostatectomy: an overview of the current literature. BJU Int. 2020;125(2):206–214. doi: 10.1111/bju.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchelouche K, Choyke PL, Capala J. Prostate specific membrane antigen—a target for imaging and therapy with radionuclides. Discov Med. 2010;9(44):55–61. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Zang S, Zhang C, Fu Y, Lv X, Zhang Q, Deng Y, Zhang C, Luo R, Zhao X, et al. Comparison of (68)Ga-PSMA-11 PET-CT with mpMRI for preoperative lymph node staging in patients with intermediate to high-risk prostate cancer. J Transl Med. 2017;15(1):230. doi: 10.1186/s12967-017-1333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaxley JW, Raveenthiran S, Nouhaud FX, Samartunga H, Yaxley AJ, Coughlin G, Delahunt B, Egevad L, McEwan L, Wong D. Outcomes of primary lymph node staging of intermediate and high risk prostate cancer with (68)Ga-PSMA positron emission tomography/computerized tomography compared to histological correlation of pelvic lymph node pathology. J Urol. 2019;201(4):815–820. doi: 10.1097/JU.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 14.Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, Debus N, Eder M, Eisenhut M, Schafer M, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44(8):1258–1268. doi: 10.1007/s00259-017-3711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nuclear Med Mol Imaging. 2012;39(6):1085–1086. doi: 10.1007/s00259-012-2069-0. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Hamza TH, Arends LR, van Houwelingen HC, Stijnen T. Multivariate random effects meta-analysis of diagnostic tests with multiple thresholds. BMC Med Res Methodol. 2009;9:73. doi: 10.1186/1471-2288-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song F, Khan KS, Dinnes J, Sutton AJ. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol. 2002;31(1):88–95. doi: 10.1093/ije/31.1.88. [DOI] [PubMed] [Google Scholar]
- 20.Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, Gildehaus FJ, Stief CG, Gratzke C, Fendler WP. (68)Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016;70(4):553–557. doi: 10.1016/j.eururo.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 21.Obek C, Doganca T, Demirci E, Ocak M, Kural AR, Yildirim A, Yucetas U, Demirdag C, Erdogan SM, Kabasakal L, et al. The accuracy of (68)Ga-PSMA PET/CT in primary lymph node staging in high-risk prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44(11):1806–1812. doi: 10.1007/s00259-017-3752-y. [DOI] [PubMed] [Google Scholar]
- 22.Gupta M, Choudhury PS, Hazarika D, Rawal S. A comparative study of (68)Gallium-prostate specific membrane antigen positron emission tomography-computed tomography and magnetic resonance imaging for lymph node staging in high risk prostate cancer patients: an initial experience. World J Nucl Med. 2017;16(3):186–191. doi: 10.4103/1450-1147.207272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz B, Turkay R, Colakoglu Y, Baytekin HF, Ergul N, Sahin S, Tugcu V, Inci E, Tasci AI, Cermik TF. Comparison of preoperative locoregional Ga-68 PSMA-11 PET-CT and mp-MRI results with postoperative histopathology of prostate cancer. Prostate. 2019;79(9):1007–1017. doi: 10.1002/pros.23812. [DOI] [PubMed] [Google Scholar]
- 24.Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, Wester HJ, Heck M, Kubler H, Beer AJ, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195(5):1436–1443. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Budaus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, Graefen M, Steuber T, Rosenbaum C. Initial Experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69(3):393–396. doi: 10.1016/j.eururo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 26.van Leeuwen PJ, Emmett L, Ho B, Delprado W, Ting F, Nguyen Q, Stricker PD. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017;119(2):209–215. doi: 10.1111/bju.13540. [DOI] [PubMed] [Google Scholar]
- 27.Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, Fossati N, Bianchi M, Boorjian SA, Sun M, Karakiewicz PI, et al. Predicting survival of patients with node-positive prostate cancer following multimodal treatment. Eur Urol. 2014;65(3):554–562. doi: 10.1016/j.eururo.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Abdollah F, Sun M, Thuret R, Budäus L, Jeldres C, Graefen M, Briganti A, Perrotte P, Rigatti P, Montorsi F, et al. Decreasing rate and extent of lymph node staging in patients undergoing radical prostatectomy may undermine the rate of diagnosis of lymph node metastases in prostate cancer. Eur Urol. 2010;58(6):882–892. doi: 10.1016/j.eururo.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Galper SL, Chen MH, Catalona WJ, Roehl KA, Richie JP, D'Amico AV. Evidence to support a continued stage migration and decrease in prostate cancer specific mortality. J Urol. 2006;175(3 Pt 1):907–912. doi: 10.1016/S0022-5347(05)00419-2. [DOI] [PubMed] [Google Scholar]
- 30.Kawakami J, Meng MV, Sadetsky N, Latini DM, Duchane J, Carroll PR. Changing patterns of pelvic lymphadenectomy for prostate cancer: results from CaPSURE. J Urol. 2006;176(4 Pt 1):1382–1386. doi: 10.1016/j.juro.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Mandel P, Tilki D, Chun FK, Pristupa E, Graefen M, Klutmann S, Budaus L, Steuber T. Accuracy of (68)Ga-prostate-specific membrane antigen positron emission tomography for the detection of lymph node metastases before salvage lymphadenectomy. Eur Urol Focus. 2020;6(1):71–73. doi: 10.1016/j.euf.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Grivas N, van den Bergh RCN, Brouwer OR, KleinJan GH, Ramirez-Backhaus M, Wilthagen EA, van der Poel HG. Pelvic lymph node distribution and metastases of prostate and bladder cancer: a systematic literature review and template proposal. World J Urol. 2020 doi: 10.1007/s00345-020-03281-1. [DOI] [PubMed] [Google Scholar]
- 33.Luchini C, Fleischmann A, Boormans JL, Fassan M, Nottegar A, Lucato P, Stubbs B, Solmi M, Porcaro A, Veronese N, et al. Extranodal extension of lymph node metastasis influences recurrence in prostate cancer: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2374. doi: 10.1038/s41598-017-02577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motterle G, Ahmed ME, Andrews JR, Karnes RJ. The role of radical prostatectomy and lymph node dissection in clinically node positive patients. Front Oncol. 2019;9:1395. doi: 10.3389/fonc.2019.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosiello G, Bandini M, Briganti A. Salvage pelvic lymph node dissection for lymph node recurrent prostate cancer. Curr Opin Urol. 2019;29(6):629–635. doi: 10.1097/MOU.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 36.Hövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, Severens JL, Barentsz JO. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63(4):387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analysed during this study are included in this published article.