Abstract
Background
Cancer outcomes are complex, involving prevention, early detection and optimal multidisciplinary care. Postoperative infection and surgical site‐infection (SSI) are not only uncomfortable for patients and costly, but may also be associated with poor oncological outcomes. A meta‐analysis was undertaken to assess the oncological effects of SSI in patients with colorectal cancer.
Methods
An ethically approved PROSPERO‐registered meta‐analysis was conducted following PRISMA guidelines. PubMed and Scopus databases were searched for studies published between 2007 and 2017 reporting the effects of postoperative infective complications on oncological survival in colorectal cancer. Results were separated into those for SSI and those concerning anastomotic leakage. Articles with a Methodological Index for Non‐Randomized Studies score of at least 18 were included. Hazard ratios (HRs) with 95 per cent confidence intervals were computed for risk factors using an observed to expected and variance fixed‐effect model.
Results
Of 5027 articles were reviewed, 43 met the inclusion criteria, with a total of 154 981 patients. Infective complications had significant negative effects on overall survival (HR 1·37, 95 per cent c.i. 1·28 to 1·46) and cancer‐specific survival (HR 2·58, 2·15 to 3·10). Anastomotic leakage occurred in 7·4 per cent and had a significant negative impact on disease‐free survival (HR 1·14, 1·09 to 1·20), overall survival (HR 1·34, 1·28 to 1·39), cancer‐specific survival (HR 1·43, 1·31 to 1·55), local recurrence (HR 1·18, 1·06 to 1·32) and overall recurrence (HR 1·46, 1·27 to 1·68).
Conclusion
This meta‐analysis identified a significant negative impact of postoperative infective complications on overall and cancer‐specific survival in patients undergoing colorectal surgery.
Postoperative infective complications have significant negative effects on oncological outcomes in colorectal cancer. Anastomotic leakage has a negative effect on every measured outcome. New registries should include oncological outcomes and complications to facilitate future research and optimize outcomes.

Colorectal cancer surgery infectious complications affect overall and cancer‐specific survival
Antecedentes
Los resultados del cáncer son complejos, implican prevención, detección precoz y atención multidisciplinaria óptima. La infección postoperatoria y la infección del sitio quirúrgico (surgical site infection, SSI) no solo son inconvenientes para los pacientes y costosas, sino que también pueden estar asociadas con malos resultados oncológicos. Este estudio realizó un metaanálisis para evaluar los efectos oncológicos de la SSI en pacientes con cáncer colorrectal.
Métodos
Se realizó un metaanálisis registrado en PROSPERO, aprobado por el comité ético, siguiendo las pautas de PRISMA y utilizando las bases de datos PubMed y Scopus para estudios entre 2007‐2017 que describían los efectos de las complicaciones infecciosas postoperatorias en la supervivencia oncológica en el cáncer colorrectal. Los resultados se separaron para el grupo de infección del sitio quirúrgico (SSI) y de fuga anastomótica. Se incluyeron los artículos con una puntuación ≥ 18 según el índice MINORS. Para los factores de riesgo se calcularon los cocientes de riesgos instantáneos (hazard ratios, HR) mediante un modelo de efectos aleatorios y el método de Mantel‐Haenszel con los i.c. del 95% utilizando el programe RevMan5.
Resultados
Se revisaron 5.027 artículos de los cuales 43 cumplieron con los criterios de inclusión. En total fueron 154.981 pacientes en los cuales las complicaciones infecciosas tuvieron efectos negativos significativos en la supervivencia global (HR: 1,37 i.c. del 95%: 1,28‐1,46) y la supervivencia específica relacionada con el cáncer (HR: 2,58 i.c. del 95%: 2,15‐3,10). La fuga anastomótica ocurrió en un 7,4% de los casos e impactó negativa y significativamente en la supervivencia libre de enfermedad (HR: 1,14 i.c. del 95%: 1,09‐1,20), en la supervivencia global (HR: 1,34 i.c. del 95%: 1,28‐1,39), en la supervivencia específica relacionada con el cáncer (HR: 1,43 i.c. del 95% 1.31‐1.55), en la recidiva local (HR: 1,18 i.c. del 95%: 1,06‐1,32) y en la recidiva global (HR: 1,46 i.c. del 95%: 1,27‐1,68).
Conclusión
Este metaanálisis identificó un impacto negativo significativo en la supervivencia global y en la supervivencia específica relacionada con el cáncer en pacientes con complicaciones postoperatorias infecciosas sometidos a cirugía colorrectal.
Introduction
Colorectal cancer affects 17 people per 100 000 worldwide and 30 per 100 000 in Europe 1 , with an average 5‐year survival rate of 65 per cent 2 . Optimizing cancer outcomes is a complex interaction involving key strategies: prevention, early detection and optimal management 3 . Many treatment paradigm shifts in both surgical and oncological treatment have improved cancer outcomes. Recurrence, which affects over 40 per cent of patients, has classically been associated with tumour stage, grade, emergency presentation and resection margin status 4 , 5 .
Surgical‐site infections (SSIs), including superficial, deep and organ space infections, are coming increasingly under the spotlight, causing discomfort for patients and family, anxiety for surgeons, and cost to healthcare systems 6 . In addition, they are associated with potential delay in, or omission of, adjuvant therapy.
A recent long‐term analysis from the German Rectal Cancer Trial 7 suggested that surgical complications were associated with both oncological and overall outcomes. Immunological forces influence survival 8 . As SSI occurs in approximately 15 per cent of patients undergoing colorectal surgery, a clear understanding of any adverse relationship is important 9 .
Although surgeons and patients alike fear the morbidity and mortality associated with postoperative complications, their potential negative impact on oncological outcomes is not widely understood or reported routinely 10 , 11 . A meta‐analysis was undertaken to determine the impact of postoperative infections on oncological outcomes in colorectal cancer surgery.
Methods
A study was conducted to assess the impact of postoperative infective complications on oncological outcomes in colorectal cancer surgery. The study was registered with PROSPERO (registration number: 42017069038) and followed PRISMA guidelines 12 . PubMed and Scopus were searched for studies that met the eligibility criteria. Original articles, published between June 2007 and May 2017, which reported the effect of infective complications on oncological survival in both colonic and rectal cancer were identified. The search strategy used the following keywords: Colon Cancer, Colorectal Cancer, Rectal Cancer, Complication, Infection, Oncological Outcomes, Anastomotic Leak, Survival and SSI. Animal studies, review articles, non‐English papers, duplicate data sets and results published only in abstracts were excluded. Details of the search strategy and data management are available in Tables S1 and S2 (supporting information).
Data extraction and quality assessment
The abstracts were screened by one author and full texts by three authors. The descriptive and quantitative data from the screened studies were extracted and papers were graded using the Methodological Index for Non‐Randomized Studies (MINORS) 13 . The MINORS criteria have been designed to assess the quality of comparative and non‐comparative surgical studies using a three‐point scale (0, not reported; 1, reported but inadequate; 2, reported and adequate), with assessment of eight items for non‐comparative studies and 12 items for comparative studies. The ideal global scores for comparative and non‐comparative studies are 24 and 16 respectively.
Articles were graded by three reviewers initially, and only those that scored at least 18 of 24 were included in the statistical analysis. If there was disagreement on whether a paper should be included or not, another reviewer graded it and made the final decision. At the outset both rectal and colonic cancer procedures were grouped into a single category.
Results were separated into two key categories: infective complications (SSI, organ space infections, infectious complications, sepsis) and anastomotic leakage. SSI was defined according to the Centers for Disease Control and Prevention 14 definition, whereas anastomotic leak was defined as reported in each article.
Overall survival, disease‐free survival, cancer‐specific survival and cancer recurrence data were analysed for each outcome where data were available and applicable. Survival terms were defined in accordance with National Institutes of Health–National Cancer Institute definitions 15 .
Statistical analysis
For oncological outcomes, hazard ratios (HRs) were taken from papers or calculated using the MedCalc® statistical calculator (MedCalc, Ostend, Belgium). Observed minus expected (O‐E) values and variance were calculated 16 , and used to compute statistical values for use in the analysis.
Statistical analysis was performed in Review Manager (RevMan) version 5 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) using O‐E and variance, a fixed‐effect model for analysis and HR as effect measure, with 95 per cent confidence intervals. Significance was assessed at the two‐sided 5 per cent level using HRs. The complication has a significant effect on the measured oncological outcome if the 95 per cent confidence interval of the HR does not include 1·00.
Results
A total of 5027 individual articles were reviewed in this study (Fig. 1 ), of which 145 were found to be relevant and underwent MINORS grading. Forty‐three articles 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 met all inclusion criteria and were used in the data analysis, with a total cohort size of 154 981 patients (Table 1 ). Publications were from the USA (7), Korea (5), the UK (4), Japan (4), China (4), Germany (4) and other countries (15). There were 23 retrospective and 20 prospective studies in this meta‐analysis. Ten studies were from multicentre databases (6 prospective, 4 retrospective).
Figure 1.
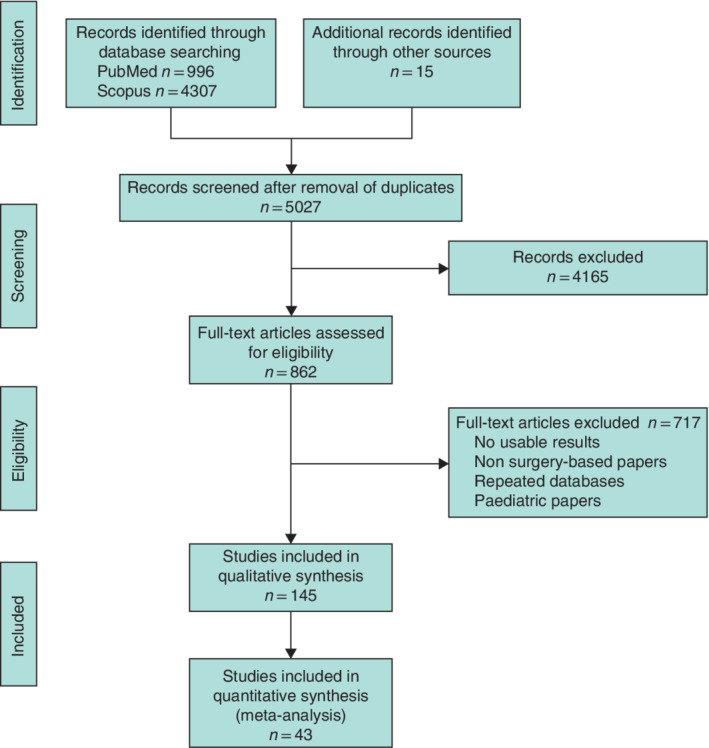
PRISMA flow chart showing selection of articles for review
Table 1.
Study characteristics
| Reference | Country | Study design | Multicentre database study | No. of patients | Anastomotic leak |
|---|---|---|---|---|---|
| Bertelsen et al. 17 | Denmark | Prospective | Yes | 1494 | 163 (10·9) |
| Cone et al. 18 | USA | Prospective | Yes | 24 730 | |
| Espín et al. 19 | Spain | Prospective | Yes | 1181 | 100 (8·5) |
| Jörgren et al. 20 | Sweden | Prospective | Yes | 1977 | 172 (8·7) |
| Krarup et al. 21 | Denmark | Prospective | Yes | 9333 | 593 (6·4) |
| Kube et al. 22 | Germany | Prospective | Yes | 28 271 | 844 (3·0) |
| Aquina et al. 23 | USA | Retrospective | Yes | 24 426 | |
| Artinyan et al. 24 | USA | Retrospective | Yes | 12 075 | |
| Chu et al. 25 | USA | Retrospective | Yes | 528 | |
| Nordholm‐Carstensen et al. 26 | Denmark | Retrospective | Yes | 774 | 71 (9·2) |
| Boccola et al. 27 | Australia | Prospective | No | 1576 | 110 (7·0) |
| Duron et al. 28 | France | Prospective | No | 3322 | |
| Eberhardt et al. 29 | USA | Prospective | No | 177 | 59 (33·3) |
| Gong et al. 30 | China | Prospective | No | 460 | 35 (7·6) |
| Gupta et al. 31 | Nepal | Prospective | No | 272 | 18 (6·6) |
| Jannasch et al. 32 | Germany | Prospective | No | 17 867 | 2134 (11·9) |
| Law et al. 33 | China | Prospective | No | 1657 | 47 (2·8) |
| Law et al. 34 | China | Prospective | No | 1580 | 60 (3·8) |
| Platt et al. 35 | UK | Prospective | No | 454 | |
| Ptok et al. 36 | Germany | Prospective | No | 2044 | 303 (14·8) |
| Richards et al. 37 | UK | Prospective | No | 423 | 18 (4·3) |
| Smith et al. 38 | USA | Prospective | No | 1127 | 40 (3·5) |
| Smith et al. 39 | USA | Prospective | No | 184 | 12 (6·5) |
| Thorgersen et al. 40 | Norway | Prospective | No | 540 | |
| Attiê et al. 41 | Brazil | Retrospective | No | 106 | |
| Ebinger et al. 42 | Switzerland | Retrospective | No | 584 | 64 (11·0) |
| Goto et al. 43 | Japan | Retrospective | No | 3364 | 85 (2·5) |
| Haruki et al. 44 | Japan | Retrospective | No | 77 | |
| Huang et al. 45 | China | Retrospective | No | 215 | |
| Jung et al. 46 | Korea | Retrospective | No | 1391 | 35 (2·5) |
| Kang et al. 47 | Korea | Retrospective | No | 1083 | 69 (6·4) |
| Katoh et al. 48 | Japan | Retrospective | No | 1101 | |
| Kerin Povšič et al. 49 | Slovenia | Retrospective | No | 186 | |
| Kulu et al. 50 | Germany | Retrospective | No | 570 | 51 (8·9) |
| Lee et al. 51 | Korea | Retrospective | No | 1278 | 51 (4·0) |
| Lim et al. 52 | Korea | Retrospective | No | 2510 | 141 (5·6) |
| Marra et al. 53 | Switzerland | Retrospective | No | 445 | 12 (2·7) |
| McMillan et al. 54 | UK | Retrospective | No | 920 | 24 (2·6) |
| Miccini et al. 55 | Italy | Retrospective | No | 479 | 34 (7·1) |
| Mrak et al. 56 | Austria | Retrospective | No | 811 | 54 (6·7) |
| Nachiappan et al. 57 | UK | Retrospective | No | 1048 | 99 (9·4) |
| Noh et al. 58 | Korea | Retrospective | No | 1258 | 101 (8·0) |
| Tsujimoto et al. 59 | Japan | Retrospective | No | 1083 | 29 (2·7) |
| Total | 154 981 |
7·4 (2·5–33·3)%* |
Values in parentheses are percentages unless indicated otherwise; *values are mean (range).
Non‐anastomotic infective complications
Sixteen papers reported SSI data that allowed meaningful analysis. Of these, 11 of 16 papers contained data on overall survival. Three 37 , 40 , 44 of 11 articles reported disease‐free survival and two 23 , 41 of 11 articles cancer‐specific survival. Infective complications were shown to have a significant negative effect on overall survival (HR 1·37, 95 per cent c.i. 1·28 to 1·46) (Fig. 2 ) and cancer‐specific survival (HR 2·58, 2·15 to 3·10). However, there was no significant association between infective complications and disease‐free survival (HR 0·89, 0·74 to 1·08).
Figure 2.
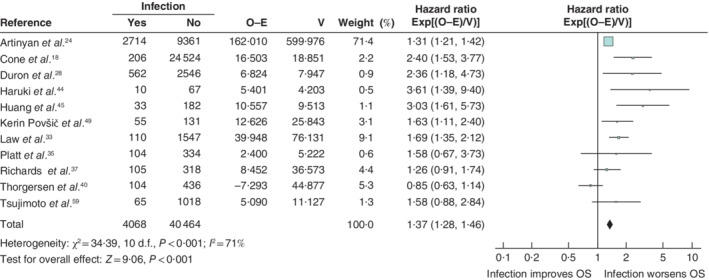
Impact of surgical‐site infection on overall survival Hazard ratios are shown with 95 per cent confidence intervals. A fixed‐effect model was used for meta‐analysis. O‐E, observed to expected; V, variance; OS, overall survival.
Anastomotic leakage
Anastomotic leakage data were suitable for analysis in 31 publications. The mean leak rate was 7·4 (range 2·5–33·3) per cent (Table 1 ). The effect of anastomotic leakage on overall survival could be assessed in 24 articles, and its effect on disease‐free survival in ten of 31 studies. Cancer‐specific survival was reported in ten of 31 articles. Nineteen of the 31 articles reported on local recurrence and ten on overall recurrence.
Anastomotic leakage had a negative impact on overall survival (HR 1·34, 95 per cent c.i. 1·28 to 1·39) (Fig. 3 ), disease‐free survival (HR 1·14, 1·09 to 1·20) (Fig. 4 ), cancer‐specific survival (HR 1·43, 1·31 to 1·55) (Fig. 5), local recurrence (HR 1·18, 1·06 to 1·32) (Fig. 6 ) and overall recurrence (HR 1·46, 1·27 to 1·68) (Fig. 7 ).
Figure 3.
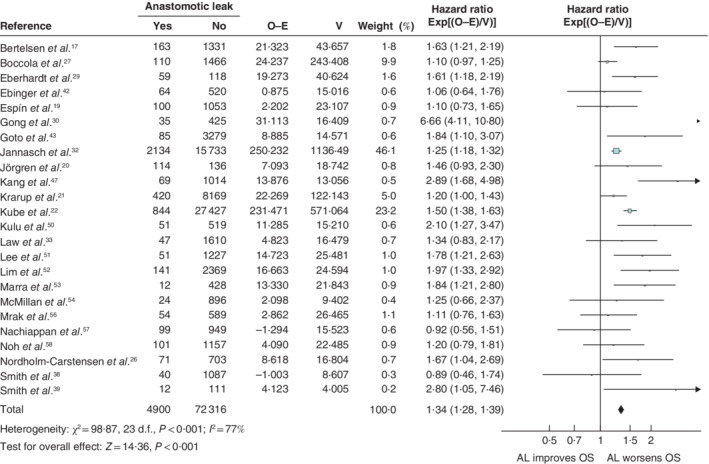
Impact of anastomotic leakage on overall survival Hazard ratios are shown with 95 per cent confidence intervals. A fixed‐effect model was used for meta‐analysis. O‐E, observed to expected; V, variance; OS, overall survival.
Figure 4.
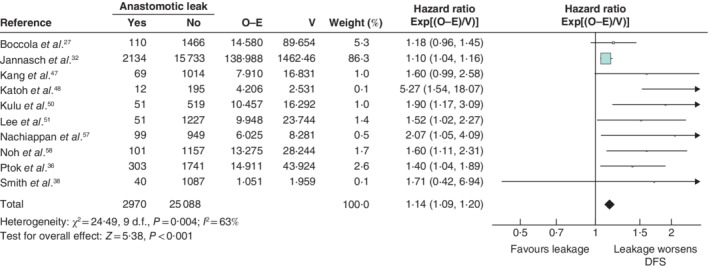
Impact of anastomotic leakage on disease‐free survival Hazard ratios are shown with 95 per cent confidence intervals. A fixed‐effect model was used for meta‐analysis. O‐E, observed to expected; V, variance; AL, anastomotic leak; DFS, disease‐free survival.
Figure 5.
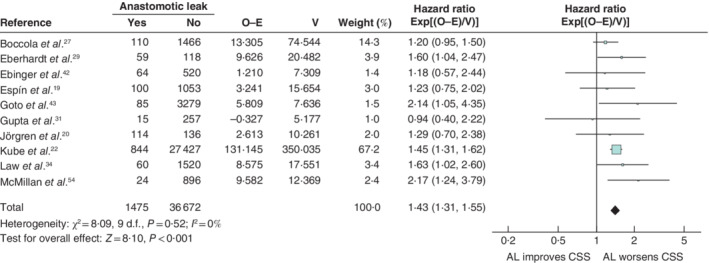
Impact of anastomotic leakage on cancer‐specific survival Hazard ratios are shown with 95 per cent confidence intervals. A fixed‐effect model was used for meta‐analysis. O‐E, observed to expected; V, variance; AL, anastomotic leak; CSS, cancer‐specific survival.
Figure 6.
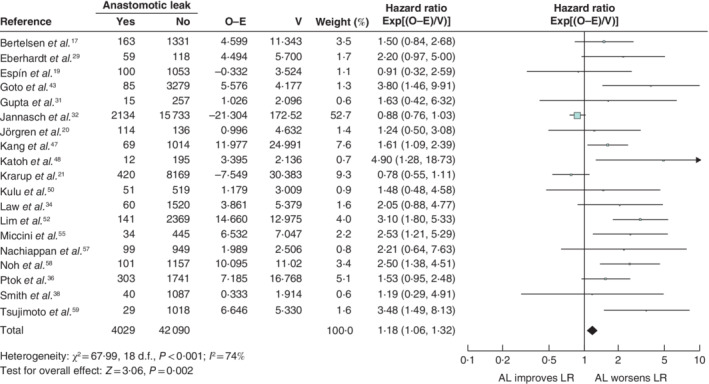
Impact of anastomotic leakage on local recurrence Hazard ratios are shown with 95 per cent confidence intervals. A fixed‐effect model was used for meta‐analysis. O‐E, observed to expected; V, variance; AL, anastomotic leak; LR, local recurrence.
Figure 7.
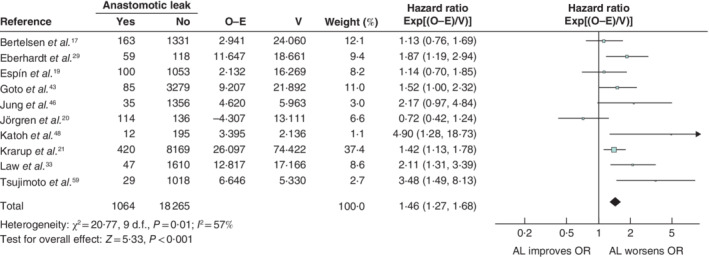
Impact of anastomotic leakage on overall recurrence Hazard ratios are shown with 95 per cent confidence intervals. A fixed‐effect model was used for meta‐analysis. O‐E, observed to expected; V, variance; AL, anastomotic leak.
Discussion
This meta‐analysis of 154 981 patients in 43 studies evaluated the impact of both wound‐related non‐anastomotic infective complications and anastomotic leakage, and identified a statistically significant negative oncological effect.
From the outset of this extensive literature review there were a number of limitations. In the overall cohort, narrowed by the quality of data and MINORS analysis, there was significant heterogeneity. SSI definitions are problematic, with variation from study to study. This is unfortunately common in all forms of surgery. In a 20‐year period up to 2015, only 18 per cent of the top 50 cited peer‐reviewed publications on ventral hernia were found to use a standardized definition of SSI and surgical‐site occurrence after ventral hernia repair 60 , 61 . The absence of a common language impedes comparisons in the literature and accurate metrics of hospital quality measures 60 . In addition, the period of surveillance used to report SSI varies between 30 and 60 days 42 , 60 . Anastomotic leak itself has a heterogeneous spectrum of presentation, depending on the effort made to detect leakage and the criteria used, whether based on combined clinical, radiological or endoscopic features. This may give rise to heterogeneity representing a potential limitation of this meta‐analysis. Few articles, in general, addressed the effect of SSI on oncological outcomes; some evaluated overall survival, a few reported disease‐free survival and none considered the recurrence rate. Furthermore, owing to the limited numbers of papers, it was not possible to undertake a subset analysis for different stages of colorectal cancer, nor to differentiate between colonic and rectal cancers.
The mean leak rate was 7·4 per cent across the 31 articles included in the analysis of anastomotic leak; this is in keeping with the mean leak rate in international data 62 . Anastomotic leakage is increasingly topical; there have been paradigm shifts in surgical, prehabilitation, intraoperative and postoperative approaches to reducing leakage 62 , 63 , 64 .
This meta‐analysis reinforces the findings of a meta‐analysis 65 in 2016, which showed that complication severity had a significant impact on both disease‐free and overall survival. Three other studies 66 , 67 , 68 identified a negative impact of anastomotic leakage on long‐term cancer‐specific survival, particularly noting an increase in local recurrence. Current efforts at SSI management after colorectal surgery focus on compliance with guidelines and evaluation of infection rates, but Gantz and colleagues 69 recently suggested that improvement is needed. Martinez et al. 70 suggested establishing national SSI bundles. Historically, mechanical and oral bowel preparations were favoured, but then bowel preparation went out of vogue. Now there is the potential for reintroduction of bowel cleansing and recognition of the importance of other factors including those relating to the gut microbiome. The gut microbiome potentially has an effect on infection and also a separate oncological effect. A variety of environmental factors, including diet, antibiotics, bowel preparation and surgical stress, act on the microbiome, altering its architecture and function, with a negative effect on oncological outcomes after surgery 71 . It is clear from the present data that anastomotic leakage is associated with increased local recurrence and decreased overall survival. The recent German rectal trial CAO/ARO/AIO‐94 7 showed that surgical complications are significantly associated with reduced overall survival. Patients with complications are more likely to have distant metastasis and local recurrences. The reason for this is somewhat unclear, although it is known that cancer cells shed from the bowel may embed themselves on stapling devices, leading to enhanced tumour dissemination in the event of anastomotic leak or reoperation. Exfoliated cancer cells have been detected in the colonic lumen and on stapling devices, suggesting that anastomotic leakage could enhance dissemination 72 , 73 .
There are many confounders to the potential negative oncological effects of infection. Systemic inflammation has been shown to promote micrometastasis 74 . An infection‐led inflammatory cascade will activate cytokines, and cell‐ and humoral‐mediated immunity.
Local recurrence is an important clinical outcome for patients with colorectal cancer; many treatment modalities have been investigated with the aim of reducing pelvic occurrence from total mesorectal excision to neoadjuvant chemoradiotherapy. The present study has identified that additional measures and routine use of SSI prevention bundles need to be implemented to reduce infective complications 75 . Infection prevention should become a potential target for oncological improvement; opportunities to reduce deep wound infection need to be revisited, incorporating wound bundles, intraoperative protective measures such as use of wound protectors, potential antibiotic solution and rectal washouts, and closer monitoring with intra‐abdominal pressure measurement after surgery.
This study had a number of limitations. An initial trawl of the literature identified almost 13 000 potential publications. On deeper analysis, including qualitative evaluation using the MINORS criteria, it was found that many of these papers lacked a definition of either SSI or anastomotic leakage 60 , 61 and, most importantly, no relationship between adverse events and oncological outcome was reported. In contrast, it is increasingly being recognized in other fields of oncology, such as breast cancer, that there may be a relationship between infection and cancer recurrence 76 . Surprisingly SSI data have not been included in cancer registries. Uniform data definitions and data analysis would make analysis easier. The small number of papers reporting infective complications may have led to bias in the present results. Subset analysis of SSI effects at different cancer stages was not possible.
This meta‐analysis has identified a statistically significant association between both anastomotic leak and wound infection/SSI and adverse oncological outcomes. Oncological registries incorporating infective and adverse events as part of their outcome analysis may help in understanding the relationship between SSI and oncological outcomes. Reduction in SSI may prove to be a noteworthy part of adjuvant cancer therapy, and wound bundles should become mandatory. There needs to be greater adoption and monitoring of strategies that might reduce SSIs and their negative impact.
Acknowledgements
M.B. is supported by the European Union's INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB) and the Dr George Moore Endowment for Data Science at Ulster University.
Disclosure: The authors declare no conflict of interest.
Supporting information
Table S1 Advanced search strategies and Boolean characters used across the various databases
Table S2 Data Management
Funding information
No funding
References
- 1. Dušek L, Mužík J, Malúšková D, Šnajdrová L. Epidemiology of Colorectal Cancer: International Comparison ; 2015. http://www.crcprevention.eu/index.php?pg=colorectal‐cancer‐epidemiology [accessed 4 January 2019].
- 2. National Cancer Institute . Surveillance, epidemiology, and End Results Program. Cancer Stat Facts: Colorectal Cancer. https://seer.cancer.gov/statfacts/html/colorect.html [accessed 4 January 2019]. [Google Scholar]
- 3. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C et al Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO‐94 randomized phase III trial after a median follow‐up of 11 years. J Clin Oncol 2012; 30: 1926–1933. [DOI] [PubMed] [Google Scholar]
- 4. Renouf DJ, Woods R, Speers C, Hay J, Phang PT, Fitzgerald C et al Improvements in 5‐year outcomes of stage II/III disease for rectal cancer relative to colon cancer. Am J Clin Oncol 2013; 36: 558–564. [DOI] [PubMed] [Google Scholar]
- 5. Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998; 133: 894–898. [DOI] [PubMed] [Google Scholar]
- 6. Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017; 96: 1–15. [DOI] [PubMed] [Google Scholar]
- 7. Sprenger T, Beißbarth T, Sauer R, Tschmelitsch J, Fietkau R, Liersch T et al Long‐term prognostic impact of surgical complications in the German Rectal Cancer Trial CAO/ARO/AIO‐94. Br J Surg 2018; 105: 1510–1518. [DOI] [PubMed] [Google Scholar]
- 8. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357: 539–545. [DOI] [PubMed] [Google Scholar]
- 9. Hawkins AT, Berger DL, Shellito PC, Sylla P, Bordeianou L. Wound dehiscence after abdominoperineal resection for low rectal cancer is associated with decreased survival. Dis Colon Rectum 2014; 57: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin D, Hübner M, Moulin E, Pache B, Clerc D, Hahnloser D et al Timing, diagnosis and treatment of surgical site infections after colonic surgery – prospective surveillance of 1263 patients. J Hosp Infect 2018; 100: 393–399. [DOI] [PubMed] [Google Scholar]
- 11. Sparreboom CL, Wu Z, Lingsma HF, Menon AG, Kleinrensink GJ, Nuyttens JJ et al; Dutch ColoRectal Audit Group. Anastomotic leakage and interval between preoperative short‐course radiotherapy and operation for rectal cancer. J Am Coll Surg 2018; 227: 223–231. [DOI] [PubMed] [Google Scholar]
- 12. PRISMA . Preferred Reporting Items for Systematic Reviews and Meta‐Analyses . http://prisma‐statement.org/ [accessed 24 June 2017].
- 13. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 14. Network National Healthcare Safety, Centers for Disease Control and Prevention. Surgical Site Infection (SSI) Event; 2017. https://web.archive.org/web/20170211232908/https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf [accessed 25 January 2017]. [Google Scholar]
- 15. National Cancer Institute . NCI Dictionary of Cancer Terms . http://www.cancer.gov/publications/dictionaries/cancer‐terms [accessed 24 July 2018].
- 16. Tierney J, Stewart L, Ghersi D, Burdett S, Sydes M. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bertelsen CA, Andreasen AH, Jørgensen T, Harling H; Danish Colorectal Cancer Group . Anastomotic leakage after curative anterior resection for rectal cancer: short and long‐term outcome. Colorectal Dis 2010; 12: e76–e81. [DOI] [PubMed] [Google Scholar]
- 18. Cone MM, Herzig DO, Diggs BS, Rea JD, Hardiman KM, Lu KC. Effect of surgical approach on 30‐day mortality and morbidity after elective colectomy: a NSQIP study. J Gastrointest Surg 2012; 16: 1212–1217. [DOI] [PubMed] [Google Scholar]
- 19. Espín E, Ciga MA, Pera M, Ortiz H, Lujan J, Fraccalvieri D et al; Spanish Rectal Cancer Project. Oncological outcome following anastomotic leak in rectal surgery. Br J Surg 2015; 102: 416–422. [DOI] [PubMed] [Google Scholar]
- 20. Jörgren F, Johansson R, Damber L, Lindmark G. Anastomotic leakage after surgery for rectal cancer: a risk factor for local recurrence, distant metastasis and reduced cancer‐specific survival? Colorectal Dis 2011; 13: 272–283. [DOI] [PubMed] [Google Scholar]
- 21. Krarup PM, Nordholm‐Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long‐term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg 2014; 259: 930–938. [DOI] [PubMed] [Google Scholar]
- 22. Kube R, Mroczkowski P, Granowski D, Benedix F, Sahm M, Schmidt U et al; Study group Qualitätssicherung Kolon/Rektum‐Karzinome (Primärtumor). Quality assurance in primary colorectal carcinoma. Anastomotic leakage after colon cancer surgery: a predictor of significant morbidity and hospital mortality, and diminished tumour‐free survival. Eur J Surg Oncol 2010; 36: 120–124. [DOI] [PubMed] [Google Scholar]
- 23. Aquina CT, Mohile SG, Tejani MA, Becerra AZ, Xu Z, Hensley BJ et al The impact of age on complications, survival, and cause of death following colon cancer surgery. Br J Cancer 2017; 116: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long‐term survival in patients undergoing curative surgery for colorectal cancer: a study of 12 075 patients. Ann Surg 2015; 261: 497–505. [DOI] [PubMed] [Google Scholar]
- 25. Chu DI, Schlieve CR, Colibaseanu DT, Simpson PJ, Wagie AE, Cima RR et al Surgical site infections (SSIs) after stoma reversal (SR): risk factors, implications, and protective strategies. J Gastrointest Surg 2015; 19: 327–334. [DOI] [PubMed] [Google Scholar]
- 26. Nordholm‐Carstensen A, Rolff HC, Krarup PM. Differential impact of anastomotic leak in patients with stage IV colonic or rectal cancer: a nationwide cohort study. Dis Colon Rectum 2017; 60: 497–507. [DOI] [PubMed] [Google Scholar]
- 27. Boccola MA, Buettner PG, Rozen WM, Siu SK, Stevenson AR, Stitz R et al Risk factors and outcomes for anastomotic leakage in colorectal surgery: a single‐institution analysis of 1576 patients. World J Surg 2011; 35: 186–195. [DOI] [PubMed] [Google Scholar]
- 28. Duron JJ, Duron E, Dugue T, Pujol J, Muscari F, Collet D et al Risk factors for mortality in major digestive surgery in the elderly: a multicenter prospective study. Ann Surg 2011; 254: 375–382. [DOI] [PubMed] [Google Scholar]
- 29. Eberhardt JM, Kiran RP, Lavery IC. The impact of anastomotic leak and intra‐abdominal abscess on cancer‐related outcomes after resection for colorectal cancer: a case control study. Dis Colon Rectum 2009; 52: 380–386. [DOI] [PubMed] [Google Scholar]
- 30. Gong J, Yang L, Huang X, Sun B, Zhou J, Yu D et al Outcomes based on risk assessment of anastomotic leakage after rectal cancer surgery. Asian Pac J Cancer Prev 2014; 15: 707–712. [DOI] [PubMed] [Google Scholar]
- 31. Gupta RK, Agrawal CS, Pathania OP, Bajracharya A, Sah SP, Sah PL. Anterior resection for rectal cancer with mesorectal excision: institutional review. Indian J Surg 2013; 75: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jannasch O, Klinge T, Otto R, Chiapponi C, Udelnow A, Lippert H et al Risk factors, short and long term outcome of anastomotic leaks in rectal cancer. Oncotarget 2015; 6: 36 884–36 893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on long‐term outcomes following curative resection for colorectal cancer. Ann Surg Oncol 2007; 14: 2559–2566. [DOI] [PubMed] [Google Scholar]
- 34. Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long‐term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg 2007; 11: 8–15. [DOI] [PubMed] [Google Scholar]
- 35. Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, Horgan PG et al C‐reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol 2012; 19: 4168–4177. [DOI] [PubMed] [Google Scholar]
- 36. Ptok H, Marusch F, Meyer F, Schubert D, Gastinger I, Lippert H. Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br J Surg 2007; 94: 1548–1554. [DOI] [PubMed] [Google Scholar]
- 37. Richards C, Platt J, Anderson J, McKee R, Horgan P, McMillan D. The impact of perioperative risk, tumor pathology and surgical complications on disease recurrence following potentially curative resection of colorectal cancer. Ann Surg 2011; 254: 83–89. [DOI] [PubMed] [Google Scholar]
- 38. Smith JD, Paty PB, Guillem JG, Temple LK, Weiser MR, Nash GM. Anastomotic leak is not associated with oncologic outcome in patients undergoing low anterior resection for rectal cancer. Ann Surg 2012; 256: 1034–1038. [DOI] [PubMed] [Google Scholar]
- 39. Smith JD, Butte JM, Weiser MR, D'Angelica MI, Paty PB, Temple LK et al Anastomotic leak following low anterior resection in stage IV rectal cancer is associated with poor survival. Ann Surg Oncol 2013; 20: 2641–2646. [DOI] [PubMed] [Google Scholar]
- 40. Thorgersen EB, Goscinski MA, Spasojevic M, Solbakken AM, Mariathasan AB, Boye K et al Deep pelvic surgical site infection after radiotherapy and surgery for locally advanced rectal cancer. Ann Surg Oncol 2017; 24: 721–728. [DOI] [PubMed] [Google Scholar]
- 41. Attiê R, Chinen LT, Yoshioka EM, Silva MC, de Lima VC. Acute bacterial infection negatively impacts cancer specific survival of colorectal cancer patients. World J Gastroenterol 2014; 20: 13 930–13 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ebinger SM, Warschkow R, Tarantino I, Schmied BM, Marti L. Anastomotic leakage after curative rectal cancer resection has no impact on long‐term survival: a propensity score analysis. Int J Colorectal Dis 2015; 30: 1667–1675. [DOI] [PubMed] [Google Scholar]
- 43. Goto S, Hasegawa S, Hida K, Uozumi R, Kanemitsu Y, Watanabe T et al; Study Group for Nomogram of the Japanese Society for Cancer of the Colon and Rectum. Multicenter analysis of impact of anastomotic leakage on long‐term oncologic outcomes after curative resection of colon cancer. Surgery 2017; 162: 317–324. [DOI] [PubMed] [Google Scholar]
- 44. Haruki K, Shiba H, Fujiwara Y, Furukawa K, Wakiyama S, Ogawa M et al Negative impact of surgical site infection on long‐term outcomes after hepatic resection for colorectal liver metastases. Anticancer Res 2013; 33: 1697–1703. [PubMed] [Google Scholar]
- 45. Huang TS, Hu FC, Fan CW, Lee CH, Jwo SC, Chen HY. A simple novel model to predict hospital mortality, surgical site infection, and pneumonia in elderly patients undergoing operation. Dig Surg 2010; 27: 224–231. [DOI] [PubMed] [Google Scholar]
- 46. Jung SH, Yu CS, Choi PW, Kim DD, Park IJ, Kim HC et al Risk factors and oncologic impact of anastomotic leakage after rectal cancer surgery. Dis Colon Rectum 2008; 51: 902–908. [DOI] [PubMed] [Google Scholar]
- 47. Kang J, Choi GS, Oh JH, Kim NK, Park JS, Kim MJ et al Multicenter analysis of long‐term oncologic impact of anastomotic leakage after laparoscopic total mesorectal excision: the Korean laparoscopic colorectal surgery study group. Medicine 2015; 94: 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Katoh H, Yamashita K, Wang G, Sato T, Nakamura T, Watanabe M. Anastomotic leakage contributes to the risk for systemic recurrence in stage II colorectal cancer. J Gastrointest Surg 2011; 15: 120–129. [DOI] [PubMed] [Google Scholar]
- 49. Kerin Povšič M, Ihan A, Beovič B. Post‐operative infection is an independent risk factor for worse long‐term survival after colorectal cancer surgery. Surg Infect (Larchmt) 2016; 17: 700–712. [DOI] [PubMed] [Google Scholar]
- 50. Kulu Y, Tarantio I, Warschkow R, Kny S, Schneider M, Schmied BM et al Anastomotic leakage is associated with impaired overall and disease‐free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg Oncol 2015; 22: 2059–2067. [DOI] [PubMed] [Google Scholar]
- 51. Lee WS, Yun SH, Roh YN, Yun HR, Lee WY, Cho YB et al Risk factors and clinical outcome for anastomotic leakage after total mesorectal excision for rectal cancer. World J Surg 2008; 32: 1124–1129. [DOI] [PubMed] [Google Scholar]
- 52. Lim SB, Yu CS, Kim CW, Yoon YS, Park IJ, Kim JC. The types of anastomotic leakage that develop following anterior resection for rectal cancer demonstrate distinct characteristics and oncologic outcomes. Int J Colorectal Dis 2015; 30: 1533–1540. [DOI] [PubMed] [Google Scholar]
- 53. Marra F, Steffen T, Kalak N, Warschkow R, Tarantino I, Lange J et al Anastomotic leakage as a risk factor for the long‐term outcome after curative resection of colon cancer. Eur J Surg Oncol 2009; 35: 1060–1064. [DOI] [PubMed] [Google Scholar]
- 54. McMillan DC, McArdle CS, Morrison DS. A clinical risk score to predict 3‐, 5‐and 10‐year survival in patients undergoing surgery for Dukes B colorectal cancer. Br J Cancer 2010; 103: 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miccini M, Borghese O, Scarpini M, Cassini D, Gregori M, Amore Bonapasta S et al Anastomotic leakage and septic complications: impact on local recurrence in surgery of low rectal cancer. Ann Ital Chir 2011; 82: 117–123. [PubMed] [Google Scholar]
- 56. Mrak K, Eberl T, Laske A, Jagoditsch M, Fritz J, Tschmelitsch J. Impact of postoperative complications on long‐term survival after resection for rectal cancer. Dis Colon Rectum 2013; 56: 20–28. [DOI] [PubMed] [Google Scholar]
- 57. Nachiappan S, Askari A, Malietzis G, Giacometti M, White I, Jenkins JT et al The impact of anastomotic leak and its treatment on cancer recurrence and survival following elective colorectal cancer resection. World J Surg 2015; 39: 1052–1058. [DOI] [PubMed] [Google Scholar]
- 58. Noh GT, Ann YS, Cheong C, Han J, Cho MS, Hur H et al Impact of anastomotic leakage on long‐term oncologic outcome and its related factors in rectal cancer. Medicine (Baltimore) 2016; 95: e4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsujimoto H, Ueno H, Hashiguchi Y, Ono S, Ichikura T, Hase K. Postoperative infections are associated with adverse outcome after resection with curative intent for colorectal cancer. Oncol Lett 2010; 1: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Haskins IN, Horne CM, Krpata DM, Prabhu AS, Tastaldi L, Perez AJ et al A call for standardization of wound events reporting following ventral hernia repair. Hernia 2018; 22: 729–736. [DOI] [PubMed] [Google Scholar]
- 61. DeBord J, Novitsky Y, Fitzgibbons R, Miserez M, Montgomery A. SSI, SSO, SSE, SSOPI: the elusive language of complications in hernia surgery. Hernia 2018; 22: 737–738. [DOI] [PubMed] [Google Scholar]
- 62. Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 2009; 208: 269–278. [DOI] [PubMed] [Google Scholar]
- 63. de Lacy FB, Chadi SA, Berho M, Heald RJ, Khan J, Moran B et al The future of rectal cancer surgery: a narrative review of an international symposium. Surg Innov 2018; 25: 525–535. [DOI] [PubMed] [Google Scholar]
- 64. Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH et al Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg 2016; 20: 2035–2051. [DOI] [PubMed] [Google Scholar]
- 65. McSorley ST, Horgan PG, McMillan DC. The impact of the type and severity of postoperative complications on long‐term outcomes following surgery for colorectal cancer: a systematic review and meta‐analysis. Crit Rev Oncol Hematol 2016; 97: 168–177. [DOI] [PubMed] [Google Scholar]
- 66. Wang S, Liu J, Wang S, Zhao H, Ge S, Wang W. Adverse effects of anastomotic leakage on local recurrence and survival after curative anterior resection for rectal cancer: a systematic review and meta‐analysis. World J Surg 2017; 41: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lu ZR, Rajendran N, Lynch AC, Heriot AG, Warrier SK. Anastomotic leaks after restorative resections for rectal cancer compromise cancer outcomes and survival. Dis Colon Rectum 2016; 59: 236–244. [DOI] [PubMed] [Google Scholar]
- 68. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta‐analysis. Ann Surg 2011; 253: 890–899. [DOI] [PubMed] [Google Scholar]
- 69. Gantz O, Zagadailov P, Merchant AM. The cost of surgical site infections after colorectal surgery in the United States from 2001 to 2012: a longitudinal analysis. Am Surg 2019; 85: 142–149. [PubMed] [Google Scholar]
- 70. Martinez C, Omesiete P, Pandit V, Thompson E, Nocera M, Riall T et al A protocol‐driven reduction in surgical site infections after colon surgery. J Surg Res 2020; 246: 100–105. [DOI] [PubMed] [Google Scholar]
- 71. Gaines S, Shao C, Hyman N, Alverdy JC. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br J Surg 2018; 105: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gertsch P, Baer HU, Kraft R, Maddern GJ, Altermatt HJ. Malignant cells are collected on circular staplers. Dis Colon Rectum 1992; 35: 238–241. [DOI] [PubMed] [Google Scholar]
- 73. Jenner DC, de Boer WB, Clarke G, Levitt MD. Rectal washout eliminates exfoliated malignant cells. Dis Colon Rectum 1998; 41: 1432–1434. [DOI] [PubMed] [Google Scholar]
- 74. Bohle B, Pera M, Pascual M, Alonso S, Mayol X, Salvado M et al Postoperative intra‐abdominal infection increases angiogenesis and tumor recurrence after surgical excision of colon cancer in mice. Surgery 2010; 147: 120–126. [DOI] [PubMed] [Google Scholar]
- 75. Weiser MR, Gonen M, Usiak S, Pottinger T, Samedy P, Patel D et al; Memorial Sloan Kettering Multidisciplinary Surgical-Site Infection Reduction Team. Effectiveness of a multidisciplinary patient care bundle for reducing surgical‐site infections. Br J Surg 2018; 105: 1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Beecher SM, O'Leary DP, McLaughlin R, Kerin MJ. The impact of surgical complications on cancer recurrence rates: a literature review. Oncol Res Treat 2018; 41: 478–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Advanced search strategies and Boolean characters used across the various databases
Table S2 Data Management


