Abstract
Pathogenicity of visceral adipose tissue (VAT) has been linked to the metabolic stress of enlarging mature adipocytes and a limited ability to recruit new adipocytes. One of the major distinguishing features of VAT preadipocytes is the high expression of the zinc metalloprotease, pregnancy-associated plasma protein-A (PAPP-A), when compared to subcutaneous adipose tissue (SAT). In this study we used 2 different approaches to investigate the effect of PAPP-A inhibition on different fat depots in mice on a high-fat diet (HFD) for 15 weeks. Conditional knockdown of PAPP-A gene expression in female adult mice resulted in significant decreases of 30% to 40% in adipocyte size in VAT (mesenteric and pericardial depots) compared to control mice. There was no effect on SAT (inguinal) or intra-abdominal perigonadal fat. Liver lipid was also significantly decreased without any effect on heart and skeletal muscle lipid. We found similar effects when using a pharmacological approach. Weekly injections of a specific immunoneutralizing monoclonal antibody (mAb-PA 1/41) or isotype control were given to male and female wild-type mice on HFD for 15 weeks. Adipocyte size was significantly decreased (30%-50%) only in VAT with mAb-PA 1/41 treatment. In this model, cell number was significantly increased in mesenteric fat in mice treated with mAb-PA 1/41, suggesting hyperplasia along with reduced hypertrophy in this VAT depot. Gene expression data indicated a significant decrease in F4/80 (macrophage marker) and interleukin-6 (proinflammatory cytokine) and a significant increase in adiponectin (anti-inflammatory adipokine with beneficial metabolic effects) in mesenteric fat compared to inguinal fat in mice treated with mAb-PA 1/41. Furthermore, there was significantly decreased liver lipid content with mAb-PA 1/41 treatment. Thus, using 2 different models systems we provide proof of principle that PAPP-A inhibition is a potential therapeutic target to prevent visceral obesity and its metabolic sequelae, such as fatty liver.
Keywords: PAPP-A, obesity, preadipocytes, insulin-like growth factor
Obesity is a complex multifactorial disease with extensive impact on morbidity, mortality, and health care costs. With the most recent Centers for Disease Control and Prevention data (2017~2018) identifying 42.4% of adult Americans as obese and 9.2% as severely obese, it is a modern-day epidemic (www.cdc.gov). Adipose tissue is a heterogeneous endocrine organ with tremendous capability for expansion. This expansion occurs primarily through 2 processes: accumulation of lipid in existing adipocytes (hypertrophy) and recruitment and differentiation of adipocyte precursors (hyperplasia) (1). Within the realm of adipose tissue depots, subcutaneous adipose tissue (SAT) tends to be protective in nature by providing a source of energy storage and regulating appetite (1-4). In contrast, visceral adipose tissue (VAT) has far greater proinflammatory characteristics, and the accrual of VAT has been linked to dyslipidemia, chronic inflammation, insulin resistance or type 2 diabetes, fatty liver, and increased risk of cardiovascular disease (5). In cross-sectional human studies, it has been shown that the enlargement of visceral fat generally occurs through hypertrophy, whereas subcutaneous fat can expand through hypertrophy and hyperplasia (6, 7). It has been well established that enlarged adipocyte size and impaired adipogenic differentiation in VAT are key characteristics of dysfunctional adipose tissue, which further propagate metabolic derangement and chronic inflammation associated with obesity (1, 8).
Adipogenic differentiation is a process that is tightly regulated by a complex interplay of multiple transcription factors in response to insulin and insulin-like growth factors (IGFs), resulting in the conversion of preadipocytes, also referred to as adipose-derived stem cells, into mature adipocytes (9, 10). The role of IGFs in adipogenesis is well established in vitro (11-13). IGF-I is an important mediator of preadipocyte proliferation, differentiation, and survival (13). Circulating levels and local activities of IGFs are regulated by a family of 6 high-affinity IGF-binding proteins (IGFBPs) (14). In turn, certain of the IGFBPs can undergo controlled proteolysis, which can have major consequences on IGF availability (15). The best known of the specific IGFBP proteases is pregnancy-associated plasma protein-A (PAPP-A). PAPP-A is a zinc metalloprotease that is expressed by many cell types, including preadipocytes (16). The secreted protein can then associate with the cell surface in an autocrine or paracrine fashion. Although PAPP-A can cleave IGFBP-4 and -5 (and in some circumstances IGFBP-2), PAPP-A is the only physiological protease for IGFBP-4 (17, 18). IGFBP-4 has a higher affinity for IGF ligand than its cognate receptor, and is considered an inhibitor of local IGF action. Importantly, IGFBP-4 is a substrate for PAPP-A only when it is bound with IGF. PAPP-A specifically cleaves the IGF/IGFBP-4 complex, releasing IGF, making it more bioavailable in the pericellular environment for receptor engagement and downstream signaling (16). This ability of PAPP-A to enhance local IGF action through IGFBP-4 proteolysis has been established in vitro and in vivo (16).
Tchkonia et al identified PAPP-A as one of the most distinctive early developmental genes involved in adipogenesis, with messenger RNA (mRNA) expression levels in preadipocytes from human VAT significantly greater than those from human SAT (19). Elevated PAPP-A protein levels in conditioned medium of preadipocytes from human VAT were later confirmed (12, 20). Similarly, preadipocytes from mouse VAT express more PAPP-A than those from SAT (21). It is of note that mature adipocytes do not express PAPP-A (20, 21). PAPP-A–deficient mice on a high-fat diet (HFD) had restrained viscerally stored fat, that is, reduced adipocyte size, as compared to wild-type (WT) mice. There was no significant effect on SAT (22). These findings led us to hypothesize that PAPP-A plays an important role in modulating the effects of IGF-I on depot-specific adipocyte development associated with obesity. However, the PAPP-A knockout (KO) mice used in this previous study (22) were generated by homologous recombination in mouse embryonic stem cells and were born as proportional dwarfs 60% the size of WT littermates and maintained this smaller size throughout life (23).
In the present study, we investigated the effects of genetic and pharmacologic inhibition of PAPP-A in normal-size adult mice on HFD. We found that the primary effect of PAPP-A inhibition was significantly decreased visceral adipocyte size in mice on an HFD; adipocyte size was not affected in SAT. Liver lipid was also significantly reduced in these 2 in vivo models of PAPP-A inhibition. Thus, we have identified PAPP-A inhibition as a potential antiobesity therapeutic approach.
Materials and Methods
Reagents
Tamoxifen (Tam) and methylene blue were from MilliporeSigma; collagenase type II was from Worthington Biochemical Corporation; and HEPES was from Gibco. Trizol and a SuperScript III First-Strand Synthesis System were purchased from Life Technologies, and an iQ SYBR green PCR Master Mix from Bio-Rad. The HFD (21% by weight [42% of calories] fat and 0.15% by weight cholesterol) was from Tekland.
Conditional pregnancy-associated plasma protein-A knockout mice
Floxed PAPP-A (fPAPP-A) mice were provided by Lexicon Pharmaceuticals, Inc. These mice were crossed with transgenic mice having a Tam-inducible Cre-mediated recombination system driven by the chicken β-actin promoter/enhancer coupled with the cytomegalovirus immediate-early enhancer (The Jackson Laboratory). This mating produced mice heterozygous for fPAPP-A and positive (pos) or negative (neg) for Tam-Cre. These mice were then mated to produce offspring with the genotypes used in this study: homozygous fPAPP-A/pos and homozygous fPAPP-A/neg. Mice were further checked for “leaky Cre,” that is, evidence of residual Cre recombinase activity. Only those mice without leaky Cre (65%) were used in experiments. See Conover et al (24) for genotyping details. Eight-week-old mice, both fPAPP-A/pos and fPAPP-A/neg, were given Tam (15 mg/kg) daily for 5 consecutive days and started on an HFD at age 10 weeks for a total of 15 weeks. At the end of the study, SAT (inguinal), VAT (mesenteric, pericardial), liver, heart, and quadriceps were harvested, weighed, and prepared for analyses.
Pharmacological in vivo pregnancy-associated plasma protein-A inhibition
WT mice were on a mixed C57BL/6 and 129/SvE background. A neutralizing monoclonal PAPP-A antibody (mAb-PA 1/41) was kindly provided by Ansh Laboratories. mAb-PA1/41 specifically inhibits PAPP-A–mediated IGFBP-4 proteolysis in vivo and in vitro (25-28). Control mice were given an immunoglobulin G isotype (IgG2a, Bio X Cell). Mice were started on HFD at age 10 weeks and at the same time treated weekly with mAb-PA 1/41 (30 mg/kg) or IgG2a via intraperitoneal delivery for 15 weeks. At the end of the study, SAT (inguinal), VAT (mesenteric, pericardial), liver, heart, and quadriceps were harvested, weighed, and prepared for analyses.
All procedures involving animals complied with the standards stated in the Guide for the Care and Use of Laboratory Animals and were approved by Mayo Clinic’s Institutional Animal Care and Use Committee.
Adipocyte size and number
Fresh samples of inguinal, pericardial, mesenteric, and perigonadal murine fat were digested with collagenase in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid) in a 37°C shaking water bath for 8 to 10 minutes or until fat globules were no longer present (7). Following centrifugation, the floating layer of cells, consisting of adipocytes, was carefully extracted and added to methylene blue for staining. Sizes of the dispersed adipocytes were determined using a Nikon Labophot-2, with Coolpix camera and software Analyze/Cell Counting and Application Processing. Circularity, diameter, and microgram (μg) lipid-per-cell measurements were computed. Images were analyzed until approximately 300 cells were sized. The number of fat cells in adipose tissue was obtained by dividing the total depot weight by mean fat cell volume times the density of triolein (29).
Tissue lipid
Tissue lipid percentage in the liver, skeletal muscle, and heart was measured using the Folch method (30).
RNA isolation and quantitative real-time polymerase chain reaction
Adipose tissue depots were rapidly isolated, and a piece immediately minced in Trizol and frozen at –80°C. Once thawed, samples were homogenized by passing fat tissue through a 21-gauge needle several times. Total RNA was then isolated from each fat depot, reverse-transcribed (RT) with the SuperScript III First-Strand Synthesis System, and evaluated by RT–quantitative polymerase chain reaction (qPCR) using the iCycler iQ5 Detection System with iQ SYBR green PCR Master Mix. Amplification plots were analyzed with iQ5 Optical System Software version 2.1 (Bio-Rad). Standards were included for each target gene as well as reference gene, ribosomal protein L22 (RPL22). Relative quantification took into account RPL22 expression for each sample. Primer sequences used for measurement of mRNA expression of macrophages (F4/80), cytokines (interleukin [IL]-10, IL-6, IL-1β, tumor necrosis factor-α [TNFα], adiponectin), fatty acid synthase (FASN), and reference gene RPL22) are listed in Supplemental Table 1 (31) and as previously reported (22).
Body composition
Lean mass and fat mass of individual mice were quantified by quantitative nuclear magnetic resonance using an EchoMRI analyzer and normalized relative to body weight. Unanesthetized animals were placed in a plastic tube, which is introduced into the EchoMRI instrument. Body compositions consisting of fat mass, lean mass, free water and total water are generated in approximately 90 seconds per animal.
Statistical analysis
Results have been presented as means ± SE. Unpaired t tests were used for normally distributed data and nonparametric Z tests were used if the data were not normally distributed. Statistical significance was set at P less than .05.
Results
Genetic deletion of pregnancy-associated plasma protein-A in adult mice on a high-fat diet is protective against visceral obesity and fatty liver
Although the characterization of the PAPP-A KO murine model in our previous publication suggested that PAPP-A inhibition may be protective against HFD-induced visceral obesity, the mice themselves were smaller than WT (22). We overcame the discrepancy in size and any effect of embryologic PAPP-A deletion in this study by using a conditional PAPP-A knockdown model, wherein PAPP-A expression was knocked down in adult female mice using a Tam-inducible Cre-recombinase-mediated excision of the PAPP-A gene at age 8 weeks (24). Male mice were not used in this experiment because of the risk of gonadal toxicity in male mice treated with Tam (32). Mice homozygous for fPAPP-A and either pos or neg for Tam-Cre (tamoxifen-inducible Cre) were fed a 42% HFD for 15 weeks, starting at age 10 weeks. There was no significant difference in weight in the 2 groups of mice either at the start of or at the end of the experiment (Table 1). However, pericardial fat depots, a VAT in mice (21), were significantly reduced in fPAPP-A/pos mice compared to fPAPP-A/neg mice. None of the other depot weights showed significant differences between the 2 groups (see Table 1). Mean adipocyte size was significantly reduced in the mouse VAT depots (Table 2). Adipocytes from fPAPP-A/pos mesenteric and pericardial fat were reduced 30% and 40%, respectively, compared to fPAPP-A/neg adipocytes. Adipocytes from perigonadal and inguinal fat depots were not statistically different between the 2 groups. Adipocyte cell number did not change in any of the fat depots regardless of genotype (data not shown). Also, liver lipid was significantly lower in fPAPP-A/pos mice than fPAPP-A/neg mice (P = .005). There was no significant difference in the percentage of lipid in skeletal muscle and the heart (Table 3).
Table 1.
Body and tissue weights of conditional pregnancy-associated plasma protein-A knockout mice on a high-fat diet
| fPAPP-A/neg | fPAPP-A/pos | P | |
|---|---|---|---|
| Whole body, 10 wks | 19.8 ± 0.55 | 19.3 ± 0.47 | .50 |
| Whole body, 25 wks | 24.7 ± 1.01 | 25.6 ± 1.10 | .56 |
| Mesenteric | 0.41 ± 0.034 | 0.33 ± 0.071 | .38 |
| Pericardial | 0.049 ± 0.006 | 0.027 ± 0.005 | .015 |
| Perigonadal | 0.43 ± 0.069 | 0.35 ± 0.044 | .33 |
| Inguinal | 0.49 ± 0.066 | 0.46 ± 0.123 | .85 |
Wild-type mice were started on a high-fat diet (HFD) 1 week after tamoxifen treatment (age 10 weeks) and continued on HFD until age 25 weeks (15 weeks of HFD).
Results (grams) are mean ± SEM of 9 to 11 mice. P values less than .05 are bolded.
Abbreviations: fPAPP, floxed pregnancy-associated plasma protein-A; neg, negative; pos, positive.
Table 2.
Adipocyte size in fat depots of conditional pregnancy-associated plasma protein-A knockout mice on a high-fat diet
| fPAPP-A/neg | fPAPP-A/pos | P | |
|---|---|---|---|
| Mesenteric | 0.21 ± 0.020 | 0.13 ± 0.025 | .040 |
| Pericardial | 0.12 ± 0.013 | 0.07 ± 0.014 | .026 |
| Perigonadal | 0.46 ± 0.056 | 0.31 ± 0.047 | .06 |
| Inguinal | 0.34 ± 0.048 | 0.26 ± 0.040 | .22 |
Wild-type mice were started on a high-fat diet 1 week after tamoxifen treatment and continued for 15 weeks. P values less than .05 are bolded.
Results (microgram [μg] lipid per cell) are mean ± SEM of 9 to 11 mice.
Abbreviations: fPAPP, floxed pregnancy-associated plasma protein-A; neg, negative; pos, positive.
Table 3.
Percentage lipid in tissues of conditional pregnancy-associated plasma protein-A knockout mice on a high-fat diet
| fPAPP-A/neg | fPAPP-A/pos | P | |
|---|---|---|---|
| Liver | 15.4 ± 0.87 | 12.1 ± 0.63 | .005 |
| Heart | 5.3 ± 0.60 | 6.1 ± 0.41 | .27 |
| Quad | 6.2 ± 0.52 | 6.4 ± 0.71 | .85 |
Wild-type mice were started on a high-fat diet 1 week after tamoxifen treatment and continued for 15 weeks.
Results are mean ± SEM of 9 to 11 mice. P values less than .05 are bolded.
Abbreviations: fPAPP, floxed pregnancy-associated plasma protein-A; neg, negative; pos, positive; Quad, quadriceps.
Pharmacological inhibition of pregnancy-associated plasma protein-A in adult wild-type mice on a high-fat diet is protective against visceral obesity and fatty liver
To explore the effect of pharmacological inhibition of PAPP-A on the development of diet-induced obesity, male and female WT mice received either a neutralizing monoclonal PAPP-A antibody (mAb-PA 1/41) (25) or isotype control (IgG2a). They were injected weekly and fed an HFD for 15 weeks starting at age 10 weeks. Weights were not different at the start of the treatment, but weight gain was attenuated by approximately 12% both in male and female mice receiving the PAPP-A antibody compared to the control group. This did not reach statistical significance in the male mice. Whole-body composition of mice on the HFD was assessed by EchoMRI. There was no significant difference in percentage of lean or percentage of fat with mAb-PA 1/41 treatment (Supplemental Table 2) (31).
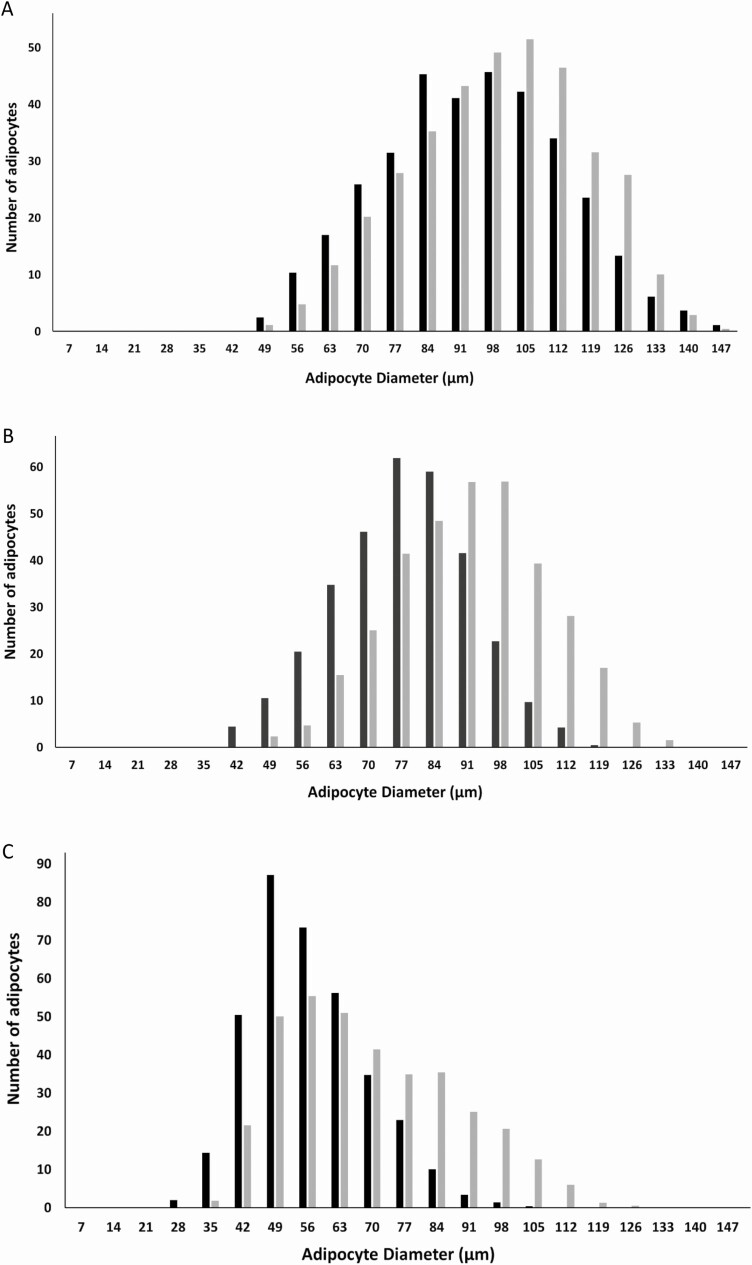
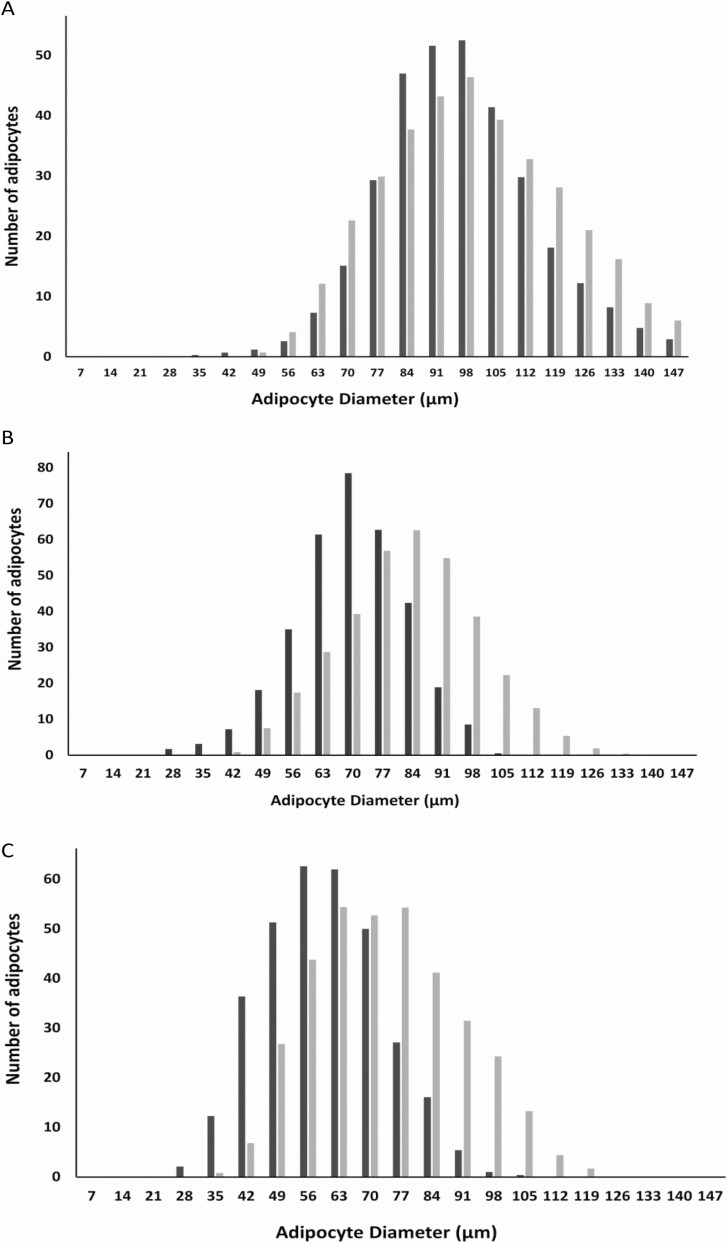
Pericardial depot weight both in male and female mice was significantly decreased in mAb-PA 1/41–treated compared to control mice. There was no significant difference in weight for the other fat depots (Table 4). However, mean adipocyte size was significantly decreased in the mesenteric and pericardial fat depots with mAb-PA 1/41 treatment (Table 5). For mesenteric adipocytes the decrease was 30% and 40% in females and males; for the pericardial adipocytes, it was 40% and 50%, respectively. There was no significant difference in mean adipocyte size of inguinal and perigonadal fat. To assess depot-specific adipocyte size further, taking into account the variability within each mouse as well as between control and mAb-PA 1/41 treatment, we analyzed frequency distribution in inguinal, mesenteric, and pericardial fat from male (Fig. 1) and female (Fig. 2) mice on the HFD. Adipocyte size frequency in inguinal fat showed no major differences between control and mAb-PA 1/41–treated male and female mice. In contrast, the adipocyte size profile was shifted to the left in mesenteric and pericardial fat both from male and female mice treated with mAb-PA 1/41, suggesting smaller overall adipocyte size in these depots. However, differences in overall frequency were not statistically significant. When we calculated the overall cell numbers in the individual depots, the antibody-treated mesenteric depots had 1.4 times the number of cells compared to controls both in males and females (P = .006 and P = .012, respectively [Table 6]). There was no significant difference in cell number in the other fat depots. Liver lipid was reduced by 10% in mAb-PA 1/41–treated mice (P = .04) with no significant changes in heart or skeletal muscle lipid.
Table 4.
Body and tissue weights of wild-type mice on a high-fat diet ± immunoneutralizing monoclonal antibody mAb-PA 1/41
| Females | IgG2a | mAb-PA 1/41 | P |
|---|---|---|---|
| Whole body | |||
| 10 wks | 21.6 ± 0.69 | 21.5 ± 0.76 | .92 |
| 25 wks | 33.0 ± 1.39 | 28.8 ± 1.24 | .036 |
| Mesenteric | 0.43 ± 0.038 | 0.36 ± 0.027 | .144 |
| Pericardial | 0.088 ± 0.009 | 0.065 ± 0.006 | .044 |
| Perigonadal | 1.76 ± 0.227 | 1.26 ± 0.014 | .071 |
| Inguinal | 0.84 ± 0.083 | 0.73 ± 0.046 | .249 |
| Males | |||
| Whole body | |||
| 10 wks | 27.2 ± 0.73 | 26.2 ± 0.44 | .25 |
| 25 wks | 41.3 ± 2.43 | 36.2 ± 1.05 | .06 |
| Mesenteric | 0.72 ± 0.096 | 0.56 ± 0.038 | .141 |
| Pericardial | 0.106 ± 0.0179 | 0.061 ± 0.003 | .025 |
| Perigonadal | 1.46 ± 0.191 | 1.16 ± 0.073 | .162 |
| Inguinal | 1.10 ± 0.141 | 0.87 ± 0.068 | .161 |
10-week-old wild-type mice on a high-fat diet were administered mAb-PA 1/41 or immunoglobulin G isotype (IgG2a) control once a week for 15 weeks.
Results (grams) are mean ± SEM of 9 or 10 mice. P values less than .05 are bolded.
Table 5.
Adipocyte size in fat depots of wild-type mice on a high-fat diet ± immunoneutralizing monoclonal antibody mAb-PA 1/41
| Females | IgG2a | mAb-PA1/41 | P |
|---|---|---|---|
| Mesenteric | 0.26 ± 0.021 | 0.16 ± 0.018 | .002 |
| Pericardial | 0.16 ± 0.019 | 0.10 ± 0.012 | .018 |
| Perigonadal | 0.62 ± 0.064 | 0.56 ± 0.057 | .194 |
| Inguinal | 0.53 ± 0.062 | 0.45 ± 0.077 | .251 |
| Males | |||
| Mesenteric | 0.37 ± 0.038 | 0.22 ± 0.021 | .004 |
| Pericardial | 0.16 ± 0.030 | 0.08 ± 0.009 | .020 |
| Perigonadal | 0.78 ± 0.078 | 0.65 ± 0.032 | .123 |
| Inguinal | 0.51 ± 0.050 | 0.47 ± 0.036 | .090 |
10-week-old wild-type mice on a high-fat diet were administered mAb-PA 1/41 or immunoglobulin G isotype (IgG2a) control once a week for 15 weeks.
Results (microgram [μg] lipid per cell) are mean ± SEM of 9 or 10 mice. P values less than .05 are bolded.
Figure 1.
Effect of pregnancy-associated plasma protein-A inhibition by an immunoneutralizing monoclonal antibody (mAb-PA 1/41) on adipocyte size distribution and frequency in male mice on a high-fat diet. A, Inguinal; B, mesenteric; C, pericardial. Gray bars, immunoglobulin G treated; black bars, mAb-PA 1/41 treated.
Figure 2.
Effect of pregnancy-associated plasma protein-A inhibition by an immunoneutralizing monoclonal antibody (mAb-PA 1/41) on adipocyte size distribution and frequency in female mice on a high-fat diet. A, Inguinal; B, mesenteric; C, pericardial. Gray bars, immunoglobulin G treated; black bars, mAb-PA 1/41 treated.
Table 6.
Cell number (× 106) in fat depots of wild-type mice on a high-fat diet ± immunoneutralizing monoclonal antibody mAb-PA 1/41
| Females | IgG2a | mAb-PA 1/41 | P |
|---|---|---|---|
| Mesenteric | 1.69 ± 0.063 | 2.30 ± 0.198 | .012 |
| Pericardial | 0.54 ± 0.017 | 0.67 ± 0.054 | .059 |
| Perigonadal | 2.31 ± 0.115 | 2.42 ± 0.233 | .388 |
| Inguinal | 1.75 ± 0.180 | 1.60 ± 0.112 | .479 |
| Males | |||
| Mesenteric | 2.89 ± 0.166 | 3.98 ± 0.198 | .006 |
| Pericardial | 0.66 ± 0.037 | 0.77 ± 0.061 | .143 |
| Perigonadal | 1.82 ± 0.083 | 1.81 ± 0.146 | .950 |
| Inguinal | 1.31 ± 0.133 | 1.27 ± 0.130 | .830 |
10-week-old wild-type mice on a high-fat diet were administered mAb-PA 1/41 or immunoglobulin G isotype (IgG2a) control once a week for 15 weeks.
Results are mean ± SEM of 9 or 10 mice. P values less than .05 are bolded.
To test if mAb-PA 1/41 treatment affected expression of macrophages, cytokines, and/or adipokines and whether such changes were depot specific, mRNA levels were assayed in inguinal and mesenteric fat depots by RT-qPCR (Table 7). mAb-PA 1/41 treatment significantly decreased expression of the macrophage marker, F4/80, and IL-6 in mesenteric fat but not inguinal fat. Antibody treatment significantly increased adiponectin expression in mesenteric fat. mAb-PA 1/41 treatment increased IL-10 expression in inguinal fat but not in mesenteric fat. There was no significant difference in TNF-α or FASN expression in inguinal or mesenteric fat with mAb-PA 1/41 treatment. IL-1β expression was below the lowest standard (102) in the assay.
Table 7.
Relative gene expression in adipose tissue from control (immunoglobulin G) and immunoneutralizing monoclonal antibody mAb-PA 1/41–treated mice after 15 weeks on a high-fat diet
| Macrophages/Cytokines | Ing | Mes | ||
|---|---|---|---|---|
| Control | mAb-PA | Control | mAb-PA | |
| F4/80 | 1.3 ± 0.11 | 1.3 ± 0.008 | 1.5 ± 0.24 | 1.0 ± 0.06a |
| IL-6 | 0.4 ± 0.03 | 0.4 ± 0.06 | 1.1 ± 0.24 | 0.5 ± 0.04a |
| IL-10 | 0.3 ± 0.05 | 0.6 ± 0.06a | 0.4 ± 0.06 | 0.2 ± 0.02 |
| TNF-α | 1.4 ± 0.08 | 1.6 ± 0.14 | 0.8 ± 0.16 | 0.8 ± 0.08 |
| Metabolism | ||||
| Adiponectin | 0.6 ± 0.07 | 0.7 ± 0.05 | 0.5 ± 0.04 | 0.7 ± 0.05a |
| FASN | 1.5 ± 0.26 | 1.2 ± 0.10 | 1.2 ± 0.41 | 0.9 ± 0.10 |
Relative gene expression was assessed by reverse transcriptase–quantitative polymerase chain reaction, as described in “Materials and Methods.”
Results are presented as mean ± SEM of 6 to 10 mice. P values less than .05 are bolded.
Abbreviations: F4/80, macrophage marker; FASN, fatty acid synthase; IL, interleukin; Ing, inguinal; Mes, mesenteric; TNF-α, tumor necrosis factor α.
a P less than .05.
Discussion
Using 2 entirely different in vivo models of targeted PAPP-A inhibition—genetic knockdown and specific immunoneutralization of proteolytic activity against IGFBP-4—we demonstrated significantly reduced adipocyte size in mesenteric and pericardial fat depots (30%-50%) compared to controls, as well as a significant decrease in the percentage of liver lipid both in male and female mice on an HFD. Subcutaneous inguinal and intra-abdominal perigonadal fat depots were not affected in either mouse model.
We had previously reported that PAPP-A KO mice on an HFD showed preferential reduction in lipid accumulation in VAT with little or no effect on SAT (22). This made sense because PAPP-A expression is much higher in VAT than in SAT, and this is true both for mice and humans (12, 19, 21). However, these PAPP-A KO mice are phenotypically smaller and we wanted to assess the effects of PAPP-A without any effect of embryologic PAPP-A deletion or overall body size. Indeed, female PAPP-A KO mice on a chow diet had reduced white adipose tissue weights compared to WT, suggesting a possible developmental influence (22). Therefore, for the present study we conditionally knocked down PAPP-A expression in adult mice using a Tam-inducible Cre system (24). It is important to note that both fPAPP-A/pos and fPAPP-A/neg mice received Tam injections, so any differences should be unrelated to the Tam per se. We found significant differences between fPAPP-A/pos and fPAPP-A/neg mice on HFD in pericardial depot weight and in pericardial and mesenteric adipocyte size. There was no difference in either parameter for inguinal and perigonadal depots. It was somewhat surprising that there was no difference in mesenteric depot weight because there was a significant decrease in mesenteric weight in PAPP-A KO mice after 20 weeks on the HFD (22). However, the different models and different duration of the HFD may explain, in part, the different findings.
In a previous study, we documented pericardial fat as a VAT depot in mice with high PAPP-A expression in preadipocytes (21). Interestingly, human epicardial preadipocytes secrete 15-fold more PAPP-A than subcutaneous preadipocytes from the same individual (20). Increased epicardial fat in humans is associated with several cardiovascular disorders as well as fatty liver disease. PAPP-A gene deletion in mice with decreased VAT resulted in significantly decreased fatty liver (22). Previous studies by Hill et al demonstrated the protective effect of PAPP-A gene deletion on glucose homeostasis and insulin resistance in mice on an HFD, which could favorably affect glucose and lipid homeostasis (33).
The availability of a monoclonal antibody that specifically inhibits PAPP-A–mediated IGFBP-4 proteolysis (25, 26) provided us with a potentially translatable model. As in the genetic model of PAPP-A inhibition, mAb-PA 1/41 treatment of mice on an HFD resulted in a 40% to 50% decrease in pericardial and mesenteric adipocyte size both in male and female mice. There was no significant effect on inguinal and perigonadal fat. mAb-PA 1/41 treatment also significantly reduced fatty liver. Interestingly, cell number was significantly increased in mesenteric fat from male and female mice treated with mAb-PA 1/41, suggesting recruitment of new adipocytes by adipogenic differentiation. Neoadipogenesis may explain the smaller size and increased cellularity of VAT depots and a decrease in the metabolic sequelae such as hepatic steatosis.
The mAb-PA 1/41–treated mice were still able to gain subcutaneous fat on the HFD, which is inherently protective in nature. Enlarged adipocytes and impaired differentiation of VAT can lead to metabolic dysfunction and chronic inflammation (5, 8). Gene expression data showed a significant increase in adiponectin, which is anti-inflammatory and associated with beneficial metabolic effects, and a significant decrease in macrophages and proinflammatory IL-6 in mesenteric fat with mAb-PA 1/41 treatment, which could contribute to attenuated chronic inflammation in the liver. Casagrande and colleagues reported that hepatic inflammation with ensuing steatosis is mediated by visceral fat accumulation in rats fed an HFD (34). Surprisingly, IL-10, which has pleiotropic immunological effects and is generally considered an anti-inflammatory cytokine, was increased with antibody treatment in inguinal but not mesenteric fat. Further studies both in vitro and in vivo will be necessary to determine the mechanism by which PAPP-A affects these processes in different fat depots.
There was an overall decrease in weight gain over the duration of the mAb-PA 1/41 treatment, which was significant only for the females. However, whole-body composition was not altered with mAb-PA 1/41 treatment in either male or female mice. There were no other differences between male and female mice in regard to PAPP-A inhibition, at least as far as the parameters tested.
We have focused on PAPP-A here, but IGFBP-4 is also an important player in this system. IGFBP-4 is highly expressed in adipose tissue, and it is a substrate for PAPP-A–mediated proteolysis only if bound with IGF (16, 35). By inhibiting PAPP-A genetically or pharmacologically, there would be more intact IGFBP-4 to bind and sequester local IGF from receptor activation. Several studies have explored the duality of IGFBP4 in vivo and in vitro. In vitro, IGFBP-4 inhibits IGF signaling (36); however, in vivo it sequesters IGF in complex, stabilizing it and prolonging its half-life (37). Preadipocytes harvested from Igfbp4 KO mice had decreased adipogenic potential, suggesting that IGFBP4 is required for adipogenesis (38). We and others have proposed that “inhibitory” IGFBP-4 acts as a local reservoir for IGF (38, 39). Thus, PAPP-A inhibition may prevent IGFBP-4 proteolysis, thereby increasing the local IGF reservoir that could preferentially affect adipose tissue biology in a depot-specific manner.
In summary, using 2 different model systems we have provided proof of principle that PAPP-A inhibition is a potential therapeutic target to prevent visceral obesity.
Acknowledgments
The authors thank Hanne Lucier for her help with this manuscript.
Financial Support: This work was supported by the National Institutes of Health (Grant AG028141 to C.A.C.).
Glossary
Abbreviations
- FASN
fatty acid synthase
- fPAPP-A
floxed pregnancy-associated plasma protein-A
- HFD
high-fat diet
- IGF
insulin-like growth factor
- IGFBP
insulin-like growth factor–binding protein
- IgG2a
immunoglobulin G isotype
- IL
interleukin
- KO
knockout
- mRNA
messenger RNA
- PAPP-A
pregnancy-associated plasma protein-A
- qPCR
quantitative polymerase chain reaction
- SAT
subcutaneous adipose tissue
- Tam
tamoxifen
- TNF-α
tumor necrosis factor-α
- VAT
visceral adipose tissue
- WT
wild-type
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Vishvanath L, Gupta RK. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest. 2019;129(10):4022-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirkland JL, Tchkonia T, Giorgadze N, Pirtskhalava T. Adipose tissue as an endocrine organ: regional differences in adipocyte endocrine. In: Medeiros-Neto G, Halpern A, Bouchard C, eds. Proceedings of the 9th International Congress on Obesity. Vol 9. Sao Paulo: John Libbey Eurotext; 2003:87-95.
- 3. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548-2556. [DOI] [PubMed] [Google Scholar]
- 4. Tchkonia T, Corkey BE, Kirkland JL. Current views of the fat cell as an endocrine cell: lipotoxicity. In: Bray GA, Ryan DH, eds. Overweight and the Metabolic Syndrome: From Bench to Bedside. Boston, MA: Springer US; 2006:105-123. [Google Scholar]
- 5. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881-887. [DOI] [PubMed] [Google Scholar]
- 6. DiGirolamo M, Fine JB, Tagra K, Rossmanith R. Qualitative regional differences in adipose tissue growth and cellularity in male Wistar rats fed ad libitum. Am J Physiol. 1998;274(5):R1460-R1467. [DOI] [PubMed] [Google Scholar]
- 7. Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107(42):18226-18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58(7):1550-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45(2):115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosen ED. The transcriptional basis of adipocyte development. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):31-34. [DOI] [PubMed] [Google Scholar]
- 11. Boney CM, Moats-Staats BM, Stiles AD, D’Ercole AJ. Expression of insulin-like growth factor-I (IGF-I) and IGF-binding proteins during adipogenesis. Endocrinology. 1994;135(5):1863-1868. [DOI] [PubMed] [Google Scholar]
- 12. Davidge-Pitts C, Escande CJ, Conover CA. Preferential expression of PAPPA in human preadipocytes from omental fat. J Endocrinol. 2014;222(1):87-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garten A, Schuster S, Kiess W. The insulin-like growth factors in adipogenesis and obesity. Endocrinol Metab Clin North Am. 2012;41(2):283-295, v. [DOI] [PubMed] [Google Scholar]
- 14. Baxter RC, Twigg SM. Actions of IGF binding proteins and related proteins in adipose tissue. Trends Endocrinol Metab. 2009;20(10):499-505. [DOI] [PubMed] [Google Scholar]
- 15. Conover CA. Insulin-like growth factor (IGF) binding protein proteases: key regulators of IGF action. CML-GH and Growth Factors. 2004;19(3):57- 63. [Google Scholar]
- 16. Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab. 2012;23(5):242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laursen LS, Overgaard MT, Søe R, et al. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504(1-2):36-40. [DOI] [PubMed] [Google Scholar]
- 18. Monget P, Mazerbourg S, Delpuech T, et al. Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: identification of cleavage site and characterization of IGFBP-2 degradation. Biol Reprod. 2003;68(1):77-86. [DOI] [PubMed] [Google Scholar]
- 19. Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292(1):E298-E307. [DOI] [PubMed] [Google Scholar]
- 20. Conover CA, Bale LK, Frye RL, Schaff HV. Cellular characterization of human epicardial adipose tissue: highly expressed PAPP-A regulates insulin-like growth factor I signaling in human cardiomyocytes. Physiol Rep. 2019;7(4):e14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bale LK, West SA, Conover CA. Characterization of mouse pericardial fat: regulation by PAPP-A. Growth Horm IGF Res. 2018;42-43:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conover CA, Harstad SL, Tchkonia T, Kirkland JL. Preferential impact of pregnancy-associated plasma protein-A deficiency on visceral fat in mice on high-fat diet. Am J Physiol Endocrinol Metab. 2013;305(9):E1145-E1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conover CA, Bale LK, Overgaard MT, et al. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131(5):1187-1194. [DOI] [PubMed] [Google Scholar]
- 24. Conover CA, Bale LK, Powell DR. Inducible knock out of pregnancy-associated plasma protein-a gene expression in the adult mouse: effect on vascular injury response. Endocrinology. 2013;154(8):2734-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claus Oxvig, Aarhus University, catalog No. Conover_mAb-PA, RRID:AB_2861192.
- 26. Mikkelsen JH, Resch ZT, Kalra B, et al. Indirect targeting of IGF receptor signaling in vivo by substrate-selective inhibition of PAPP-A proteolytic activity. Oncotarget. 2014;5(4):1014-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Becker MA, Haluska P Jr, Bale LK, Oxvig C, Conover CA. A novel neutralizing antibody targeting pregnancy-associated plasma protein-A inhibits ovarian cancer growth and ascites accumulation in patient mouse tumorgrafts. Mol Cancer Ther. 2015;14(4):973-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conover CA, Bale LK, Oxvig C. Targeted inhibition of pregnancy-associated plasma protein-A activity reduces atherosclerotic plaque burden in mice. J Cardiovasc Transl Res. 2016;9(1):77-79. [DOI] [PubMed] [Google Scholar]
- 29. Parlee SD, Lentz SI, Mori H, MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014;537:93-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iverson SJ, Lang SL, Cooper MH. Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids. 2001;36(11):1283-1287. [DOI] [PubMed] [Google Scholar]
- 31. Ramakrishna A, Bale LK, West SA, Conover CA. Data from: genetic and pharmacological inhibition of PAPP-A protects against visceral obesity in mice. Harvard Dataverse Repository. Deposited July 24, 2020, 10.7910/DVN/4YFLHY [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel SH, O’Hara L, Atanassova N, et al. Low-dose tamoxifen treatment in juvenile males has long-term adverse effects on the reproductive system: implications for inducible transgenics. Sci Rep. 2017;7(1):8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hill CM, Arum O, Boparai RK, et al. Female PAPP-A knockout mice are resistant to metabolic dysfunction induced by high-fat/high-sucrose feeding at middle age. Age (Dordr). 2015;37(3):9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Casagrande BP, de Souza DV, Ribeiro DA, Medeiros A, Pisani LP, Estadella D. Hepatic inflammation precedes steatosis and is mediated by visceral fat accumulation. J Endocrinol. 2020;245(3):369-380. [DOI] [PubMed] [Google Scholar]
- 35. Hjortebjerg R. IGFBP-4 and PAPP-A in normal physiology and disease. Growth Horm IGF Res. 2018;41:7-22. [DOI] [PubMed] [Google Scholar]
- 36. Byun D, Mohan S, Yoo M, Sexton C, Baylink DJ, Qin X. Pregnancy-associated plasma protein-A accounts for the insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) proteolytic activity in human pregnancy serum and enhances the mitogenic activity of IGF by degrading IGFBP-4 in vitro. J Clin Endocrinol Metab. 2001;86(2):847-854. [DOI] [PubMed] [Google Scholar]
- 37. Hjortebjerg R, Berryman DE, Comisford R, et al. Depot-specific and GH-dependent regulation of IGF binding protein-4, pregnancy-associated plasma protein-A, and stanniocalcin-2 in murine adipose tissue. Growth Horm IGF Res. 2018;39:54-61. [DOI] [PubMed] [Google Scholar]
- 38. Maridas DE, DeMambro VE, Le PT, et al. IGFBP-4 regulates adult skeletal growth in a sex-specific manner. J Endocrinol. 2017;233(1):131-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ning Y, Schuller AG, Conover CA, Pintar JE. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol Endocrinol. 2008;22(5):1213-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”