ABSTRACT
Background
High postprandial glucose excursions may increase risk for disease. Individuals have widely varying glucose responses to different meals, and precision nutrition approaches often seek to personalize diets to minimize postprandial glycemic responses as measured by continuous glucose monitors (CGMs). However, it is unknown whether different CGM devices result in concordant meal rankings according to postprandial glycemic excursions.
Objective
We explored whether meal rankings according to postprandial glycemic excursions differ between 2 simultaneously worn CGMs.
Methods
We collected 27,489 simultaneous measurements from Dexcom G4 Platinum and Abbott Freestyle Libre Pro CGMs during 28 inpatient days in 16 adults without diabetes. Simultaneous glucose measurements obtained for 2 h following 760 ad libitum meals were used to compare within-subject meal rankings between the CGM devices according to their incremental glucose response.
Results
Postprandial responses to ad libitum meals were highly variable, with the Abbott and Dexcom systems resulting in within-subject incremental mean ± SD glucose CVs of 91.7 ± 1.9% and 94.2 ± 2.7%, respectively. Within-subject meal rankings for incremental glycemic responses were relatively discordant between CGMs, with a mean Kendall rank correlation coefficient of 0.43 ± 0.05. Meals in the bottom compared with those in the top half of incremental glycemic responses ranked by Abbott resulted in 50 ± 10% (P = 0.0002) less glycemic reduction as measured by Dexcom, and vice versa. The missing glycemic reduction by eating meals ranked according to the discordant CGM was inversely correlated with each subject's Kendall rank correlation coefficient (r = −0.95; P < 0.0001).
Conclusions
Precision nutrition approaches that use CGMs to personalize meal recommendations for minimizing glycemic excursions may be premature given the discordance of within-subject meal rankings between simultaneous CGM devices. More research is needed to clarify the source of this imprecision. This trial was registered at clinicaltrials.gov as NCT03407053.
Keywords: continuous glucose monitor, glycemia, glucose variability, personalized nutrition, precision nutrition
Introduction
Postprandial glucose is believed to play an important role in health and disease (1, 2). Different meals result in widely varying postprandial glucose excursions that may depend on differences between individuals as well as differences between meals. Precision nutrition approaches are being developed to personalize an individual's diet to minimize postprandial glycemic responses measured using continuous glucose monitors (CGMs) (3, 4). However, such approaches have been criticized (5) and it is unclear to what extent different CGMs might measure different glycemic responses to the same meals in the same individual. In other words, it is unknown whether different CGMs might result in discordant meal rankings, such that personalized diets that minimize glucose excursions according to measurements by one CGM may not result in similarly low postprandial glucose obtained using a different CGM.
To explore this issue, we analyzed CGM data in adults without diabetes who simultaneously wore Dexcom G4 Platinum and the Abbott Freestyle Libre Pro CGM systems during a 28-d inpatient study at the NIH Clinical Center. We previously reported the primary outcome of the study, which was the difference in ad libitum energy intake between 2 experimental diets provided in random order for 2 wk each (6). Here, we explored the simultaneous CGM measurements to investigate whether within-subject meal rankings according to their postprandial glycemic responses were CGM dependent and whether meals chosen to minimize glycemic responses using one CGM also minimized the glycemic responses as determined by the other CGM.
Methods
The study protocol was approved by the institutional review board of the National Institute of Diabetes & Digestive & Kidney Diseases. This trial was registered at clinicaltrials.gov as NCT03407053. Eligible subjects were between 18 and 50 y old with a BMI (in kg/m2) >18.5 and were weight stable (weight change <5% increase or decrease during the past 6 mo) with no history of illness or psychiatric disease or dietary restrictions, and were not taking prescription medications that could influence metabolism (6).
The subjects were admitted as inpatients for 28 d to the Metabolic Clinical Research Unit at the NIH Clinical Center. At the end of each diet period, on study days 14 and 28, each subject completed an oral glucose tolerance test (OGTT). After fasting overnight, subjects consumed 75 g oral glucose, and we compared the CGM measurements to the glucose measurements from venous blood samples at 0, 10, 20, 30, 60, 90, and 120 min postconsumption.
Twenty subjects wore the Dexcom G4 Platinum, and 16 subjects also simultaneously wore the Abbott Freestyle Libre Pro. Each device consisted of a small sensor, a transmitter, and a hand-held receiver. The sensor was inserted subcutaneously to measure interstitial glucose concentrations, which the transmitter would send wirelessly to the receiver. Subjects were blinded to their glucose readings obtained by the monitors. The Dexcom sensor was inserted in the lower abdomen and sampled glucose concentrations every 5 min. Per manufacturer specifications, the device was calibrated using capillary blood finger stick measurements after insertion and each morning and night. The sensor was changed every 7 d, following the manufacturer-specified lifetime. The Abbott Freestyle Libre Pro was inserted in the back of the upper arm and sampled glucose concentrations every 15 min with a minimum glucose reading of 40 mg/dL. No finger stick calibrations were required, and the sensor was changed after 14 d. Both CGM systems were removed during MRI procedures and the Dexcom was removed for DXA scans once a week. The data for each monitor were downloaded at the end of each inpatient stay.
In total, 27,489 simultaneous CGM measurements (excluding the OGTT periods) were obtained in 16 subjects by pairing the time stamps of the 2 devices with the closest match. The maximum gap within a pair was set to 150 s. Not all subjects had simultaneous CGM data during the entire study due to scheduling of procedures and the sensor lifetime of each monitor. We restricted our analysis of postprandial data to those subjects with simultaneous CGM measurements of ≥105 min after the meal or OGTT and with a minimum of 7 data points for the Abbott system and 20 data points for the Dexcom system. We analyzed 760 ad libitum meals in 16 subjects and 22 OGTTs from 15 subjects who had simultaneous CGM measurements meeting these criteria. The baseline glucose measurement was defined as the first CGM measurement obtained ≤5 min following ingestion. The mean postprandial glucose was expressed as the incremental area under the glucose curves, calculated using the trapezoid rule with respect to the baseline glucose measurement, divided by the duration of the postprandial measurements. We did not exclude periods of glucose below the baseline. The mean within-subject CV in postprandial glucose was calculated using the CVs for each subject weighted by the number of meals contributing simultaneous CGM data, which ranged from 5 to 67 meals per subject.
We quantified the fractional reduction in the postprandial glycemic response that was missed by ranking meals based on the discordant CGM as follows:
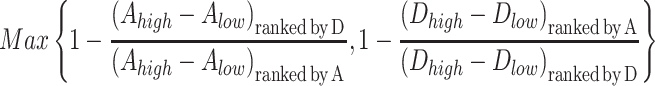 |
(1) |
where Ahigh and Dhigh are the mean within-subject incremental glucose responses measured by the Abbott and Dexcom systems, respectively, to the half of meals ranked highest in glycemic response; Alow and Dlow are the mean within-subject incremental glucose responses measured by the Abbott and Dexcom systems, respectively, to the half of meals ranked lowest in glycemic response. The subscript “ranked by” A or D refers to whether the Abbott or Dexcom system was used to rank the meals by their glycemic response, respectively.
According to this mathematical expression, when the postprandial glycemic rankings of the meals are completely concordant between the devices, the numerators and denominators of the quotients are equal and the output of the overall expression is zero, indicating that there is no missing reduction in postprandial glycemia when the discordant CGM is used to rank the meals. In contrast, if ranking the meals by the discordant CGM results in no mean difference in postprandial glycemia between the top and bottom half of ranked meals then the numerators of the quotients are zero and the output of the overall expression is 1, indicating that the fraction of postprandial glycemic reduction that was missed by the discordant ranking is 100%.
Statistical analyses were performed using SAS (version 9.4; SAS Institute Inc.). Kendall rank correlation coefficients were calculated using PROC CORR KENDALL. The data are presented as means ± SEs. Paired t-tests were used to compare the CGM results, and significance was declared at the threshold P < 0.05.
Results
We collected CGM data from 7 male and 9 female adult subjects without diabetes (mean ± SE age: 31 ± 2 y; BMI: of 26 ± 1.4) who simultaneously monitored glucose using the Dexcom G4 Platinum and the Abbott Freestyle Libre Pro systems while they resided continuously for a 28-d period as inpatients at the NIH Clinical Center.
Overall comparison of simultaneous CGM data
We measured 27,489 simultaneous CGM glucose values with both the Abbott and Dexcom systems (excluding the OGTT periods) in 16 subjects. Mean glucose was significantly higher with the Dexcom (93.4 ± 0.12 mg/dL) compared with the Abbott system (80.5 ± 0.12 mg/dL; P < 0.0001). The Abbott CGM measurements were 12.9 ± 0.1 mg/dL (P < 0.0001) lower than the Dexcom system. The glucose values were significantly correlated between the CGMs (r = 0.57; P < 0.0001). A small, but significant linear bias was present such that for every 1-mg/dL increment in mean glucose, the Abbott measurements were progressively 0.03 ± 0.006 mg/dL lower than the Dexcom measurements (P < 0.0001). The mean within-subject discrepancy between the Abbott and Dexcom systems increased significantly with percentage of body fat (r = 0.56; P = 0.02), such that for each 1% increase in body fat the Abbott system resulted in a 0.49 ± 0.19 mg/dL lower mean glucose than the Dexcom system.
Despite the significant bias between the simultaneous CGM measurements described above, it is possible that the incremental CGM response to ingestion of meals may be comparable. To investigate this possibility, we measured incremental glucose excursions for 2 h following 22 OGTTs in 15 subjects and compared these simultaneous CGM measurements with incremental glucose responses calculated using venous glucose measurements. The mean incremental glucose response during the OGGTs was 38 ± 3 mg/dL, as determined by the venous measurements, which was not significantly different from the results of the Abbott (38 ± 3 mg/dL; P = 0.98) and Dexcom (43 ± 3 mg/dL; P = 0.19) CGM measurements. The mean incremental glucose during the OGTTs was significantly correlated with the venous measurements for both the Abbott ( r = 0.69; P = 0.0004) and Dexcom (r = 0.76; P < 0.0001) systems, which were also significantly correlated with each other (r = 0.59; P = 0.004). Therefore, mean incremental glucose measurements obtained with OGTTs were, on average, comparable with those obtained with the CGM devices.
We analyzed postprandial glucose responses to 760 ad libitum meals in 16 subjects, with each subject having simultaneous CGM measurements for 47.5 ± 5 meals. The mean incremental glucose response following each meal was 16.4 ± 0.5 mg/dL using the Abbott system compared with 14.5 ± 0.5 mg/dL using the Dexcom system ( P < 0.0001). Across all 16 subjects, the CGMs provided moderately correlated measurements for mean incremental glucose following the meals ( r = 0.68, P < 0.0001).
Individual variability of postprandial glycemic responses
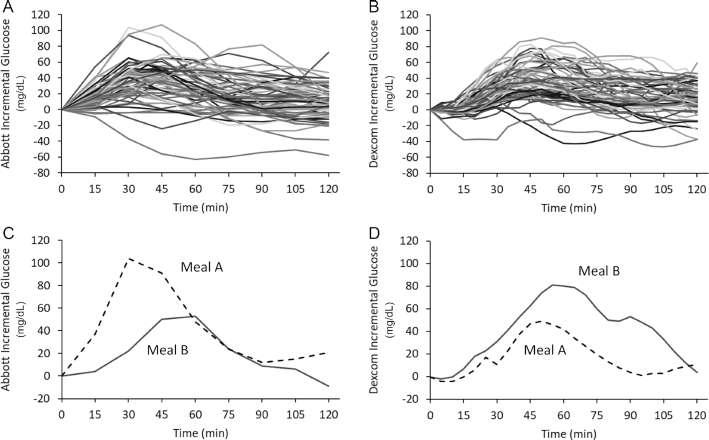
Within-subject postprandial responses to ad libitum meals were highly variable, with the Abbott and Dexcom systems having incremental glucose CVs of 91.7 ± 1.9% and 94.2 ± 2.7%, respectively. Figure 1A and B show the incremental glucose measurements of an example subject in response to 64 ad libitum meals using the Abbott and Dexcom systems with CVs of 77.3% and 68.8%, respectively. Figure 1C illustrates the same subject's incremental glucose responses to 2 different breakfasts measured by the Abbott system. Meal A was a berry and walnut quinoa cereal and meal B was a plain bagel with cream cheese and turkey bacon. Figure 1D shows discordant simultaneous Dexcom measurements in the same subject in response to the same meals. In other words, the different CGMs ranked these 2 meals quite differently regarding their impact on postprandial glycemia for this subject.
Figure 1.
Abbott continuous glucose measurements (A) in an example subject consuming 64 ad libitum meals illustrating incremental glucose responses with a CV of 77.3%. Dexcom continuous glucose measurements (B) simultaneously obtained in the same subject showing incremental glucose responses with a CV of 68.8%. Example of incremental glucose responses to meals A and B as measured by the Abbott system (C). (Meal A was a berry and walnut quinoa cereal and meal B was a plain bagel with cream cheese and turkey bacon.) The Dexcom system (D) measured comparatively discordant incremental glycemic responses to the same 2 meals simultaneously measured in the same subject.
Ranking meals by CGM glycemic responses
To investigate the within-subject concordance of meal rankings according to the glycemic responses simultaneously measured by the Abbott and Dexcom systems, we calculated the Kendall rank correlation coefficient which has a value of 1 for identical meal rankings between CGMs, a value of −1 for completely discordant rankings, and a value of 0 indicates an equal number of discordant and concordant rankings. We found a mean Kendall rank correlation coefficient of 0.43 ± 0.05 for incremental glucose response, indicating a relatively low degree of concordance in the meal rankings between simultaneous CGMs.
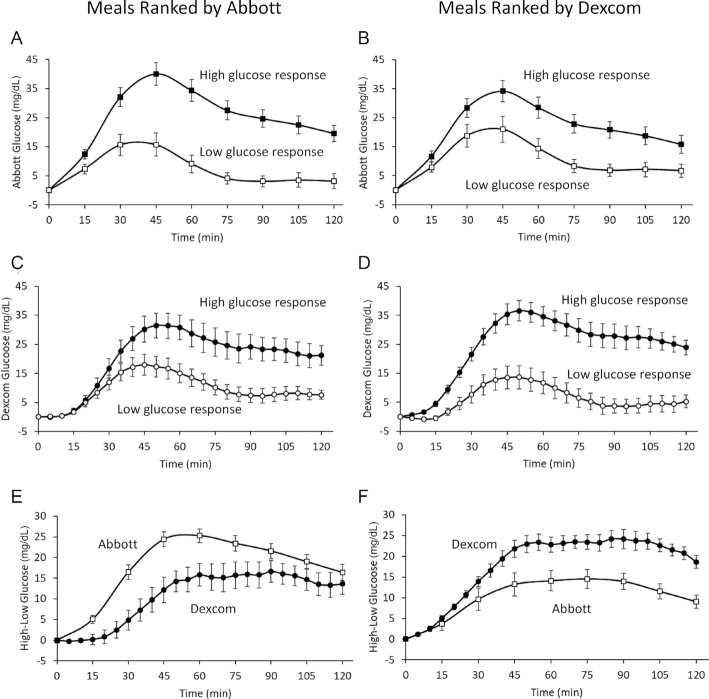
The relatively low concordance of meal rankings between CGMs may not necessarily result in meaningful glycemic differences. To investigate the magnitude of the discordant CGM effect, we calculated within-subject differences in the mean incremental glucose responses to the top half compared with the bottom half of meals ranked and measured by the concordant CGM in comparison to the differences measured by the discordant CGM. The left column of Figure 2 presents CGM data when meals were ranked according to the Abbott system and the right column shows the results when the same meals were ranked by the Dexcom system. Figure 2A is a plot of the mean incremental glucose response measured by the Abbott system in response to the top compared with the bottom half of 760 meals ranked according to the Abbott system. In this case, the concordant Abbott CGM measured a 17.9 ± 1.1 mg/dL difference in mean incremental glucose between the top and bottom ranked meals (P < 0.0001). Figure 2B shows that when the same meals were ranked by the Dexcom system, the difference in the mean incremental glucose between the top and bottom half of ranked meals was 10.7 ± 1.6 mg/dL (P < 0.0001) as measured by the discordant Abbott system, which was significantly lower than when the meals were ranked by the Abbott system (P < 0.0001).
Figure 2.
Mean Abbott glucose responses (A) to the high- compared with the low-ranked half of 760 ad libitum meals according to the Abbott system. Mean Abbott glucose responses (B) in the same 16 subjects to the same high-ranked compared with the low-ranked meals according to the Dexcom system. Mean Dexcom glucose responses (C) to the meals ranked as high glycemic compared with those ranked as low glycemic by the Abbott system. Mean Dexcom glucose responses (D) to the meals ranked as high glycemic compared with those ranked as low glycemic by the Dexcom system. Difference between the Abbott- and Dexcom-measured glucose responses (E) for the meals ranked as high glycemic compared with those ranked as low glycemic by the Abbott system. Difference between the Abbott- and Dexcom-measured glucose responses (F) for the meals ranked as high glycemic compared with those ranked as low glycemic by the Dexcom system.
Conversely, Figure 2C shows the mean incremental glucose measurements obtained by the discordant Dexcom system in response to the meals ranked high and low according to the Abbott system. The difference in mean incremental glucose was 10.7 ± 2.0 mg/dL (P < 0.0001). Figure 2D shows the mean Dexcom incremental glucose measurements in response to meals ranked by the Dexcom system and illustrates the 17.9 ± 0.9 mg/dL difference in mean glucose between high- and low-ranked meals (P < 0.0001), which was significantly greater than the difference for meals ranked by the Abbott system (P < 0.0001).
Figure 2E and F show that the mean incremental glucose differences measured by the Abbott system between the top- and bottom-ranked meals according to Abbott were greater than the differences in incremental glucose determined by the Dexcom system, and vice versa. The mean fraction of missing glycemic reduction determined by the discordant CGMs was 50 ± 10% (P = 0.0002) with respect to the incremental glucose responses. The missing glycemic reduction by eating meals according to the discordant CGM was inversely correlated with each subject's Kendall rank correlation coefficient for incremental glucose responses (r = −0.95; P < 0.0001).
The Abbott system measured 3.4 ± 0.6 mg/dL higher mean incremental glucose in response to meals ranked low by the Dexcom system compared with the meals ranked low by the Abbott system (P < 0.0001). Similarly, the Dexcom system measured 3.4 ± 0.8 mg/dL higher mean incremental glucose in response to the lower half of meals ranked by the Abbott system compared with the meals ranked lowest by the Dexcom system (P = 0.001).
Discussion
Different ad libitum meals resulted in a wide range of postprandial glucose responses regardless of the CGM. However, the within-subject rankings of the same meals according to one CGM did not necessarily correspond to the rankings measured simultaneously by the other CGM. Rather, we found substantial differences in glycemic responses that depended on the CGM used to make the meal rankings. The difference in glycemic response between the top half of meals compared to the bottom half of meals ranked by one CGM was significantly greater than the glycemic differences measured simultaneously by the other CGM.
The reasons for the differences in meal ranking according to CGM are unclear. The different anatomical locations of the CGM sensors and the influence of subcutaneous fat on interstitial glucose may have played a role. Previous studies have demonstrated that simultaneous capillary glucose measurements at different anatomical locations can indicate distinct glucose kinetics (7–9). However, a recent study in subjects with diabetes reported similar accuracy of glucose measurements from simultaneous Dexcom sensors inserted on the upper arm in comparison to the manufacturer-recommended abdominal location (10). Perhaps random variation in the glucose measurements, performance differences between CGM systems, or a combination thereof were responsible for the discordance. But regardless of the reason, our data indicate that the meals minimizing postprandial glycemic excursions were somewhat inconsistent between the CGMs.
Our results have implications for precision nutrition approaches that seek to personalize an individual's diet to minimize CGM-measured glycemic responses to meals (3, 4). An implicit assumption that has not previously been investigated is that the ranking of an individual's meals by glycemic response is relatively independent of the CGM used to make the measurements. Our results suggest that this assumption may be incorrect, at least in people without diabetes.
Precision nutrition approaches seeking to personalize meals to minimize glycemic responses via one CGM may not necessarily recommend the same meals according to another CGM. More research is needed to clarify the source of this imprecision. Misclassifying meals or foods as “bad” or “good” in terms of their CGM responses may affect overall eating behaviors with potentially deleterious consequences. For example, if diet recommendations are based on imprecise CGM measures that disregard other aspects of optimal nutrition, then individuals may make poor dietary choices to achieve potentially illusory glycemic benefits.
Supplementary Material
Acknowledgments
The authors' contributions were as follows: RH, JG, and KDH: designed the study, analyzed the data, and wrote the manuscript; KDH: had primary responsibility for the final content; and all authors: read and approved the final manuscript. Author disclosures: The authors report no conflicts of interest.
Notes
Supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes & Digestive & Kidney Diseases.
Data Sharing: Data described in the manuscript and analytic code will be made publicly and freely available without restriction at https://osf.io/rx6vm/.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CGM, continuous glucose monitor; OGTT, oral glucose tolerance test.
Contributor Information
Rebecca Howard, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
Juen Guo, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
Kevin D Hall, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
References
- 1. Hall H, Perelman D, Breschi A, Limcaoco P, Kellogg R, McLaughlin T, Snyder M. Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol. 2018;16(7):e2005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blaak EE, Antoine JM, Benton D, Bjorck I, Bozzetto L, Brouns F, Diamant M, Dye L, Hulshof T, Holst JJ et al. Impact of postprandial glycaemia on health and prevention of disease. Obes Rev. 2012;13(10):923–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mendes-Soares H, Raveh-Sadka T, Azulay S, Ben-Shlomo Y, Cohen Y, Ofek T, Stevens J, Bachrach D, Kashyap P, Segal L et al. Model of personalized postprandial glycemic response to food developed for an Israeli cohort predicts responses in Midwestern American individuals. Am J Clin Nutr. 2019;110(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–94. [DOI] [PubMed] [Google Scholar]
- 5. Wolever TM. Personalized nutrition by prediction of glycaemic responses: fact or fantasy?. Eur J Clin Nutr. 2016;70(4):411–13. [DOI] [PubMed] [Google Scholar]
- 6. Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey V et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food. Cell Metab. 2019;30(1):67–77. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellison JM, Stegmann JM, Colner SL, Michael RH, Sharma MK, Ervin KR, Horwitz DL. Rapid changes in postprandial blood glucose produce concentration differences at finger, forearm, and thigh sampling sites. Diabetes Care. 2002;25(6):961–4. [DOI] [PubMed] [Google Scholar]
- 8. Jungheim K, Koschinsky T. Glucose monitoring at the arm: risky delays of hypoglycemia and hyperglycemia detection. Diabetes Care. 2002;25(6):956–60. [DOI] [PubMed] [Google Scholar]
- 9. Van Der Valk PR, Van Der Schatte Olivier-Steding I, Wientjes KJ, Schoonen AJ, Hoogenberg K. Alternative-site blood glucose measurement at the abdomen. Diabetes Care. 2002;25(11):2114–15. [DOI] [PubMed] [Google Scholar]
- 10. Steineck K II, Mahmoudi Z, Ranjan A, Schmidt S, Jorgensen JB, Norgaard K. Comparison of continuous glucose monitoring accuracy between abdominal and upper arm insertion sites. Diabetes Technol Ther. 2019;21(5):295–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.