Abstract
Maternal ischemic stroke and cerebral venous sinus thrombosis (CVST) are dreaded complications of pregnancy and major contributors to maternal disability and mortality. This chapter summarizes the incidence and risk factors for maternal arterial ischemic stroke (AIS) and CVST and discusses the pathophysiology of maternal AIS and CVST. The diagnosis, treatment, and secondary preventive strategies for maternal stroke are also reviewed. Special populations at high risk of maternal stroke, including women with moyamoya disease, sickle cell disease, HIV, thrombophilia, and genetic cerebrovascular disorders, are highlighted.
INTRODUCTION
Maternal stroke, defined as stroke occurring during pregnancy or the postpartum period, is increasingly recognized as a major cause of maternal morbidity and mortality. While differing definitions of “stroke” have hampered epidemiologic estimates of its incidence, most studies point to an increasing incidence of maternal strokes of all types (Kuklina et al., 2011; Liu et al., 2019a). Pregnancy-related physiologic changes raise the risk of thromboembolic events, including arterial ischemic stroke (AIS) and cerebral venous sinus thrombosis (CVST). Modern medicine has paved the way for women with increasingly complex medical issues to successfully become pregnant; however, pregnancy constitutes a stress test for the maternal cardio- and cerebrovascular system, and hypertension, underlying cardiac disease, or hematologic disorders in women have increased risk of complications, including AIS and CVST, during pregnancy and postpartum. In addition, the increasing incidence of pregnancy-induced hypertensive disorders contributes to maternal stroke risk.
Acute stroke treatment has made great strides over the last decade, and life- and function-preserving treatments are now available to additional patients (Albers et al., 2018; Nogueira et al., 2018; Thomalla et al., 2018; Ma et al., 2019). However, pregnant women have been excluded from randomized trials, and acute stroke treatment is generally off-label for this population. Nevertheless, pregnant and postpartum women may benefit enormously from acute stroke treatments and secondary prevention strategies used in other populations (Powers et al., 2018).
This chapter reviews the epidemiology, risk factors, and pathophysiology of maternal AIS and CVST, and discusses pregnancy in special populations who have baseline increased risk of stroke, including women with moyamoya disease, sickle cell disease (SCD), HIV, thrombophilia, and genetic cerebrovascular disorders. Lastly, we review current recommendations for diagnosis, treatment, and secondary prevention of AIS and CVST in pregnancy and postpartum.
EPIDEMIOLOGY OF MATERNAL ARTERIAL ISCHEMIC STROKE AND CEREBRAL VENOUS SINUS THROMBOSIS
Maternal stroke incidence
Stroke risk during pregnancy and the puerperium is an estimated threefold higher compared to nonpregnant women of childbearing age (Swartz et al., 2017). A recent meta-analysis found a crude incidence of stroke during pregnancy and the puerperium of 30 per 100,000 deliveries (95% confidence interval [95% CI] 18.8–49.4). Maternal strokes are approximately evenly divided between hemorrhagic, ischemic, and CVST (Swartz et al., 2017). The incidence of maternal stroke is variably reported from the low single digits (Wiebers and Whisnant, 1985) to greater than 60 per 100,000 deliveries (Jaigobin and Silver, 2000). The variability arises due to many reasons, including study era, patient demographics (i.e., community hospitals vs referral center, geographical area, socioeconomics), study definition of stroke, inclusion of various stroke subtypes, and defined length of postpartum period.
Impact
Despite the variability in reported rates, compelling data establish maternal stroke as a serious public health issue. Stroke is the most common cause of serious long-term disability following pregnancy (Treadwell et al., 2008; Sells and Feske, 2017) and is the seventh leading cause of death among pregnant women, accounting for 7.4% of pregnancy-related deaths, in the United States (Creanga et al., 2017). Mortality rates from maternal stroke ranges from 2.7% to 20.4% (Swartz et al., 2017). One United Kingdom study found a case fatality rate of 20% for ante-natal stroke and that 45% of stroke survivors remained significantly disabled (Scott et al., 2012). Epidemiologic studies in East Asia also show a high rate of maternal stroke, although hemorrhagic events are relatively more common (Jeng et al., 2004; Liang et al., 2006). Data on the global impact of maternal stroke are lacking, but the World Health Organization (WHO) found stroke was one of the 10 leading causes of death in women of reproductive age (15–44 years) in low-, middle-, and high-income countries (World Health Organization, 2008).
A significant proportion of strokes among women of childbearing age are pregnancy-related (Awada et al., 1995; Miller et al., 2016a), accounting for an estimated 12%–35% of strokes among women 15–45 years of age (Kittner et al., 1996). A French study of maternal strokes found most stroke-related deaths were unavoidable; however, diagnostic delay, diagnostic error, and inadequate treatment contributed in some cases (Cohen and Rossignol, 2017). Another study at the University of Tennessee-Memphis documented frequent delays in diagnosis of stroke during pregnancy because of a presumption of eclampsia as the etiology of focal neurologic symptoms (Witlin et al., 1997).
Temporal trends
While evidence is mixed, available data suggest that the rate of maternal stroke is likely increasing. An early epidemiologic study in Rochester, Minnesota using 1955–1979 data reported just 3.5 strokes per 100,000 deliveries (but did not include the postpartum period) (Wiebers and Whisnant, 1985); whereas, later studies of maternal stroke in the United States consistently find much higher rates (Sharshar et al., 1995; Kittner et al., 1996; Jaigobin and Silver, 2000; Lanska and Kryscio, 2000; James et al., 2005; Ban et al., 2017; Yoshida et al., 2017; Liu et al., 2019a). A review of US hospital discharge data demonstrated increased rates of maternal stroke from 1994 to 2007, with a 47% increase in antepartum stroke and 83% increase in postpartum stroke and unchanged risk at time of delivery (Kuklina et al., 2011). A study by the Canadian Institute of Health similarly noted an increase in strokes from 2003 to 2016 (Liu et al., 2019a). However, another meta-analysis found the rate of maternal stroke unchanged from 1990 to 2017 (Swartz et al., 2017).
The possible reasons for increased incidence include both reporting and public health trends. Improved diagnostic testing, awareness of stroke, and surveillance systems may increase the number of reported cases with or without an actual increase in incidence. The increased rate of maternal stroke in the US hospital discharge data was almost entirely attributed to the increased prevalence of hypertensive disorders in pregnancy (Kuklina et al., 2011).
Timing of stroke during pregnancy and the puerperium
The risk of arterial and venous infarcts changes throughout the antepartum, peripartum, and postpartum period. While there is some variation between studies, in general, epidemiologic data show that the highest risk periods for stroke are the third trimester, time of delivery, and early postpartum periods. One study found that almost half (48%) of strokes occurred postpartum and most of the remainder (41%) occurred at the time of delivery (Rantanen and Tatlisumak, 2013), which was similar to the Nationwide Inpatient Sample, which found that 89% of strokes occurred at the time of delivery or postpartum (James et al., 2005). A review of maternal strokes at a stroke referral center in New York City found that over 70% occurred postpartum (Miller et al., 2016a). A systematic review found slightly more strokes occurred in the antepartum and delivery period, but given most studies included about 6 weeks of postpartum data vs 9 months of pregnancy, the per-day stroke risk was significantly higher in the postpartum period (Swartz et al., 2017).
Epidemiology of cerebral venous sinus thrombosis
CVST occurs when a clot forms in the cerebral venous system or dural sinuses, which may lead to venous congestion, cerebral edema, and ischemic and/or hemorrhagic stroke. Overall, 75% of CVST occur in women (Ferro et al., 2004). Known risk factors for CVST include oral contraceptives, hypercoagulability, infection, malignancy, and pregnancy (Ferro et al., 2004). Venous strokes account for a disproportionate amount of all strokes during pregnancy (Jeng et al., 2004) with estimates ranging from 6% to 64% of all maternal strokes (Feske and Singhal, 2014). The risk of pregnancy-related CVST increases with the presence of hypertensive disorders, age, cesarean section, infections, and excessive vomiting (Lanska and Kryscio, 2000). Approximately three-quarters of pregnancy-related CVST occur in the postpartum period, with the greatest risk approximately 1–4 weeks after delivery (Jeng et al., 2004; Coutinho et al., 2009; Yoshida et al., 2017). While many epidemiologic studies of maternal stroke include only 6 weeks postpartum, the risk may be increased for 12 weeks (Kamel et al., 2014) or up to 1 year postpartum (Cheng et al., 2017).
RISK FACTORS FOR MATERNAL STROKE
Risk factors for maternal stroke include general risk factors for stroke in young adults, and unique risk factors specific to pregnancy, summarized in Table 1.1. This section reviews general risk factors first, followed by pregnancy-specific factors.
Table 1.1.
Risk factors for maternal stroke
| Nonpregnancy-specific | Pregnancy-specific | |
|---|---|---|
| Modifiable •Smoking and other drug use • Diabetes • Hypertension • Cardiac disease (structural, arrhythmias) • Arterial disease • Patent foramen ovale • Migraine • Infection • Malignancy |
Nonmodifiable • Age • Race • Socioeconomic status • Genetic stroke and hypercoagulable syndromes |
• Hypertensive disorders of pregnancy (gestational hypertension, preeclampsia/eclampsia, HELLP) • Assisted reproductive technology (ovarian hyperstimulation syndrome) • Cesarean section • Postpartum cerebral angiopathy • Peripartum cardiomyopathy • Amniotic fluid embolism • Sheehan syndrome • Metastatic choriocarcinoma • Age • Race/socioeconomic status • Genetic stroke and hypercoagulable syndromes |
General risk factors that affect risk of maternal stroke
Age
Maternal stroke is more common in older pregnant women (Lanska and Kryscio, 2000; Katsuragi et al., 2018; Liu et al., 2019a). The absolute risk is lowest among women aged 20–34 and highest among women aged 40 and over (James et al., 2005). In a logistic regression model designed to distinguish stroke from stroke mimickers during pregnancy, age was a strong predictor of stroke (Meyer et al., 2018). Interestingly, a population-based analysis of New York State data suggested that while the absolute risk of maternal stroke increases with age, the incidence risk ratio (IRR) for pregnancy-related stroke compared to nonpregnancy-related stroke was higher in younger age groups: the IRR in women aged 18–24 was 2.2 (95% CI 1.9–2.6), and for ages 25–34 was 1.6 (95% CI 1.4–1.7), whereas in women aged 35–44 the IRR was 1.1 (95% CI 0.9–1.2). Of note, maternal strokes accounted for 18% of all strokes in women aged 18–35. However, the absolute risk of maternal stroke was more than three times higher in the oldest group (46.9 per 100,000 deliveries) compared to the youngest group (14 per 100,000 deliveries) (Miller et al., 2016b).
Race/ethnicity
Health disparities affect risk of maternal stroke. In the National Inpatient Sample from 2000 to 2001, Black women had nearly twice the risk of maternal stroke compared to Hispanic and white women (James et al., 2005). Recent data from the National Inpatient Sample showed a significant interaction between hypertensive status and race on the risk of maternal ischemic stroke: among women without hypertension, only Black women had increased risk of ischemic stroke compared to whites (adjusted RR 1.41, 95% CI 1.07–1.85). However, among women with pregnancy-induced hypertension, Black, Hispanic, and Asian/Pacific Islander women all had higher risk of ischemic stroke, with the highest risk seen in Black women (adjusted RR 2.44, 95% CI 2.00–2.97) (Zambrano et al., 2019).
Heart disease
Cardiac disease, including structural and rhythm abnormalities, is a well-known stroke risk factor. Many types of heart disease have been described as risk factors for maternal stroke. US inpatient data found an odds ratio of 13.2 (95% CI 10.2–17.0) for maternal stroke with heart disease, including congenital and acquired causes (James et al., 2005). Congenital heart disease encompasses a diverse range of cardiac anomalies present from birth, which may predispose to stroke in children and adults. Canadian inpatient data found a greater than 35 times risk of maternal stroke in women with congenital heart disease (Liu et al., 2019a). Globally, acquired heart disease may account for a greater proportion of maternal stroke; a Taiwanese study found rheumatic heart disease present in almost half of patients with ischemic stroke and CVST in pregnancy and the puerperium (Jeng et al., 2004). Other intrinsic cardiac anomalies, including a reported case of cardiac papillary fibroelastoma, may lead to maternal stroke (Binhas et al., 2019).
Patent foramen ovale (PFO)
A PFO occurs when the fetal connection between the cardiac atria fails to fuse after birth. About a quarter of all adults have a PFO, according to autopsy studies (Hagen et al., 1984). When present, blood can shunt from the right atrium to the left atrium, particularly during Valsalva maneuvers, and may lead to ischemic stroke via paradoxical emboli. Pregnancy and the puerperium are associated with high risk of venous thromboembolism and therefore, unsurprisingly, maternal stroke may result from paradoxical emboli (Daehnert et al., 2001; Giberti et al., 2005; Miller et al., 2015; Chen et al., 2016). Additionally, straining during labor may predispose to paradoxical embolization. In a review of reported cases of PFO-related strokes during pregnancy, key factors included high-risk PFO morphology (atrial septal aneurysm), larger right-to-left shunt, multiple gestation, and concurrent hypercoagulability (Chen et al., 2016).
The risk of paradoxical embolism score can help clinicians assess whether cryptogenic strokes are likely to be the result of a PFO vs an alternative etiology by assessing the presence of traditional risk factors for stroke (hypertension, diabetes, smoking, prior transient ischemic attack (TIA)/strokes, and age) and imaging characteristics (Kent et al., 2013). One study comparing maternal stroke due to PFO vs alternative etiologies found that women with PFO-related strokes were statistically more likely to have hypercoagulable state, migraine with aura, and iliac vein compression syndrome (May–Thurners syndrome) (Chen et al., 2016). Clinical equipoise persists regarding PFO closure, but several randomized clinical trials guide recommendations (Furlan et al., 2012; Carroll et al., 2013; Mas et al., 2017; Søndergaard et al., 2017) and suggest that closure, in cases of large interatrial shunts or associated atrial septal aneurysms, may reduce risk of recurrent stroke vs antiplatelet therapy alone. Medical management (including antiplatelet and/or anticoagulation) and surgical closure for women with PFO-related maternal stroke requires consideration of her medical history and PFO characteristics. Whether women with a known PFO and no history of thromboembolic events should be treated prophylactically during pregnancy with antiplatelet or anticoagulation has not been studied.
Tobacco and other drug use
The WHO recommends all healthcare providers screen pregnant women for tobacco use. In the United States, more than 7% of women self-report smoking during pregnancy (Drake et al., 2018). Internationally, tobacco use during pregnancy varies from <1% to as high as 18% and secondhand smoking exposure is often much higher (Bloch et al., 2008). In a Swedish cohort, the relative risk for maternal stroke in smokers was 2.4 times (95% CI 1.9–3.0) that of nonsmokers (Ros et al., 2002). In a hospital database in the United States, pregnant smokers were also found to have much greater risk of stroke, odds ratio 1.7 (95% CI 1.2–2.5) (Roelands et al., 2009). In addition to the association between tobacco use and maternal stroke, pregnancy may be a “teachable moment” to help women, and potentially their partners, quit smoking early in life to help reduce lifetime stroke risk.
The impact of illicit drugs on maternal stroke is not well studied. A review of hospital discharges for pregnant women in the United States from 2002 to 2014 found an alarming 300% increase in opioid use over the period, while amphetamine use remained stable and cocaine use declined, reflecting trends in the general population (Salihu et al., 2018). While the study did not examine strokes, acute cardiac events increased with opioid use. A case report documents the use of thrombolysis for a left posterior cerebral artery ischemic stroke in a 33-year-old pregnant woman with known tobacco, cocaine, cannabis, heroin, and amphetamine use (Khan et al., 2017).
Infection
Infections contribute to stroke risk, including during pregnancy. A case–control study of inpatient data in California, Florida, and New York found a statistically significant 1.74 times (95% CI 1.29–2.35) peripartum stroke risk for women with an infection at the time of admission for delivery compared to uninfected women. Stroke risk was highest in sepsis and with genitourinary infections, and the relationship persisted after controlling for vascular risk factors, including hypertensive disorders of pregnancy (Miller et al., 2018). Among women with preeclampsia, presence of an infection at the time of admission triples the risk of maternal stroke, according to inpatient data from the state of New York (Miller et al., 2017). A review of nationwide inpatient data similarly found postpartum infection increased maternal stroke risk (James et al., 2005). A more recent study using data from the US Nationwide Readmissions Database found that women with infections had higher risk of readmission for postpartum ischemic stroke (adjusted risk ratio [RR] 1.75, 95% CI 1.37–2.22) but not for postpartum hemorrhagic stroke (adjusted RR 0.96, 95% CI 0.75–1.23) (Miller et al., 2019a). Case reports also document instances of maternal stroke attributable to reactivation of varicella zoster virus (McNamara et al., 2016) and meningovascular syphilis (Bowring et al., 2008). CVST in pregnancy is also highly associated with infection (Lanska and Kryscio, 2000). Efforts for prevention, earlier/increased detection, and appropriate treatment of infections at the time of labor and delivery may potentially reduce maternal strokes.
Migraine
Migraines are highly prevalent in women of childbearing age (Lipton et al., 2007). While many women may have a decrease in migraine frequency and/or severity during pregnancy, a migraine history is a well-established risk factor for maternal stroke (James et al., 2005; Scott et al., 2012; Feske and Singhal, 2014). In a review of national US inpatient data, migraine had the highest association with maternal stroke with an odds ratio of 16.9 (95% CI 9.7–29.5) (James et al., 2005). However, the diagnosis of stroke is not standardized nationally, and the association may be confounded to some degree by complex migraines and migraine auras being misdiagnosed as TIA or stroke. Nonetheless, preconception counseling for female migraineurs should aim to minimize other stroke risk factors, particularly smoking and elevated blood pressure.
While a discussion of migraine management in pregnancy is reviewed elsewhere in this collection, clinicians should note that many preventive and abortive headache medications, particularly vasoactive agents, may have adverse fetal drug effects. Vasoconstrictive medications should be avoided in women with a history of cerebrovascular disease. In addition, new-onset headaches in pregnancy or postpartum should prompt consideration of a broad differential diagnosis, including preeclampsia, ischemic or hemorrhagic stroke, and CVST, in addition to migraine and other primary headache disorders.
Intimate partner violence
While literature review did not identify any reported cases during pregnancy, strangulation is a reported etiology of cervical artery dissection (Malek et al., 2000). Intimate partner violence is more prevalent during pregnancy, including physical abuse, and all women should be screened (Bailey and Daugherty, 2007).
Maternal stroke risk factors specific to pregnancy
Hypertensive disorders of pregnancy
Hypertensive disorders of pregnancy, including chronic hypertension, gestational hypertension, preeclampsia, and eclampsia, are well-established risk factors for maternal stroke (Sharshar et al., 1995; Scott et al., 2012; Liu et al., 2019a). The disorders are classified based on the degree of blood pressure elevation and the presence of systemic involvement. Gestational hypertension is the development of new-onset elevated blood pressure after 20 weeks of gestation in the absence of other systemic manifestations and is classified as severe if systolic blood pressure is persistently over 160 mmHg or diastolic over 110 mmHg, or both, according to the American College of Obstetricians and Gynecologists (American College of Obstetricians and Gynecologists and Task Force on Hypertension in Pregnancy, 2013). A study of inpatient data from 1994 to 2011 in the United States found that women with hypertensive disorders of pregnancy were 5.2 times more likely (95% CI 4.9–5.6) to have a stroke (hemorrhagic or ischemic) than those without (Leffert et al., 2015). Many other studies have also identified hypertension as a risk factor for maternal stroke (Cheng et al., 2017; Yoshida et al., 2017; Katsuragi et al., 2018; Lappin et al., 2018; Liu et al., 2019a). Chronic hypertension, too, has been shown to increase the risk of maternal stroke (Too et al., 2018) and augments stroke risk in women with preeclampsia, although the level of blood pressure used to define chronic hypertension cannot be determined from administrative data (Miller et al., 2017). Among women with preeclampsia, those who suffer a stroke are more likely to have severe preeclampsia or eclampsia and to have coexisting prothrombotic states, coagulopathies, infections, and/or chronic hypertension (Miller et al., 2017).
Hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome is a severe variant of preeclampsia. Neurologic complications in HELLP syndrome are common and may include posterior reversible encephalopathy syndrome, subarachnoid hemorrhage, and hemorrhagic and ischemic stroke (Paul et al., 2013). In a study of maternal death due to stroke in Japan, more than half of the cases of stroke related to hypertensive disorders of pregnancy were complicated by HELLP syndrome (Hasegawa et al., 2015).
Hypertensive disorders of pregnancy can begin in the postpartum period. A retrospective study of readmissions for stroke within 60 days of delivery discharge found women with chronic hypertension and hypertensive diseases of pregnancy are at higher risk of postpartum stroke; most occur within 10 days of discharge (Too et al., 2018).
In addition, hypertensive disorders of pregnancy may predict future cardiovascular disease, including stroke. Gestational hypertension and preeclampsia are associated with increased incidence of future renal, cardiovascular, and cerebrovascular disease even after adjusting for common vascular risk factors, suggesting they are independent predictors of overall vascular health (Schokker et al., 2015; Wu et al., 2017). In the California Teachers Study, women with a history of hypertensive disorders of pregnancy were at 1.3 times greater risk (95% CI 1.2—1.4) of stroke later in life (Miller et al., 2019b).
Gestational diabetes
Limited data suggest gestational diabetes mellitus may be associated with increased maternal stroke risk: a case–control study found a more than 20-fold increase in stroke risk in women with gestational diabetes, but small numbers precluded precise estimates (Scott et al., 2012). Administrative data from Canada showed a crude odds ratio of 1.7 (95% CI 1.2–2.3) for maternal stroke in women with gestational diabetes, but the association was no longer significant after adjustment for other risk factors (Liu et al., 2019b). In addition, gestational diabetes mellitus is a risk factor for developing cardiovascular and cerebrovascular disease later in life after controlling for subsequent nongestational diabetes and other traditional vascular risk factors (Fadl et al., 2014).
Cesarean section
Higher rates of postpartum stroke may occur in women who deliver via cesarean section vs vaginal delivery (Lanska and Kryscio, 2000; Lin et al., 2008). One Taiwanese study found a 1.49 times risk (95% CI 1.27–1.76) of stroke within 12 months of delivery via cesarean section (Lin et al., 2008). However, delineating an increased stroke risk from the underlying indication for the cesarean section and an increased risk from the procedure is difficult. Notably, in the Taiwanese study, the elevated stroke risk did not persist when the cesarean was “maternally requested” and the association was largely driven by cesarean deliveries for preeclampsia/eclampsia. One hypothesis regarding the association between cesarean section and maternal stroke is that women who have cesarean sections may have a priori health conditions predisposing to stroke; however, the procedure itself may also predispose to arterial and venous stroke through greater blood loss and hypotension, inflammatory or infectious sequela of the procedure, and increased length of inactivity and venous stasis postoperatively.
Peripartum cardiomyopathy
Peripartum cardiomyopathy, left ventricular dysfunction in the last month of pregnancy or within 5 months postpartum in women without preexisting cardiac disease or alternative etiology, is another possible cause of maternal cardioembolic stroke, with a few documented cases in the literature (Dyken and Biller, 1994; Lappin et al., 2018). A review from the University of Iowa found cardiomyopathy occurred about once per 8000 deliveries and there was one known postpartum stroke among five postpartum cardiomyopathy cases (Dyken and Biller, 1994).
Assisted reproductive technology (ART)
ART is an umbrella term for reproductive techniques including in vitro fertilization (IVF), artificial insemination, surrogacy, and fertility medications. IVF involves medically stimulating the ovaries to release eggs that are then extracted and fertilized in vitro prior to returning to the uterus. Thrombosis occurs in 0.2% of IVF cycles but when IVF is complicated by ovarian hyperstimulation syndrome (OHSS), the incidence of thrombosis rises to around 10% (Kasum et al., 2014). Of the thrombotic events, most are venous, including CVST; among the arterial events, most are ischemic strokes (Alatri et al., 2011). OHSS is an iatrogenic complication of fertility treatments where the hyperstimulation of ovaries leads to high levels of human chorionic gonadotropin (hCG), overexpression of vascular endothelial growth factor and activation of the renin–angiotensin–aldosterone system, leading to hemoconcentration and hypercoagulability. A meta-analysis comparing pregnancies via ovulation induction to controls found a trend toward increased risk of ischemic stroke and TIA (pooled hazard ratio of 1.25, 95% CI 0.96–1.63) (Dayan et al., 2017). One case of successful intraarterial thrombolysis for a right proximal middle cerebral artery stroke occurring due to OHSS has been reported (Elford et al., 2002). In addition to OHSS, pregnancies conceived with ART may be at higher risk for stroke due to maternal factors such as advanced age and multiple gestation.
In addition, infertility may result from an underlying risk of vascular disease. A prospective cohort study compared successful and unsuccessful ART and found women who failed ART, over 8 years follow-up were more likely to have an adverse cardiovascular event, including stroke, regardless of the number of IVF treatment cycles (Udell et al., 2017). The results suggest that infertility may reflect an underlying predisposition to vascular disease and pregnancy can unmask this predisposition in women undergoing IVF or other forms of ART.
PATHOPHYSIOLOGY OF MATERNAL THROMBOTIC STROKE
Pregnancy is associated with several physiologic changes that increase risk of thrombotic stroke including (1) coagulation factor changes (see Fig. 1.1); (2) connective tissue changes, increasing venous compliance; (3) cardiac and hemodynamic changes; and (4) immunological and inflammatory changes, modulating endothelial cell function. Pregnancy is also associated with several rare pathophysiologic stroke mechanisms, such as postpartum angiopathy, pituitary apoplexy due to Sheehan syndrome, and metastatic choriocarcinoma.
Fig 1.1.
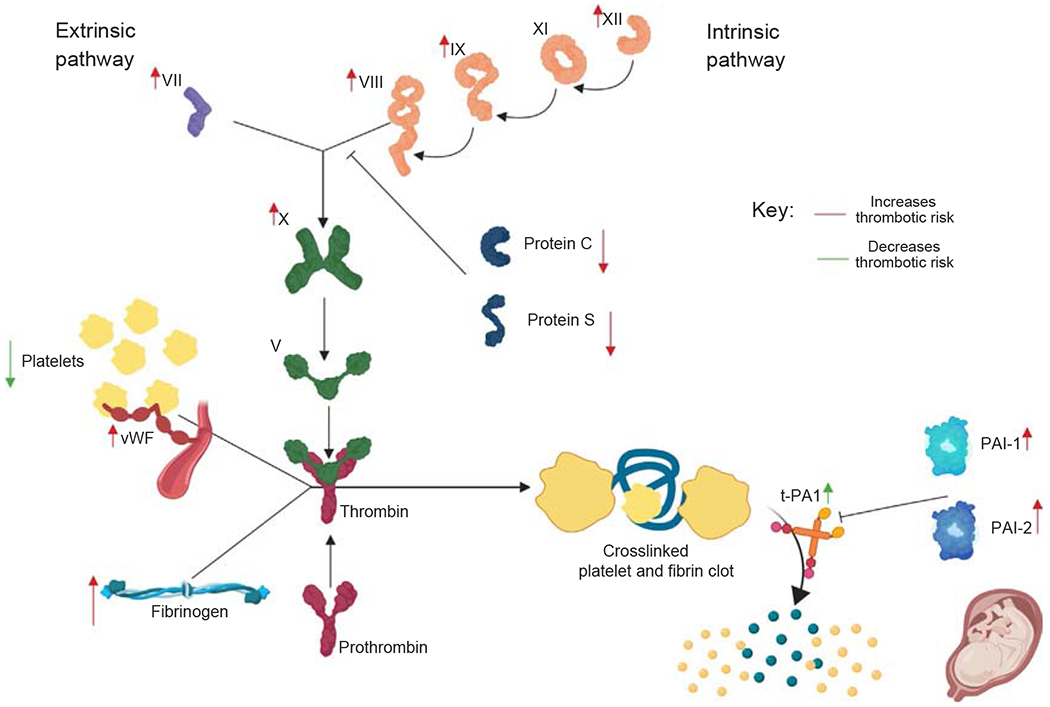
Many changes occur in the coagulation system during pregnancy. The red arrows represent changes that predispose toward a prothrombotic state, including increases in intrinsic and extrinsic clotting factors (factors VII, VIII, IX, X, XI and XII), decreases in intrinsic anticoagulation factors (protein C and protein S), and increases in PAI-1 and PAI-2, including placental production of PAI-2. Increases in von Willebrand factor and fibrinogen also increase clotting risk during pregnancy. However, other changes, indicated by green arrows, including pregnancy-related thrombocytopenia and increases in t-PA1 later in pregnancy also occur.
Changes in the maternal coagulation system
During pregnancy, concentrations of coagulation factors V, VII, VIII, IX, X, XII, and von Willebrand factor rise, accompanied by an increase in fibrinogen levels up to twofold compared to prepregnancy values (Stirling et al., 1984; Brenner, 2004). Von Willebrand factor and factor VIII increase in late gestation (Stirling et al., 1984; Brenner, 2004), promoting a prothrombotic state, particularly during the third trimester and postpartum (Pomp et al., 2008).
Intrinsic anticoagulation factors are also altered during pregnancy. Protein S acts as a cofactor of protein C to inactivate Factors Va and VIIIa, preventing thrombosis. Total protein S (both active and inactive), as well as the ratio of active to inactive protein S, decreases during pregnancy (Castoldi and Hackeng, 2008). There is also evidence that resistance against protein C develops during pregnancy (Cumming et al., 1995). Similarly, activated protein C (APC) resistance can be induced via oral contraceptive or hormone replacement therapy (Fleischer et al., 2009). Decreased sensitivity to APC is associated with preeclampsia, as well as pregnancy loss and placental abruption (De Stefano et al., 2003; Fleischer et al., 2009).
Endogenous tissue plasminogen activator (tPA) activity, produced by endothelial cells, decreases during the first trimester of pregnancy but then increases during the third trimester (Coolman et al., 2006). At 35 weeks, plasminogen activator inhibitor-1 (PAI-1) values were fivefold higher than in the 12th week of pregnancy (Lecander and Åstedt, 1986). In addition, the placenta produces a unique antifibrinolytic, PAI-2, with variable levels depending on the amount of placental tissue (Lecander and Åstedt, 1986). PAI-2 plays an important part in the vascular remodeling necessary for successful placental implantation, replacing maternal endothelial cells with trophoblast cells that have reduced capacity to lyse fibrin (Sheppard and Bonnar, 1999).
Changes in vascular compliance
Total intravascular volume is significantly higher in pregnant than in nonpregnant women (Goulart et al., 2013). Elevated estrogen levels increase venous compliance and reduce systemic vascular resistance during pregnancy (Gherman et al., 1998, 1999). Progesterone and relaxin are both elevated during pregnancy and contribute to systemic vasodilation and increased venous capacitance (Sanghavi and Rutherford, 2014). In conjunction with the overall hypervolemic state, increased vascular compliance promotes venous stasis and predisposes to formation of venous thromboses, especially when coupled with mechanical compression of the iliac veins by the gravid uterus and delivery-associated endothelial damage, completing Virchow’s triad of stasis, hypercoagulability, and endothelial damage. In addition, sex hormones may contribute to aneurysm growth or rupture; see also Chapter 2 of this volume for a detailed discussion of this.
Hemodynamic changes during pregnancy
The growing fetus puts high demands on the maternal cardiovascular system. Beginning at approximately 6 weeks’ gestation, increasing levels of estrogen stimulate renin production in the kidneys, uterus, and liver (Hsueh et al., 1982; Shufelt and Bairey Merz, 2009). Increased levels of renin stimulate aldosterone production, resulting in an increase in total body water. Concurrently, progesterone, placental chorionic somatomammotropin, and prolactin lead to increased erythropoiesis (Jepson, 1968). On average, plasma volume increases 45% but red blood cell production increases only 30%, resulting in physiologic hemodilution of pregnancy and an overall decrease in hemoglobin concentration, as low as 11–12g/dL (Pritchard, 1965; Chesley, 1972). Oral iron supplementation can decrease severity of this anemia; however, its clinical utility is unknown (Milman, 2006). Other hormones, including deoxycorticosterone, prostaglandins, prolactin, placental lactogen, growth hormone, and adrenocorticotrophic hormone, also contribute to the hypervolemic state (Duvekot and Peeters, 1994).
Prior studies have linked anemia with AIS in the general population; the odds ratio of morbidity, hospitalization, and mortality in patients with anemia is similar to those of smoking, diabetes mellitus, hypertension, and hypercholesterolemia (Spence, 2010). A larger population-based study found, after controlling for other stroke risk factors, the adjusted odds ratio of prior iron deficiency anemia for cases with ischemic stroke was 1.45 (95% CI 1.34–1.58) compared to controls (Chang et al., 2013). Anemia may also impair stroke recovery (Chan and Ganasekaran, 2015). The mechanism by which anemia confers additional stroke risk is undetermined. One potential mechanism is that increased erythropoietin levels lead to a reactive thrombocytosis as a result of similarities between erythropoietin and thrombopoietin (Stohlawetz et al., 2000). However, how physiologic anemia of pregnancy contributes to maternal stroke risk and recovery is unknown.
Blood pressure starts to decline around the 7th week of pregnancy, reaching its nadir near the 20th week of pregnancy, before gradually rising again to prepregnancy levels by term (MacGillivray et al., 1969; Morganti et al., 1980). The decline in blood pressure is driven primarily by a decrease in systemic vascular resistance (Capeless and Clapp, 1989). Excluding hypertensive disorders of pregnancy, systolic blood pressure returns to near prepregnancy levels (Morganti et al., 1980) or up to 10mmHg higher (MacGillivray et al., 1969) by term, depending on the study.
The heart undergoes temporary remodeling during pregnancy that may predispose to formation of an intracardiac thrombus. Mild four chamber dilatation, right atrium and ventricle more so than the left-sided chambers, is a normal transthoracic echocardiographic finding in pregnancy (Bredy et al., 2018). The dilatation is thought to occur due to hypervolemia and decreased systemic vascular resistance. The cardiac structural changes increase the risk of atrial arrhythmias and right-to-left shunting (Bredy et al., 2018), which in turn increase risk of AIS due to cardioembolism, including paradoxical embolism. Although development of atrial fibrillation is uncommon in pregnancy (Butt and Latif, 2018), it may contribute to increased stroke risk in patients with preexisting heart conditions.
Immunologic and inflammatory changes
Pregnancy-related changes in inflammatory cytokine levels may contribute to increased risk of stroke, although this has not been well studied. The first trimester is characterized by a high level of proinflammatory T helper (Th)-1 activity and subsequent elevation of interleukin-6 (IL-6), C-reactive protein (CRP) and tumor necrosis factor alpha (TNFα) (Mor et al., 2011). Several large epidemiologic studies have consistently found higher circulating levels of CRP (induced by IL-6) to be associated with an increased risk of stroke. IL-6, CRP, and TNFα act on endothelial cells and acutely can induce thrombus formation (Esenwa and Elkind, 2016).
Pathophysiologic mechanisms of maternal stroke
Common mechanisms for maternal ischemic stroke include cardioembolism and paradoxical embolism; atherosclerotic causes are uncommon in this age group (Table 1.2). In addition, the following uncommon pregnancy-related conditions may result in maternal ischemic stroke.
Table 1.2.
Mechanisms of arterial ischemic stroke in pregnancy
| Mechanism | Presentation | Risk factors |
|---|---|---|
| Cardiogenic embolism | Acute onset focal deficit determined by vessel distribution, more likely to have cortical signs (e.g., aphasia, neglect) More frequently associated with altered mental status given involvement of cortical structures |
Cardiac arrhythmia (e.g., atrial fibrillation) Mechanical heart valves Reduced ejection fraction Cardiac thrombus or vegetation Congenital heart disease |
| Paradoxical embolism | Similar to cardiogenic embolism | Patent foramen ovale Pulmonary shunt (may be associated with hereditary hemorrhagic telangiectasia syndrome) |
| Artery-to-artery embolism | Acute onset focal deficit determined by vessel distribution, more likely to have cortical signs (e.g., aphasia, neglect) Limited to single artery territory |
Hypertension Hyperlipidemia Diabetes Tobacco use Prior head and neck radiation (radiation vasculopathy) |
| Cerebral small vessel disease | Acute onset Less frequently associated with altered mental status |
Hypertension Hyperlipidemia Diabetes Tobacco use |
| Cervical artery dissection (carotid or vertebral) | Similar to artery-to-artery embolism Often associated with neck pain or headache |
Atherosclerosis Hypercholesterolemia Prior head and neck radiation (radiation vasculopathy) Connective tissue abnormalities: Ehlers-Danlos syndrome IV (EDS IV) Marfan syndrome Osteogenesis imperfecta type I Fibromuscular dysplasia Head or neck trauma – Victim of domestic violence – Chiropractic manipulation – Motor vehicle accident |
| Reversible cerebral vasoconstriction syndrome (RCVS) | Recurrent, sudden onset, severe thunderclap headache Transient acute onset focal deficits that may resolve within minutes or hours Persistent acute onset focal deficits if infarction occurs Seizure may be initial symptom |
Migraines Hypertension Exposure to serotonergic and adrenergic drugs Postpartum state |
Postpartum cerebral angiopathy
Postpartum cerebral angiopathy, sometimes referred to as Call–Fleming syndrome, is a form of the reversible cerebral vasoconstriction syndrome (RCVS) (Call et al., 1988). RCVS most often presents as a single or recurrent thunderclap headache, commonly occurring within 1 week of delivery, and may cause ischemic stroke via vasospasm (Konstantinopoulos et al., 2004; Gupta et al., 2016). Postpartum angiopathy is more common in women with preeclampsia and HELLP, although most cases occur in women with uncomplicated pregnancy and delivery (Fugate et al., 2012). Other arteriopathies, including Takayasu arteritis, can also cause maternal ischemic stroke (Zhang et al., 2017).
Sheehan syndrome
Sheehan syndrome is pituitary infarction secondary to severe postpartum hemorrhage and may present as persistent hypotension and tachycardia despite adequate volume resuscitation, as hypoglycemia, or in lactation failure (Sheehan, 1971).
Amniotic fluid embolism
Amniotic fluid embolism is a reported cause of maternal stroke via paradoxical cerebral embolism (Sharshar et al., 1995) and may present with respiratory failure, cardiogenic shock, and disseminated intravascular coagulation.
Metastatic choriocarcinoma
Metastatic choriocarcinoma has been reported to invade cerebral vessels, leading to local thrombosis and distal tumor embolization (Saad et al., 2006). hCG levels may be included as part of a postpartum stroke work-up, as markedly elevated levels may lead to additional work-up for choriocarcinoma.
SPECIAL POPULATIONS AT RISK FOR MATERNAL STROKE
Moyamoya disease and syndrome
Moyamoya disease is a progressive, stenoocclusive cerebrovascular disease affecting the proximal arteries, particularly the internal carotid arteries, which predisposes affected individuals to ischemic and hemorrhagic strokes. Primary moyamoya disease occurs for idiopathic reasons, likely genetic, and is most common in Asian populations (Burke et al., 2009). Secondary moyamoya syndromes may occur due to trisomy 21, SCD, brain radiation, and neurofibromatosis type 1, as well as severe intracranial atherosclerotic disease. Moyamoya disease is more common in women and often presents in early adulthood. A systematic review of 54 articles related to moyamoya and pregnancy found that over 95% of women diagnosed prior to pregnancy had good outcomes (Maragkos et al., 2018). In patients who were first diagnosed during pregnancy, most were diagnosed in the second half of pregnancy and more than two-thirds presented with intracerebral hemorrhage, with a high rate of maternal and fetal morbidity and mortality. In those diagnosed with moyamoya following a postpartum stroke, the vast majority occurred within 3 days of delivery and were predominately ischemic strokes (Maragkos et al., 2018). The review concluded that moyamoya disease is not a contraindication to pregnancy if hemodynamics are properly managed. They also found no compelling evidence to perform bypass surgery prior to pregnancy or to prefer cesarean section over vaginal delivery (Maragkos et al., 2018). With regard to the mode of delivery, a Japanese-based study of maternal and fetal outcomes comparing vaginal delivery with epidural analgesia and cesarean section found no significant difference between the two groups, but the number of patients was small (Sato et al., 2015). Another retrospective study of moyamoya during pregnancy and the puerperium suggested that frequent prior TIAs or strokes, along with imaging showing severely reduced regional blood flow, predicted a higher risk of neurologic deterioration during pregnancy. The authors suggest that surgical revascularization prior to pregnancy may be beneficial for carefully selected patients (Park et al., 2018).
Sickle cell disease (SCD)
SCD is an inherited red blood cell disorder that causes erythrocytes to become deformed (sickle) in the deoxygenated state. Patients with SCD are hypercoagulable and at risk for many vascular and thrombotic complications, including AIS and CVST. Risk factors for SCD-related stroke include prior TIA, low hemoglobin, hypertension, and acute chest syndrome (Ohene-Frempong et al., 1998). Pregnancy in women with SCD confers a high rate of both maternal and fetal morbidity, including a higher risk of preeclampsia/eclampsia, vaso-occlusive crises, acute chest syndrome, and stroke (Boga and Ozdogu, 2016). Elevated cerebral blood flow velocity on transcranial Doppler (TCD) is the best known predictor of stroke risk in young patients with SCD (Adams et al., 1992). Prophylactic red blood cell transfusion is standard of practice for stroke prevention in children with elevated TCD velocities. The role of transfusion in pregnancy is uncertain but may be beneficial (Asma et al., 2015). Hydroxyurea, which may lower TCD velocities and provide primary and secondary stroke prevention in patients with SCD, is teratogenic and should not be used during pregnancy (Ware and Helms, 2012; Ware et al., 2016). Transfusions in pregnant women are recommended for those who develop stroke, preeclampsia/eclampsia, acute chest syndrome, or significant anemia and should be considered in women with severe SCD; for women on a long-term transfusion program, transfusions should be continued during pregnancy (Boga and Ozdogu, 2016). Notably, in acute stroke management, SCD is not a contraindication to intravenous thrombolysis (Powers et al., 2018).
Human immunodeficiency virus (HIV)
HIV is a retrovirus spread via sexual contact and blood products, which causes immunosuppression and can lead to the acquired immunodeficiency syndrome (AIDS), causing hosts to be susceptible to opportunistic infections, neoplasms, and many neurologic conditions. HIV/AIDS confers an increased risk of stroke after controlling for other traditional vascular risk factors (Gutierrez et al., 2017). The etiology for increased stroke rates in HIV is likely multifactorial and includes HIV-associated vasculopathy, opportunistic infections, antiretroviral (ART) therapy side effects, and metabolic syndromes (Gutierrez et al., 2017). In terms of ART therapy, the risk of stroke is highest in the first 6 months after starting medication, which is hypothesized to be due to an inflammatory process; however, in the long term, higher CD4 counts are associated with a reduced stroke risk, suggesting that ART therapy is overall beneficial for stroke prevention among patients with HIV.
According to the WHO, over 1.5 million pregnant women have HIV globally (World Health Organization, 2014). Research specifically examining maternal stroke among women with HIV is lacking. However, a Boston-based retrospective study found increased stroke rates among HIV-infected patients with a greater relative risk of ischemic stroke in women and young patients with HIV (Chow et al., 2012). After controlling for traditional vascular and sex-specific stroke risk factors, women living with HIV had a statistically significant hazard ratio of 1.89 (95% CI 1.28–2.81) compared to women without HIV (Chow et al., 2018). A study using transthoracic echocardiography in women with HIV at term found reduced left and right ventricular systolic function and increased ventricular dilation compared to HIV-negative controls in South Africa (Dennis et al., 2015). These data suggest that pregnant women with HIV may have increased risk of stroke, but more research is needed.
Antiphospholipid syndrome (APLS) and other rheumatologic syndromes
As many autoimmune conditions commonly present in young women, considering the impact of them on maternal stroke risk is relevant. APLS is an autoimmune disorder associated with persistent antiphospholipid antibodies (APLA) and predisposes to arterial and venous thrombosis and pregnancy morbidity, including miscarriages. A systemic review suggested that among patients with maternal stroke, 13.5% of patients had APLA positivity (Andreoli et al., 2013).
Systemic lupus erythematous (SLE) is an autoimmune condition with protean multisystemic involvement, including neuropsychiatric symptoms. In a review of national US inpatient data, women with SLE had an odds ratio of 15.2 (95% CI 7.4–31.2) for maternal stroke compared to women without SLE (James et al., 2005). Another cohort study in Sweden found that women with SLE were more likely to have worse maternal and fetal outcomes, including higher rates of preeclampsia, infection, and maternal stroke (Arkema et al., 2016).
Thrombophilias
Thrombophilias are a group of inherited or acquired hematologic disorders that predispose to abnormal coagulation and thrombosis. Canadian epidemiologic data suggest that a known thrombophilia confers a 4.2 times (95% CI 1.5–12.1) greater risk of maternal stroke (Liu et al., 2019a). In US inpatient data, thrombophilia was associated with an odds ratio of 16.0 (95% CI 9.4–27.2) for maternal stroke (James et al., 2005). A single-center study of inherited thrombophilia in pregnant women found that thrombotic events (including deep vein thrombosis, pulmonary embolism, CVST, and ischemic stroke) were 2.66 times (95% CI 0.96–7.37) as common in women with a factor V Leiden (FVL) mutation (grouping heterozygotes and homozygotes), which occurred most often in the third trimester and postpartum (Coriu et al., 2014). Several studies have reported that patients with FVL mutations are at higher risk of developing CVST (Ginsberg et al., 1989; Rizk et al., 1990). A study of venous thromboembolism during pregnancy and the puerperium (not maternal stroke) found FVL mutation homozygotes were several-fold more likely to develop thromboses than women who were heterozygotes for the FVL mutation (Zotz et al., 2003).
A meta-analysis of risk of AIS in thrombophilia found the strongest relative risk in the young female demographic, though pregnancy status was not delineated in the pooled results (Kim and Becker, 2003). Women with a family history of thrombosis were also more than twice as likely to have a thrombosis during pregnancy. Instances of maternal stroke with other hereditary thrombophilia, including homozygous type-II HBS antithrombin deficiency, have also been reported (Kovac et al., 2016).
Hypercoagulable disorders such as FVL-related thrombophilia and APLS share similar mechanisms in favoring a prothrombotic state. In people with FVL mutation, amino acid deletion renders the cleavage site of Factor V impervious to APC activity. Similarly, APLA-positive women with a history of thromboembolism and/or repeated fetal loss have a reduced response to APC when compared with APLA-negative women (Brenner, 2004).
Genetic stroke syndromes
While genetic cerebrovascular diseases are rare causes of stroke, these patients require highly specialized care during pregnancy.
Mitochondrial disease
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) is a rare mitochondrial disorder and only a handful of case reports address MELAS during pregnancy. The available literature documents clinical deterioration in the form of new-onset status epilepticus, neuropathy, myopathy, diabetes, pulmonary edema, and obstetrical complications (Yanagawa et al., 1998; Kovilam et al., 1999; Sikdar et al., 2007; Bell et al., 2017). Although successful deliveries are reported and sample sizes are small, women with mitochondrial disorders are believed to be at high risk for pregnancy-related complications due to the high energy demands.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)
CADASIL, a genetic disorder due to autosomal dominant inheritance of a NOTCH3 gene mutation, leads to migraine, mood disorders, recurrent lacunar strokes, and vascular dementia. A single-center review of CADASIL during pregnancy, including 50 patients and a total of 93 pregnancies, found no ischemic strokes occurred during pregnancy (Donnini et al., 2017). However, another retrospective review including 25 women with CADASIL and 43 pregnancies found high rates of neurologic symptoms, particularly postpartum, although some may have presented with migraine with aura (Roine et al., 2005).
Fibromuscular dysplasia (FMD)
FMD is an angiopathy of medium-sized vessels, which often occurs in women of childbearing age and commonly affects the cervical arteries. A case report of a woman with FMD and history of left middle cerebral artery stroke due to internal carotid artery dissection documents a spontaneous vaginal delivery at 34 weeks gestation without maternal complications; she was maintained on low-molecular weight heparin (LMWH) and aspirin during pregnancy (Cunningham et al., 2018).
DIAGNOSIS AND MANAGEMENT OF ARTERIAL ISCHEMIC STROKE IN PREGNANCY
AIS is defined as an episode of acute neurologic dysfunction attributable to focal infarction of the brain, spinal cord, or retina in a defined arterial distribution (Sacco et al., 2013). While AIS maybe confirmed via radiologic evidence, AIS can be diagnosed clinically if neurologic symptoms can be localized to an area of the central nervous system served by a single vascular territory.
Acute management of maternal AIS
Current guidelines regarding management of maternal strokes recommend treatment very similar to that of the general population (Table 1.3). The clinician must establish time of symptom onset (or “last known well”) and obtain a history focused on exclusion criteria for intravenous thrombolysis (Table 1.4) (Powers et al., 2018). Additional pertinent history for pregnant patients includes weeks of gestation and any pregnancy complications that may increase the risk of bleeding. Early collaboration between the stroke and obstetrics teams is of critical importance.
Table 1.3.
Principles of acute ischemic stroke management in pregnancy
| Management step | General population | Additional considerations due to pregnancy status |
|---|---|---|
| Initial history | • Last known well • Brief history focusing on tPA exclusion criteria |
•Determine gestational age •Early involvement of obstetrics team |
| Initial studies | • Blood glucose by fingerstick • Complete blood count, basic metabolic panel, and coagulation studies • EKG and troponin Note: only blood glucose by fingerstick is needed prior to tPA unless there is suspicion for thrombocytopenia or coagulopathy |
• Urine or serum beta-HCG test (for all women of childbearing age) • Fetal monitoring when >24 weeks gestation (particularly when abnormal maternal blood pressure) |
| Other considerations | • Magnesium sulfate administration in cases of severe preeclampsia | |
| tPA candidates | • Clinical diagnosis of stroke with disabling deficits • Defined onset and able to start treatment within 4.5h of onset • Brain imaging without evidence of hemorrhage, most commonly by noncontrast head CT • No contraindications to tPA by history and labs |
• No additional inclusion or exclusion criteria • CT head without contrast acceptable with counseling on imaging-associated risks • MRI also acceptable, but imaging modality should be determined by whichever modality most readily available |
| Thrombectomy | • Vessel imaging, most commonly by CT angiography, showing a large vessel occlusion • Defined onset and able to start treatment within 6h of onset • If onset within 6–24 h, perfusion imaging showing a large area of salvageable brain tissue relative to the core infarct (with additional criteria per DEFUSE-3 and DAWN studies) |
• CT angiography (head and neck) acceptable vessel imaging • MRA also acceptable, but imaging modality should be determined by whichever imaging modality most readily available • If perfusion imaging required for extended-window thrombectomy eligibility, CT perfusion should be used rather than MR perfusion to avoid use of gadolinium contrast |
Table 1.4.
Main contraindications for intravenous thrombolysis
| Unclear time of onset Unwitnessed symptom onset |
|
| Time of onset | Last known normal >4.5 ha |
| CT | Intracranial hemorrhage identified by CT Hypodensity >1/3 cerebral hemisphere |
| Vitals | Uncontrolled hypertension above 185/110 mmHg, despite use of antihypertensive agents |
| Anticoagulation | Therapeutic enoxaparin in the previous 24h (prophylactic dosing is not a contraindication) Current use of direct thrombin or factor Xa inhibitors (within 48 h) |
| History | Prior ischemic stroke within 3 months Severe head trauma within 3 months Intracranial/intraspinal surgery within 3 months Major surgery within past 14 days (including cesarean delivery) GI bleeding within 21 days Arterial puncture at noncompressible site within 7 days Note: lumbar puncture is NOT an absolute contraindication |
| Labs | Platelet <100,000/μL INR >1.7 PT > 15s aPTT >40s |
| Comorbidities | Infective endocarditis Intraaxial neoplasm Aortic arch dissection Structural GI malignancy |
The recently published EXTEND trial (Ma et al., 2019) and a subsequent meta-analysis (Campbell et al., 2014) suggest a benefit for thrombolysis in selected patients beyond the 4.5 h window, based on identification of patients with perfusion mismatch on imaging. However, this is not yet guideline-based standard of care.
If a focal neurologic deficit is identified (Table 1.5), brain imaging should be obtained as with any nonpregnant patient. Current guidelines aim for noncontrast computed tomography (NCCT) of the brain within 20 min of arrival to the emergency department or symptom discovery (if stroke occurs in hospital) (Powers et al., 2018).
Table 1.5.
Common arterial ischemic stroke syndromes
| Artery | Presentation |
|---|---|
| Left internal carotid artery | Right face/arm/leg weakness; left gaze deviation; aphasia (typically global) |
| Right internal carotid artery | Left face/arm/leg weakness; right gaze deviation; neglect of left side |
| Left middle cerebral artery | Aphasia; right face, arm > leg weakness; right sensory loss; left gaze deviation |
| Right middle cerebral artery | Neglect of left side; left face, arm > leg weakness; right gaze deviation; anosognosia; agitation |
| Anterior cerebral artery | Contralateral leg weakness; abulia; occasionally can present with bilateral leg weakness |
| Posterior cerebral artery | Contralateral homonymous hemianopsia; contralateral sensory loss |
| Posterior inferior cerebellar artery | Vertigo, nausea, hiccups, headache, direction-changing nystagmus Dysarthria, ipsilateral deviation of tongue “Crossed” motor deficits: ipsilateral facial and contralateral limb/trunk numbness Horner syndrome (ptosis, miosis, anhidrosis) |
| Anterior inferior cerebellar artery | Ataxia; contralateral weakness/numbness; acute hearing loss |
| Superior cerebellar artery | Ataxic gait; vertigo; nystagmus, dysarthria |
| Basilar artery and basilar perforators | Locked-in syndrome: quadriplegia with preserved consciousness, preserved vertical eye movements and blinking |
Imaging for acute stroke
When an acute stroke is suspected, the standard of care is to obtain brain imaging within minutes of arrival at the hospital, most often with computed tomography (CT) (Powers et al., 2018). A frequent concern is radiation exposure causing harm to the developing fetus (Ratnapalan et al., 2004). Based on currently available evidence, the ionizing radiation exposure from NCCT in pregnant patients does not expose the fetus to levels of radiation that are associated with increased risks of miscarriage, malformation, or other adverse pregnancy outcomes (Tremblay et al., 2012). The growing fetus is most vulnerable to teratogens between the 8th and 15th week of gestation (Brent, 2009), during which ionizing radiation in the 50–500 milliGray (mGy) range is associated with increased risk of intellectual disability and may increase the risk of major malformations and fetal growth restriction (International Commission on Radiological Protection, 2000). The dose of radiation to the fetus from a single NCCT is just 0.01–0.001 mGy (Jain, 2019). Currently, the American College of Radiology (ACR) states that doses below 50–100 mGy do not meet the necessary threshold to induce any developmental abnormalities (American College of Radiology, 2013) and similarly, the American College of Obstetricians and Gynecologist (ACOG) states that doses below 100 mGy represent minimal risk to the fetus (Jain, 2019).
Computed tomographic angiography (CTA) of the head and neck confers approximately 2–3 times more radiation but is still well below the accepted risk threshold. Studies have shown that small doses of iodinated contrast can cross the placenta and enter fetal circulation or pass directly into amniotic fluid (Puac et al., 2017). There are theoretical concerns for neonatal hypothyroidism from contrast, based on animal studies where contrast was introduced directly into the amniotic cavity (Kodzwa, 2017; Puac et al., 2017); however, intravenous iodinated contrast has not been shown to affect neonatal thyroid activity (Bona et al., 1992). ACR and ACOG state that the risk of intravascular iodinated contrast during pregnancy is unknown (Chen et al., 2008). Despite the uncertainty, CTA head and neck with intravenous contrast is recommended for pregnant patients with suspected stroke who may be candidates for acute intervention, as the potential benefit outweighs the risk (Grear and Bushnell, 2013; Powers et al., 2018). Regarding iodinated contrast and breastfeeding, human studies found very low doses in breast milk, less than 1% of the dose given to the mother (Kodzwa, 2017), and studies have shown no adverse effects from these doses. Current guidelines from ACOG do not recommend interrupting breastfeeding due to the administration of intravenous iodinated contrast (Jain, 2019).
In some clinical centers, magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) are obtained in lieu of CTA for evaluation of thrombectomy candidacy. MRI and MRA have the advantage of no ionizing radiation and are regarded as very safe for pregnancy (Jain, 2019). Though gadolinium is rarely needed during acute stroke management, the effects of gadolinium on human pregnancies are poorly characterized; a 2016 retrospective study of gadolinium exposure during the first trimester found an increased rate of stillbirths (Ray et al., 2016). Current guidelines from ACR and ACOG recommend against the routine use of gadolinium in pregnant patients. Thus, if perfusion-based imaging is desired for identification of potential extended-window thrombolysis or thrombectomy candidates, CTA with perfusion would be preferable to MRI/MRA with perfusion, which requires gadolinium.
In cases of suspected angiopathy or in cases of AIS due to large vessel occlusion, cerebral angiography (threading a catheter into the large vessels, injecting iodinated contrast, and using X-ray to image the cerebral vessels) may be used for diagnostic or therapeutic purposes. When maternal stroke is due to large vessel occlusion, mechanical thrombectomy (using angiography to deploy suction and/or stent retriever to remove the clot) may be an option for acute treatment. While the quantity of fetal radiation exposure from cerebral angiography is difficult to estimate, the neuro-interventionist can help limit radiation exposure to the fetus through proper positioning, abdominal shielding, and limiting fluoroscopy in proximity to the uterus (Meyers et al., 2000). While potential fetal risks from iodinated contrast, radiation, arterial puncture, and anesthesia are present, the significant potential benefit to the mother and fetus from treatment of a large vessel occlusion is felt to outweigh the theoretical exposure risk to the fetus (Ladhani et al., 2018). The risk-benefit of performing diagnostic cerebral angiography in the setting of suspected arteriopathy should be decided on a case-by-case basis. In pregnant and postpartum patients with a clinical history and imaging consistent with RCVS, cerebral angiography to exclude primary central nervous system vasculitis is not necessary (Singhal et al., 2016).
Intravenous tissue plasminogen activator (tPA)
Pregnant women were excluded from all trials of tPA use for ischemic stroke, so studies evaluating the use of tPA in the pregnant population have been limited to case reports and series. Though data are limited, the percentage of good outcomes and of complications is commensurate with those reported in the nonpregnant population, despite the fact that pregnant women given tPA had more severe strokes (Sousa Gomes et al., 2019). As of 2019, more than two dozen cases of maternal stroke treated with thrombolysis are published; cases are summarized in Table 1.6. Currently, tPA is recommended for acute stroke management in pregnant patients with disabling deficits when the benefits are felt to outweigh the risks (Tversky et al., 2016; Powers et al., 2018; Pacheco et al., 2019). Contraindications to tPA are identical to those in the nonpregnant population (Powers et al., 2018). The tPA dose is unchanged in pregnant patients (0.9 mg/kg IV, with maximum dose of 90mg, 10% as a bolus and the remainder as a drip over 60min); however, whether prepregnancy or actual body weight is preferred is unknown (Peksa et al., 2019). Tenecteplase is a newer alternative thrombolytic for ischemic stroke with longer half-life, greater fibrin specificity, and easier administration than tPA (Burgos and Saver, 2019); no reported cases of its use in maternal stroke or other thromboses are known (Sousa Gomes et al., 2019).
Table 1.6.
Reported cases of thrombolysis and thrombectomy in pregnancy
| Author (year) | Maternal age (year) | Gestational age | Signs/symptoms | Risk factors/comorbidities | Imaging | Thrombolysis and/or thrombectomy (time after symptom onset) | Potential complications | Secondary prevention | Mode of delivery | Maternal outcome | Fetal outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rodrigues et al. (2019) | 29 | 27 weeks | R-sided weakness/numbness, hemianopsia, and aphasia | NA | Stroke code CTH/CTA: unremarkable Follow-up CTH: L MCA/ACA stroke |
IV tPA | NA | Aspirin and LMW heparin | C-section | Persistent expressive aphasia and R-sided weakness | Healthy |
| Blythe et al. (2019) | 29 | 39 weeks | L-sided weakness and neglect | Factor XI deficiency, gestational thrombocytopenia | CTA with R M1/2 occlusion | Thrombectomy (no tPA due to low platelets) | NA | Not reported | C-section | Asymptomatic | Healthy |
| Peksa et al. (2019) | 35 | 9 weeks | L-sided weakness and L visual field deficit and neglect | Prior history of preeclampsia, GDM | CTH with hyperdense R MCA, CTA with M1 occlusion | IV tPA | NA | Aspirin and LMW heparin | Vaginal | Mild residual symptoms (NIHSS 0, mRS 0) | Healthy |
| Watanabe et al. (2019) | 36 | 21 weeks | R-sided weakness, dysarthria, headache | NA | MRA: L ICA occlusion | IV tPA+ thrombectomy | NA | Not reported | Not reported | Mild residual weakness | Healthy |
| Landais et al. (2018) | 32 | 13 weeks | Aphasia and R-hand numbness | NA | MRI DWI restriction in L MCA territory and chronic R MCA stroke | IV tPA | NA | Aspirin, transitioned to warfarin postpartum | Vaginal | Residual aphasia | Healthy |
| Zhu et al. (2018) | 28 | 9 weeks | R-sided weakness and numbness and dysarthria | Previous miscarriage | CTH: early L MCA infarct signs and hyperdense MCA CTA: L MCA occlusion |
IV tPA+ thrombectomy | NA | LMW heparin | Vaginal | Asymptomatic at 1 year follow-up | Healthy |
| Shah et al. (2018) | 37 | 9 weeks | Recurrent L-sided weakness and R gaze preference 2 days apart | Hypertension, hyperlipidemia, and dilated cardiomyopathy | (1) CTH: early loss of gay-white differentiation in R MCA territory and hyperdense R MCA CTA: inferior M2 cutoff (2) CTH/CTA: R proximal M1 cutoff |
(1) Initial occurrence tPA with improvement (2) Thrombectomy after recurrence 2 days later |
NA | LMW heparin | Published prior to delivery | Mild residual weakness | Fetus healthy at follow-up |
| Jiang and Hu (2018) | 26 | 31 weeks | R-sided weakness and dysarthria | History of rheumatic fever with mitral regurgitation and prolapse | CTH: unremarkable MRI: multiple bilateral acute infarcts (R caudate, L BG, L CR) |
IV tPA | Small L cerebellar and R temporal hemorrhage | LMW heparin, then long-term warfarin | Vaginal | Asymptomatic | Healthy |
| Bhogal et al. (2017)(A) | 38 | 24 weeks | R-sided weakness/numbness, L gaze deviation, and aphasia | Drug abuse, PFO | CTH: hyperdense L MCA MRI: L lentiform nucleus and insular diffusion restriction Angio: terminal L ICA occlusion |
Thrombectomy (outside tPA window once transferred to referral center) | NA | Clopidogrel + aspirin for 3 month and then aspirin indefinitely | Vaginal | Mild residual weakness | Healthy |
| Bhogal et al. (2017) (B) | 36 | 25 weeks | Headache, nausea and vomiting, progressing to stupor | Prior type A aortic dissection | CTH: dense basilar CTA: distal basilar occlusion |
IV tPA + thrombectomy | NA | Ticagrelor+aspirin, then switched to prasugrel+aspirin | Published prior to delivery | Internuclear ophthalmoplegia only | Fetus healthy at follow-up |
| Khan et al. (2017) | 33 | 9 weeks | R-sided weakness/numbness and visual field cut | Prior miscarriages (11), tobacco and substance use disorder | CTH: unremarkable Repeat CTH: PCA infarct |
IV tPA | Fetal hemorrhage/demise | Aspirin, then long-term clopidogrel | D&C | Residual visual symptoms and weakness | First trimester loss |
| Reining-Festa et al. (2017) | 37 | 5 weeks | L-sided weakness | HTN, obesity, history of rheumatic fever, prior second semester miscarriage | MRI: R MCA diffusion restriction | IV tPA | NA | Aspirin, then LMW heparin | C-section | Residual sensory symptoms | Healthy |
| Tversky et al. (2016) | 31 | 5 weeks | R-sided weakness and dysarthria | Protein C and S deficiencies, DVT, PFO (presumed paradoxical embolism) | CTH unremarkable, MRI with L thalmocapsular DWI restriction | IV tPA | NA | LMW heparin | NA (published prior to delivery) | Asymptomatic at discharge | No evidence of abnormalities in second trimester |
| Bereczki Jr et al. (2016) | 40 | 2 weeks postpartum | R-sided weakness and aphasia | Cervical artery dissection, migraine, childhood epilepsy | CTH: R MCA hyperdensity CTA: RM1 occlusion, b/l ICA dissections |
IV tPA + thrombectomy (and stended R ICA) | NA | LMW heparin and clopidogrel | Vaginal | Asymptomatic at 6 weeks | Healthy |
| Boyko et al. (2016) | 32 | 37 weeks | R-sided weakness/numbness and aphasia | Hypothyroidism, obesity, and obstructive sleep apnea | CTH: hyperdense L MCA with early loss of gray-white differentiation CTA: L M1 occlusion |
IV tPA + thrombectomy | Asymptomatic hemorrhage | Aspirin | C-section | Residual facial droop | Healthy |
| Aaron et al. (2016) (A) | 24 | Third trimester | L-sided weakness | Rheumatic fever, mitral valve replacement (on LMWH) | MRI/A: diffusion restriction involving lateral lenticulostriate territory; R M1 occlusion | Thrombectomy | NA | Oral anticoagulation | Vaginal | Mild residual weakness | Healthy |
| Aaron et al. (2016) (B) | 28 | 37 weeks | L-sided weakness | Rheumatic fever, mitral valve replacement (on LMWH), severe IUGR | MRI/A: diffusion restriction in R putamen and R M1 occlusion | Thrombectomy | NA | LMW heparin, then oral anticoagulants postpartum | C-section | Residual weakness | Healthy |
| Pongracz et al. (2015) | 21 | 21 weeks | L-sided weakness | Tobacco use | CTH: unremarkable CTA: R M1 cutoff |
IA tPA | NA | LMW heparin | C-section | Mild residual weakness | Healthy |
| Ritchie et al. (2015) | 28 | 39 weeks | L-sided weakness/numbness | NA | MRI: small R MCA stroke | IV tPA | NA | Aspirin until delivery, clopidogrel and tinzaparin postpartum | Vaginal (forceps-assisted) | Full recovery at 8 months | Healthy |
| Ritter et al. (2014) | 32 | 36 weeks | R-sided weakness/numbness, dysarthria, neglect | Migraine with aura | CTA: L lower M2 occlusion | IV tPA | NA | Aspirin until delivery, clopidogrel postpartum | C-section | Residual weakness | Healthy |
| Tassi et al. (2013) | 28 | 16 weeks | R-sided weakness/numbness and aphasia | PFO, factor V Leiden | MRI/A: L MCA territory ischemic stroke | IV tPA | NA | Aspirin | Vaginal | Mild residual deficits | Healthy |
| Hori et al. (2013) | 35 | 13 weeks | L-sided weakness and visual field cut | Protein S deficiency | R PCA occlusion | IV tPA | NA | IV heparin—> warfarin at 15 weeks | C-section (w/general anesthesia) | Residual visual deficits | Healthy |
| Li et al. (2012) | 24 | 11 and 13 weeks | R-sided weakness and dysarthria at 11 weeks and facial numbness at 13 weeks | PFO, pulmonary AVM | L MCA ischemic stroke at 11 weeks and vertebrobasilar territory at 13 weeks | IA tPA | NA | Aspirin and enoxaparin | Vaginal | Residual weakness | Healthy |
| Yamaguchi et al. (2010) | 36 | 18 weeks | R-sided weakness/numbness and aphasia | Hashimoto disease | MRI/A: L MCA occlusion and high intensity areas | IV tPA | NA | Aspirin and heparin | Vaginal | Asymptomatic | Healthy |
| Méndez et al. (2008) | 37 | 15h after delivery | L-sided weakness/numbness, dysarthria and visual field cut | migraine | CTH: unremarkable Angiogram distal R M1 occlusion |
IA urokinase | NA | LMW heparin | C-section | Asymptomatic | Healthy |
| Wiese et al. (2006) | 33 | 13 weeks | R-sided weakness, aphasia | Prosthetic mitral valve thrombois, prior GDM | CTH: L BG/IC hypodensity | IV tPA | NA | LMW heparin | C-section | Residual weakness | Healthy |
| Murugappan et al. (2006) (A) | 37 | 12 weeks | NIHSS = 19 | Mitral valve replacement | R MCA occlusion | IV tPA | Intrauterine hematoma | NA | NA | Healthy | Medical termination of pregnancy |
| Murugappan et al. (2006) (B) | 31 | 4 weeks | Not reported | Protein S deficiency | L MCA occlusion | IV tPA | NA | NA | NA | Healthy | Medical termination of pregnancy |
| Murugappan et al. (2006) (C) | 29 | 6 weeks | NIHSS = 13 | Aortic valve replacement | R MCA occlusion | IV tPA | Arterial dissection during angioplasty | NA | NA | Death | Death |
| Murugappan et al. (2006) (D) | 43 | 37 weeks | NIHSS = 25 | Antithrombin 3, protein C and S deficiencies | L MCA occlusion | IA tPA | NA | NA | Not reported | Healthy | Healthy |
| Murugappan et al. (2006) (E) | 28 | 6 weeks | Not reported | Protein C and S deficiency, PFO | Basilar occlusion | IA urokinase | Buttock hematoma | NA | Not reported | Healthy | Healthy |
| Murugappan et al. (2006) (F) | 40 | 6 weeks | Not reported | PCV, essential thrombolysis | Superior sagittal sinus thrombosis | Local urokinase | Partial recanalization | NA | NA | Healthy | Fetal demise, chromosomal abnormality |
| Murugappan et al. (2006) (G) | 21 | 8 weeks | Not reported | +dRVVT, antiphospholipid antibodies | Cerebral venous thrombosis | Local urokinase | Enlargement of IVC-related hemorrhage | NA | NA | Healthy | Medical termination of pregnancy |
| Murugappan et al. (2006) (H) | 25 | First trimester | Not reported | Bacterial endocarditis | L MCA occlusion | Local urokinase | NA | NA | NA | Healthy | Spontaneous abortion |
| Leonhardt et al. (2006) | 26 | 23 weeks | R-sided weakness | Antiphospholipid syndrome | MRI: L BG diffusion restriction and L M1 occlusion | IV tPA | NA | LMW heparin | Vaginal | Residual weakness | Healthy premature |
| Johnson et al. (2005) | 39 | 37 weeks | L-sided weakness, dysarthria, field cut, and neglect | Chronic hypertension | CTH: unremarkable Angiogram: R M1 occlusion |
IA tPA | NA | Not reported | Vaginal (forceps-assisted) | Asymptomatic | Healthy |
| Weatherby et al. (2003) | 29 | 9 weeks | L-sided weakness and papilledema bilaterally | NA | CTH: increased attenuation of superior sagittal sinus with associated edema MRI/MRV: absence of flow in SSS | Direct thrombolysis | NA | Dalteparin | Vaginal | Asymptomatic | Healthy |
| Elford et al. (2002) | 28 | 3–4 weeks (7 days embryo transfer of IVF) | L-sided weakness, dysarthria | Ovarian hyperstimulation syndrome | CTH: early R perisylvian/frontal/parietal changes CTA: R M1 occlusion |
IA tPA | Small R BG hemorrhage | Dalteparin | Vaginal | Mild residual symptoms | Healthy |
| Dapprich and Boessenecker (2002) | 31 | 12 weeks | R-sided weakness and aphasia | Protein S deficiency | CTH: early L BG hypodensity; TCD w/L M1 occlusion MRI: L BG hemorrhagic transformation |
IV tPA | Small L BG hemorrhage | Aspirin 100mg and LMW heparin | Not reported | Mild residual symptoms | Healthy |
Major obstetric concerns regarding use of thrombolytics are whether their use increases the risk for premature labor, placental abruption, miscarriage, peripartum uterine bleeding, postpartum hemorrhage, and fetal demise (Wiese et al., 2006; Landais et al., 2018). A review of thrombolytic use in pregnancy for thromboembolic diseases including ischemic stroke, pulmonary embolism, deep venous thrombosis, and mechanical valve thrombosis (Sousa Gomes et al., 2019) identified 141 cases and found the following rates of adverse events: 2.8% maternal mortality, 8.5% major bleeding, 9.2% mild/moderate bleeding, 6.4% miscarriage, 9.9% preterm delivery, 1.4% fetal death, and 0.7% neonatal death. tPA for acute stroke treatment in pregnant women is off-label and the risks and benefits must be thoroughly discussed with the patient and her family, with close involvement of the obstetrical team.
The safety of thrombolysis in the early postpartum period (<2 weeks following delivery) is not well established (Powers et al., 2018). One successful case of IV tPA and mechanical thrombectomy without complications at 2 weeks postpartum has been reported (Bereczki Jr et al., 2016). However, a small retrospective review of 13 cases of postpartum thrombolytic administration within 48 h of delivery (predominately for acute pulmonary embolism but one AIS included) found that bleeding complications were the norm: 92% required blood transfusion and 38% required laparotomy to control bleeding. In the review, all five cases requiring laparotomy followed cesarean deliveries. However, no maternal deaths occurred (Akazawa and Nishida, 2017).
Mechanical thrombectomy
Similar to thrombolysis, all randomized trials establishing efficacy of early endovascular intervention for large vessel arterial occlusion excluded pregnant women, so data regarding efficacy in the pregnant population is currently limited to case reports (Campbell et al., 2014; Berkhemer et al., 2015; Goyal et al., 2015, 2016; Molina et al., 2015; Saver et al., 2015). To date, 10 case reports have been published regarding use of thrombectomy in pregnant patients (Aaron et al., 2016; Bereczki Jr et al., 2016; Boyko et al., 2016; Bhogal et al., 2017; Shah et al., 2018; Zhu et al., 2018; Blythe et al., 2019; Kristiansen et al., 2019; Watanabe et al., 2019). In this small group, there was only one case of asymptomatic hemorrhage, no cases of symptomatic hemorrhage, and no reports of dissection or vasospasm. Given that the majority of pregnant patients are young, healthy, and fully functional at baseline, pregnancy is not a reason to delay or forgo mechanical thrombectomy (Ladhani et al., 2018). Obstetric anesthesiologists should be involved in guiding appropriate anesthetic use, hemodynamic monitoring, and fetal monitoring during the procedure.
There are no specific contraindications to mechanical thrombectomy in the postpartum period.
Blood pressure management
The involvement of experts in obstetrics, particularly maternal-fetal medicine, is recommended for ongoing assessment in determining blood pressure goals for the maternal-placental-fetal unit. Blood pressure goals that optimize maternal cerebral perfusion while minimizing risk of placental abruption are undetermined, and blood pressure targets must be determined on a case-by-case basis. Canadian guidelines recommend acute management of severe hypertension (SBP > 160mmHg or DBP > 110mmHg) for all pregnant women presenting with focal neurologic deficits (Ladhani et al., 2018) but neurologic exam, fetal monitoring, and other maternal comorbidities should be assessed when adjusting blood pressure parameters.
DIAGNOSIS AND MANAGEMENT OF CEREBRAL VENOUS SINUS THROMBOSIS IN PREGNANCY AND POSTPARTUM
Presentation
The most common presenting symptom of CVST is headache (90%–97% of CVST presentations in the general population and 73% in the pregnant population). During pregnancy and the puerperium, CVST can also present with obtundation (40%), motor weakness (35%), seizures (48%), and visual disturbance (27%) (de Bruijn et al., 2001; Kashkoush et al., 2017). Presentation with headache only was associated with a favorable prognosis (Kashkoush et al., 2017). Onset is often gradual; a systematic review of CSVT in pregnant patients found mean duration of symptom onset to diagnosis to be 5.9 days (95% CI 4.2–7.6) (Kashkoush et al., 2017). The diagnosis is frequently delayed because symptoms may be erroneously attributed to preeclampsia or postdural puncture headaches. Approximately 28% of cases present with a syndrome resembling benign intracranial hypertension with headache, papilledema, and visual disturbances (Villringer et al., 1994). The headache of CVST is most commonly described as diffuse and often progresses in severity over days to weeks. Case reports have described thunderclap headache as a presentation, though this is uncommon (de Bruijn et al., 1996). Most pregnancy-related CVST occurs in the third trimester or puerperium. Seven of eight cases of CVST among the 50,700 admissions for delivery in Canada occurred postpartum (Jaigobin and Silver, 2000).
Imaging and diagnostic studies
Diagnosis can be confirmed with either CT venography or MR venography (Stam, 2005). Clot involvement of the superior sagittal sinus was seen in 67% of cases involving pregnant patients, transverse/sigmoid in 64% and deep venous system in 15% of cases (Kashkoush et al., 2017). Lumbar puncture is not typically helpful in cases with focal neurologic abnormalities and radiographic confirmation of the diagnosis of CVST (Furie et al., 2011). Elevated opening pressure may help diagnose CVST in patients who present to the emergency department with headaches, and cerebrospinal fluid abnormalities can also be seen, elevated cell count (~50%) and elevated protein (~35%) (Ferro et al., 2004). Fibrin split products (D-dimer) may support the diagnosis, but if there is a strong clinical suspicion of CVST, a normal D-dimer level should not preclude further evaluation.
Management of CVST
One prospective study of CVST outcomes enrolled 624 patients, of which 77 were pregnant (Ferro et al., 2004). Subgroup analysis of pregnant patients were not included but following CVST, 79% of the total cohort had a modified Rankin Score (mRS 0–1); 10% had amRS 2–3; and 2% had a mRS 4–5. A 2017 systematic review of all published cases of CVST in pregnancy found that 91% were treated with anticoagulation, while a minority of patients were treated with thrombolysis and/or thrombectomy. Intracranial hemorrhage occurred in 4 of the 66 patients; all of them had received both anticoagulation and thrombolysis (Kashkoush et al., 2017). Importantly, CVST with associated intracranial hemorrhage should still be treated with anticoagulation (Masuhr and Einhaupl, 2008).
Unfractionated heparin is associated with teratogenicity and increased fetal bleeding (Saposnik et al., 2011). LMWH is the recommended anticoagulant during pregnancy and the puerperium (Ferro et al., 2017). European guidelines also recommend prophylactic LMWH during pregnancy for women with prior history of CVST (Ferro et al., 2017).
POSTSTROKE MANAGEMENT OF THE PREGNANT OR POSTPARTUM PATIENT
The goal of poststroke management is (1) to prevent poststroke complications and (2) to determine the etiology of stroke to guide secondary prevention. In pregnant and postpartum women, special considerations apply.
Prevention of immediate poststroke complications
Pregnant patients are at risk for the same poststroke complications as the general population, including dysphagia-related aspiration, infections, and thromboembolic events. General stroke guidelines should be followed to prevent these complications, including mechanical venous thromboembolismprophylaxis, early mobilization if there are no contraindications, dysphagia screening and evaluation, oral hygienic care, and limiting use of urinary catheters and other lines as able (Powers et al., 2018). In addition, obstetric management must also be considered poststroke. Increased fetal surveillance may be warranted depending on maternal and fetal health (Ladhani et al., 2018). We believe maternal stroke patients should be managed on a specialized stroke unit to allow close neurologic checks with close involvement of the obstetrical and/or maternal-fetal medicine team.
American Stroke Association guidelines recommend initiating an enteral diet within 7 days of admission for acute stroke in the general population and consideration of nutritional supplements for those at risk of malnourishment (Powers et al., 2018). However, as pregnant and postpartum patients have additional caloric and nutrient requirements, early involvement of a nutritionist, particularly for patients with significant dysphagia, should be considered. A lactation specialist should be consulted and should collaborate with occupational therapists, to aid with initiation of breastfeeding.
As in the general stroke population, rehabilitation should be started early, as tolerated, in the acute care hospital (Powers et al., 2018).
Diagnostic evaluation after maternal stroke
Poststroke diagnostic evaluation aims to establish the stroke mechanism to guide secondary prevention. Evaluation may include additional brain parenchyma imaging, cervical and cerebral vessel imaging, blood or cerebrospinal fluid testing, and cardiac evaluation. Of note, the serum lipid profile may be difficult to interpret during pregnancy due to physiologic elevations in both cholesterol and triglycerides (Wiznitzer et al., 2009). Cardiac evaluation may include transthoracic and/or transesophageal echocardiogram to assess for intracardiac thrombi/vegetation, valvular disease, shunting, or other structural abnormalities. Prolonged heart rhythm monitoring to assess for occult arrhythmia may be pursued for suspected cardioembolic strokes. If a PFO is identified by cardiac imaging, sources of venous thromboembolism should be investigated with Doppler ultrasound of the extremities and magnetic resonance venography of the pelvis. If no mechanism is readily identified, additional testing for an underlying hypercoagulable, autoimmune, or genetic condition should be pursued, as these conditions are associated with higher rates of maternal stroke (James et al., 2005; Andreoli et al., 2013; Liu et al., 2019a). However, testing for hypercoagulable conditions, particularly protein S, may be unreliable in the setting of pregnancy and a hematologic consultation should be considered (Faught et al., 1995).
Secondary stroke prevention after maternal ischemic stroke
Secondary prevention strategies differ depending on the mechanism of the stroke. After maternal ischemic stroke, additional considerations apply, as some medications used in nonpregnant patients have poor or unknown safety profiles in pregnancy.
Aspirin
The majority of systematic reviews of randomized controlled trials have found no increase in hemorrhagic complications associated with low-dose aspirin during pregnancy (Askie et al., 2007; LeFevre, 2014; Duley et al., 2019). ACOG guidelines currently state that low-dose aspirin (81 mg/day) use in pregnancy is considered safe and is associated with low likelihood of serious maternal or fetal complications (American College of Obstetricians and Gynecologists, 2018). In fact, prophylactic low-dose aspirin use in women at high risk for preeclampsia protects against preeclampsia and other adverse health effects (Henderson et al., 2014). During the postpartum period, while there is a theoretical risk of Reye’s syndrome from breastfeeding, no confirmed reports associated with maternal low-dose aspirin use are known (Swartz et al., 2018).
Other antiplatelet agents
The safety of clopidogrel during pregnancy, with regard to both teratogenicity and bleeding risk, is unknown and data are limited to case reports (De Santis et al., 2011; Myers et al., 2011). Similarly, the teratogenicity of other adenosine diphosphate receptor inhibitors and glycoprotein IIb/IIIa inhibitors is unknown (Ismail et al., 2017).
Anticoagulation
When anticoagulation is indicated for secondary stroke prophylaxis, LMWH is generally the preferred agent during pregnancy (Swartz et al., 2018). Warfarin is potentially teratogenic and should typically be avoided, particularly during the first trimester; however, when a strong indication for warfarin exists (such as a mechanical heart valve), its use may be considered after consultation with vascular neurology, obstetrics, and cardiology and appropriate patient counseling. The American Heart Association valvular heart disease guidelines support the use of warfarin at doses less than or equal to 5mg/day throughout pregnancy for women with mechanical heart valves and recommend substitution with LMWH during the first trimester if therapeutic levels are not reached with that dose; however, a clear multidisciplinary management plan should be in place for pregnant women with mechanical heart valves (Alshawabkeh et al., 2016). The safety of direct oral anticoagulants is unknown (Swartz et al., 2018).
Statins
Statins, a mainstay of secondary stroke prevention therapy, are generally contraindicated in pregnancy because of concern for higher rates of birth defects (Bateman et al., 2015). However, a recent systematic review found no evidence to support an association between statin use and teratogenicity, although the authors recommend avoiding statin use in the first trimester (Karalis et al., 2016). Interestingly, a small randomized phase 2 pilot study found that pravastatin use starting in the second trimester appeared safe and may reduce the risk of preeclampsia (Costantine et al., 2016). A larger phase 3 study is currently planned (Costantine et al., 2016).
STROKE RECOVERY: SPECIAL CONSIDERATIONS
Delivery considerations after stroke
Maternal stroke is not an absolute contraindication to vaginal delivery. As summarized in the Canadian Stroke guidelines, an interdisciplinary team involving vascular neurology, obstetrics, maternal-fetal medicine, obstetric anesthesiology, and neonatology, together with the patient and her family, should make decisions regarding method and timing of delivery based on maternal and fetal condition (Ladhani et al., 2018). If cesarean delivery is planned, obstetric anesthesiology guidance regarding regional vs general anesthesia and blood pressure management are required. Cesarean delivery should be considered in women with elevated intracranial pressure and at high risk of hemorrhagic conversion. If delivering vaginally, neuraxial anesthesia may be beneficial to reduce Valsalva efforts (active pushing during the second stage of labor) and sympathetic response; however, mass effect, increased intracranial pressure, or active anticoagulation may present contraindications to neuraxial anesthesia. A history of stroke makes continuous fetal monitoring during labor appropriate (Ladhani et al., 2018).
In women using antiplatelet and/or anticoagulation for secondary prevention following maternal stroke, the decision and timing for holding or adjusting agents prior to delivery, given the bleeding risk, should be decided by a multidisciplinary team that considers delivery mode, stroke mechanism and recurrence risk, obstetrical history, medical conditions, and patient preference.
Breastfeeding considerations after stroke
Decisions regarding breastfeeding after stroke should consider maternal condition and comorbidities, medicationuse, and patient preferences. While low-dose aspirin, LMWH, and warfarin are considered safe during breastfeeding, the safety of direct oral anticoagulants and other antiplatelet agents is unknown (Swartz et al., 2018). Accommodations should be made to facilitate breastfeeding including availability of a lactation consultant and nutritionist. Depending on the disability from the stroke, she may need additional assistance from physical and occupational therapy or rehabilitation medicine for pumping breast milk and breastfeeding the infant.
Poststroke depression in the setting of pregnancy/postpartum
Depression in the months following stroke is common and is associated with increased mortality. The rate of poststroke depression increases with stroke severity, prior depression, and female gender (Jørgensen et al., 2016). Similarly, postpartum depression is a significant public health issue. One review found women with a history of neurologic disorders were at increased risk of peripartum mental illness (adjusted pooled OR 1.45, 95% CI 1.19–1.77) compared to peripartum women without chronic medical conditions (Brown et al., 2018). Therefore, patients with maternal stroke are at high risk for depression and should all be screened (Ladhani et al., 2018). Early referral to psychiatry (with specialized perinatal mental health services as available) should be expedited when indicated.
RISK OF RECURRENT ARTERIAL ISCHEMIC STROKE OR VENOUS SINUS THROMBOSIS IN FUTURE PREGNANCIES
For physicians treating women of childbearing age with a stroke history, preconception counseling may include the risk of recurrent stroke during potential future pregnancies. The risk varies considerably based on the etiology of the initial stroke, and data in the area are limited, but a few studies have addressed this question. A French observational study following 373 women with a history of AIS found that over the next 5 years, 13 women had recurrent strokes, of which two were pregnancy-related (Lamy et al., 2000). The FUTURE study of stroke in the young found that women with a prior stroke had higher rates of pregnancy loss and pregnancy-related complications, such as gestational hypertension, but no recurrent strokes during pregnancy were identified (van Alebeek et al., 2018). In another observational study of 26 pregnancies following a diagnosis of TIA, AIS, or CVST, no recurrent strokes occurred during pregnancy or the postpartum period (Cruz-Herranz et al., 2015). Another study on the risk of CVST recurrence in future pregnancies calculated an overall rate of 2.2% per subsequent pregnancy (Aguiar de Sousa et al., 2017). In women with a history of stroke, guidelines recommend secondary prevention based on stroke mechanism and aggressive management of additional stroke risk factors, such as hypertensive disorders of pregnancy (Swartz et al., 2018).
CONCLUSION
Maternal stroke constitutes a major public health concern and is a leading cause of maternal disability. Understanding of the heightened risk of stroke that pregnancy confers and education of patients with known risk factors about stroke signs and symptoms have the potential to reduce maternal stroke morbidity. In addition, current acute stroke treatment options can dramatically reduce disability after stroke, especially in young patients. Stroke diagnostic and therapeutic procedures should be offered to pregnant and postpartum patients, with a candid discussion of the risks and unknowns. Optimal treatment of maternal stroke requires close collaboration with a multidisciplinary team that includes vascular neurologists, maternal–fetal medicine specialists, obstetric anesthesiologists, neurointerventionists, and rehabilitation medicine specialists.
References
- Aaron S et al. (2016). Mechanical thrombectomy for acute ischemic stroke in pregnancy using the penumbra system. Ann Indian Acad Neurol 19: 261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists (2018). ACOG Committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol 132: e44–e52. [DOI] [PubMed] [Google Scholar]
- Adams RJ et al. (1992). Transcranial Doppler correlation with cerebral angiography in sickle cell disease. Stroke 23: 1073–1077. [DOI] [PubMed] [Google Scholar]
- de Sousa D Aguiar et al. (2017). Safety of pregnancy after cerebral venous thrombosis: results of the ISCVT (International Study on Cerebral Vein and Dural Sinus Thrombosis)-2 pregnancy study. Stroke 48: 3130–3133. [DOI] [PubMed] [Google Scholar]
- Akazawa M, Nishida M (2017). Thrombolysis with intravenous recombinant tissue plasminogen activator during early postpartum period: a review of the literature. Acta Obstet Gynecol Scand 96: 529–535. [DOI] [PubMed] [Google Scholar]
- Alatri A et al. (2011). Thrombotic risk in assisted reproductive technology. Rev Med Suisse 7: 357–360. [PubMed] [Google Scholar]
- Albers GW et al. (2018). Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshawabkeh L, Economy KE, Valente AM (2016). Anticoagulation during pregnancy: evolving strategies with a focus on mechanical valves. J Am Coll Cardiol 68: 1804–1813. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy (2013). Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol 122: 1122. [DOI] [PubMed] [Google Scholar]
- American College of Radiology (2013). ACR-SPR practice parameter for imaging pregnant or potentially pregnant adolescents and women with ionizing radiation In: ACR practice parameter, 2015, American College of Radiology; on their website: https://www.acr.org/Clinical-Resources/Radiology-Safety/Radiation-Safety. [Google Scholar]
- Andreoli L et al. (2013). Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res 65: 1869–1873. [DOI] [PubMed] [Google Scholar]
- Arkema EV et al. (2016). What to expect when expecting with systemic lupus erythematosus (SLE): a population-based study of maternal and fetal outcomes in SLE and Pre-SLE. Arthritis Care Res 68: 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askie LM et al. (2007). Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 369: 1791–1798. [DOI] [PubMed] [Google Scholar]
- Asma S et al. (2015). Prophylactic red blood cell exchange may be beneficial in the management of sickle cell disease in pregnancy. Transfusion 55: 36–44. [DOI] [PubMed] [Google Scholar]
- Awada A et al. (1995). Stroke and pregnancy. Int J Gynecol Obstet 48: 157–161. [DOI] [PubMed] [Google Scholar]
- Bailey BA, Daugherty RA (2007). Intimate partner violence during pregnancy: incidence and associated health behaviors in a rural population. Matern Child Health J 11: 495. [DOI] [PubMed] [Google Scholar]
- Ban L, Sprigg N, Abdul Sultan A et al. (2017). Incidence of first stroke in pregnant and nonpregnant women of childbearing age: a population-based cohort study from England. J Am Heart Assoc 6 (4). 10.1161/JAHA.116.004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman BT et al. (2015). Statins and congenital malformations: cohort study. BMJ 350: h1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JD et al. (2017). Anesthetic management of mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS syndrome) in a high-risk pregnancy: a case report. A A Case Rep 9: 38–41. [DOI] [PubMed] [Google Scholar]
- Bereczki D Jr et al. (2016). Systemic thrombolysis and endovascular intervention in postpartum stroke. Ideggyogy Sz 69: 129–132. [DOI] [PubMed] [Google Scholar]
- Berkhemer OA et al. (2015). A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372: 11–20. [DOI] [PubMed] [Google Scholar]
- Bhogal P et al. (2017). Mechanical thrombectomy in pregnancy: report of 2 cases and review of the literature. Interv Neurol 6: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binhas M et al. (2019). Cardiac papillary fibroelastoma and stroke during pregnancy: case report. Ann Cardiol Angeiol 68: 129–131. [DOI] [PubMed] [Google Scholar]
- Bloch M et al. (2008). Tobacco use and secondhand smoke exposure during pregnancy: an investigative survey of women in 9 developing nations. Am J Public Health 98: 1833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blythe R et al. (2019). Mechanical thrombectomy for acute ischemic stroke in pregnancy. J Stroke Cerebrovasc Dis 28: e75–e76. [DOI] [PubMed] [Google Scholar]
- Boga C, Ozdogu H (2016). Pregnancy and sickle cell disease: a review of the current literature. Crit Rev Oncol Hematol 98: 364–374. [DOI] [PubMed] [Google Scholar]
- Bona G et al. (1992). Effects of iopamidol on neonatal thyroid function. Eur J Radiol 14: 22–25. [DOI] [PubMed] [Google Scholar]
- Bowring J et al. (2008). Stroke in pregnancy associated with syphilis. J Obstet Gynaecol Res 34: 405–407. [DOI] [PubMed] [Google Scholar]
- Boyko M et al. (2016). Decision making and the limits of evidence: a case study of acute stroke in pregnancy. Neurohospitalist 6: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy C et al. (2018). Pregnancy in adults with repaired/unrepaired atrial septal defect. J Thorac Dis 10: S2945–S2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B (2004). Haemostatic changes in pregnancy. Thromb Res 114: 409–414. [DOI] [PubMed] [Google Scholar]
- Brent RL (2009). Saving lives and changing family histories: appropriate counseling of pregnant women and men and women of reproductive age, concerning the risk of diagnostic radiation exposures during and before pregnancy. Am J Obstet Gynecol 200: 4–24. [DOI] [PubMed] [Google Scholar]
- Brown HK et al. (2018). Chronic medical conditions and peripartum mental illness: a systematic review and meta-analysis. Am J Epidemiol 187: 2060–2068. [DOI] [PubMed] [Google Scholar]
- Burgos AM, Saver JL (2019). Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke 50: 2156–2162. [DOI] [PubMed] [Google Scholar]
- Burke GM et al. (2009). Moyamoya disease: a summary. Neurosurg Focus 26: E11. [DOI] [PubMed] [Google Scholar]
- Butt MI, Latif M (2018). Atrial fibrillation in pregnancy. a safe approach and literature review. Cardiovasc Ther 10: 555787. [Google Scholar]
- Call G et al. (1988). Reversible cerebral segmental vasoconstriction. Stroke 19: 1159–1170. [DOI] [PubMed] [Google Scholar]
- Campbell BC et al. (2014). A multicenter, randomized, controlled study to investigate extending the time for thrombolysis in emergency neurological deficits with intraarterial therapy (EXTEND-IA). Int J Stroke 9: 126–132. [DOI] [PubMed] [Google Scholar]
- Capeless EL, Clapp J (1989). Cardiovascular changes in early phase of pregnancy. Am J Obstet Gynecol 161:1449–1453. [DOI] [PubMed] [Google Scholar]
- Carroll JD et al. (2013). Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 368: 1092–1100. [DOI] [PubMed] [Google Scholar]
- Castoldi E, Hackeng TM (2008). Regulation of coagulation by protein S. Curr Opin Hematol 15: 529–536. [DOI] [PubMed] [Google Scholar]
- Chan T, Ganasekaran G (2015). The effect of anemia on the functional outcomes of the stroke patients and the efficiency of their stroke rehabilitation. J Stroke Cerebrovasc Dis 24: 1438–1442. [DOI] [PubMed] [Google Scholar]
- Chang YL et al. (2013). Association between ischemic stroke and iron-deficiency anemia: a population-based study. PLoS One 8: e82952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MM et al. (2008). Guidelines for computed tomography and magnetic resonance imaging use during pregnancy and lactation. Obstet Gynecol 112: 333–340. [DOI] [PubMed] [Google Scholar]
- Chen L et al. (2016). Patent foramen ovale (PFO), stroke and pregnancy. J Investig Med 64: 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CA et al. (2017). Pregnancy increases stroke risk up to 1 year postpartum and reduces long-term risk. QJM 110: 355–360. [DOI] [PubMed] [Google Scholar]
- Chesley LC (1972). Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol 112: 440–450. [DOI] [PubMed] [Google Scholar]
- Chow FC et al. (2012). Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. JAIDS 60: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow FC et al. (2018). Elevated ischemic stroke risk among women living with HIV infection. AIDS 32: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Rossignol M (2017). Maternal death by stroke. Results from the French enquiry into maternal deaths, 2010–2012. Gynecol Obstet Fertil 45: S65–S70. [DOI] [PubMed] [Google Scholar]
- Coolman M et al. (2006). Concentrations of plasminogen activators and their inhibitors in blood preconceptionally, during and after pregnancy. Eur J Obstet Gynecol Reprod Biol 128: 22–28. [DOI] [PubMed] [Google Scholar]
- Coriu L et al. (2014). Hereditary thrombophilia and thrombotic events in pregnancy: single-center experience. J Med Life 7: 567–571. [PMC free article] [PubMed] [Google Scholar]
- Costantine MM et al. (2016). Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 214: 720.e1–720.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho JM et al. (2009). Cerebral venous and sinus thrombosis in women. Stroke 40: 2356–2361. [DOI] [PubMed] [Google Scholar]
- Creanga AA et al. (2017). Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol 130: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Herranz A et al. (2015). Recurrence of stroke amongst women of reproductive age: impact of and on subsequent pregnancies. Eur J Neurol 22: 681–e42. [DOI] [PubMed] [Google Scholar]
- Cumming A et al. (1995). Development of resistance to activated protein C during pregnancy. Br J Haematol 90: 725–727. [DOI] [PubMed] [Google Scholar]
- Cunningham TK, Draper H, Rajesh U (2018). Management of a pregnancy with underlying fibromuscular dysplasia with a history of stroke and carotid artery dissection. J Obstet Gynaecol 39: 1–3. [DOI] [PubMed] [Google Scholar]
- Daehnert I et al. (2001). Echocardiographically guided closure of a patent foramen ovale during pregnancy after recurrent strokes. J Interv Cardiol 14: 191–192. [DOI] [PubMed] [Google Scholar]
- Dapprich M, Boessenecker W (2002). Fibrinolysis with alteplase in a pregnant woman with stroke. Cerebrovasc Dis 13: 290. [DOI] [PubMed] [Google Scholar]
- Dayan N et al. (2017). Cardiovascular risk following fertility therapy: systematic review and meta-analysis. J Am Coll Cardiol 70: 1203–1213. [DOI] [PubMed] [Google Scholar]
- de Bruijn SF, Stam J, Kappelle LJ (1996). Thunderclap headache as first symptom of cerebral venous sinus thrombosis. CVST Study Group. Lancet 348: 1623–1625. [DOI] [PubMed] [Google Scholar]
- de Bruijn SF, de Haan RJ, Stam J (2001). Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. For The Cerebral Venous Sinus Thrombosis Study Group. J Neurol Neurosurg Psychiatry 70: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis M et al. (2011). Clopidogrel treatment during pregnancy: a case report and a review of literature. Intern Med 50: 1769–1773. [DOI] [PubMed] [Google Scholar]
- De Stefano V, Rossi E, Leone G (2003). Inherited thrombophilia, pregnancy, and oral contraceptive use: clinical implications. Semin Vasc Med 3: 47–60. [DOI] [PubMed] [Google Scholar]
- Dennis A et al. (2015). Transthoracic echocardiographic assessment of haemodynamics in severe pre-eclampsia and HIV in South Africa. Anaesthesia 70: 1028–1038. [DOI] [PubMed] [Google Scholar]
- Donnini I et al. (2017). Pregnancy in CADASIL. Acta Neurol Scand 136: 668–671. [DOI] [PubMed] [Google Scholar]
- Drake P, Driscoll AK, Mathews T (2018). Cigarette smoking during pregnancy: United States, 2016, CDC Stacks. [PubMed] [Google Scholar]
- Duley L, Meher S, Hunter KE et al. (2019). Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev 2019 (10): CD004659 10.1002/14651858.CD004659.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvekot JJ, Peeters LL (1994). Renal hemodynamics and volume homeostasis in pregnancy. Obstet Gynecol Surv 49: 830–839. [DOI] [PubMed] [Google Scholar]
- Dyken ME, Biller J (1994). Peripartum cardiomyopathy and stroke. Cerebrovasc Dis 4: 325–328. [Google Scholar]
- Elford K et al. (2002). Stroke in ovarian hyperstimulation syndrome in early pregnancy treated with intra-arterial rt-PA. Neurology 59: 1270–1272. [DOI] [PubMed] [Google Scholar]
- Esenwa CC, Elkind M (2016). Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol 12: 594. [DOI] [PubMed] [Google Scholar]
- Fadl H et al. (2014). Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG 121: 1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faught W et al. (1995). Changes in protein C and protein S levels in normal pregnancy. Am J Obstet Gynecol 172: 147–150. [DOI] [PubMed] [Google Scholar]
- Ferro JM et al. (2004). Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 35: 664–670. [DOI] [PubMed] [Google Scholar]
- Ferro JM et al. (2017). European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—endorsed by the European Academy of Neurology. Eur J Neurol 24: 1203–1213. [DOI] [PubMed] [Google Scholar]
- Feske SK, Singhal A (2014). Cerebrovascular disorders complicating pregnancy. Continuum 20: 80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer K et al. (2009). Effects of the contraceptive patch, the vaginal ring and an oral contraceptive on APC resistance and SHBG: a cross-over study. Thromb Res 123: 429–435. [DOI] [PubMed] [Google Scholar]
- Fugate JE et al. (2012). Variable presentations of postpartum angiopathy. Stroke 43: 670–676. [DOI] [PubMed] [Google Scholar]
- Furie KL et al. (2011). Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 227–276. [DOI] [PubMed] [Google Scholar]
- Furlan AJ et al. (2012). Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 366: 991–999. [DOI] [PubMed] [Google Scholar]
- Gherman RB et al. (1998). Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol 5: 155–156. [DOI] [PubMed] [Google Scholar]
- Gherman RB et al. (1999). Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol 94: 730–734. [DOI] [PubMed] [Google Scholar]
- Giberti L, Bino G, Tanganelli P (2005). Pregnancy, patent foramen ovale and stroke: a case of pseudoperipheral facial palsy. Neurol Sci 26: 43–45. [DOI] [PubMed] [Google Scholar]
- Ginsberg JS et al. (1989). Risks to the fetus of radiologic procedures used in the diagnosis of maternal venous thromboembolic disease. Thromb Haemost 61: 189–196. [PubMed] [Google Scholar]
- Goulart VB et al. (2013). Anatomical and physiological changes in the venous system of lower limbs in pregnant women and findings associated with the symptomatology. Arch Gynecol Obstet 288: 73–78. [DOI] [PubMed] [Google Scholar]
- Goyal M et al. (2015). Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- Goyal M et al. (2016). Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- Grear KE, Bushnell CD (2013). Stroke and pregnancy: clinical presentation, evaluation, treatment, and epidemiology. Clin Obstet Gynecol 56: 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Rohatgi A, Patel S (2016). Post-Partum angiopathy presenting as ischemic stroke. J Assoc Physicians India 64: 89–90. [PubMed] [Google Scholar]
- Gutierrez J, Albuquerque ALA, Falzon L (2017). HIV infection as vascular risk: a systematic review of the literature and meta-analysis. PLoS One 12: e0176686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen PT, Scholz DG, Edwards WD (1984). Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 59: 17–20. Elsevier. [DOI] [PubMed] [Google Scholar]
- Hasegawa J et al. (2015). Maternal death due to stroke associated with pregnancy-induced hypertension. Circ J 79: 1835–1840. [DOI] [PubMed] [Google Scholar]
- Henderson JT, Whitlock EP, O’Conner E et al. (2014). Low-dose aspirin for the prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force [Internet], Agency for Healthcare Research and Quality (US), Rockville (MD), (Evidence Syntheses, No. 112; ) Available from: https://www.ncbi.nlm.nih.gov/books/NBK196392/. [PubMed] [Google Scholar]
- Hori H et al. (2013). Intravenous recombinant tissue plasminogen activator therapy in a 14-week pregnant woman with embolic stroke due to protein S deficiency. Clin Neurol 53: 212–216. [DOI] [PubMed] [Google Scholar]
- Hsueh WA et al. (1982). Changes in active and inactive renin throughout pregnancy. J Clin Endocrinol Metab 54: 1010–1016. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (2000). Pregnancy and medical radiation. Ann ICRP 30: iii–viii.11108925 [Google Scholar]
- Ismail S et al. (2017). ST-elevation acute myocardial infarction in pregnancy: 2016 update. Clin Cardiol 40: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaigobin C, Silver FL (2000). Stroke and pregnancy. Stroke 31: 2948–2951. [DOI] [PubMed] [Google Scholar]
- Jain C (2019). ACOG Committee opinion no. 723: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol 133: 186. [DOI] [PubMed] [Google Scholar]
- James AH et al. (2005). Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol 106: 509–516. [DOI] [PubMed] [Google Scholar]
- Jeng J-S, Tang S-C, Yip P-K (2004). Incidence and etiologies of stroke during pregnancy and puerperium as evidenced in Taiwanese women. Cerebrovasc Dis 18: 290–295. [DOI] [PubMed] [Google Scholar]
- Jepson JH (1968). Endocrine control of maternal and fetal erythropoiesis. Can Med Assoc J 98: 844–847. [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Hu Z (2018). Remote intracerebral hemorrhage following intravenous thrombolysis in pregnancy at 31 weeks gestation: a case report and review of the literature. Neurologist 23: 19–22. [DOI] [PubMed] [Google Scholar]
- Johnson DM et al. (2005). Thrombolytic therapy for acute stroke in late pregnancy with intra-arterial recombinant tissue plasminogen activator. Stroke 36: e53–e55. [DOI] [PubMed] [Google Scholar]
- Jørgensen TS et al. (2016). Incidence of depression after stroke, and associated risk factors and mortality outcomes, in a large cohort of Danish patients. JAMA Psychiat 73: 1032–1040. [DOI] [PubMed] [Google Scholar]
- Kamel H et al. (2014). Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med 370: 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis DG et al. (2016). The risks of statin use in pregnancy: a systematic review. J Clin Lipidol 10: 1081–1090. [DOI] [PubMed] [Google Scholar]
- Kashkoush AI et al. (2017). Cerebral venous sinus thrombosis in pregnancy and puerperium: a pooled, systematic review. J Clin Neurosci 39: 9–15. [DOI] [PubMed] [Google Scholar]
- Kasum M et al. (2014). Thrombosis following ovarian hyperstimulation syndrome. Gynecol Endocrinol 30: 764–768. [DOI] [PubMed] [Google Scholar]
- Katsuragi S et al. (2018). Analysis of preventability of stroke-related maternal death from the nationwide registration system of maternal deaths in Japan. J Matern Fetal Neonatal Med 31: 2097–2104. [DOI] [PubMed] [Google Scholar]
- Kent DM et al. (2013). An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 81: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A et al. (2017). Lesson of the month 2: use of thrombolysis for ischaemic stroke in pregnancy—a case report and review of literature. Clin Med 17: 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RJ, Becker RC (2003). Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J 146: 948–957. [DOI] [PubMed] [Google Scholar]
- Kittner SJ et al. (1996). Pregnancy and the risk of stroke. N Engl J Med 335: 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodzwa R (2017). Updates to the ACR manual on contrast media. Radiol Technol 89: 186–189. [PubMed] [Google Scholar]
- Konstantinopoulos PA et al. (2004). Postpartum cerebral angiopathy: an important diagnostic consideration in the postpartum period. Am J Obstet Gynecol 191: 375–377. [DOI] [PubMed] [Google Scholar]
- Kovac M et al. (2016). Pregnancy related stroke in the setting of homozygous type-II HBS antithrombin deficiency. Thromb Res 139: 111–113. [DOI] [PubMed] [Google Scholar]
- Kovilam OP et al. (1999). Pregnancy with mitochondrial encephalopathy, lactic acidosis, and strokelike episodes syndrome. Obstet Gynecol 93: 853. [DOI] [PubMed] [Google Scholar]
- Kristiansen ES et al. (2019). Acute ischemic stroke in late pregnancy treated with intravenous thrombolysis and endovascular therapy. Case Rep Neurol 11: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklina EV et al. (2011). Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke 42: 2564–2570. [DOI] [PubMed] [Google Scholar]
- Ladhani NNN et al. (2018). Canadian stroke best practice consensus statement: acute stroke management during pregnancy. Int J Stroke 13: 743–758. [DOI] [PubMed] [Google Scholar]
- Lamy C et al. (2000). Ischemic stroke in young women: risk of recurrence during subsequent pregnancies. Neurology 55: 269–274. [DOI] [PubMed] [Google Scholar]
- Landais A, Chaumont H, Dellis R (2018). Thrombolytic therapy of acute ischemic stroke during early pregnancy. J Stroke Cerebrovasc Dis 27: e20–e23. [DOI] [PubMed] [Google Scholar]
- Lanska DJ, Kryscio RJ (2000). Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke 31: 1274–1282. [DOI] [PubMed] [Google Scholar]
- Lappin JM et al. (2018). Fatal stroke in pregnancy and the puerperium. Stroke 49: 3050–3053. [DOI] [PubMed] [Google Scholar]
- Lecander I, Åstedt B (1986). Isolation of a new specific plasminogen activator in hibitor from pregnancy plasma. Br J Haematol 62: 221–228. [DOI] [PubMed] [Google Scholar]
- LeFevre ML (2014). Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med 161: 819–826. [DOI] [PubMed] [Google Scholar]
- Leffert LR et al. (2015). Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol 125: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt G et al. (2006). Thrombolytic therapy in pregnancy. J Thromb Thrombolysis 21: 271–276. [DOI] [PubMed] [Google Scholar]
- Li Y et al. (2012). Thrombolytic therapy for ischemic stroke secondary to paradoxical embolism in pregnancy: a case report and literature review. Neurologist 18: 44–48. [DOI] [PubMed] [Google Scholar]
- Liang CC et al. (2006). Stroke complicating pregnancy and the puerperium. Eur J Neurol 13: 1256–1260. [DOI] [PubMed] [Google Scholar]
- Lin SY et al. (2008). Increased risk of stroke in patients who undergo cesarean section delivery: a nationwide population-based study. Am J Obstet Gynecol 198: 391.e1–391.e7. [DOI] [PubMed] [Google Scholar]
- Lipton RB et al. (2007). Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68: 343–349. [DOI] [PubMed] [Google Scholar]
- Liu S et al. (2019a). Stroke and cerebrovascular disease in pregnancy: incidence, temporal trends, and risk Factors. Stroke 50: 13–20. [Google Scholar]
- Liu S, et al. for the Canadian Perinatal Surveillance System (Public Health Agency of Canada) (2019b). Stroke and cerebrovascular disease in pregnancy. Stroke 50: 13–20. [Google Scholar]
- Ma H et al. (2019). Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med 380: 1795–1803. [DOI] [PubMed] [Google Scholar]
- MacGillivray I, Rose GA, Rowe B (1969). Blood pressure survey in pregnancy. Clin Sci 37: 395–407. [PubMed] [Google Scholar]
- Malek AM et al. (2000). Patient presentation, angiographic features, and treatment of strangulation-induced dissection of the internal carotid artery: report of three cases. J Neurosurg 92: 481–487. [DOI] [PubMed] [Google Scholar]
- Maragkos GA et al. (2018). Moyamoya disease in pregnancy: a systematic review. Acta Neurochir 160: 1711–1719. [DOI] [PubMed] [Google Scholar]
- Mas J-L et al. (2017). Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med 377: 1011–1021. [DOI] [PubMed] [Google Scholar]
- Masuhr F, Einhäupl K (2008). Treatment of cerebral venous and sinus thrombosis, handbook on cerebral venous thrombosis, Karger Publishers; 132–143. [DOI] [PubMed] [Google Scholar]
- McNamara JF et al. (2016). Re-activation of varicella zoster virus associated with anterior spinal cord stroke in pregnancy. Infect Dis 48: 705–707. [DOI] [PubMed] [Google Scholar]
- Méndez JC et al. (2008). Successful intra-arterial thrombolysis for acute ischemic stroke in the immediate postpartum period: case report. Cardiovasc Intervent Radiol 31: 193–195. [DOI] [PubMed] [Google Scholar]
- Meyer C, Murillo J, De Havenon AJS (2018). Abstract NS4: predictors of ischemic stroke and stroke mimic during pregnancy. Stroke 49: ANS4. [Google Scholar]
- Meyers PM et al. (2000). Endovascular treatment of cerebral artery aneurysms during pregnancy: report of three cases. Am J Neuroradiol 21: 1306–1311. [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Strbian D, Sundararajan S (2015). Stroke in the young: patent foramen ovale and pregnancy. Stroke 46: e181–e183. [DOI] [PubMed] [Google Scholar]
- Miller EC et al. (2016a). Mechanisms and outcomes of stroke during pregnancy and the postpartum period: a cross-sectional study. Neurol Clin Pract 6: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EC et al. (2016b). Risk of pregnancy-associated stroke across age groups in New York State. JAMA Neurol 73: 1461–1467. [DOI] [PubMed] [Google Scholar]
- Miller EC et al. (2017). Risk factors for pregnancy-associated stroke in women with preeclampsia. Stroke 48:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EC et al. (2018). Infections and risk of peripartum stroke during delivery admissions. Stroke 49: 1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EC et al. (2019a). Infection during delivery hospitalization and risk of readmission for postpartum stroke. Stroke 50: 2685–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EC et al. (2019b). Aspirin reduces long-term stroke risk in women with prior hypertensive disorders of pregnancy. Neurology 92: e305–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman N (2006). Iron and pregnancy—a delicate balance. Ann Hematol 85: 559–565. [DOI] [PubMed] [Google Scholar]
- Molina CA et al. (2015). REVASCAT: a randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke 10: 619–626. [DOI] [PubMed] [Google Scholar]
- Mor G et al. (2011). Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 1221: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti AA et al. (1980). Blood pressure, the renin-aldosterone system and sex steroids throughout normal pregnancy. Am J Med 68: 97–104. [DOI] [PubMed] [Google Scholar]
- Murugappan A et al. (2006). Thrombolytic therapy of acute ischemic stroke during pregnancy. Neurology 66: 768–770. [DOI] [PubMed] [Google Scholar]
- Myers GR et al. (2011). Clopidogrel use throughout pregnancy in a patient with a drug-eluting coronary stent. Obstet Gynecol 118: 432–433. [DOI] [PubMed] [Google Scholar]
- Nogueira RG et al. (2018). Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378: 11–21. [DOI] [PubMed] [Google Scholar]
- Ohene-Frempong K et al. (1998). Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 91: 288–294. [PubMed] [Google Scholar]
- Pacheco LD et al. (2019). Acute management of ischemic stroke during pregnancy. Obstet Gynecol 133: 933–939. [DOI] [PubMed] [Google Scholar]
- Park W et al. (2018). Neurologic deterioration in patients with moyamoya disease during pregnancy, delivery, and puerperium. World Neurosurg 111: e7–e17. [DOI] [PubMed] [Google Scholar]
- Paul BS et al. (2013). Spectrum of neurological complications in HELLP syndrome. Neurol India 61: 467. [DOI] [PubMed] [Google Scholar]
- Peksa GD, Ostrem J, Davis T (2019). Intravenous tissue plasminogen activator for ischemic stroke in early pregnancy dosed by actual body weight. SAGE Open Med Case Rep 7: 2050313x19828247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomp E et al. (2008). Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost 6: 632–637. [DOI] [PubMed] [Google Scholar]
- Pongracz E et al. (2015). Intra-arterial thrombolysis in second trimester of pregnancy. A case report. J Crit Care Med (Targu Mures) 1: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ et al. (2018). 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49: e46–e99. [DOI] [PubMed] [Google Scholar]
- Pritchard JA (1965). Changes in the blood volume during pregnancy and delivery. Anesthesiology 26: 393–399. [DOI] [PubMed] [Google Scholar]
- Puac P et al. (2017). Safety of contrast material use during pregnancy and lactation. Magn Reson Imaging Clin N Am 25: 787–797. [DOI] [PubMed] [Google Scholar]
- Rantanen K, Tatlisumak T (2013). Stroke in women-oral contraception, pregnancy, and hormone replacement therapy. Curr Vasc Pharmacol 11: 58–73. [PubMed] [Google Scholar]
- Ratnapalan S et al. (2004). Physicians’ perceptions of teratogenic risk associated with radiography and CT during early pregnancy. Am J Roentgenol 182: 1107–1109. [DOI] [PubMed] [Google Scholar]
- Ray JG et al. (2016). Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 316: 952–961. [DOI] [PubMed] [Google Scholar]
- Reining-Festa A et al. (2017). Intravenous thrombolysis of stroke in early pregnancy: a case report and review of the literature. J Neurol 264: 397–400. [DOI] [PubMed] [Google Scholar]
- Ritchie J, Lokman M, Panikkar J (2015). Thrombolysis for stroke in pregnancy at 39 weeks gestation with a subsequent normal delivery. BMJ Case Rep 2015: bcr2015209563 10.1136/bcr-2015-209563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter LM et al. (2014). Successful thrombolysis of stroke with intravenous alteplase in the third trimester of pregnancy. J Neurol 261: 632–634. [DOI] [PubMed] [Google Scholar]
- Rizk B, Meagher S, Fisher AM (1990). Severe ovarian hyperstimulation syndrome and cerebrovascular accidents. Hum Reprod 5: 697–698. [DOI] [PubMed] [Google Scholar]
- Rodrigues R et al. (2019). Acute ischemic stroke in pregnancy. Case Rep Neurol 11: 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelands J et al. (2009). Consequences of smoking during pregnancy on maternal health. J Women’s Health 18: 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roine S et al. (2005). Neurologic symptoms are common during gestation and puerperium in CADASIL. Neurology 64: 1441–1443. [DOI] [PubMed] [Google Scholar]
- Ros HS et al. (2002). Pulmonary embolism and stroke in relation to pregnancy: how can high-risk women be identified? Am J Obstet Gynecol 186: 198–203. [DOI] [PubMed] [Google Scholar]
- Saad N et al. (2006). Metastatic choriocarcinoma: a rare cause of stroke in the young adult. Australas Radiol 50: 481–483. [DOI] [PubMed] [Google Scholar]
- Sacco RL et al. (2013). An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44: 2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihu HM et al. (2018). Opioid drug use and acute cardiac events among pregnant women in the United States. Am J Med 131: 64–71. e1. [DOI] [PubMed] [Google Scholar]
- Sanghavi M, Rutherford JD (2014). Cardiovascular physiology of pregnancy. Circulation 130: 1003–1008. [DOI] [PubMed] [Google Scholar]
- Saposnik G et al. (2011). Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 1158–1192. [DOI] [PubMed] [Google Scholar]
- Sato K et al. (2015). Vaginal delivery under epidural analgesia in pregnant women with a diagnosis of moyamoya disease. J Stroke Cerebrovasc Dis 24: 921–924. [DOI] [PubMed] [Google Scholar]
- Saver JL et al. (2015). Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- Schokker SA et al. (2015). Cerebrovascular, cardiovascular and renal hypertensive disease after hypertensive disorders of pregnancy. Pregnancy Hypertens 5: 287–293. [DOI] [PubMed] [Google Scholar]
- Scott CA et al. (2012). Incidence, risk factors, management, and outcomes of stroke in pregnancy. Obstet Gynecol 120: 318–324. [DOI] [PubMed] [Google Scholar]
- Sells CM, Feske SK (2017). Stroke in pregnancy. Semin Neurol 37: 669–678. [DOI] [PubMed] [Google Scholar]
- Shah SS et al. (2018). Transradial mechanical thrombectomy for proximal middle cerebral artery occlusion in a first trimester pregnancy: case report and literature review. World Neurosurg 120: 415–419. [DOI] [PubMed] [Google Scholar]
- Sharshar T, Lamy C, Mas J (1995). Incidence and causes of strokes associated with pregnancy and puerperium: a study in public hospitals of Ile de France. Stroke 26: 930–936. [DOI] [PubMed] [Google Scholar]
- Sheehan HL (1971). The recognition of chronic hypopituitarism resulting from postpartum pituitary necrosis. Am J Obstet Gynecol 111: 852–854. [DOI] [PubMed] [Google Scholar]
- Sheppard BL, Bonnar J (1999). Uteroplacental hemostasis in intrauterine fetal growth retardation. Semin Thromb Hemost 25: 443–446. [DOI] [PubMed] [Google Scholar]
- Shufelt CL, Bairey Merz CN (2009). Contraceptive hormone use and cardiovascular disease. J Am Coll Cardiol 53: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikdar S et al. (2007). Pregnancy-precipitated status epilepticus: a rare presentation of MELAS syndrome. Neurol India 55: 82. [DOI] [PubMed] [Google Scholar]
- Singhal AB et al. (2016). Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol 79: 882–894. [DOI] [PubMed] [Google Scholar]
- Søndergaard L et al. (2017). Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 377: 1033–1042. [DOI] [PubMed] [Google Scholar]
- Sousa Gomes M, Guimaraes M, Montenegro N (2019). Thrombolysis in pregnancy: a literature review. J Matern Fetal Neonatal Med 32: 2418–2428. [DOI] [PubMed] [Google Scholar]
- Spence RK (2010). The economic burden of anemia in heart failure. Heart Fail Clin 6: 373–383. [DOI] [PubMed] [Google Scholar]
- Stam J (2005). Thrombosis of the cerebral veins and sinuses. N Engl J Med 352: 1791–1798. [DOI] [PubMed] [Google Scholar]
- Stirling Y et al. (1984). Haemostasis in normal pregnancy. Thromb Haemost 52: 176–182. [PubMed] [Google Scholar]
- Stohlawetz PJ et al. (2000). Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood 95: 2983–2989. [PubMed] [Google Scholar]
- Swartz RH et al. (2017). The incidence of pregnancy-related stroke: a systematic review and meta-analysis. Int J Stroke 12: 687–697. [DOI] [PubMed] [Google Scholar]
- Swartz RH et al. (2018). Canadian stroke best practice consensus statement: secondary stroke prevention during pregnancy. Int J Stroke 13: 406–419. [DOI] [PubMed] [Google Scholar]
- Tassi R et al. (2013). Systemic thrombolysis for stroke in pregnancy. Am J Emerg Med 31: 448.e1–448.e3. [DOI] [PubMed] [Google Scholar]
- Thomalla G et al. (2018). MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med 379: 611–622. [DOI] [PubMed] [Google Scholar]
- Too G et al. (2018). Timing and risk factors of postpartum stroke. Obstet Gynecol 131: 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadwell S, Thanvi B, Robinson T (2008). Stroke in pregnancy and the puerperium. Postgrad Med J 84: 238–245. [DOI] [PubMed] [Google Scholar]
- Tremblay E et al. (2012). Quality initiatives: guidelines for use of medical imaging during pregnancy and lactation. Radiographics 32: 897–911. [DOI] [PubMed] [Google Scholar]
- Tversky S et al. (2016). Thrombolysis for ischemic stroke during pregnancy: a case report and review of the literature. J Stroke Cerebrovasc Dis 25: e167–e170. [DOI] [PubMed] [Google Scholar]
- Udell JA, Lu H, Redelmeier DA (2017). Failure of fertility therapy and subsequent adverse cardiovascular events. CMAJ 189: E391–E397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alebeek ME et al. (2018). Increased risk of pregnancy complications after stroke: the FUTURE Study (follow-up of transient ischemic attack and stroke patients and unelucidated risk factor evaluation). Stroke 49: 877–883. [DOI] [PubMed] [Google Scholar]
- Villringer A, Mehraein S, Einhaupl KM (1994). Pathophysiological aspects of cerebral sinus venous thrombosis (SVT). J Neuroradiol 21: 72–80. [PubMed] [Google Scholar]
- Ware RE, Helms RW (2012). Stroke with transfusions changing to hydroxyurea (SWiTCH). Blood 119: 3925–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware RE et al. (2016). Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia—TCD with transfusions changing to hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet 387: 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe TT, Ichijo M, Kamata T (2019). Uneventful pregnancy and delivery after thrombolysis plus thrombectomy for acute ischemic stroke: case study and literature review. J Stroke Cerebrovasc Dis 28: 70–75. [DOI] [PubMed] [Google Scholar]
- Weatherby S et al. (2003). Good outcome in early pregnancy following direct thrombolysis for cerebral venous sinus thrombosis. J Neurol 250: 1372–1373. [DOI] [PubMed] [Google Scholar]
- Wiebers DO, Whisnant JP (1985). The incidence of stroke among pregnant women in Rochester, Minn, 1955 through 1979. JAMA 254: 3055–3057. [PubMed] [Google Scholar]
- Wiese KM et al. (2006). Intravenous recombinant tissue plasminogen activator in a pregnant woman with cardioembolic stroke. Stroke 37: 2168–2169. [DOI] [PubMed] [Google Scholar]
- Witlin AG et al. (1997). Cerebrovascular disorders complicating pregnancy—beyond eclampsia. Am J Obstet Gynecol 176: 1139–1148. [DOI] [PubMed] [Google Scholar]
- Wiznitzer A et al. (2009). Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol 201: 482.e1–482.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2008). The global burden of disease: 2004 update, WHO. [Google Scholar]
- World Health Organization (2014). Global update on the health sector response to HIV, 2014, WHO. [Google Scholar]
- Wu P et al. (2017). Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 10: e003497. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y et al. (2010). Intravenous recombinant tissue plasminogen activator in an 18-weekpregnant woman with embolic stroke. Clin Neurol 50: 315–319. [DOI] [PubMed] [Google Scholar]
- Yanagawa T et al. (1998). Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes with deterioration during pregnancy. Intern Med 37: 780–783. [DOI] [PubMed] [Google Scholar]
- Yoshida K et al. (2017). Strokes associated with pregnancy and puerperium: a nationwide study by the Japan Stroke Society. Stroke 48: 276–282. [DOI] [PubMed] [Google Scholar]
- Zambrano MD et al. (2019). Abstract WP354: racial and ethnic disparities in risk for pregnancy-related stroke: the national inpatient sample. Stroke 50: AWP354. [Google Scholar]
- Zhang Y, Li Y, Zhang J (2017). Clinical analysis: 13 cases of pregnancy complicated with Takayasu arteritis. Ginekol Pol 88: 654–661. [DOI] [PubMed] [Google Scholar]
- Zhu F et al. (2018). Combined reperfusion therapy to treat cryptogenic acute ischemic stroke during the first trimester of pregnancy: case report and literature review. Ther Clin Risk Manag 14: 1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotz RB et al. (2003). Inherited thrombophilia and gestational venous thromboembolism. Best Pract Res Clin Haematol 16: 243–259. [DOI] [PubMed] [Google Scholar]


