Abstract
The term “neuro-obstetrics” refers to a multidisciplinary approach to the care of pregnant women with neurologic comorbidities, both preconceptionally and throughout pregnancy. General preconception care should be offered to all women, including women with neurologic disease. Women with neurologic comorbidities should also be offered specialist preconception care by an obstetrician who consults with a neurologist, anesthesiologist, and if indicated clinical geneticist and/or other specialists.
In women with neurologic comorbidities, neurologic sequelae may influence the course of the pregnancy and delivery. Also, pregnancy may influence the severity of the neurologic condition, depending on the type of disease. Physiologic adaptations during pregnancy and altered pharmacokinetics may cause altered blood serum levels of drugs, leading to decreased or increased drug effects. When administering drugs to a woman who wishes to conceive, it is important to consider possible teratogenic effects and possible secretion in breast milk. Tailoring medication regimens should be considered, preferably preconceptionally. In this chapter, we review general principles of neuro-obstetric care, as well as some specific considerations for neurologists, obstetricians, and anesthesiologists caring for pregnant women with common neurologic conditions.
INTRODUCTION
The overlap of obstetrics and neurology may not be immediately apparent, and practitioners in both fields may question the need for two entire volumes devoted to the topic of what we have termed “neuro-obstetrics.” On the one hand, the field of obstetrics is defined by the care concerning pregnancy. The majority of women will experience pregnancy at some point in their lives; it is a highly complex and unique physiologic state that carries the potential for myriad complications. On the other hand, the field of neurology is defined anatomically, encompasses a broad array of conditions, and provides care that a much smaller percentage of women will ever seek. But the very high lifetime incidence of pregnancy ensures that obstetricians will treat numerous patients with chronic neurologic conditions and that neurologists will treat women who are pregnant or wish to become pregnant. A thorough understanding of the interdisciplinary interface is crucial for practitioners in both specialties, for reasons outlined here and explained in detail in Section II of Volume 172.
First, certain neurologic conditions commonly present in and preferentially affect women of childbearing age. Examples include multiple sclerosis (MS), migraine, and certain types of stroke. The diagnosing neurologist must consider reproductive planning when initiating treatment for new diagnoses in this patient demographic. Further, birth control, fertility treatments, and pregnancy itself can all lower the threshold for development of neurologic phenomena. Hormonal therapies can increase risk of ischemic stroke, the classic example being combined oral contraceptive therapy in patients who have migraine with aura. Cardiac output increases in pregnancy, and cerebral autoregulation must occur to ensure that cerebral perfusion is maintained within a narrow window. At the end of pregnancy, cerebral arteries must adapt to counter arterial vasoconstriction (Cipolla, 2013). Preeclampsia/eclampsia, posterior reversible encephalopathy syndrome (PRES), and reversible cerebral vascular syndrome (RCVS) are perhaps the most dramatic manifestations of impaired cerebral autoregulation, the exact causes and mechanisms of which remain unclear. Expression of inhibitory gamma-aminobutyric acid type A (GABAa) receptor subunits is decreased in the cerebral cortex in normal pregnancy, resulting in a lowering of the seizure threshold (Johnson et al., 2015). In these and other ways, women who are pregnant or wish to become pregnant are at unique risk for developing neurologic conditions.
Second, the many physiologic changes that occur in pregnancy can dramatically affect the course of preexisting neurologic diseases and the safety and efficacy of therapies used to treat them. Autoimmune/autoinflammatory conditions such as MS commonly become less active in pregnancy and can flare up in the postpartum period. Most of the medications used for migraine prophylaxis have not been shown to be safe in pregnancy. Many antiepileptic medications carry some risk of teratogenicity, while maternal seizures during pregnancy can have similarly devastating effects on the fetus. Changes in the profile of serum-binding proteins during and after pregnancy can cause wide fluctuations in the bioavailability of medications such as lamotrigine, requiring careful monitoring and dose titration. The neurologist should therefore prioritize counseling regarding birth control and conception planning for these patients in coordination with the obstetrician.
Finally, both neurologists and obstetricians frequently serve as the de facto primary care physicians for their patients, and thus are tasked with the responsibility of ensuring continuity and coordination of care at all stages of the reproductive process. Preconception planning for neurologic patients is crucial for the reasons described earlier; obstetricians providing preconception care should be alert to the indications for referral to a neurologist, including lapse of neurologic care for patients with neurologic conditions and history of stroke or other hypercoagulable disorders. Similar principles apply during pregnancy; while all pregnant patients should be under the care of an obstetrician without referral by a neurologist, this obstetrician should be alert to development of signs and symptoms requiring neurologic referral, such as a new headache type or new neurologic deficits. Coordinated delivery planning may be warranted in patients with elevated intracranial pressure in whom Valsalva may be dangerous or in those with a neuromuscular disorder, motor neuron disorder, or spinal cord injury who may not tolerate vaginal delivery. Postpartum interdisciplinary care is equally critical, for example, to ensure prioritization of ongoing blood pressure monitoring and management for the preeclamptic patient, and may require communication between the obstetrician and the neurologist. Finally, both specialists should be aware of the need to involve other specialists, such as anesthesiologists, ophthalmologists, clinical geneticists, neonatologists, and pediatricians, at various stages of the reproductive process.
This chapter will provide an overview of the many ways in which neurology and obstetrics interface clinically, organized by categories of neurologic disease and with an emphasis on the most important considerations to guide practitioners in providing interdisciplinary care.
The following points should be part of every specialist preconception consultation:
Effects of the neurologic disease on pregnancy and pregnancy outcome.
Effects of pregnancy on the course of preexisting neurologic disease.
Effects of pregnancy on the risk of developing new neurologic disease.
Considerations regarding use of medications required to treat neurologic diseases during pregnancy (possible teratogenicity, changes in pharmacokinetics and pharmacodynamics, etc.).
Considerations regarding delivery and postpartum care.
PRECONCEPTION CARE: SPECIAL CONSIDERATIONS
All women should have access to general preconception care that includes lifestyle advice and the assessment of possible risk factors in the medical and family history. Neurologic disease may signal increased and/or unique risks for both mother and fetus. For example, prior stroke may indicate metabolic disease or a hypercoagulable disorder that can worsen in pregnancy and cause harm to mother and fetus. History of a genetic epilepsy syndrome or neurodegenerative condition may have implications for heritability. Women taking certain antiepileptics or immunomodulating therapies may be at increased risk of folate deficiency, which is detrimental to fetal neural tube development. In addition, a number of the routine considerations for all patients (with or without a diagnosed neurologic condition) concern either the use of neuroactive substances (caffeine, tobacco, alcohol, illicit drugs, etc.), the effects of various factors on fetal neurologic development (congenital infections, general nutritional supplementation), or both. While factors related to preexisting neurologic conditions will be discussed in greater detail further on, we here present some of the neurologic implications of general risk factor management.
General lifestyle advice for women prior to and during pregnancy
From an obstetric point of view, general preconception recommendations are of great value for all pregnant women, but especially for those with chronic neurologic morbidity. General advice concerning additional supplements to be taken preconceptionally and during pregnancy is presented in Table 7.1.
Table 7.1.
Nutritional supplements in pregnancy (World Health Organization, 2013; Youngblood et al., 2013; Hofmeyr et al., 2014; Khaskheli et al., 2016; Mills, 2017; Looman et al., 2018)
| Nutritional supplement | Preventative effect | Indication | Special dosages and indications |
|---|---|---|---|
| Folic acid, 400–800 mcg daily | Neural tube defects | For all women, 4 weeks preconception until 10 weeks of pregnancy to reduce the risk of neural tube defects | 4–5 mg daily in women with a previous child with a neural tube defect |
| Vitamin D, 10 mcg daily | Poor bone growth, preeclampsia and gestational diabetes, low birth weight, recurrent wheeze, asthma | For all women preconception | 20 mcg daily for women with dark skin and low sunlight exposure |
| Calcium, 1.52 g daily | Preeclampsia | For women in areas with low dietary calcium intake | |
| Iron, 200 mg daily | Iron-deficiency anemia | For women with iron deficiency |
Alcohol
All alcohol consumption during pregnancy causes fetal alcohol exposure, which is associated with low birth weight, birth abnormalities such as fetal alcohol syndrome, and fetal alcohol spectrum disorders as well as fetal and infant death. When alcohol intake persists during pregnancy, risks increase (Witt et al., 2015; Denny et al., 2017).
Alcohol consumption in both women and men influences fertility and may pose challenges in conception (Bouchery et al., 2011). Also, exposure to alcohol may induce epigenetic alterations in both male and female fetuses (Shukla and Aroor, 2006).
All risks concerning conception, pregnancy, and pregnancy outcome associated with alcohol consumption are dose-dependent, i.e., increase with increased alcohol intake (Bouchery et al., 2011).
Since preconception counseling leads to a significant decrease in alcohol consumption during the first trimester, such counseling concerning alcohol use both before and during pregnancy is desired in all women of reproductive potential (Lassi et al., 2014; Witt et al., 2015).
Tobacco
Cigarette smoking during pregnancy is the most important etiologic determinant of fetal growth restriction in developed countries (Bergmann et al., 2008). Other abnormalities associated with cigarette smoking are gastroschisis, placenta previa, placental abruption, stillbirth, and preterm delivery. Risks remain high even after cessation of smoking in the first trimester (Gould et al., 2017; Perry et al., 2019). However, risks do decrease the earlier cessation occurs, and therefore counseling and appropriate support to motivate cessation are recommended (Blatt et al., 2015).
Caffeine
Caffeine is a widely consumed substance among people of all ages, including women of reproductive age. It is usually found in beverages such as coffee or energy drinks. There is evidence that consumption of up to 300 mg caffeine per day in healthy pregnant women is generally not associated with adverse reproductive and developmental effects (Wikoff et al., 2017).
Illicit drugs
The use of illicit drugs such as cocaine, morphine, and marijuana during pregnancy is associated with maternal complications and adverse outcomes for infants and children. Different illicit drugs are associated with different complications; however, due to poly drug use (also combined with/including alcohol and tobacco), exact associations are difficult to make in evaluating the literature. Table 7.2 shows problems related to the consumption of commonly used illicit drugs during pregnancy. Disease-specific drug use before and during pregnancy and during lactation will be discussed per disease in section “Specialist Individual Preconception Care”.
Table 7.2.
Adverse effects of illicit drugs in pregnancy
| Illicit drug | Adverse effects |
|---|---|
| Opiates (e.g., morphine, fentanyl) | Neonatal abstinence syndrome (NAS), neural tube defects, congenital heart defects, gastroschisis, miscarriage, stillbirth, preterm delivery, low birth weight (Minozzi et al., 2013) |
| Cocaine | Preterm birth, premature rupture of membranes, placental abruption and infarction, preeclampsia, growth restriction (Cressman et al., 2014), gastroschisis (Draper et al., 2008) |
| Amphetamines (e.g., crystal meth) | Gastroschisis (Draper et al., 2008), clefting, cardiac anomalies, fetal growth reduction deficits (Plessinger, 1998) |
| Cannabis (e.g., marijuana) | Conflicting evidence, possible effect on intellectual development in young children (Draper et al., 2008; Albright and Rayburn, 2009); seems to enhance the teratogenic effects of ethanol (Hansen et al., 2008) |
Preconception risk factor assessment
To develop an appropriate preconception plan, the first preconception consultation should consist of thorough history taking of both partners. Elements that should be discussed are set out in Table 7.3. Depending on the findings, consultation with a specialist should be considered. In patients with existing chronic neurologic morbidity, it is important to consider additional risk factors and comorbidities that should be taken into account.
Table 7.3.
Risk factor assessment
| Medical history |
| Anemia (e.g., sickle cell anemia, hemoglobinopathy) |
| Autoimmune diseases (e.g., systemic lupus erythematosus; Kesler and Kupferminc, 2013) |
| Cardiovascular diseases (e.g., hypertension, congenital heart disease, deep venous thrombosis) |
| Diabetes (type I, type II, gestational diabetes) |
| Gastrointestinal diseases (e.g., Crohn’s disease, ulcerative colitis) |
| Gynecological diseases (e.g., myomas, pelvic inflammatory disease) |
| Infectious diseases (and risk behavior) (e.g., human immunodeficiency virus (HIV), syphilis, hepatitis A/B/C/D/E, human papilloma virus (HPV), group-B streptococcus, group-A streptococcus, zika virus, gonorrhea) |
| Neurological disorders (e.g., epilepsy, stroke, multiple sclerosis (MS)) |
| Oncological diseases (e.g., leukemia, breast cancer) |
| Orthopedic diseases (e.g., hip dysplasia, rickets) |
| Psychiatric diseases (e.g., pica, schizophrenia, depression) |
| Pulmonary diseases (e.g., asthma, pulmonary embolism, pulmonary hypertension, cystic fibrosis) |
| Thyroid disorders (e.g., Hashimoto, Graves, thyroiditis) |
| Urogenital diseases (e.g., hypospadias, obstructive renal disease, dysplastic renal disease) |
| Previous surgical interventions (abdominal: e.g., appendectomy, gastric bypass, cesarean section; urogenital: e.g., conization, myomectomy) |
| Medication use (prescribed and “over-the-counter”) |
| Reproductive history |
| Number of pregnancies |
| Number of deliveries |
| Course of pregnancies (e.g., previous preeclampsia), deliveries, and postpartum period |
| Neonatal course postpartum |
| Uterine or cervical defects |
| Miscarriages |
| Abortions |
| Premature birth |
| Intrauterine fetal death (IUFD) |
| Low birth weight (<2500 g) |
| Children with congenital anomalies |
| Family history |
| Congenital disorders |
| Consanguinity |
| Ethnicity |
| Social history |
| Home (accommodation, physical and mental abuse, involvement of authorities) |
| Family and friends (relationship, social safety net) |
| Education |
| Employment and income |
| Lifestyle |
| Alcohol |
| Tobacco |
| Drugs (e.g., opiates, cocaine, amphetamines, cannabis, hallucinogens) |
| Exposure to chemicals and radiation |
| Exercise |
| BMI |
| Nutrition |
| Diet (e.g., vegetarian, vegan) |
| Bulimia or anorexia nervosa |
| Vitamin supplementation (folate, zinc) |
| Lactose intolerance |
SPECIALIST INDIVIDUAL PRECONCEPTION CARE
A multidisciplinary individual approach to manage pregnancy and delivery is required to optimize outcome. All women with comorbidities, including neurologic disease, are at increased risk of complications before or during pregnancy as well as during the puerperium. Cooperation and alignment between involved healthcare providers is of utmost importance.
Neurologists are often the first to encounter fertile women with a neurologic morbidity and should stay alert to the possibility that these patients may wish to become pregnant. The key question “would you like to become pregnant in the next year” should always be considered. All women who do should be referred for specialist preconception counseling. Those with neurologic comorbidities should have preconception and/or prenatal consultation with an anesthesiologist, regardless of the expected mode of delivery or wish for analgesia, to prepare for unforeseen emergency situations. For women with a congenital or genetic neurologic disease, questions concerning heredity should be discussed, preferably preconceptionally or in early pregnancy. Consultation with a clinical geneticist should also be considered. Women with neurologic diseases or traumatic brain or spinal cord injury in their history may have serious neurologic sequelae. Resulting disability may require need for extra support or interventions during pregnancy and caring for the infant.
Coordination of care
Various specialists may be included in the care of women with a neurologic comorbidity when they wish to conceive or are pregnant. These specialists include neurologists, obstetricians, anesthesiologists, clinical geneticists, neonatologists, pediatricians, and others depending on the underlying morbidity. As cases might include rare diseases and extraordinary presentation, multidisciplinary consultation is required to allow for optimal care.
The organization for multidisciplinary care may be a challenge due to busy schedules and irregular working schemes that are usually not synchronized. Of course, digital platforms may offer solutions to help facilitate collaboration. Multidisciplinary care in preconception can be organized as presented in Fig. 7.1.
Fig. 7.1.
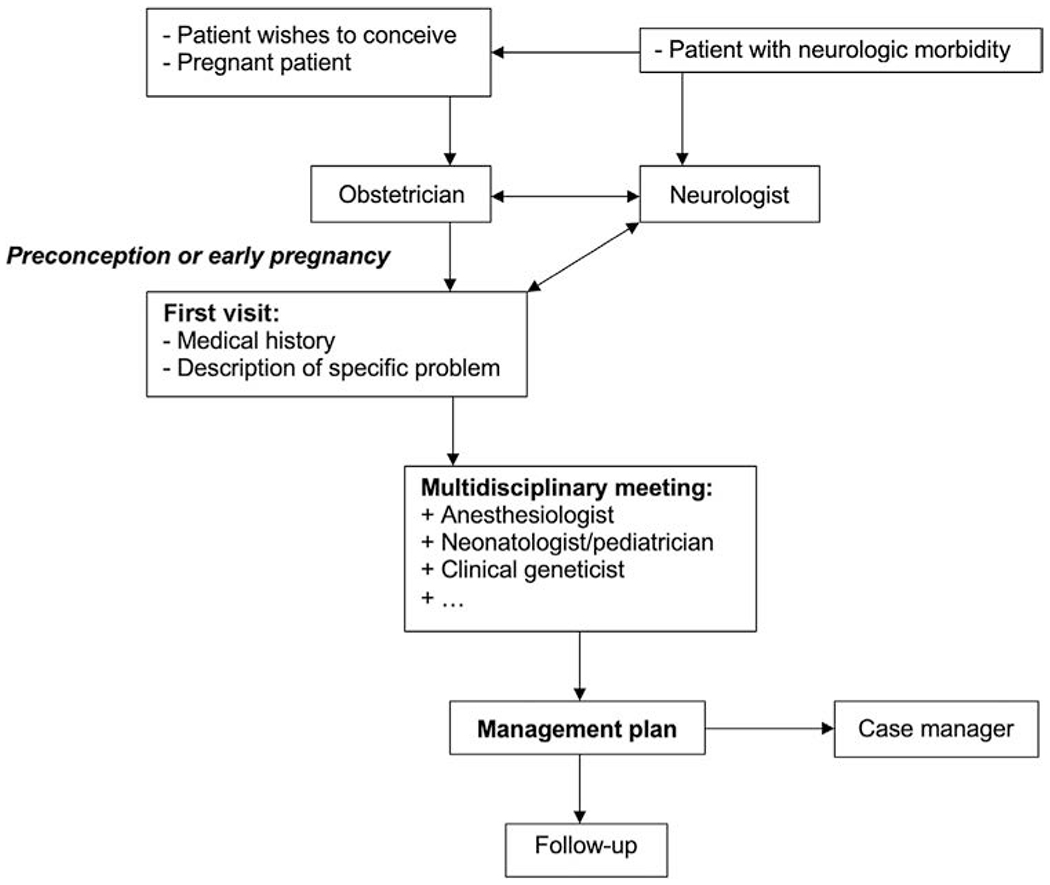
Organization of multidisciplinary preconception care.
Depending on the population of a hospital or region, multidisciplinary meetings could be planned on a regular basis. Another option could be a multidisciplinary outpatient clinic. Both options allow for improved patient-centered care. In multidisciplinary outpatient clinics, the direct involvement of the patient allows for improved shared decision-making.
In multidisciplinary meetings, cases with neurologic comorbidity, preconceptionally or while pregnant, should be evaluated based on risks and treatment options. To make such multidisciplinary meetings work, it is vital that a representative of all involved specialties is present so that all perspectives on the morbidity and treatment plan are accounted for. For continuity of care, a case manager is recommended.
Based on the most frequently occurring neurologic diseases among women in their reproductive years, the rest of this section will discuss these groups and present an overview of management considerations for obstetricians, neurologists, and anesthesiologists.
Cerebrovascular disease
The incidence of maternal stroke has risen in the past 2 decades (Kuklina et al., 2011), and this has been attributed in part to a higher percentage of patients initiating their pregnancies with one or more comorbidities (Tate and Bushnell, 2011). The complexity of these cases in many instances necessitates the participation of the cardiologist, neurologist, and anesthesiologist early in the course of the pregnancy.
Management considerations for obstetricians
A multidisciplinary approach is crucial in the prevention and management of cerebrovascular diseases. Obstetricians have a fundamental role in the prevention of neurologic complications as they are usually the first point of contact for the pregnant woman. They should identify high-risk patients and form an integrated healthcare team based on the patient’s specific needs, to adequately monitor and treat conditions that could increase the risk of cerebrovascular complications. Pregnant women are at higher risk for ischemic strokes even in the absence of other risk factors; this is primarily due to a hypercoagulable state that results from the hemodynamic and hormonal changes occurring during gestation reaching their peak during the third trimester and continuing postpartum. Headache and visual changes need to be carefully investigated, as the risk of secondary headaches is significantly higher during pregnancy. Preeclampsia, reversible vasoconstriction syndrome, and venous sinus thrombosis should always be on the differential.
Pregnancy is not contraindicated in women with a history of ischemic and/or hemorrhagic strokes or cerebral venous sinus thrombosis (CVST). However, women with a history of stroke or thrombosis are at an increased risk during pregnancy. It is important to be familiar with the origin of the previous cerebrovascular event to assess for increased risk. If not previously analyzed, screening for inherited and acquired thrombophilia, coagulopathies (i.e., antiphospholipid syndrome, protein C or S deficiency), or cardiac origin (embolisms) is recommended (Marjot et al., 2011).
Prophylactic anticoagulant therapy should be considered, particularly in patients with a personal or familial history of thrombophilia (Scheres et al., 2019). The risk of recurrence of cerebral venous thrombosis is low, but prophylactic antithrombotic medication should be administered and continued during pregnancy and postpartum (Ciron et al., 2013). Consultation with a hematologist may be warranted. In case of a history of CVST, the preferred treatment during pregnancy is low molecular weight heparin (LMWH) given its superior safety profile (Podymow and August, 2008). The neurologist should always involve the obstetrician in the decision to initiate anticoagulation therapy. Warfarin is considered safe during breastfeeding (Warfarin, 2006).
In women with a history of ischemic stroke, aspirin is recommended for secondary stroke prophylaxis (Oza et al., 2017). Although rare but serious complications due to low-dose aspirin cannot be excluded, it appears to be well tolerated (Ahrens et al., 2016).
For patients with a history of thrombophilia, unfractionated and LMWH treatment in both prophylactic and therapeutic dosages can be safely prescribed throughout the entire pregnancy and postpartum. Vitamin K antagonists and direct oral anticoagulant are absolutely contraindicated in the first trimester of pregnancy.
In patients with a history of stroke, a cesarean section should only be performed based on obstetric indications. Induction of labor is sometimes preferred in cases wherein the patient is receiving anticoagulation, to allow for better control. Unfractionated heparin and LMWH should be stopped 24h before labor induction and continued 24 h after birth, if no contraindications have developed (Katsuragi et al., 2018).
Women with known vascular lesions, including aneurysms and AVMs, have higher rates of cesarean deliveries. In retrospective studies, women with unknown arteriovenous malformations who had vaginal deliveries were not found to have increased numbers of AVM ruptures. Therefore the increased rate of cesarean deliveries may not be justified (Kim et al., 2013). In the case of lesions with a high risk for rupture, pushing may be contraindicated. Instrumental second stage of labor should be considered before deciding to perform a cesarean section.
Hypertension
One out of 10 pregnancies in the United States is complicated by hypertension (Report of the National High Blood Pressure Education Program, 2000; von Dadelszen and Magee, 2005), which is the greatest risk factor for maternal stroke. Patients with a history of chronic hypertension are more likely to develop pregnancy-induced hypertensive disorders. Deciding what the blood pressure target should be during pregnancy and when to treat have been the biggest challenges. Especially concerning milder forms of hypertension, either chronic or pregnancy-induced, there is insufficient evidence to accurately determine risks and benefits of treatment (Abalos et al., 2001).
Hypertension can be managed with a wide variety of antihypertensive drugs. The agent of first choice is methyldopa. Other agents to be considered include labetalol, nifedipine, hydralazine, b-receptor blockers, hydrochlorothiazide, and diazoxide.
The use of ACE inhibitors and angiotensin type 1-receptor antagonists is absolutely contraindicated during pregnancy due to associations with fetal loss, cardiac defects, oligohydramnios, growth restriction, renal agenesis, and neonatal anuric renal failure. If women of reproductive age use these agents, preconception switches to other agents should be considered (Podymow and August, 2008).
Management considerations for neurologists
Patients with a known history of vascular lesions, including arteriovenous malformations and intracranial aneurysms, should optimally get a preconceptional evaluation by a neurologist. In the case of high-risk lesions, a surgical consultation is warranted and can offer women the opportunity to opt for surgical treatment before conception with the objective of avoiding potential complications derived from the hyperdynamic state characteristic of pregnancy and risks related to surgery during pregnancy (Karlsson et al., 2018).
In the case of known vascular lesions without complications, current recommendations favor delaying any interventions until after delivery (Kim et al., 2013; Lv and Li, 2015). In intracranial or subarachnoid hemorrhage secondary to these lesions, all appropriate treatment interventions should be given. Computed tomography and even direct cerebral subtraction angiography use ionizing radiation; however, in emergent situations, these modalities are often preferred. The radiation exposure with proper shielding for both procedures is considerably below teratogenic levels. Surgical or endovascular interventions should be performed to secure these lesions if required, as this directly correlates with maternal and fetal outcomes (Agarwal et al., 2014).
Management considerations for anesthesiologists
Contraindications for neuraxial analgesia as pain management during vaginal delivery or cesarean section have not been published in patients with a history of ischemic stroke.
Anticoagulant therapy should be carefully evaluated according to anesthesiology guidelines before proceeding with epidural or spinal analgesia (Leffert and Schwamm, 2013; Miller and Leffert, 2020).
Epilepsy
Management considerations for obstetricians
Studies have shown conflicting results with trends toward both worsening and improvement of seizure frequency during pregnancy in women with epilepsy (Sabers et al., 1998; Harden et al., 2009; Thomas et al., 2017; Cagnetti et al., 2014). The main predictor of breakthrough seizures during pregnancy is a history of severe or uncontrolled epilepsy before gestation (EURAP Study Group, 2006; Thomas et al., 2017).
Often, breakthrough seizures during pregnancy are attributed to modifiable risk factors, including medication noncompliance or subtherapeutic serum levels of antiepileptic drugs (AEDs). Noncompliance in this group is often associated with fears of congenital malformations, making it fundamental to educate mothers-to-be about potential consequences of seizures during gestation, including direct trauma, hypoxia, and metabolic changes, which often result in significant morbidity and mortality (MacDonald et al., 2015; Christensen et al., 2018). However, it is also important to consider the risks of continuing AED administration during pregnancy and involve the pregnant woman in the decision-making to improve compliance. An overview of various AEDs is presented in Table 7.4, where pregnancy advices and teratogenic risks are portrayed.
Table 7.4.
Antiepileptic drugs (Tomson et al., 2011a, b; Bromley et al., 2013; Gooneratne et al., 2017; Stadelmaier et al., 2017; Koc et al., 2018)
| Drug | Pregnancy advice | Risk | Frequent teratogenic effects | Malformation risk (%) | Neurobehavioral effects |
|---|---|---|---|---|---|
| Sodium valproate | Absolute contraindication, dose-dependent teratogenic effect; if inevitable: lowest effective dose and monotherapy | Teratogenic effects, risk for fetal valproate syndrome | Neural tube defects, heart defects, oral cleft, hypospadias, craniosynostosis, dysmorphic facial features (e.g., epicanthic folds, broad nasal bridge, small nose with anteverted nostrils) | 4.7–10 | Up to 66.7%a |
| Phenytoin | 10–20 mg vitamin K supplementation after 36 weeks of gestationb | Teratogenic effects | Dysmorphic craniofacial features (e.g., wide philtrum, long upper lip) | 2.9–6.7 | Yes |
| Phenobarbital | 10–20 mg vitamin K supplementation after 36 weeks of gestationb | Teratogenic effects | Dysmorphic craniofacial features (e.g., wide philtrum, long upper lip), heart defects, hypospadias, neural tube defects | 5.5–7.4 | Yes |
| Carbamazepine | Dose-dependent teratogenic effect; if inevitable: lowest effective dose and monotherapy; 10–20 mg vitamin K supplementation after 36 weeks of gestationb |
Teratogenic effects | Dysmorphic craniofacial features, heart defects, hypospadias | 2.6–5.6 | Yes |
| Lamotrigine | Dose-dependent teratogenic effect; daily doses up to 300 mg not associated with increased malformation risk | Teratogenic effects when used in second and third trimesters | Heart defects, hypospadias, orofacial clefts | 2.0–3.4 | Unknown |
| Topiramate | 10–20 mg vitamin K supplementation after 36 weeks of gestationb | Teratogenic effects | Orofacial clefts | 4.2–7.7 | Unknown |
| Levetiracetam | Relatively safe as monotherapy | Teratogenic effects | Heart defects, neural tube defects | 0–2.4 | Unknown |
| Oxcarbazepine | 10–20 mg vitamin K supplementation after 36 weeks of gestationb | Teratogenic effects when used in second and third trimesters | Orofacial clefts | 1.8–3.3 | Unknown |
Permanent disorders in behavior and learning.
Due to reduced vitamin K dependent clotting factors in the newborn and the related increased risk of hemorrhagic disease in the newborn.
In all described AEDs, the risk for occurrence of malformations increases with increased doses.
Breastfeeding should be encouraged in all women with epilepsy. The levels of AEDs in breast milk are lower than what the fetus was exposed to in utero. In addition, lactation prevents withdrawal symptoms in the newborn, although this condition is rare with newer AEDs.
The risk of having a major seizure while caring for the newborn should be minimized. Safety strategies, including bathing the baby in shallow water and setting a nursery station on the bedroom floor, should be discussed prior to discharge.
Sleep deprivation may pose an extra risk for seizures in women with epilepsy (Schmidt et al., 1983). Close monitoring of blood levels of AEDs is indicated to maintain optimal seizure control and avoid toxicity during the puerperium.
Management considerations for neurologists
The management of epilepsy during pregnancy begins before conception. All women of childbearing age need to receive clear information about contraception, drug interactions, and teratogenesis during their first visit to a neurologist. It is important to repeat this conversation and consider initiating this conversation when patients transfer from different clinics or from pediatric neurology to adult neurology.
Approximately 55% of all pregnancies in women with epilepsy are unintended (Johnson et al., 2018). Patients who are not looking to become pregnant need to be advised that some AEDs may interact with hormonal oral contraceptives, decreasing their efficacy. The rate of pregnancy in women with epilepsy on oral contraceptives is 3.1 per 100 woman-years, which is higher than that in women with no history of epilepsy (0.7 per 100 woman-years). Alternative measures for contraception, such as devices (IUD) and implants, are preferred and should be offered in all cases (Reimers, 2016; Herzog et al., 2017).
In women who prefer oral contraceptives, special considerations need to be observed. Medications that act as inducers of cytochrome P450 (carbamazepine, phenytoin, and phenobarbital) will cause increased metabolism of both estrogen and progesterone, which can result in contraceptive failure. Alternative agents such as lamotrigine, which has minimal effects on oral contraceptives (Curtis et al., 2016; Gooneratne et al., 2017), should be favored.
Newer AEDs, including oxcarbazepine, parampanel, and felbamate, are also known to decrease the hormone serum levels due to increased metabolism. In addition, topiramate decreases ethinylestradiol levels by 30% by a different mechanism (Rosenfeld et al., 1997). For these patients, combined oral contraceptives with an estrogen dose of 0.50mcg or higher is recommended. Folic acid supplementation has been a topic of debate; however, the current recommendation by the American Academy of Neurology is to prescribe at least 0.4 mg daily prior to conception and through pregnancy (class C) (Harden et al., 2009; Stephen et al., 2019).
In women with epilepsy the neurologist should emphasize the importance of family planning. Young women with optimal seizure control may lose contact with their epilepsy service (Leach et al., 2017). Thus all women should receive education during their first visit about possible complications derived from inadequate seizure control and fetal malformations linked to their medications.
If the patient is planning a pregnancy, individual circumstances, including epilepsy severity, current seizure control, and family history of congenital malformations, need to be considered to develop a therapeutic plan. The aim will always be achieving optimal seizure control while minimizing the exposure to AEDs. Monotherapy at the lowest effective dose is preferred. Drug levels need to be followed carefully through pregnancy due to changes in pharmacodynamics to ensure an adequate plasma concentration of the drug. If changes in the medication are necessary, the patient should take an active role in the decision-making process (Lagana et al., 2016).
Of all patients with epilepsy, 30% experience seizures despite optimal medical management. In patients with inadequate seizure control, it is recommended to counsel them to delay pregnancy until at least a year after acceptable seizure control has been achieved (EURAP Study Group, 2006). Careful analysis is needed to minimize the seizure frequency and severity while controlling for AED exposure and associated teratogenic risks (Leach et al., 2017). Status epilepticus is rare; however, it can lead to serious complications such as aspiration pneumonia, respiratory insufficiency, and metabolic acidosis (Sveberg et al., 2015) resulting in poor outcomes for the mother and the fetus.
Management considerations for anesthesiologists
There is evidence that the risk of seizures is higher peripartum. This has been attributed to inconsistent administration of AEDs, sleep deprivation, and stressors associated with labor and delivery (Schmidt et al., 1983). It is recommended that the patient continue with her home medication; in some cases, epidural anesthesia has been suggested to decrease the degree of physical stress. Intravenous lorazepam needs to be available in the labor and delivery rooms.
Demyelinating diseases
Demyelinating diseases, including neuromyelitis optica, acute disseminated encephalomyelitis, progressive multifocal leukoencephalopathy, and MS, are frequently seen among young individuals. MS is the most common form of demyelinating disease, most often first presenting in women at the age group of 20–40, where pregnancy is more common; therefore this section will focus on the approach to MS during pregnancy.
Management considerations for obstetricians
Urinary tract infections (UTIs) are common in patients with MS and frequently attributed to detrusor-sphincter dyssynergia (Mahadeva et al., 2014). In addition, patients are at a higher risk of all infections due to the immunosuppression caused by their therapy. UTIs in this population tend to be asymptomatic and may result in premature labor or, in some cases, pseudoexacerbations, which manifest with focal neurologic deficits. Timely diagnosis and appropriate treatment of UTIs are required.
MS is no contraindication for vaginal delivery. Cesarean section should be performed based only on obstetric indications.
A protective effect against postpartum relapses has been described with breastfeeding. These findings need to be carefully interpreted due to the potential bias resulting from the fact that patients with a higher rate of relapses are more likely to restart their disease-modifying therapy (DMT) immediately after pregnancy, and the findings could potentially reflect patients with milder forms who chose to breastfeed (Langer-Gould et al., 2017; Bove et al., 2018).
Management considerations for neurologists
Most women with MS are diagnosed before pregnancy, which allows time for appropriate counseling by their neurologist before conception. During her first visit to the MS clinic, a patient usually receives education about the disease, including the available pharmacologic and nonpharmacologic interventions. It is important to talk about the significance of family planning early on, particularly as teratogenic effects have been linked to the majority of MS medications (DMT) and the risk of miscarriage and malformations does not stop as soon as the medication is discontinued, requiring a washout period that may last months (Corp. G, 2016; Coyle, 2016; Corp. NP, 2018; Coyle et al., 2019). There is limited experience with DMTs during pregnancy. Therefore it is important to analyze the patient’s disease status and evaluate the need for therapy. Recommendations regarding the use of DMTs before and during pregnancy are summarized in Table 7.5. Due to long half-life of most DMTs and their teratogenic origin, stopping these therapies before conception is often recommended. Adequate contraceptives should be considered and advised during washout periods to prevent pregnancy and possible exposure to teratogenic agents.
Table 7.5.
DMTs: advice for usage preconception and during pregnancy (Corp. G, 2016; Coyle, 2016; Corp. NP, 2018; Coyle et al., 2019)
| Drug | Preconception | Pregnancy |
|---|---|---|
| Fingolimod | Stop, long half-life | Stop |
| Teriflunomide | Stop, long half-life | Stop |
| Cladribine | Stop, washout until 6 months after last dose | Stop |
| Rituximab | Stop, washout until 12 months | Stop |
| Ocrelizumab | Continue | May be continued, check B-cell counts in newborns before administering live or live-attenuated vaccines (risk of B-cell depletion) |
| Natalizumab | Continue | May be continued |
| Glatiramer acetate (GA) | Continue | No negative effects reported |
| Interferon beta (IFNβ) | Continue | No negative effects reported |
| Alemtuzumab | Continue | May be continued, thyroid function monitoring due to risk of hypothyroidism and associated risks |
Pulses of glucocorticoids should be considered in patients with a high risk of relapses who choose to discontinue their DMT before pregnancy (Bove et al., 2014). This treatment strategy can be continued during pregnancy, with the consideration that glucocorticoid use has been associated with fetal growth restriction (Busada and Cidlowski, 2017).
As a general rule, contraceptive methods should be offered to all patients. If patients already use contraceptive methods, refilling of prescriptions if required is recommended. If patients wish to start on contraception, they need to be informed about the different options with regard to their preferences. Consultation with general practitioner or gynecologists may be warranted. In women with limited mobility, hormonal contraceptives are strongly discouraged due to the potential risk of thromboembolism along with decreased bone mineral density (Curtis et al., 2016).
Patients who are planning a pregnancy frequently ask their neurologist about the potential risk of their children developing the disease. In general, the risk of inheriting MS is low as the mode of inheritance is polygenic with at least 200 genetic susceptibility loci identified (Bove et al., 2018; Coyle et al., 2019). In addition, other factors, including HLA-associated variants, have been associated with the condition. The risk of MS for a child with one affected parent is 2%—5%, whereas if both parents are affected, the risk increases to 20%. Unfortunately, at the moment, there are no tests available to predict the disease. Furthermore, the influence of environmental exposures has an important role in the development of MS.
Pregnancy by itself does not appear to increase the risk of exacerbations; on the contrary, the rate of relapses decreases as pregnancy advances with the lowest rates during the third trimester (Coyle et al., 2019). However, women with active disease prior to pregnancy have a relatively higher risk of exacerbations during pregnancy and the postpartum period (Coyle et al., 2019). Special considerations apply to the first three months postpartum as an increased risk of relapses has been observed in this period.
Patients should be encouraged to exercise, as it has been shown to slow the progression of the disease (Wesselink et al., 2017).
Management considerations for anesthesiologists
There are no restrictions concerning obstetric anesthesia. Nonetheless, a careful history of preexisting neurologic deficits needs to be recorded in the chart of patients with MS by the anesthesiologist, as neurologic deficits may be observed after the use of various anesthetics in the delivery room, which can be mistakenly interpreted as an MS exacerbation or a stroke; this can result in unnecessary diagnostic tests or potentially harmful interventions.
Neuromuscular diseases
Neuromuscular diseases include conditions that affect nerves, the neuromuscular junction, and muscle. This section will provide a broad overview of management considerations in patients with these diseases, focusing on myasthenia gravis (MG) and myotonic dystrophy (MD) as two representative diagnoses.
Management considerations for obstetricians
The main considerations for obstetricians treating women with neuromuscular disease involve counseling about the heritability of diseases and potential adverse effects of pharmacotherapies on mother and fetus.
Myasthenia gravis
Autoimmune MG is a multifactorial neuromuscular disease characterized by fluctuating weakness of extraocular and proximal limb muscles. As women typically present with the condition in their 20s and 30s, many with undiagnosed disease may become pregnant. Notably, severe exacerbations most frequently occur in the first year after presentation. Any woman complaining of intermittent double vision, difficulty swallowing food, or weakness of arms or legs with sustained use (e.g., difficulty lifting the arms after styling hair) should therefore be referred to a neurologist for diagnostic evaluation.
Those with the diagnosis need to be reassured that MG does not affect fertility (Waters, 2019). While the disease is markedly influenced by genetic factors, it exhibits limited heritability. Because of the frequent co-occurrence of MG and autoimmune thyroid disease, the provider should test thyroid antibody status and thyroid function prior to conception. Patients receiving mycophenolate mofetil or methotrexate should be referred to a neurologist for adjustment of therapy prior to conception. If a woman becomes pregnant while using methotrexate, she should receive folic acid and be immediately taken off the drug.
The obstetrician should be aware that prednisolone, azathioprine, cyclosporine, and tacrolimus can contribute to gestational diabetes, leukopenia, liver function abnormalities, and decreased renal function and should monitor accordingly. Transplacental passage of antibodies to the fetal y-subunit of the AchR subunit may cause a severe and often fatal form of fetal arthrogryposis that may be detected on ultrasound as poor fetal movement and polyhydramnios. Currently, there is no in utero treatment for fetal MG. Transient neonatal MG occurs at an incidence rate of 9%-30%. Onset may be somewhere between immediate to several months after birth. There is a high recurrence rate of transient neonatal MG in subsequent pregnancies (Chaudhry et al., 2012). It is good practice to monitor closely infants born to mothers with MG for at least 72h postpartum.
Importantly, a number of common treatments for complications of pregnancy can have undesired effects in patients with MG. Magnesium sulfate is relatively contraindicated due to muscle relaxation. It has been studied that the use of levetiracetam in preeclampsia/eclampsia as anticonvulsant is effective in most cases, when magnesium sulfate is contraindicated or ineffective. If levetiracetam or other anticonvulsants are ineffective, the use of magnesium should be balanced against the risks (Satia et al., 2016). In addition, not all antihypertensive drugs are suitable for MG patients. β-Adrenoceptor blockers and calcium-channel blockers both have the potential to worsen weakness. Therefore in this patient group, methyldopa or oral hydralazine may be the initial drugs of choice in nonsevere hypertension (Ozcan et al., 2015).
There is no contraindication for vaginal term delivery in patients with MG, but this should always be hospital based with neurologic and neonatal backup available. The course of the first stage of labor in MG patients is not altered because the condition does not affect smooth muscle. The second stage of labor could be complicated by weakened expulsive efforts. Instrumental vaginal delivery may be indicated.
Myotonic dystrophy
MD is a degenerative neuromuscular and neuroendocrine disease. It is associated with cardiac dystrophy, presenting as conduction defects, arrhythmias, or congestive heart failure. There are two types of the disease (MD types 1 and 2); though they share many of the same phenotypic features, they vary in clinical severity and genetic basis. MD type 1 is an autosomal dominant trinucleotide repeat inherited disorder, with the affected gene located on chromosome 19. MD type 2 is autosomal dominant, with an expansion of the CNBP gene (Dalton et al., 2013).
The obstetrician should obtain baseline echocardiography and pulmonary function tests before conception in patients with either form of the disease and should alert the patient to symptoms of arrhythmia. In severely affected women, pregnancy is rare. Referral to a geneticist for preconception counseling may be warranted for consideration of in vitro fertilization and preimplantation genetic diagnosis to avoid giving birth to an affected infant (particularly for patients with MD type 1). Infants born to these mothers may be affected with congenital MD, characterized by severe generalized hypotonia and weakness, respiratory issues, difficulty sucking and swallowing, talipes, arthrogryposis, and learning difficulties. Some of these features may be detectable on prenatal ultrasound. Prenatal diagnosis is possible with DNA analysis from amniocentesis or chorionic villus sampling. MD type 1 is associated with insulin resistance (Takeshima et al., 2018); the obstetrician should monitor closely for gestational diabetes.
Patients with MD may have weakened abdominal and uterine muscles, requiring instrumental vaginal delivery techniques. In case of uterine inertia, oxytocin is a safe pharmacotherapy. Due to possible failure of contraction of the uterus, there is a higher risk for postpartum hemorrhage in these patients.
Management considerations for neurologists
Major considerations for neurologists treating women with neuromuscular disease include fluctuations in disease activity during and after pregnancy and the safety and efficacy of the medications used to treat these conditions during pregnancy.
Myasthenia gravis
Patients taking mycophenolate mofetil and methotrexate should change therapies before conception as these medications are teratogenic and contraindicated in pregnancy. Drugs that have been proven safe during pregnancy are pyridostigmine, prednisolone, azathioprine, cyclosporine/tacrolimus, and intravenous immunoglobulin. While safe, these drugs do carry potential side effects (gestational diabetes, leukopenia, and liver function abnormalities), warranting use of the lowest effective dose. Drug pharmacokinetics may change during pregnancy, causing subtherapeutic serum levels of medication and increased disease activity. This may be particularly relevant late in pregnancy when diaphragm elevation can further compromise respiratory function. The course of MG may alternate in different pregnancies in the same patient; close monitoring of disease activity in each successive pregnancy is therefore crucial. Close postpartum monitoring is also important given that 30% of patients experience exacerbations of MG after delivery.
Myotonic dystrophy
Mexiletine is used in some patients with MD as antimyotonic drug. There is very limited data available concerning mexiletine use during pregnancy; careful monitoring during pregnancy and postpartum to maintain therapeutic drug levels is advised (Gregg and Tomich, 1988). Significant worsening of the symptoms of MD (myotonia and weakness) can occur at any point during pregnancy but is most common in the third trimester. These exacerbations typically abate after delivery.
Management considerations for anesthesiologists
The main consideration for anesthesiologists in delivering infants of mothers with neuromuscular disease is management of potential weakness, particularly of respiratory muscles.
Myasthenia gravis
Anesthetic challenges may arise when patients with MG require general anesthesia. There is always an increased postsurgical risk of respiratory failure (Blichfeldt-Lauridsen and Hansen, 2012). Due to lower levels of nAChRs in patients with MG and the use of cholinesterase inhibitors (pyridostigmine), altered reactions to neuromuscular blocking agents (succinylcholine) may occur. MG patients are at risk for prolonged blockade, and reversion of residual block may be unsuccessful. Regional anesthesia is preferred, as epidural or spinal analgesia may reduce the need for muscle relaxants and offer pain control during delivery or postcesarean section (Blichfeldt-Lauridsen and Hansen, 2012; Norwood et al., 2014).
Myotonic dystrophy
General anesthesia and respiratory depressants should be avoided due to the risk of exacerbation of pulmonary hypoventilation. Nondepolarizing neuromuscular blocking agents should also be avoided.
Headache
Neurologists classify headaches as either primary, due to primary pathology in the nerves in the scalp and other cranial tissues, or secondary, due to a separate pathology that affects these nerves. The first of these categories includes migraine and the trigeminal autonomic cephalalgias, among others. The second includes headaches due to elevated intracranial pressure, infection, vasculitis, and other vascular pathologies. Primary headache disorders (specifically, migraines) are the most common headache types overall and tend to cause minimal complications in pregnancy, though their treatments may require modification (Allais et al., 2019). Secondary headache syndromes, however, may reflect serious illnesses for which pregnant and postpartum women are particularly at risk. It is important to recognize the features of these headaches to minimize morbidity.
Management considerations for obstetricians
Migraine
There is an association of migraine with both preeclampsia and maternal stroke. However, it is not clear whether this is a causal effect (Bushnell and Chireau, 2011). Patients with rare migraines, especially those who are not on daily prophylactic pharmacotherapy, may not require referral to a neurologist. Many prophylactic medications (beta blockers, tricyclic antidepressants, antiepileptics, and abortives such as the triptans) are either contraindicated or poorly studied in pregnancy; patients taking these should confer with their neurologist regarding therapy modification prior to conception (Allais et al., 2019).
Idiopathic intracranial hypertension (IIH)
IIH is a secondary headache syndrome caused by elevated intracranial pressure. Weight gain can adversely affect disease activity, and thus pregnant women should aim not to exceed maximum weight gain recommendations. The primary risk of inadequately treated IIH is visual loss; any patient reporting change in visual acuity or visual fields should be promptly referred to a neurologist or ophthalmologist. First-line pharmacotherapies for IIH include acetazolamide and topiramate. Acetazolamide may cause adverse fetal effects, and topiramate use during pregnancy is contraindicated (Tomson et al., 2011a). Alternative therapies include the use of glucocorticoids or lumbar puncture (Martin and Foley, 2005).
Posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vascular syndrome (RCVS)
These conditions comprise a spectrum of diseases resulting from endothelial dysfunction and subsequent vascular dilatation and leakage (PRES) or vasospasm (RCVS). There is considerable overlap between the two syndromes: the risk of both conditions increases significantly during pregnancy and in the postpartum period, both are associated with hypertension and preeclampsia/eclampsia, and both can cause ischemic stroke or intracranial hemorrhage. The obstetrician should be alerted to the following characteristic symptoms and refer patients promptly to a neurologist or the emergency room if they develop any of the following: visual field deficits, blurred vision, episodes of seizure, focal neurologic deficits, and so-called thunderclap headaches that reach maximal intensity within seconds of onset. If these symptoms develop in the setting of hypertension, it is crucial to lower blood pressure and obtain an MRI and MRA of the brain.
Cerebral venous sinus thrombosis (CVST)
Thrombosis of any of the intracranial veins or venous sinuses can cause headache due to elevated intracranial pressure. If untreated, increased venous pressure can eventually cause intracranial hemorrhage. Women are at increased risk of venous thromboembolic events during pregnancy and in the postpartum period due to hormone-mediated alterations in coagulation pathways. The obstetrician should be alerted to the following characteristic symptoms and refer patients promptly to a neurologist or the emergency room if they develop any of the following: headache that is worse when supine, maximal in the morning, or wakes the patient from sleep; significant nausea/vomiting associated with headaches; and focal neurologic deficits. MR venography can assess for the presence of CVST, which requires treatment using anticoagulation therapy.
For further information concerning CVST, please find this in section “Cerebrovascular disease”.
Management considerations for neurologists
Migraine
Observational studies have shown that migraine tends to improve during pregnancy; however, worsening of symptoms and frequency of episodes has also been described (Sacco and Ripa, 2015; Tepper, 2015; Petrovski et al., 2018). Data regarding the safety of many migraine prophylactics and abortives in pregnancy is scarce; see Chapter 11 of Volume 172 for a detailed review of management options.
Idiopathic intracranial hypertension (IIH)
As mentioned earlier, acetazolamide is of unclear safety in pregnancy and topiramate is contraindicated due to teratogenicity. Symptoms and visual fields should be monitored closely during pregnancy, as weight gain can cause worsening of disease. Lumbar puncture with large-volume CSF removal should be considered for management during pregnancy.
Posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vascular syndrome (RCVS)
It is crucial for the neurologist to remember that women are at increased risk for these conditions in the postpartum period as well as during pregnancy. In the setting of preeclampsia/eclampsia, the choice of antihypertensive agent may be different during pregnancy than in the general population due to the safety of these drugs for the fetus; preferred medications include nifedipine, magnesium (also for seizure prophylaxis), nicardipine, and hydralazine. Transcranial Doppler offers a noninvasive method for monitoring the response to therapy in women with RCVS (Marsh et al., 2016). Verapamil has been used successfully to treat RCVS-related headaches and is considered safe when breastfeeding (Verapamil, 2006).
Neuro-oncology
Brain tumors are rare, and the overall incidence of primary brain tumors is the same in pregnant and nonpregnant women of childbearing age. They may be either primary (e.g., gliomas, glioblastomas, meningiomas, pituitary adenomas) or secondary metastases of a primary extracranial malignancy. The systemic cancers that most commonly metastasize to the brain are breast, lung, colon, kidney, and melanoma. All patients with a primary brain neoplasm should be under the care of a neurologist with subspecialty training in neuro-oncology; those with metastatic disease from an extracranial primary should be cared for by a medical oncologist as well as either a neuro-oncologist or a general neurologist. Important factors guiding management of pregnant women with brain tumors are the type of neoplasm, its rate of growth, its location in the brain, and the degree to which it causes symptoms.
Many treatments of brain tumors (radiation, surgical resection, and certain chemotherapeutics such as methotrexate) can be harmful to the fetus and are either contraindicated or high risk in pregnancy. In addition, certain chemotherapeutic agents, especially alkylating chemotherapies, such as cyclophosphamide, busulfan, melphalan procarbazine, and chemotherapeutic combinations that include alkylating chemotherapies, can affect a woman’s future fertility (Waimey et al., 2015). For these reasons, interdisciplinary planning is of the utmost importance, starting before conception if at all possible and continuing throughout pregnancy. Other specialists that may need to be involved in these discussions include radiation oncologists, neurosurgeons, anesthesiologists, and, in the case of brain metastases of extracranial primary malignancy, medical oncologists.
Management considerations for obstetricians
Brain tumors can cause secondary neurologic processes that are potentially dangerous; the obstetrician should be aware of these complications and their common symptoms. The tumor may expand and associated edema may increase, causing mass effect and, if severe, herniation and compression of part of the brain. As noted earlier, certain hormone-responsive tumors (prolactinomas and some meningiomas) may grow at an increased rate during pregnancy. Symptoms of increased mass effect include headache, nausea, vomiting, decreased alertness, confusion, and focal neurologic deficits (e.g., weakness, language impairment, and visual changes). Headaches due to increased intracranial pressure are typically worse when the patient is supine and may be present when she wakes in the morning. Hemorrhage into a tumor may cause symptoms similar to those described earlier. Glioblastoma, renal cell carcinoma, and melanoma are particularly prone to hemorrhage. Brain tumors can cause seizure due to cortical irritation; the patient may report intermittent sensory changes or rhythmic movements in one part of the body, periods of language disturbance, or loss of awareness. If a patient reports any of these symptoms during a visit, she should be referred promptly to her neurologist for evaluation.
In case of increased intracranial pressure, vaginal delivery may be assisted with ventouse or forceps to minimize pushing, which can further raise pressure, or cesarean section may be performed. However, there is a lack of empirical data to guide these decisions.
Management considerations for neurologists
The safety of chemotherapeutic agents commonly used to treat brain cancers will be discussed in more detail in a following section of this volume; initiation and continuation of chemotherapy during pregnancy should be pursued only in conversation with the obstetrician. Use of glucocorticoids to treat tumor-associated vasogenic edema also requires careful consideration and discussion with the obstetrician, as glucocorticoid use during pregnancy is associated with fetal growth restriction (Busada and Cidlowski, 2017). If prolonged high-dose steroid treatment is required, prednisone is the first agent of choice. When considering how frequently to monitor tumor growth during pregnancy, the neurologist should keep in mind that certain hormone-responsive tumors, such as prolactinomas and some meningiomas, may grow at an accelerated rate due to hormonal shifts. If there is significant mass effect or obstructive hydrocephalus from tumor, the risk of dural puncture leading to herniation may be too high to safely administer intrapartum neuraxial anesthesia. An alternative plan should be discussed with the obstetrician and anesthesiologist prior to delivery.
Management considerations for anesthesiologists
Any plan for neuraxial anesthesia during delivery should first be discussed with the neurologist to identify any risk of herniation from increased intracranial pressure or tumor location.
CONCLUSION
The importance of coordinated, multidisciplinary care for pregnant women with neurologic comorbidities is increasingly recognized. We propose the term “neuro-obstetrics” to describe the complex care required for these women. All neurologists and obstetricians should be familiar with the basic principles of neuro-obstetric care, including the need for preconception counseling and close collaboration between specialties to optimize maternal and fetal outcomes for women with chronic or acute neurologic conditions.
References
- Abalos E, Duley L, Steyn DW et al. (2001). Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev CD002252. [DOI] [PubMed] [Google Scholar]
- Agarwal N, Guerra JC, Gala NB et al. (2014). Current treatment options for cerebral arteriovenous malformations in pregnancy: a review of the literature. World Neurosurg 81: 83–90. [DOI] [PubMed] [Google Scholar]
- Ahrens KA, Silver RM, Mumford SL et al. (2016). Complications and safety of preconception low-dose aspirin among women with prior pregnancy losses. Obstet Gynecol 127: 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright BB, Rayburn WF (2009). Substance abuse among reproductive age women. Obstet Gynecol Clin North Am 36: 891–906, xi–xii. [DOI] [PubMed] [Google Scholar]
- Allais G, Chiarle G, Sinigaglia S et al. (2019). Migraine during pregnancy and in the puerperium. Neurol Sci 40: 81–91. [DOI] [PubMed] [Google Scholar]
- Bergmann RL, Bergmann KE, Dudenhausen JW (2008). Undernutrition and growth restriction in pregnancy. Nestle Nutr Workshop Ser Pediatr Program 61: 103–121. [DOI] [PubMed] [Google Scholar]
- Blatt K, Moore E, Chen A et al. (2015). Association of reported trimester-specific smoking cessation with fetal growth restriction. Obstet Gynecol 125: 1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blichfeldt-Lauridsen L, Hansen BD (2012). Anesthesia and myasthenia gravis. Acta Anaesthesiol Scand 56: 17–22. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ et al. (2011). Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med 41: 516–524. [DOI] [PubMed] [Google Scholar]
- Bove R, Alwan S, Friedman JM et al. (2014). Management of multiple sclerosis during pregnancy and the reproductive years: a systematic review. Obstet Gynecol 124 (6): 1157–1168. 10.1097/AOG.0000000000000541. [DOI] [PubMed] [Google Scholar]
- Bove R, Rankin K, Chua AS et al. (2018). Oral contraceptives and MS disease activity in a contemporary real-world cohort. Mult Scler 24 (2): 227–230. 10.1177/1352458517692420. [DOI] [PubMed] [Google Scholar]
- Bromley RL, Mawer GE, Briggs M et al. (2013). The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry 84: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busada JT, Cidlowski JA (2017). Mechanisms of glucocorticoid action during development. Curr Top Dev Biol 125: 147–170. [DOI] [PubMed] [Google Scholar]
- Bushnell C, Chireau M (2011). Preeclampsia and stroke: risks during and after pregnancy. Stroke Res Treat 2011: 858134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnetti C, Lattanzi S, Foschi N et al. (2014). Seizure course during pregnancy in catamenial epilepsy. Neurology 83 (4): 339–344. 10.1212/WNL.0000000000000619. Epub 2014 Jun 18. [DOI] [PubMed] [Google Scholar]
- Chaudhry SA, Vignarajah B, Koren G (2012). Myasthenia gravis during pregnancy. Can Fam Physician 58:1346–1349. [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Vestergaard C, Hammer Bech B (2018). Maternal death in women with epilepsy: smaller scope studies. Neurology 91: e1716–e1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ (2013). The adaptation of the cerebral circulation to pregnancy: mechanisms and consequences. J Cereb Blood Flow Metab 33: 465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciron J, Godeneche G, Vandamme X et al. (2013). Obstetrical outcome of young women with a past history of cerebral venous thrombosis. Cerebrovasc Dis 36: 55–61. [DOI] [PubMed] [Google Scholar]
- Corp. G (2016). Available: http://products.sanofi.us/aubagio/aubagio.html [Accessed 24–05-2019].
- Corp. NP (2018). Available: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/gilenya.pdf [Accessed 24-05-2019].
- Coyle PK (2016). Management of women with multiple sclerosis through pregnancy and after childbirth. Ther Adv Neurol Disord 9: 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle PK, Oh J, Magyari M et al. (2019). Management strategies for female patients of reproductive potential with multiple sclerosis: an evidence-based review. Mult Scler Relat Disord 32: 54–63. [DOI] [PubMed] [Google Scholar]
- Cressman AM, Natekar A, Kim E et al. (2014). Cocaine abuse during pregnancy. J Obstet Gynaecol Can 36: 628–631. [DOI] [PubMed] [Google Scholar]
- Curtis KM, Tepper NK, Jatlaoui TC et al. (2016). U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 65: 1–103. [DOI] [PubMed] [Google Scholar]
- Dalton JC, Ranum LPW, Day JW (2013). Myotonic dystrophy type 2, University of Washington, Seattle, Seattle (WA), [Google Scholar]
- Denny L, Coles S, Blitz R (2017). Fetal alcohol syndrome and fetal alcohol spectrum disorders. Am Fam Physician 96: 515–522. [PubMed] [Google Scholar]
- Draper ES, Rankin J, Tonks AM et al. (2008). Recreational drug use: a major risk factor for gastroschisis? Am J Epidemiol 167: 485–491. [DOI] [PubMed] [Google Scholar]
- EURAP Study Group (2006). Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology 66: 354–360. [DOI] [PubMed] [Google Scholar]
- Gooneratne IK, Wimalaratna M, Ranaweera AKP et al. (2017). Contraception advice for women with epilepsy. BMJ 357: j2010. [DOI] [PubMed] [Google Scholar]
- Gould GS, Lim LL, Mattes J (2017). Prevention and treatment of smoking and tobacco use during pregnancy in selected indigenous communities in high-income countries of the United States, Canada, Australia, and New Zealand: an evidence-based review. Chest 152: 853–866. [DOI] [PubMed] [Google Scholar]
- Gregg AR, Tomich PG (1988). Mexilitene use in pregnancy. J Perinatol 8: 33–35. [PubMed] [Google Scholar]
- Hansen HH, Krutz B, Sifringer M et al. (2008). Cannabinoids enhance susceptibility of immature brain to ethanol neurotoxicity. Ann Neurol 64: 42–52. [DOI] [PubMed] [Google Scholar]
- Harden CL, Hopp J, Ting TY et al. (2009). Practice parameter update: management issues for women with epilepsy—focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 73: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Mandle HB, Cahill KE et al. (2017). Predictors of unintended pregnancy in women with epilepsy. Neurology 88: 728–733. [DOI] [PubMed] [Google Scholar]
- Hofmeyr GJ, Belizan JM, von Dadelszen P et al. (2014). Low-dose calcium supplementation for preventing preeclampsia: a systematic review and commentary. BJOG 121: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Nagle KJ, Tremble SM et al. (2015). The contribution of normal pregnancy to eclampsia. PLoS One 10: e0133953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EL, Burke AE, Wang A et al. (2018). Unintended pregnancy, prenatal care, newborn outcomes, and breastfeeding in women with epilepsy. Neurology 91: e1031–e1039. [DOI] [PubMed] [Google Scholar]
- Karlsson B, Johansson AV, Yang HC et al. (2018). A novel method to determine the natural course of unruptured brain arteriovenous malformations without the need for follow-up information. J Neurosurg 129: 10–16. [DOI] [PubMed] [Google Scholar]
- Katsuragi S, Yoshimatsu J, Tanaka H et al. (2018). Management of pregnancy complicated with intracranial arteriovenous malformation. J Obstet Gynaecol Res 44: 673–680. [DOI] [PubMed] [Google Scholar]
- Kesler A, Kupferminc M (2013). Idiopathic intracranial hypertension and pregnancy. Clin Obstet Gynecol 56: 389–396. [DOI] [PubMed] [Google Scholar]
- Khaskheli MN, Baloch S, Sheeba A et al. (2016). Iron deficiency anaemia is still a major killer of pregnant women. Pak J Med Sci 32: 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YW, Neal D, Hoh BL (2013). Cerebral aneurysms in pregnancy and delivery: pregnancy and delivery do not increase the risk of aneurysm rupture. Neurosurgery 72: 143–149; discussion 150. [DOI] [PubMed] [Google Scholar]
- Koc G, Keskin Guler S, Karadas O et al. (2018). Fetal safety of levetiracetam use during pregnancy. Acta Neurol Belg 118: 503–508. [DOI] [PubMed] [Google Scholar]
- Kuklina EV, Tong X, Bansil P et al. (2011). Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke 42: 2564–2570. [DOI] [PubMed] [Google Scholar]
- Laganà AS, Triolo O, D’Amico V et al. (2016). Management of women with epilepsy: from preconception to postpartum. Arch Gynecol Obstet 293 (3): 493–503. 10.1007/s00404-015-3968-7. Epub 2015 Nov 30. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A, Smith JB, Hellwig K et al. (2017). Breastfeeding, ovulatory years, and risk of multiple sclerosis. Neurology 89 (6): 563–569. 10.1212/WNL.0000000000004207. Epub 2017 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassi ZS, Imam AM, Dean SV et al. (2014). Preconception care: caffeine, smoking, alcohol, drugs and other environmental chemical/radiation exposure. Reprod Health 11: S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JP, Smith PE, Craig J et al. (2017). Epilepsy and pregnancy: for healthy pregnancies and happy outcomes. Suggestions for service improvements from the Multispecialty UK Epilepsy Mortality Group. Seizure 50: 67–72. [DOI] [PubMed] [Google Scholar]
- Leffert LR, Schwamm LH (2013). Neuraxial anesthesia in parturients with intracranial pathology: a comprehensive review and reassessment of risk. Anesthesiology 119: 703–718. [DOI] [PubMed] [Google Scholar]
- Looman M, van den Berg C, Geelen A et al. (2018). Supplement use and dietary sources of folate, vitamin D, and n-3 fatty acids during preconception: the GLIMP2 study. Nutrients 10: 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X, Li Y (2015). The clinical characteristics and treatment of cerebral AVM in pregnancy. Neuroradiol J 28: 385–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SC, Bateman BT, McElrath TF et al. (2015). Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol 72: 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadeva A, Tanasescu R, Gran B (2014). Urinary tract infections in multiple sclerosis: under-diagnosed and undertreated? A clinical audit at a large university hospital. Am J Clin Exp Immunol 3: 57–67. [PMC free article] [PubMed] [Google Scholar]
- Marjot T, Yadav S, Hasan N et al. (2011). Genes associated with adult cerebral venous thrombosis. Stroke 42:913–918. [DOI] [PubMed] [Google Scholar]
- Marsh EB, Ziai WC, Llinas RH (2016). The need for a rational approach to vasoconstrictive syndromes: transcranial Doppler and calcium channel blockade in reversible cerebral vasoconstriction syndrome. Case Rep Neurol 8: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SR, Foley MR (2005). Approach to the pregnant patient with headache. Clin Obstet Gynecol 48: 2–11. [DOI] [PubMed] [Google Scholar]
- Miller EC, Leffert L (2020). Stroke in pregnancy: a focused update. Anesth Analg 130 (4): 1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JL (2017). Strategies for preventing folate-related neural tube defects: supplements, fortified foods, or both? JAMA 317: 144–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Bellisario C et al. (2013). Maintenance agonist treatments for opiate-dependent pregnant women. Cochrane Database Syst Rev CD006318. [DOI] [PubMed] [Google Scholar]
- Norwood F, Dhanjal M, Hill M et al. (2014). Myasthenia in pregnancy: best practice guidelines from a U.K. multispecialty working group. J Neurol Neurosurg Psychiatry 85: 538–543. [DOI] [PubMed] [Google Scholar]
- Oza R, Rundell K, Garcellano M (2017). Recurrent ischemic stroke: strategies for prevention. Am Fam Physician 96: 436–440. [PubMed] [Google Scholar]
- Ozcan J, Balson IF, Dennis AT (2015). New diagnosis myasthenia gravis and preeclampsia in late pregnancy. BMJ Case Rep 2015. 10.1136/bcr-2014-208323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MF, Mulcahy H, DeFranco EA (2019). Influence of periconception smoking behavior on birth defect risk. Am J Obstet Gynecol 220 (6): 588.e1–588.e7. [DOI] [PubMed] [Google Scholar]
- Petrovski BE, Vetvik KG, Lundqvist C et al. (2018). Characteristics of menstrual versus non-menstrual migraine during pregnancy: a longitudinal population-based study. J Headache Pain 19: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessinger MA (1998). Prenatal exposure to amphetamines. Risks and adverse outcomes in pregnancy. Obstet Gynecol Clin North Am 25: 119–138. [DOI] [PubMed] [Google Scholar]
- Podymow T, August P (2008). Update on the use of antihypertensive drugs in pregnancy. Hypertension 51: 960–969. [DOI] [PubMed] [Google Scholar]
- Reimers A (2016). Contraception for women with epilepsy: counseling, choices, and concerns. Open Access J Contracept 7: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Report of the National High Blood Pressure Education Program (2000). Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol 183 (1): S1–S22. [PubMed] [Google Scholar]
- Rosenfeld WE, Doose DR, Walker SA et al. (1997). Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy. Epilepsia 38: 317–323. [DOI] [PubMed] [Google Scholar]
- Sabers A, aRogvi-Hansen B, Dam M et al. (1998). Pregnancy and epilepsy: a retrospective study of 151 pregnancies. Acta Neurol Scand 97 (3): 164–170. 10.1111/j.1600-0404.1998.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Sacco S, Ripa P (2015). Migraine in pregnancy. J Headache Pain 16: A24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satia MN et al. (2016). A study of the obstetric and perinatal outcomes of eclampsia and the use of levetiracetam in its management. Int J Reprod Contracept Obstet Gynecol 5 (12): 4266–4270. https://www.ijrcog.org/index.php/ijrcog/article/view/34/34. [Google Scholar]
- Scheres LJJ, Bistervels IM, Middeldorp S (2019). Everything the clinician needs to know about evidence-based anticoagulation in pregnancy. Blood Rev 33: 82–97. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Canger R, Avanzini G et al. (1983). Change of seizure frequency in pregnant epileptic women. J Neurol Neurosurg Psychiatry 46: 751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla SD, Aroor AR (2006). Epigenetic effects of ethanol on liver and gastrointestinal injury. World J Gastroenterol 12: 5265–5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmaier R, Nasri H, Deutsch CK et al. (2017). Exposure to sodium valproate during pregnancy: facial features and signs of autism. Birth Defects Res 109: 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen LJ, Harden C, Tomson T et al. (2019). Management of epilepsy in women. Lancet Neurol 18: 481–491. [DOI] [PubMed] [Google Scholar]
- Sveberg L, Svalheim S, Taub0ll E (2015). The impact of seizures on pregnancy and delivery. Seizure 28: 35–38. 10.1016/j.seizure.2015.02.020. Epub 2015 Feb 21 [DOI] [PubMed] [Google Scholar]
- Takeshima K, Ariyasu H, Ishibashi T et al. (2018). Myotonic dystrophy type 1 with diabetes mellitus, mixed hypogonadism and adrenal insufficiency. Endocrinol Diabetes Metab Case Rep 2018: pii: 17-0143 10.1530/EDM-17-0143. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate J, Bushnell C (2011). Pregnancy and stroke risk in women. Womens Health (Lond) 7: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper D (2015). Pregnancy and lactation—migraine management. Headache 55: 607–608. [DOI] [PubMed] [Google Scholar]
- Thomas S, Hovinga ME, Rai D et al. (2017). Brief report: prevalence of co-occurring epilepsy and autism spectrum disorder: The U.S. National Survey of Children’s Health 2011–2012. J Autism Dev Disord 47 (1): 224–229. 10.1007/s10803-016-2938-7. [DOI] [PubMed] [Google Scholar]
- Tomson T, Battino D, Bonizzoni E et al. (2011a). Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 10: 609–617. [DOI] [PubMed] [Google Scholar]
- Tomson T, Battino D, Perucca E (2011b). Major birth defects after exposure to newer-generation antiepileptic drugs. JAMA 306: 826–827, author reply 827. [DOI] [PubMed] [Google Scholar]
- Verapamil (2006). SourceDrugs and Lactation Database (LactMed) [Internet]. Bethesda (MD): National Library of Medicine (US). [Google Scholar]
- von Dadelszen P, Magee LA (2005). Antihypertensive medications in management of gestational hypertension-preeclampsia. Clin Obstet Gynecol 48: 441–459. [DOI] [PubMed] [Google Scholar]
- Waimey KE, Smith BM, Confino R et al. (2015). Understanding fertility in young female cancer patients. J Womens Health (Larchmt) 24: 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfarin (2006), SourceDrugs and Lactation Database (LactMed) [Internet]. Bethesda (MD): National Library of Medicine (US). [Google Scholar]
- Waters J (2019). Management of myasthenia gravis in pregnancy. Neurol Clin 37: 113–120. [DOI] [PubMed] [Google Scholar]
- Wesselink AK, Rothman KJ, Hatch EE et al. (2017). Age and fecundability in a North American preconception cohort study. Am J Obstet Gynecol 217: 667, e661–667 e668. https://www.ncbi.nlm.nih.gov/pubmed/28917614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff D, Welsh BT, Henderson R et al. (2017). Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxicol 109: 585–648. [DOI] [PubMed] [Google Scholar]
- Witt WP, Mandell KC, Wisk LE et al. (2015). Predictors of alcohol and tobacco use prior to and during pregnancy in the US: the role of maternal stressors. Arch Womens Ment Health 18: 523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization; (2013). Guideline: calcium supplementation in pregnant woman, WHO. [PubMed] [Google Scholar]
- Youngblood ME, Williamson R, Bell KN et al. (2013). 2012 Update on global prevention of folic acid-preventable spina bifida and anencephaly. Birth Defects Res A Clin Mol Teratol 97: 658–663. [DOI] [PubMed] [Google Scholar]


