Abstract
Treatment of end-stage glenohumeral arthritis in young patients is a challenge; however, there is a lack of consensus on optimal treatment algorithms. A thorough history and physical examination are essential. Nonoperative treatments should first be attempted, whereas surgical options range from arthroscopic debridement to arthroplasty. One arthroplasty option is glenohumeral resurfacing with the objective of maintaining more native anatomy and bone stock. The described treatment includes a hemi-cap implant for the humerus and inlay polyethylene glenoid. While hemi-caps have been successfully used for decades, inlay glenoid implants are a more modern treatment, with the objective of less glenoid loosening, the typical complication and failure method in young patients. With the potential for greater longevity and preservation of anatomy, glenohumeral resurfacing for end-stage shoulder arthritis is an important treatment option to consider before total shoulder arthroplasty. This Technical Note describes resurfacing of the glenohumeral joint in a young, active patient presenting with extensive osteoarthritis on both the glenoid and humerus after a previous failed Trillat stabilization.
Treatment options for glenohumeral (GH) arthritis are a challenge in young patients. Unlike elderly patients, who typically have excellent outcomes following shoulder arthroplasty, those same treatments commonly have high failure rates in young patients due to the high demands on these patients' shoulders.1, 2, 3
An initial thorough history and physical examination are required in young patients with GH arthritis. Distinguishing other pathologies, including those of the acromioclavicular joint, rotator cuff, and labrum, are important.4 History of infection, trauma, instability, and physical labor are often essential keys. Medical history including causes of avascular necrosis as well as surgical history are also imperative. Infection should always be considered. A thorough physical examination focused on range of motion, rotator cuff strength, and axillary nerve function is crucial. A full radiologic series is also an important aspect in the workup to evaluate for GH subluxation, glenoid retroversion, osteophytes, and other abnormal anatomy. Advanced imaging including computed tomography or magnetic resonance imaging can also provide useful information.5, 6, 7
Nonoperative treatments should initially be tried, which include physical therapy for range of motion and strengthening as well as anti-inflammatory medications and activity modification.8,9 Injections such as corticosteroids, hyaluronic acid, or platelet-rich plasma also can be attempted; however, these injections should be used judiciously, as cartilage and rotator cuff damage can occur.10,11 If nonoperative treatments fail in young patients with severe GH arthritis, personalized surgical decision making and intervention can be attempted, which include arthroscopic GH debridement, hemiarthroplasty with or without glenoid resurfacing, or total shoulder arthroplasty (TSA). In severe situations with neurologic dysfunction or anatomic abnormalities, arthrodesis or resection are salvage options.
In this Technical Note, we describe the technique of GH resurfacing for younger (≤35 years) in the setting of a previous failed Trillat procedure that resulted in debilitating end-stage osteoarthritis. The described technique resurfaces the GH joint with hemi-cap humerus and inlay glenoid implants. Inlay glenoid implants have the potential for less loosening, which is the typical complication in young patients. Further, this resurfacing combination allows the surgeon to maintain more native bone stock and anatomy for future arthroplasty treatments if needed.
Surgical Technique (With Video Illustration)
A narrated video with demonstration of the following surgical technique may be reviewed (Video 1).
Indications and Preoperative Imaging
The Trillat procedure aims to restore anterior shoulder stability through the use of a coracoid closing-wedge osteotomy with the goal of placing the conjoint tendon anterior to the humeral head. However, the screws used for this technique can lead to the development or progression of GH osteoarthritis. Regardless of previous shoulder procedures, osteoarthritis of the shoulder in younger patients is a challenging entity to treat, and TSA or reverse TSA should be reserved for when all other bone-preserving resurfacing techniques have been exhausted. Preoperative radiographs with standard shoulder views, and computed tomography scans with 3-dimensional reconstructions should be obtained to assess the extent of osteoarthritis and potential retained hardware from previous procedures (Figs 1 and 2).
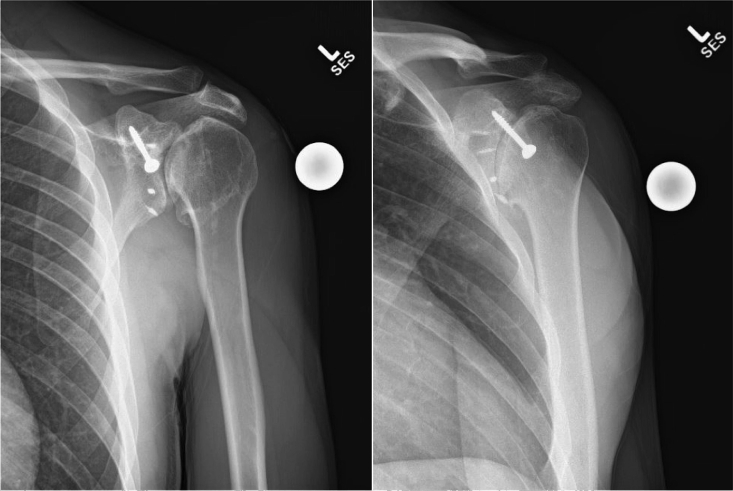
Fig 1.
Preoperative anteroposterior (AP) shoulder (left) and AP glenoid/Grashey (right) view radiographs of the patient's left shoulder demonstrating retained hardware in the coracoid from the patient's previous Trillat procedure.
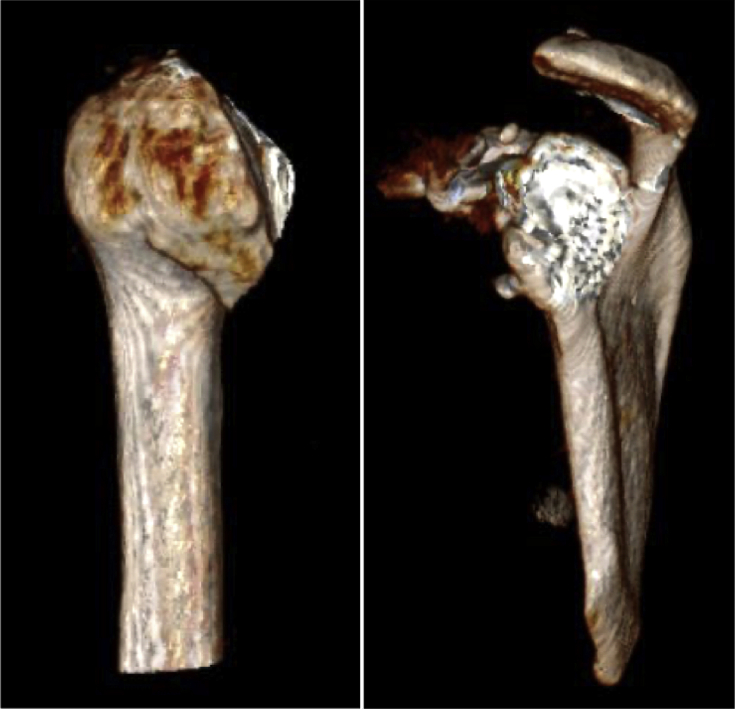
Fig 2.
Preoperative 3-dimensional computed tomography scans of the patient's left shoulder demonstrate extensive end-stage osteoarthritis of both the humeral head (left) and glenoid (right).
Surgical Approach and Hardware Removal
After regional and general anesthesia is completed, the patient is placed in the beach chair position and a well-padded Mayo stand is placed under the elbow. A standard deltopectoral approach to the shoulder is performed. In the case of revision of a Trillat procedure, the conjoint tendon is lateralized due to the previous coracoid osteotomy, making the coracohumeral interval particularly small, resulting in difficult access to the GH joint. Therefore, the coracoid hardware is removed, and the coracoid is osteotomized at its base. The nearby neurovascular structures must be protected at all times. The conjoint tendon is removed from the coracoid, then the osteotomized bone is removed. The conjoint tendon is then whipstitched then tucked medially for later repair to the coracoid base. The subscapularis is then tenotomized 3 to 4 mm lateral to the insertion, leaving a stump of healthy subscapularis tendon laterally for later repair. The capsule is then divided and released from the humerus, with care taken to protect the axillary nerve.
Humeral Preparation
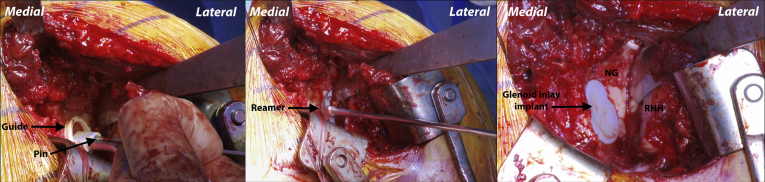
Once adequate humeral head exposure is achieved, the humeral head is measured using the Arthrosurface OVO (Arthrosurface, Franklin, MA) humeral head sizing guides. Measurements are taken from superior to inferior and anterior to posterior. A humeral head sizing trial is then placed on the humeral head in the correct version to ensure correct fit. With the humeral head sizing trial placed over the humeral head in the correct version, a central 2.5-mm pin is advanced unicortically through the central hole (Fig 3). A power drill is then used to advance the centering shaft over the 2.5-mm pin into the humeral head. It is advanced until the distal lip is flush with the articular surface. Note that if the humeral head is flattened, the centering shaft should be stopped 2 to 3 mm before the lip reaching the articular surface.
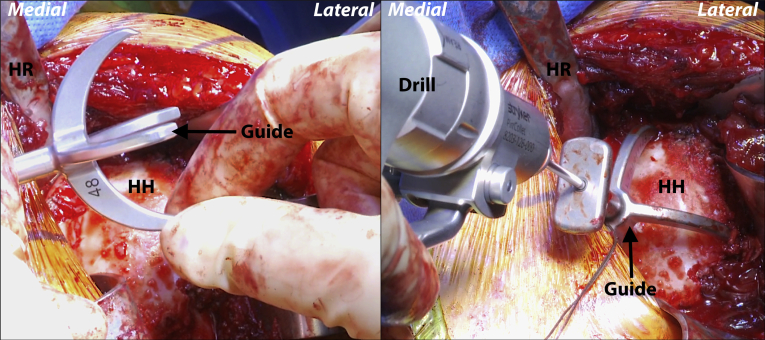
Fig 3.
Left shoulder, reclined beach chair position. With the 4-pronged humeral head sizing trial placed over the humeral head in the correct version (left), a central 2.5-mm pin is advanced unicortically through the central hole to temporarily secure the guide (right). (HH, humeral head; HR, Hohmann retractor.)
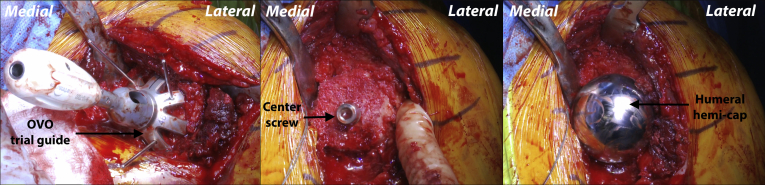
Next, the Arthrosurface OVO reamer corresponding to the anterior to posterior humeral head dimensions is advanced over the centering shaft until it bottoms out. This is then repeated with the crown reamer (Arthrosurface; Fig 4). With the humeral head reamed, the preparation trial is placed on the guide handle and secured into position on the humerus with 2 guide pins. The pilot drill is then advanced through the center of the guide handle until the laser line on the drill aligns with the back of the guide handle. The pilot drill bit is then released from the drill and left in place, and the guide handle is unscrewed and removed. Next, the step drill is advanced over the pilot drill until the proximal shoulder of the step drill is even with the height marker on the preparation trial collar. Note that if the patient has poor bone quality, the step drill should be stopped 2 to 3 mm premature. This will improve bony purchase when the taper post screw is placed. The step drill is removed and with the pilot drill still in place, the tap is advanced until the laser line is even with the height marker on the preparation trial collar. The tap and the pilot drill are then both removed.
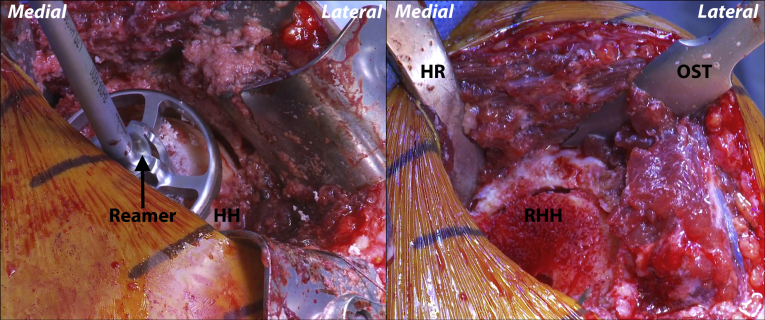
Fig 4.
Left shoulder, reclined beach chair position. Once the shaft is flush with the humeral head and all remaining osteophytes have been excised, a combination of surface and access reamers is used to remove 12.5 mm of humeral head height. (HH, humeral head; HR, Hohmann retractor; OST, osteotome; RHH, reamed humeral head.)
With all instrumentation removed except for the preparation trial (pinned in place), the pilot hole for the taper post (Arthrosurface) is pulse irrigated and all debris is removed. If the patient has poor bone quality, bone cement is placed into the pilot hole and the taper post is advanced into the cement and held until the cement cures. If the patient has excellent bone quality, the taper post is placed without the use of bone cement. The depth and alignment of the taper post is confirmed with the alignment gauge and the fit is confirmed with the reduction trial. If the reduction trial sits proud at the edge of the articular cartilage, the humerus may be reamed again until the reduction trial sits flush against the humerus.
Glenoid Preparation
Two Hohmann retractors are placed on the posterior glenoid and the humerus is retracted laterally. With the glenoid exposed, the glenoid drill guide is centered on the inferior aspect of the glenoid articular defect. A guide pin is then advanced through the hole in the drill guide to the depth of the laser line. The guide is removed, and the glenoid reamer is advanced over the guide pin until the depth stop makes contact with the guide pin (Fig 5). Confirmation that the correct depth has been reamed is then done. If the trial sits flush or just recessed relative to the adjacent articular surface of the glenoid, the flexible peg drill is advanced into the central hole.
Fig 5.
Left shoulder, reclined beach chair position. A guide pin is advanced through the hole in the glenoid component drill guide to the depth of the laser line (left). The guide is removed, and the glenoid reamer is advanced over the guide pin until the depth stop makes contact with the guide pin (middle). The inlay implant is then secured to the glenoid with cement on its posterior surface (right). (NG, native glenoid; RHH, reamed humeral head.)
If using the double glenoid implant (Arthrosurface), the glenoid trial is placed in its proper position and the guide pin advanced into the superior hole. The trial is then removed and final glenoid reaming is performed over the guide pin. Proper depth is the confirmed with the double glenoid trial. If the trial sits flush with the articular surface or is slightly recessed, the flexible peg drill is advanced into both central holes of the glenoid trial. The trial is removed and the angled gouge is used to microfracture the periphery of the glenoid fossa to aid with cement fixation. Pulse irrigation is then done, then the glenoid is dried.
Implant Insertion
A small amount of low-viscosity bone cement is applied into the glenoid recess then pressure is applied using the rubber finger impactor to ensure that cement fills the peg holes and gouge channels. A small amount of cement may also be placed on the back side of the polyethylene inlay. The glenoid inlay (Arthrosurface) is appropriately positioned and then the glenoid impactor is used. The glenoid implant should sit flush with the adjacent articular surface, or slightly recessed. Firm pressure is then held on the implant until the bone cement cures. Excess bone cement is removed from the glenoid during this time.
Attention is then turned back to the humerus and retractors are repositioned for adequate exposure. The OVO (Arthrosurface) humeral component is then aligned in its proper orientation relative to the humerus. The humeral component is impacted onto the taper post (Fig 6). It is then confirmed that it is fully seated on the Morse taper (Arthrosurface). The humerus and glenoid are pulse irrigated, and the GH joint is then reduced. Passive motion, fit, and stability are then assessed.
Fig 6.
Left shoulder, reclined beach chair position. The preparation trial of the OVO Motion head is impacted over the reamed humeral head and held flush in place with 3 guide pins (left). A centering drill is advanced through the guide followed by the centering screw (middle). Once the oval humeral hemi-cap implant is centered over the Morse taper of the centering screw, it is gently tapped into place to ensure solid fixation to the native humeral head (right).
Conjoint Tendon and Subscapularis Repair
The previously whip stitched conjoint tendon is then repaired back to the coracoid base using a 4.75-mm SwiveLock (Arthrex, Naples, FL) anchor. The subscapularis is then repaired back to its anatomic footprint. A suture tape is placed both on the upper and lower margin of the subscapularis using a Mason–Allen technique. Each end of these suture tapes is placed in the anatomic footprint of the subscapularis using a 4.75-mm SwiveLock anchor. Nonabsorbable suture tape is then used to oversew the subscapularis back to the 3- to 4-mm lateral tendon stump in an interrupted figure-of-eight fashion. Final motion and stability are assessed.
Closure
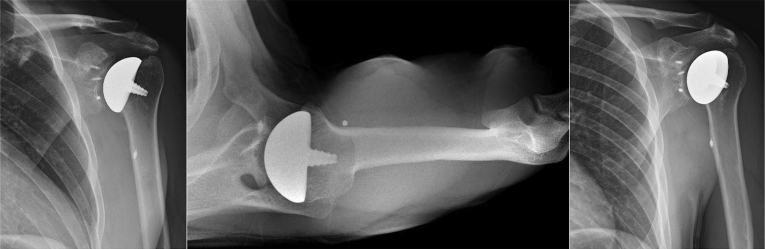
The wound is irrigated and then closed in a layered fashion. A sterile dressing is applied, and the patient is placed in a padded abduction sling. Postoperative radiographic imaging is performed to assess the newly resurfaced glenoid and humeral head (Fig 7). The pearls/pitfalls and advantages/disadvantages associated with the technique are listed in Table 1 and Table 2, respectfully.
Fig 7.
Left shoulder, reclined beach chair position. Postoperative radiographic imaging is performed on the left shoulder with anteroposterior (AP) shoulder (left), superoinferior axial (middle), and AP glenoid/Grashey views (right) to assess the newly resurfaced glenoid and humeral head hemi-cap.
Table 1.
Pearls and Pitfalls of the Described Glenohumeral Resurfacing Technique
| Pearls | Pitfalls |
|---|---|
| In cases for revision Trillat, the coracohumeral distance will be significantly decreased due to the previous lateralizing coracoid osteotomy. We recommend coracoid osteotomy at its base to allow adequate access to the glenohumeral joint. | Given the revision nature of this procedure, be mindful of excessive scarring and altered anatomy. Without special care, injury may occur to the axillary nerve at the inferior aspect of the subscapularis tenotomy or during glenoid preparation. |
| Once the coracoid osteotomy is performed, the osteotomized bone is removed and the conjoint tendon whipstitched for later repair to the coracoid base with a suture anchor. | If the glenoid depth is not confirmed with the glenoid trial, the implant with underlying cerement may sit proud in the glenoid inlay recess. |
| The inlay glenoid implant should sit flush with the native glenoid or slightly recessed. This should be visualized carefully during trialing. Further, a “snowman” type implant can be used for larger glenoid lesions. | |
| If the humeral head is flattened, the centering shaft should not be advanced the full depth, stopping 2-3 mm before the lip reaching the articular surface. This allows the centering shaft to be placed slightly proud to the surface to allow appropriate humeral implant positioning. |
Table 2.
Advantages and Disadvantages of the Described Glenohumeral Resurfacing Technique
| Advantages | Disadvantages |
|---|---|
| Restores functional range of motion by addressing end-stage osteoarthritis with the use of hemi-cap humeral and inlay glenoid implants while maintaining anatomy and bone stock. | No outcome studies have yet reported on the short- or long-term outcomes of this glenohumeral resurfacing procedure. |
| Serves as a potential alternative to hemiarthroplasty or total shoulder arthroplasty for patients with greater levels of activity or vocational demands. | Procedure does not address underlying soft-tissue pathology, such as rotator cuff deficiency. |
| Inlay glenoid reduces risk of glenoid implant loosening and reserves the use of total shoulder arthroplasty for revisional purposes if needed. | There have been no long-term outcomes studies to date that assess inlay glenoid implant longevity. |
Rehabilitation
Immediately following the procedure, and once all wounds are appropriately dressed, the patient is placed in an abduction sling for 4 weeks and will remain non-weight bearing. The patient may come out of the sling daily for passive and gentle active assist forward flexion to 120° and abduction to 60° immediately postoperatively. The patient may begin working with physical therapy in the first week following the procedure. To protect the subscapularis repair, the patient is limited in passive external rotation to 30° and no internal rotation past neutral for 4 weeks. The patient may begin progressive weight bearing at 4 weeks and strengthening may begin at 6 weeks postoperatively with gradual return to full activity thereafter.
Discussion
This Technical Note describes resurfacing of the GH joint in a young, active patient presenting with extensive osteoarthritis on both the glenoid and humerus after a prior failed Trillat stabilization. The goal of this procedure was to restore functional range of motion by addressing end-stage osteoarthritis with the use of hemi-cap humeral and inlay glenoid implants with the hope of maintaining anatomy and bone stock. Moreover, the inlay glenoid implant may decrease the risk of the fairly common complication of glenoid loosening in young active patients. This technique presents a an alternative treatment option for young patients with severe bipolar osteoarthritis, serving as a potential alternative to hemiarthroplasty or TSA for those with greater levels of activity or vocational demands.
The first step in addressing progressive GH osteoarthritis (OA) is most commonly nonoperative treatment. Although nonsteroidal anti-inflammatory drugs are commonly recommended for initial management, there have been no clinical trials to date that demonstrate a significant benefit or decrease in shoulder pain levels following their use. However, at least moderate short-term relief is reported in the majority of patients with shoulder OA.12,13 Intra-articular injections have grown to be one of the most frequently used nonoperative treatments, with some of the most studied being corticosteroid and hyaluronic acid injections.8, 9, 10 Although patients treated with corticosteroid and hyaluronic acid injections have demonstrated significant decreases in pain and significant improvements in activities of daily living following shoulder injections, the efficacy of these injections remains inconclusive, and the American Academy of Orthopaedic Surgeons is unable to recommend for or against their use to treat GH OA.10,11,14
Options for surgical management of progressive GH OA include arthroscopic debridement, hemiarthroplasty (HA), or TSA.15 Predisposing risk factors for inferior outcomes following shoulder arthroplasty are widely discussed throughout the literature, with one of the most prominent factors being younger age at the time of surgery.1, 2, 3 Sperling et al.3 followed a cohort of patients who underwent either HA or TSA and were younger than the age of 50 years for a minimum of 15 years. This 2004 study reported unsatisfactory outcomes in 60% of the HA group and in 50% of the TSA group. These are significantly worse than those previously reported for shoulder arthroplasty in older patients. The cause of these inferior results is likely multifactorial; however, they may be due in part to the greater activity levels of younger patients, postoperative athletic participation, and increased expectations in younger patients of overall TSA efficacy.16,17
Although implanting glenoid components when performing TSA has demonstrated superior results when compared with those of HA for the management of extensive GH OA, the long-term risk of glenoid component loosening may deter some surgeons from this option.18, 19, 20 The risk of such postoperative hardware complications is further exacerbated by the aforementioned increased functional demands and expectations of younger patients.1,2,16 In a 2013 study by Denard et al.,1 50 patients aged 55 years or younger who underwent TSA for OA with a keeled glenoid component were retrospectively reviewed. Overall survivorship of the glenoid component at 5 years was reported to be 98%; however, implant survival dropped to 62.5% at 10-year follow-up and glenoid components were 6 times more likely to loosen if the humeral head was not positioned anatomically.1
In the setting of severe glenoid component loosening, revision options are repeat TSA or reverse TSA. Revision of failed primary TSA has proven to be problematic, with high rates of intra- or postoperative complications and significantly worse patient satisfaction compared with primary TSA.21, 22, 23, 24, 25, 26, 27 Antoni et al.25 reported on a cohort of 37 patients treated with revision TSA at a mean time of 78.4 ± 59.7 months (range, 1-200 months) following primary TSA. Intraoperative complications occurred in 24.3% of patients and postoperative complications were reported with 29.7%, yielding an overall complication rate of 54%. Reoperation following revision TSA was found to be 21.9%.25 Similarly, inferior outcomes following revision of failed primary TSA with reverse TSA were reported by Shields et al.24 in 2019. In this matched cohort analysis, the revision group had significantly worse subjective shoulder value scores (63 ± 30 vs 79 ± 21) overall satisfaction (74% vs 90%), and more complications (31% vs 13%).24 These findings highlight the importance of appropriate patient selection for TSA.
In this Technical Note, we describe the senior author's technique for GH resurfacing with the use of hemi-cap humeral and inlay glenoid implants as an alternative to hemiarthroplasty or TSA in young and active patients. The use of an inlay glenoid implant allows for bone preservation by reaming of the glenoid and fixation of the implant within the bone, ideally resulting in less rocking on the implant and thus avoiding complications associated with glenoid loosening postoperatively. Similarly, the use of a stemless humeral implant preserves more native bone with the idea of more bone stock being available in the future if future arthroplasty is required. Although no outcome studies have yet reported on outcomes of this resurfacing procedure, the authors believe it is an important treatment option in young patients with end-stage GH OA who do not respond to nonoperative treatment.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: M.T.P. reports personal fees from Arthrex, Joint Research Foundation (JRF), SLACK, and Arthrosurface, outside the submitted work, and is an editorial board member for the Arthroscopy Association of North America, American Academy of Orthopaedic Surgeons, American Orthopaedic Society for Sports Medicine, American Shoulder and Elbow Surgeons, International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine, the San Diego Shoulder Institute, and the Society of Military Orthopaedic Surgeons. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Investigation performed at the Steadman Philippon Research Institute in Vail, Colorado, U.S.A.
Supplementary Data
Left shoulder, reclined beach chair position. In this video, we present our preferred surgical technique for glenohumeral resurfacing in an active 35-year-old male patient with end-stage osteoarthritis of the shoulder. A standard deltopectoral approach is used and the screw from the previous Trillat procedure on the coracoid is removed. The biceps tendon is tagged and sutured to the superior border of the pectoralis major. Next, a subscapularis peel is performed. The humeral head is outlined along the native articular surface and the 4-pronged guide is temporarily secured with a guide pin. The guide pin is then over-drilled with a centering shaft down to the proximal shelf to recreate the humeral height. Once the shaft is flush with the humeral head and all remaining osteophytes have been excised, a combination of surface and access reamers is used to remove 12.5 mm of humeral head height. A pointed Hohman retractor is placed posteriorly to the glenoid and the subscapularis is tagged with any remaining adhesions excised to provide good exposure and excursion. After marking the inferior two thirds of the glenoid, the 30° glenoid sizing component is introduced to the field and a centering nitinol pin is inserted. A 20-mm reamer is placed over the pin and advanced to a depth of 4mm. A second centering nitinol pin is then placed and reamed superiorly to allow for a snowman configuration glenoid inlay implant. Next, the centering pegs for the inlay glenoid are drilled with a flexible drill. The microfracture awl is then used to make microfracture holes and remove any remaining debris on the glenoid surface prior to final glenoid implant fixation. The inlay implant is then secured to the glenoid with cement on its posterior surface. The preparation trial of theOVOMotion head is impacted over the reamed native humeral head and held flush in place with 3 guide pins. A centering drill is advanced through the guide followed by the centering screw. The screw is advanced until the white screwdriver is flush with the guide. Once the oval humeral hemi-cap implant is centered over the Morse taper of the centering screw, it is gently tapped into place to ensure solid fixation to the native humeral head. Lastly, the subscapularis is meticulously repaired with a combination of rotator cuff anchors. A combination of suture anchors and tape/cylindrical sutures are used to restore the native tenotomy of the subscapularis. It is crucial to achieve excellent soft-tissue balancing and solid subscapularis closure.
References
- 1.Denard P.J., Raiss P., Sowa B., Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22:894–900. doi: 10.1016/j.jse.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Schoch B., Schleck C., Cofield R.H., Sperling J.W. Shoulder arthroplasty in patients younger than 50 years: Minimum 20-year follow-up. J Shoulder Elbow Surg. 2015;24:705–710. doi: 10.1016/j.jse.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Sperling J.W., Cofield R.H., Rowland C.M. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13:604–613. doi: 10.1016/S1058274604001296. [DOI] [PubMed] [Google Scholar]
- 4.Kelly J.D., Jr., Norris T.R. Decision making in glenohumeral arthroplasty. J Arthroplasty. 2003;18:75–82. doi: 10.1054/arth.2003.50005. [DOI] [PubMed] [Google Scholar]
- 5.Werner B.S., Hudek R., Burkhart K.J., Gohlke F. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1477–1483. doi: 10.1016/j.jse.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Li Q., Amano K., Link T.M., Ma C.B. Advanced imaging in osteoarthritis. Sports Health. 2016;8:418–428. doi: 10.1177/1941738116663922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter D.J., Arden N., Conaghan P.G., Eckstein F., Gold G. Definition of osteoarthritis on MRI: Results of a Delphi exercise. Osteoarthritis Cartilage. 2011;19:963–969. doi: 10.1016/j.joca.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons I.M.t., Weldon E.J., 3rd, Titelman R.M., Smith K.L. Glenohumeral arthritis and its management. Phys Med Rehabil Clin N Am. 2004;15:447–474. doi: 10.1016/j.pmr.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Izquierdo R., Voloshin I., Edwards S., Freehill M.Q., Stanwood W. Treatment of glenohumeral osteoarthritis. J Am Acad Orthop Surg. 2010;18:375–382. doi: 10.5435/00124635-201006000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Merolla G., Sperling J.W., Paladini P., Porcellini G. Efficacy of hylan G-F 20 versus 6-methylprednisolone acetate in painful shoulder osteoarthritis: A retrospective controlled trial. Musculoskelet Surg. 2011;95:215–224. doi: 10.1007/s12306-011-0138-3. [DOI] [PubMed] [Google Scholar]
- 11.Silverstein E., Leger R., Shea K.P. The use of intra-articular hylan G-F 20 in the treatment of symptomatic osteoarthritis of the shoulder: A preliminary study. Am J Sports Med. 2007;35:979–985. doi: 10.1177/0363546507300256. [DOI] [PubMed] [Google Scholar]
- 12.Fardet L., Messow M., Maillefert J.F., Dougados M. Primary glenohumeral degenerative joint disease: Factors predisposing to arthroplasty. Clin Exp Rheumatol. 2003;21:13–18. [PubMed] [Google Scholar]
- 13.Sinha I., Lee M., Cobiella C. Management of osteoarthritis of the glenohumeral joint. Br J Hosp Med (Lond) 2008;69:264–268. doi: 10.12968/hmed.2008.69.5.29358. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Orthopaedic Surgeons The treatment of glenohumeral joint arthritis: Guideline and evidence report. https://www.aaos.org/research/guidelines/gloguideline.pdf Published 2009. Available at:
- 15.Millett P.J., Horan M.P., Pennock A.T., Rios D. Comprehensive Arthroscopic Management (CAM) procedure: Clinical results of a joint-preserving arthroscopic treatment for young, active patients with advanced shoulder osteoarthritis. Arthroscopy. 2013;29:440–448. doi: 10.1016/j.arthro.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Henn R.F., 3rd, Ghomrawi H., Rutledge J.R., Mazumdar M., Mancuso C.A. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93:2110–2115. doi: 10.2106/JBJS.J.01114. [DOI] [PubMed] [Google Scholar]
- 17.Schumann K., Flury M.P., Schwyzer H.K., Simmen B.R., Drerup S. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38:2097–2105. doi: 10.1177/0363546510371368. [DOI] [PubMed] [Google Scholar]
- 18.Radnay C.S., Setter K.J., Chambers L., Levine W.N., LU Bigliani. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: A systematic review. J Shoulder Elbow Surg. 2007;16:396–402. doi: 10.1016/j.jse.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Sperling J.W., Cofield R.H., Rowland C.M. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am. 1998;80:464–473. doi: 10.2106/00004623-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Bryant D., Litchfield R., Sandow M., Gartsman G.M., Guyatt G. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87:1947–1956. doi: 10.2106/JBJS.D.02854. [DOI] [PubMed] [Google Scholar]
- 21.Saltzman B.M., Chalmers P.N., Gupta A.K., Romeo A.A., Nicholson G.P. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1647–1654. doi: 10.1016/j.jse.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Zumstein M.A., Pinedo M., Old J., Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: A systematic review. J Shoulder Elbow Surg. 2011;20:146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Wall B., Nove-Josserand L., O'Connor D.P., Edwards T.B., Walch G. Reverse total shoulder arthroplasty: A review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 24.Shields E., Wiater J.M. Patient outcomes after revision of anatomic total shoulder arthroplasty to reverse shoulder arthroplasty for rotator cuff failure or component loosening: A matched cohort study. J Am Acad Orthop Surg. 2019;27:e193–e198. doi: 10.5435/JAAOS-D-17-00350. [DOI] [PubMed] [Google Scholar]
- 25.Antoni M., Barthoulot M., Kempf J.F., Clavert P. Revisions of total shoulder arthroplasty: Clinical results and complications of various modalities. Orthop Traumatol Surg Res. 2016;102:297–303. doi: 10.1016/j.otsr.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Boileau P., Watkinson D., Hatzidakis A.M., Hovorka I. Neer award 2005: The Grammont reverse shoulder prosthesis: Results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Austin L., Zmistowski B., Chang E.S., Williams G.R., Jr. Is reverse shoulder arthroplasty a reasonable alternative for revision arthroplasty? Clin Orthop Relat Res. 2011;469:2531–2537. doi: 10.1007/s11999-010-1685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left shoulder, reclined beach chair position. In this video, we present our preferred surgical technique for glenohumeral resurfacing in an active 35-year-old male patient with end-stage osteoarthritis of the shoulder. A standard deltopectoral approach is used and the screw from the previous Trillat procedure on the coracoid is removed. The biceps tendon is tagged and sutured to the superior border of the pectoralis major. Next, a subscapularis peel is performed. The humeral head is outlined along the native articular surface and the 4-pronged guide is temporarily secured with a guide pin. The guide pin is then over-drilled with a centering shaft down to the proximal shelf to recreate the humeral height. Once the shaft is flush with the humeral head and all remaining osteophytes have been excised, a combination of surface and access reamers is used to remove 12.5 mm of humeral head height. A pointed Hohman retractor is placed posteriorly to the glenoid and the subscapularis is tagged with any remaining adhesions excised to provide good exposure and excursion. After marking the inferior two thirds of the glenoid, the 30° glenoid sizing component is introduced to the field and a centering nitinol pin is inserted. A 20-mm reamer is placed over the pin and advanced to a depth of 4mm. A second centering nitinol pin is then placed and reamed superiorly to allow for a snowman configuration glenoid inlay implant. Next, the centering pegs for the inlay glenoid are drilled with a flexible drill. The microfracture awl is then used to make microfracture holes and remove any remaining debris on the glenoid surface prior to final glenoid implant fixation. The inlay implant is then secured to the glenoid with cement on its posterior surface. The preparation trial of theOVOMotion head is impacted over the reamed native humeral head and held flush in place with 3 guide pins. A centering drill is advanced through the guide followed by the centering screw. The screw is advanced until the white screwdriver is flush with the guide. Once the oval humeral hemi-cap implant is centered over the Morse taper of the centering screw, it is gently tapped into place to ensure solid fixation to the native humeral head. Lastly, the subscapularis is meticulously repaired with a combination of rotator cuff anchors. A combination of suture anchors and tape/cylindrical sutures are used to restore the native tenotomy of the subscapularis. It is crucial to achieve excellent soft-tissue balancing and solid subscapularis closure.