Abstract
Purpose
Bromodomain and extraterminal (BET) proteins are key epigenetic transcriptional regulators, inhibition of which may suppress oncogene expression. We report results from 2 independent first-in-human phase 1/2 dose–escalation and expansion, safety and tolerability studies of BET inhibitors INCB054329 (study INCB 54329–101; NCT02431260) and INCB057643 (study INCB 57643–101; NCT02711137).
Patients and Methods
Patients (≥18 years) with advanced malignancies, ≥1 prior therapy, and adequate organ functions received oral INCB054329 (monotherapy) or INCB057643 (monotherapy or in combination with standard-of-care) in 21-day cycles (or 28-day cycles depending on standard-of-care combination). Primary endpoints were safety and tolerability.
Results
Sixty-nine and 134 patients received INCB054329 and INCB057643, respectively. Study INCB 54329–101 has been completed; INCB 57643–101 is currently active, but not recruiting (no patients were receiving treatment as of January 8, 2019). Terminal elimination half-life was shorter for INCB054329 versus INCB057643 (mean [SD], 2.24 [2.03] vs. 11.1 [8.27] hours). INCB054329 demonstrated higher interpatient variability in oral clearance versus INCB057643 (CV%, 142% vs. 45.5%). Most common (>20%) any-grade treatment-related adverse events were similar for both drugs (INCB054329; INCB057643): nausea (35%; 30%), thrombocytopenia (33%; 32%), fatigue (29%; 30%), decreased appetite (26%; 22%). Two confirmed complete responses and 4 confirmed partial responses with INCB057643 were reported as best responses.
Conclusions
INCB057643 exhibited a more favorable PK profile versus INCB054329; exposure-dependent thrombocytopenia was observed with both drugs which limited the target inhibition that could be safely maintained. Further efforts are required to identify patient populations that can benefit most, and an optimal dosing scheme to maximize therapeutic index.
Introduction
The bromodomain and extraterminal domain (BET) family consists of 4 proteins (BRD2, BRD3, BRD4, and BRDT), each with 2 bromodomain (BRD) modules (BD1 and BD2) that bind selectively to acetylated histone lysine residues within chromatin that interact with gene promoter and enhancer elements (1). Upon binding, BET proteins recruit transcription initiation and elongation complexes, including mediator, polymerase-associated factor complex, and super elongation complex; thereby promoting gene transcription (2, 3). In vitro and in vivo studies have demonstrated that inhibition of BRD binding downregulates key genes [e.g., MYC, BCL-2, BCL-xl, p21(C1P1/WAF1), RUNX2, and IRF4] that promote cell-cycle progression, survival, and inflammation (4, 5), leading to growth inhibition in preclinical models of solid tumors and hematologic malignancies. In these studies, tumor growth associated with dysregulation of transcription factors, such as MYC, were particularly responsive to BET inhibition (6–8). On the basis of these findings, BET inhibitors are garnering interest as potential therapeutic targets for the treatment of solid tumors and/or hematologic malignancies, either as a monotherapy or in combination with standard-of-care (SOC) and/or novel anticancer agents.
Early clinical experience with BET inhibitors revealed challenges in balancing optimal target inhibition with the management of treatment-associated toxicities (9, 10); of note, the pharmacokinetic (PK) and pharmacodynamic (PD) profile for BET inhibitors that provides an optimal therapeutic index has not been determined. INCB054329 (8) and INCB057643 are structurally distinct small-molecule BET inhibitors, with differentiated PK profiles based on experimental PK data from preclinical studies: INCB054329 with higher clearance and a shorter half-life, and INCB057643 with lower clearance and a correspondingly longer half-life (11). In preclinical in vitro and in vivo studies, both drugs showed similar PD and antitumor activities, and elicited antiproliferative activities against a panel of hematologic and solid tumor cancer cell lines. Furthermore, both were active in xenograft models of acute myelogenous leukemia (AML), multiple myeloma (MM), diffuse large B-cell lymphoma (DLBCL), and castrate-resistant prostate cancer (CRPC; refs. 8, 12, 13). Moreover, for both INCB054329 and INCB057643, inhibition of tumor growth correlated with reduction in MYC levels in xenograft models of hematologic malignancies (8, 12).
This report presents the PK, PD, and safety results obtained from 2 independent first-in-human clinical trials of INCB054329 and INCB057643, to examine whether the differentiated PK profiles of INCB054329 and INCB057643 may translate to differentiated safety profiles and therapeutic index.
Patients and Methods
Study design
In the 2 separate phase 1/2, open-label, dose escalation and dose expansion, safety and tolerability studies, patients with advanced malignancies received INCB054329 as monotherapy (study INCB 54329-101; NCT02431260; initiated June 8, 2015) or INCB057643 (study INCB 57643-101; NCT02711137; initiated May 18, 2016) as monotherapy and in combination with SOC agents in continuous 21-day cycles (or 28-day cycles depending on SOC combination regimen; Fig. 1A and B). INCB054329 was administered at oral doses ranging from 15 to 30 mg once daily (QD) and 15 to 25 mg BID, including continuous daily and intermittent dose regimens (5-days on/2-days off; 4-days on/3-days off; 7-days on/7-days off); INCB057643 was administered at oral doses ranging from 8 to 16 mg QD.
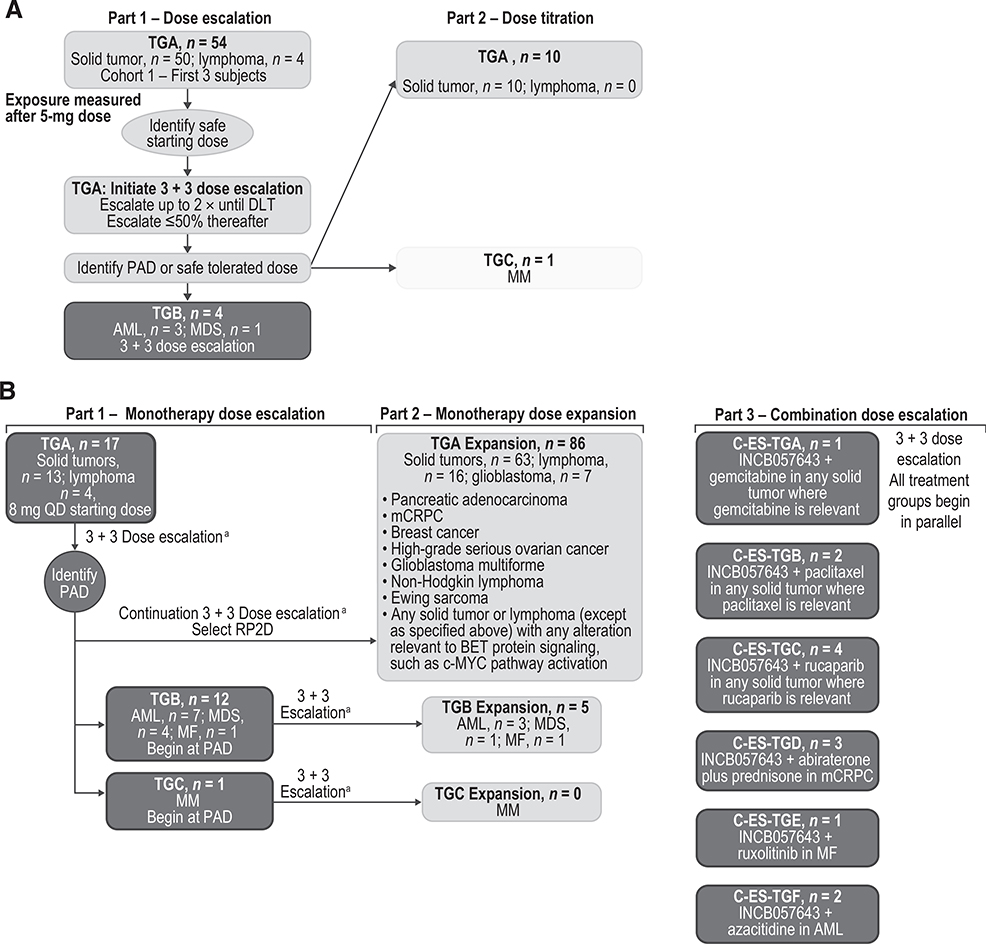
Figure 1.
Study design of study INCB 54329–101 (A) and study INCB-57643–101 (B). a Increments up to 100% until DLT occurs within a given treatment group, then up to 50% thereafter.
The dose escalation employed a 3+3 design to determine the maximum tolerated dose (MTD) or the pharmacologically active dose (PAD). The MTD was defined as 1 dose level below the non-tolerated dose at which one third or more of patients in a particular cohort reported dose-limiting toxicities (DLTs). The PAD was defined as the dose at which plasma concentrations of the BET inhibitor exceeded the IC50 for the inhibition of MYC expression for approximately 10 hours for INCB054329 and for approximately 6 to 12 hours for INCB057643. See the Supplementary Methods for further details of dose escalation and expansion, and definitions of DLTs.
Each study was conducted in accordance with the study protocol, Declaration of Helsinki, Good Clinical Practice guidelines, and applicable regulatory requirements. Study protocols and amendments were approved by the relevant institutional review boards or independent ethics committees. All patients provided written, informed consent before study participation.
Patient eligibility
For both studies, eligible patients were ages ≥18 years, had advanced malignancies confirmed by histology or cytology, had refractory or relapsed diseases following ≥1 line of prior therapy, and had no further established SOC therapy known to provide clinical benefit available to them (including patients who were intolerant to or refused the established therapy). Patients with AML had relapsed and/or refractory disease, or were ≥65 years of age and were not candidates for or declined standard chemotherapy. Patients with myelofibrosis (MF) had resistant/refractory disease, or were intolerant to prior ruxolitinib treatment. Patients with MM had relapsed or were refractory to ≥2 prior treatment regimens, including proteasome inhibitors and immunomodulatory drugs. Eligibility also required an Eastern Cooperative Oncology Group (ECOG) performance status ≤1 (study INCB 54329–101 and study INCB 57643–101 Parts 1 and 3) or ≤2 (INCB-057643 Part 2). See Supplementary Methods for key exclusion criteria for both studies.
In Part 1 (monotherapy dose escalation) of study INCB 54329–101 (Fig. 1A) or study INCB 57643–101 (Fig. 1B), patients were eligible if they had any advanced solid tumor or lymphoma, or had acute leukemia, myelodysplastic syndrome (MDS), MDS/myeloproliferative neoplasms (MPN), MF, MM (INCB 57643–101 only; see Fig. 1A and B for Treatment Group designations).
In Part 2 (dose titration) of study INCB 54329–101, patients were eligible if they had any advanced solid tumor or lymphoma (TGA) or MM (TGC; Fig. 1A). In Part 2 (monotherapy dose expansion) of study INCB 57643–101, patients were eligible if they had select solid tumors or lymphomas, or any advanced solid tumors or lymphomas with any alteration(s) relevant to BET protein signaling (such as MYC pathway activation) that were postulated to be particularly susceptible to inhibition of BET proteins (as determined by the investigator and requiring approval by the sponsor; TGA), or if they had AML, high-risk MDS (HR-MDS), MDS/MPN, or MF (TGB), or measurable/evaluable MM (TGC; Fig. 1B). In Part 3 (combination dose escalation [C-ES]) of study INCB 57643–101, eligible patients had any solid tumor for which gemcitabine (C-ES-TGA), paclitaxel (C-ES-TGB), or rucaparib (C-ES-TGC) was utilized in SOC regimens. Patients were also eligible if they had metastatic CRPC (mCRPC) eligible to receive abiraterone plus prednisone (C-ES-TGD), had any MF with an inadequate response to current ruxolitinib treatment (C-ES-TGE), or had any AML or HR-MDS eligible to receive azacitidine (C-ES-TGF; Fig. 1B). Patients with mCRPC were also required to have castrate levels of testosterone (<50 ng/dL) at screening and during the study. Inadequate response to ruxolitinib in patients with MF was defined as palpable spleen length (below the left subcostal margin on physical examination) of >10 cm or 5–10 cm with active symptoms using the Screening Symptom Form; and current treatment with ruxolitinib for ≥6 months with a stable dose for ≥8 weeks at doses ranging from 5 to 25 mg twice daily (BID).
Outcomes and assessments
The primary endpoint in each study was safety and tolerability, monitored in patients who had received ≥1 dose of study drug for the duration of the study and up to 30–37 days after the last dose, based on adverse events (AEs) assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03, clinical laboratory assessments, physical examination, vital signs, and 12-lead electrocardiograms.
The secondary endpoints were PK and PD, and efficacy. PK/PD were assessed for patients who had received ≥1 dose of study drug and had ≥1 PK and/or PD assessment. See Supplementary Methods for details of PK sample collection and assay used.
Plasma PK parameters were determined by noncompartmental analysis, using WinNonlin Enterprise version 8.0 (Pharsight Corporation). A correlative ex vivo assay was developed as a complement to the PK assessment to measure contributions of protein binding and potential metabolites to the PK of each inhibitor. This assay measured the levels of pharmacologically active INCB054329 or INCB057643 based on corresponding reductions in the levels of MYC in KMS-12-BM cells spiked into patient plasma samples. See Supplementary Methods for description of the assay used.
In addition, to explore the potential PD biomarkers associated with BET inhibitor treatment, plasma samples obtained at baseline and during treatment from 80 patients enrolled in study INCB 57643–101 were tested for the presence of approximately 1,012 proteins using the Olink multiplex proximity extension assay (Olink Bioscience AB).
Objective responses were assessed among patients who had received ≥1 dose of study drug, had measurable/evaluable disease at baseline, and had ≥1 post-baseline disease assessment, based on response criteria applicable to each malignancy.
Results
Patients
Sixty-nine patients were enrolled and treated in study INCB 54329–101 (Fig. 1A) and 134 patients were enrolled and treated in study INCB 57643–101 (Fig. 1B), as of the data cutoff dates (April 11, 2018 and September 24, 2018, respectively). Among the 134 patients in study INCB 57643–101, 13 were enrolled and treated in the combination TGs. Study INCB 54329–101 was completed on January 31, 2018; study INCB 57643–101 is currently active, but not recruiting, and no enrolled patients were receiving treatment as of January 8, 2019.
Patient demographics and baseline disease characteristics were similar across the studies (Table 1). Most patients had an ECOG performance status of ≤1 (≥95%), and most were heavily pretreated with ≥69% of patients in each study having received ≥3 prior therapies. Median durations of INCB054329 and INCB057643 (monotherapy or combination therapy) treatment were 58 (range, 7–533) days and 50.5 (range, 6–735) days, respectively. In both studies, the most common reason for treatment discontinuation was disease progression (53/69 [77%] in study INCB 54329–101 and 76/134 [57%] in study INCB 57643–101; Table 2).
Table 1.
Patient demographics and baseline characteristics across all treatment groups.
| Characteristic | Study INCB 54329-101 (n = 69) | Study INCB 57643-101 (n = 134) |
|---|---|---|
| Median (range) age (y) | 63 (18–87) | 66 (19–84) |
| Women, n (%) | 36 (52) | 67 (50) |
| Race, n (%) | ||
| White/Caucasian | 65 (94) | 109 (81) |
| Asian | 4 (6) | 0 |
| Black/African American | 0 | 16 (12) |
| American-Indian/Alaska Native | 0 | 1 (1) |
| Native Hawaiian/Pacific Islander | 0 | 1 (1) |
| Other | 0 | 6 (5) |
| Missing | 0 | 1 (1) |
| ECOG performance status, n (%) | ||
| 0 | 22 (32) | 35 (26) |
| 1 | 47 (68) | 94 (70) |
| ≥2 | 0 | 4 (3) |
| Missing | 0 | 1 (1) |
| Number of prior systemic anticancer therapiesa | ||
| 0 | 0 | 2 (2)b |
| 1 | 5 (7) | 20 (15) |
| 2 | 12 (17) | 19 (14) |
| ≥3 | 52 (75) | 93 (69) |
| Most common tumor typec n (%) | ||
| Prostate cancer | 9 (13) | 15 (11) |
| Colorectal cancer | 8 (12) | 9 (7) |
| Breast cancer | 6 (9) | 18 (13) |
| Ovarian cancer | 5 (7) | 16 (12) |
| Lymphomad | 4 (6) | 20 (15) |
| Acute myeloid leukemia | 3 | 12 (9) |
| Pancreatic cancer | 2 | 7 (5) |
| Glioblastoma | 0 (0) | 7 (5) |
| Myelodysplastic syndrome | 1 (1) | 5 (4) |
| Other | 31 (23) | 25 (19) |
Includes all treatments, potentially neoadjuvant, adjuvant, induction/consolidation, local relapse, or in metastatic setting; may also include multiple cycles for the same treatment (may or may not be considered the same line treatment).
Patients had no known SOC therapies available.
Occurring in ≥5 patients in either study.
Lymphoma subtypes according to disease history assessed at screening (study INCB 54329-101: DLBCL (n = 2), lymphoblastic lymphoma (n = 1); lymphoma subtype not reported (n = 1); study INCB 57643-101: DLBCL (n = 11), FL (n = 8), and splenic marginal zone lymphoma (n = 1).
Table 2.
Patient disposition across all treatment groups at the data cutoff date for each studya.
| Study INCB 54329-101 (n = 69) | Study INCB 57643-101 (n = 134) | |
|---|---|---|
| Patient treated, n (%) | ||
| Ongoing | 0 | 8 (6) |
| Discontinued | 69 (100) | 126 (94) |
| Primary reason for discontinuation, n (%) | ||
| Disease progression | 53 (77) | 76 (57) |
| Withdrew consent | 5 (7) | 9 (7) |
| Physician decision | 3 (4) | 10 (7) |
| Adverse event | 2 (3) | 20 (15) |
| Other | 4 (6)b | 2 (1)c |
| Lost to follow-up | 1 (1) | 0 |
| Death | 1 (1) | 9 (7) |
Data cutoff dates: study INCB 54329-101 - April 11, 2018; study INCB 57643-101-September 24, 2018.
One patient had clinical progression; 1 patient developed a new tumor; 2 patients discontinued treatment due to study closure.
One patient came off treatment as the study treatment was on hold for more than 14 days; 1 patient had clinical progression of skin lesions.
Pharmacokinetics and pharmacodynamics
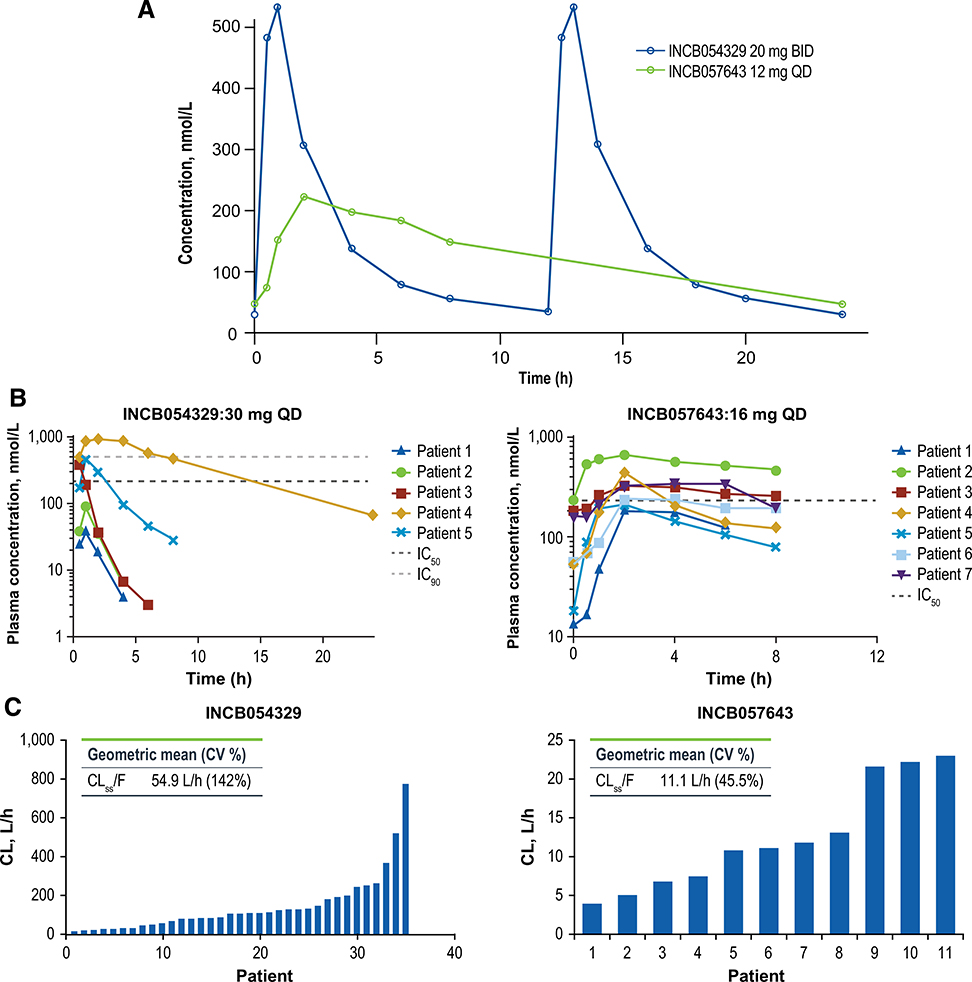
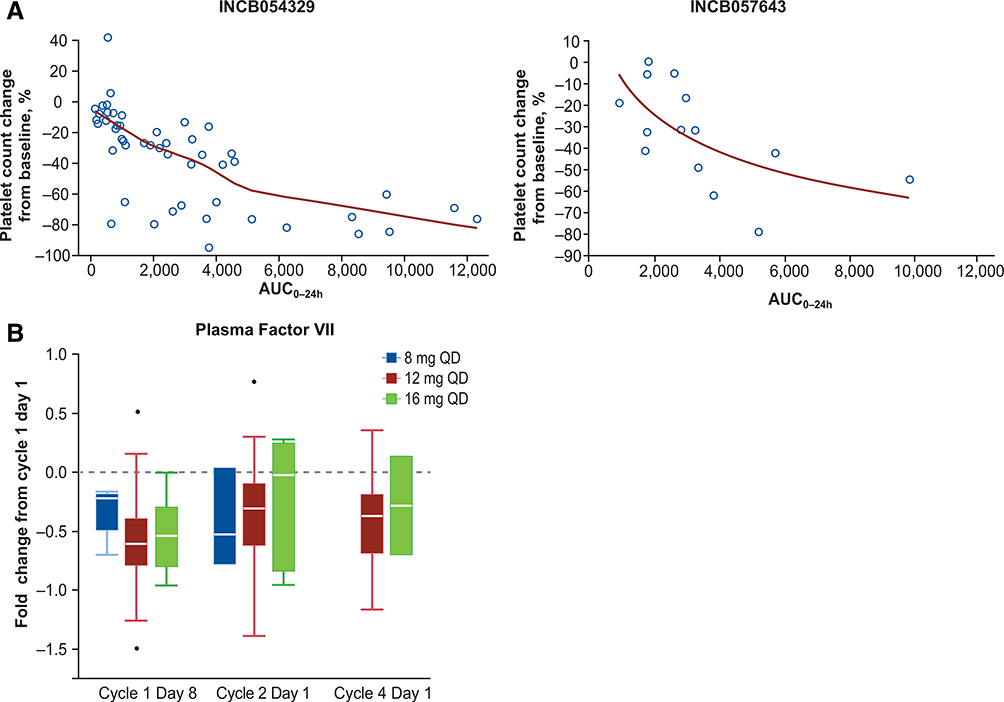
Both BET inhibitors demonstrated rapid oral absorption, with INCB054329 exhibiting much higher peak and lower trough levels compared with INCB057643 (Fig. 2A and B). INCB054329 also demonstrated much higher overall oral clearance (mean [SD] CLss/F = 95.5 [135] L/h) and shorter overall terminal elimination half-life (mean [SD] t1/2 = 2.24 [2.03] hours) versus INCB057643 (CLss/F = 11.1 [4.97] L/h; t1/2 = 11.1 [8.27] hours; Supplementary Table S1). Furthermore, the interpatient variability in oral clearance for INCB054329 was notably higher compared with INCB057643 (coefficient of variation [CV%], 142% vs. 45.5%; Fig. 2C), which is also evident from the divergent INCB054329 PK profiles obtained for individual patients receiving the same 30 mg QD starting dose of INCB054329 (Fig. 2B). This higher interpatient variability in CLss/F for INCB054329 versus INCB057643 was observed across all INCB054329 starting doses (Supplementary Table S1). For both drugs, decreases in platelet counts correlated with exposure (area under the plasma concentration–time curve [AUC]) on an individual patient basis, with higher exposures resulting in a larger percent decrease from baseline (Fig. 3A).
Figure 2.
Comparison of mean (A) and individual patient (B) concentration–time profiles and interpatient variabilities in clearance (C) for INCB054329 versus INCB057643.
Figure 3.
Platelet count change from baseline versus INCB054329 and INCB057643 exposure (A). The red line represents the best fit polynomial regression line. Fold-change versus baseline (cycle 1 day 1) in the level of Factor VII measured in plasma samples obtained from patients enrolled in study INCB 57643–101 receiving INCB057643 at doses of 8, 12, and 16 mg at cycle 1 day 8, cycle 2 day 1, and cycle 4 day 1 (B).
The effect of receiving a high-fat meal on the PK of INCB057643 was evaluated in 12 patients by comparing the PK in the fasted and fed state. Compared with the fasted state, coadministration with a high-fat meal delayed the steady-state time to maximum plasma drug concentration (Tmax) of INCB057643 from a median (range) of 2 (2–6) hours to 6 (4–24) hours, lowered the geometric mean of the maximal plasma drug concentration (Cmax) by 11% [fed vs. fasted geometric mean ratio of Cmax, 0.89; 90% confidence interval (CI), 0.78–1.01], and increased geometric mean of AUCtau (AUC over 1 dosing interval) by 13% (fed vs. fasted geometric mean ratio of AUCtau, 1.13; 90% CI, 0.99–1.29].
The concentration dependence of MYC reduction determined in KMS-12-BM cells spiked into patient plasma samples was proportional to the respective plasma concentration profiles for each drug (Supplementary Fig. S1).
To evaluate changes in circulating analytes in patients, the Olink proteomics platform was used to analyze pre- and post-treatment plasma samples from study INCB 57643–101. The results revealed differentially expressed analytes, including 50 proteins that were downregulated (absolute fold change <0.6), and 64 proteins that were upregulated (absolute fold change >1.5) post-treatment, at either cycle 1 day 8, cycle 2 day 1, or cycle 4 day 1. Among proteins that were downregulated, decreases in the levels of coagulation factor VII (Factor VII) displayed a dose-dependent trend at cycle 1 day 8 (mean [SEM] fold decrease vs. baseline: −0.32 [0.10], −0.53 [0.14], and −0.53 [0.33] with INCB057643 8, 12, and 16 mg, respectively; Fig. 3B).
INCB054329
Dose escalation
Dose escalation began in cohort 1 of Part 1 TGA following a 5-mg single-dose PK assessment at day 0 in the first 3 patients. A total of 54 patients (solid tumor, n = 50; lymphoma, n = 4) received oral INCB054329 across 11 dose cohorts of 15–30 mg QD to 15–25 mg BID, including continuous daily and intermittent dose regimens (see Supplementary Results for further details).
In Part 1 TGA, 2 DLTs occurred (both grade 4 thrombocytopenia at 30 mg QD and at 22.5 mg BID); doses greater than 20 mg BID continuous daily were not tolerated beyond cycle 1. Among patients receiving 20 mg BID continuous daily dosing, 1 experienced a grade 3 thrombocytopenia event without bleeding or transfusion during cycle 1; no DLTs or grade 4 AEs were reported; 20 mg BID continuous dosing was identified as the PAD. The MTD per protocol was not reached and no further dose escalation was conducted.
Per protocol, Part 1 TGB (AML, n = 3; MDS, n = 1) was initiated at a starting dose of 20 mg BID (PAD); treatment-related adverse events (TRAEs) led to dose interruptions in 2 patients due to grade 3 hyperglycemia. No DLTs were reported. One patient discontinued treatment due to TRAEs of nausea, stomatitis, vertigo, and vomiting (all grade 3) and neutropenia (grade 4).
Dose titration
After evaluating the overall PK, PD, and safety data in Part 1, the RP2D of INCB054329 could not be determined, mainly due to higher than expected interpatient variability in clearance and exposures at any given dose (Fig. 2B and C). Consequently, the study protocol was amended to include a dose titration cohort in Part 2 to establish an optimal dose for each individual patient. All 11 patients enrolled in Part 2 (TGA: solid tumor, n = 10; TGC: MM, n = 1) received the same starting dose of 20 mg BID continuous daily (PAD), and the dose was subsequently titrated according to protocol-specified criteria. However, the starting dose of 20 mg BID was not tolerated, with 5 patients (45%) requiring dose interruption due to TRAEs of thrombocytopenia (n = 4), anemia (n = 3), blood bilirubin increased, epistaxis, and fatigue (each n = 1), and 3 patients (27%) requiring dose reduction due to TRAEs of AST increased, fatigue, oral pain, and tremor (each n = 1). Only 1 patient was able to titrate to a higher dose (Supplementary Fig. S2). Two patients in Part 2 TGA discontinued treatment due to disease progression, and 1 patient in Part 2 TGC discontinued treatment due to physician’s decision.
INCB057643
Dose escalation (monotherapy)
A total of 17 patients (solid tumor, n = 13; lymphoma, n = 4) received INCB057643 across 3 cohorts in Part 1 TGA at dose levels of 8 mg QD, 12 mg QD, and 16 mg QD; dose escalation began in cohort 1 starting at 8 mg orally QD (continuous; see Supplementary Results for further details).
Overall in Part 1 TGA, only 1 DLT of grade 3 increased international normalized ratio (INR) occurred among the 8 patients enrolled in the 16 mg QD cohort. However, 4 patients (50%) enrolled in this 16 mg QD cohort required dose interruptions and/or reductions due to AEs during cycles 1 and 2, including DLT and TRAEs such as grade 3 INR increase, grade 3 thrombocytopenia, and grade 3 conjugated bilirubin increase. Thus, the 16 mg QD dose was deemed not tolerated; although the MTD was not reached at this dose (per protocol definition). No DLTs occurred at the 12 mg QD dose and there was only 1 dose interruption due to a TRAE (grade 2 dyspnea). Consequently, the 12 mg QD dose was selected as the RP2D for dose expansion in Part 2.
In Part 1 TGB (AML, n = 7; MDS, n = 4, MF; n = 1), doses of 8 mg and 12 mg QD were evaluated. Four patients required dose interruptions due to TRAEs of thrombocytopenia (n = 2), anemia, dehydration, diarrhea, and hyperglycemia (each n = 1). Two patients discontinued treatment due to TRAEs of anemia and thrombocytopenia; and nausea, respectively.
In Part 1 TGC (MM, n = 1), the patient received INCB057643 at a starting dose of 8 mg QD. No TRAEs were reported.
Dose expansion (monotherapy)
Part 2 TGA enrolled 86 patients (solid tumors, n = 70; lymphoma, n = 16) to receive INCB057643 12 mg QD. Among these patients, 16 had various solid tumors and lymphomas with gene alteration(s) postulated to be relevant to BET protein signaling (e.g., BRD4 and MYC amplification, alteration, overexpression, or rearrangement; Supplementary Table S2). At the data cutoff date, a total of 85 (99%) patients discontinued treatment, primarily due to disease progression (n = 53; 62%). The median (range) time on treatment was 45 (7–380) days. Thirty-one patients (36%) experienced a grade 3/4 TRAE and 11 patients (13%) experienced a serious TRAE. TRAEs led to dose interruptions and dose reductions in 30 (35%) and 9 patients (11%), respectively, most commonly due to thrombocytopenia in each case (interruptions, n = 10; reductions, n = 4). Part 2 TGB enrolled 5 patients (AML, n = 3; MF, n = 1; MDS, n = 1) to receive INCB057643 12 mg QD. The median (range) time on treatment was 37 (21–141) days. Two patients (40%) experienced a grade 3/4 TRAE and 2 experienced a serious TRAE. One patient (20%) required dose interruption due to febrile neutropenia and thrombocytopenia; no patients required dose reductions due to TRAEs.
Dose escalation (combination therapy)
Overall, 13 patients were enrolled in Part 3; all TGs received INCB057643 at the starting dose of 8 mg QD in combination with the corresponding SOC agents. TRAEs led to dose interruptions in 6 patients, most commonly due to fatigue and thrombocytopenia (each n = 2); and dose reductions in 4 patients most commonly due to thrombocytopenia (n = 2)
Overall safety
INCB054329
Overall TRAEs occurring across TGs in Parts 1 and 2 are shown in Table 3 and summarized in Supplementary Table S3. Among the 69 enrolled patients, 54 (78%) reported ≥1 any grade TRAE. The most frequently reported (≥10% incidence) TRAEs were nausea (n = 24; 35%), thrombocytopenia (n = 23; 33%), fatigue (n = 20; 29%), decreased appetite (n = 18; 26%), diarrhea (n = 12; 17%), dysgeusia (n = 9; 13%), anemia (n = 7; 10%), and vomiting (n = 7; 10%). The most common grade ≥3 TRAEs (≥3% incidence) were thrombocytopenia (n = 9; 13%), anemia (n = 4; 6%), neutropenia (n = 4; 6%), AST increased (n = 3; 4%), and hyperglycemia (n = 2; 3%). Among 7 patients experiencing serious TRAEs, the most common (occurring in ≥2 patients) were thrombocytopenia (n = 4) and anemia (n = 2).
Table 3.
TRAEs of any grade and grade ≥3 occurring across all treatment groups.
| Study INCB 54329-101 (n = 69) |
Study INCB 57643-101 (n = 134) |
|||
|---|---|---|---|---|
| Any gradea | Grade ≥3 | Any gradea | Grade ≥3 | |
| TRAEs, n (%) | 54 (78) | 16 (23) | 115 (86) | 48 (36) |
| Nausea | 24 (35) | 1 (1) | 40 (30) | 2 (2) |
| Thrombocytopeniab | 23 (33) | 9 (13) | 43 (32) | 24 (18) |
| Fatigue | 20 (29) | 0 (0) | 40 (30) | 1 (1) |
| Decreased appetite | 18 (26) | 0 (0) | 30 (22) | 0 (0) |
| Diarrhea | 12 (17) | 0 (0) | 21 (16) | 2 (2) |
| Dysgeusia | 9 (13) | 0 (0) | 22 (16) | 0 (0) |
| Anemia | 7 (10) | 4 (6) | 19 (14) | 14 (10) |
| Vomiting | 7 (10) | 1 (1) | 21 (16) | 2 (2) |
| Hyperglycemia | 3 (4)c | 2 (3) | 18 (13) | 4 (3) |
TRAEs that occurred in ≥10% of patients in either study.
MedDRA preferred terms of Thrombocytopenia and Platelet counts decreased were combined for this analysis.
Although this TRAE occurred in <10% of patients in Study INCB 54329-101, it is listed because it occurred in ≥10% of patients in Study INCB 57643-101.
Three patients (4%) discontinued treatment due to TRAEs [nausea, neutropenia, stomatitis, vertigo, and vomiting (n = 1); weight decreased (n = 1); thrombocytopenia (n = 1)]. TRAEs led to dose interruptions in 20 patients (29%), most commonly due to thrombocytopenia (17%); TRAEs led to dose reductions in 5 patients (7%; Supplementary Table S3).
Three deaths occurred during the study (septic shock, n = 1; respiratory failure, dyspnea, and hypoxia, n = 1; febrile neutropenia, n = 1), none of which was deemed treatment-related.
Overall treatment-emergent AEs across TGs in Parts 1 and 2 are shown in Supplementary Table S4.
INCB057643
Overall TRAEs occurring across all TGs in Parts 1, 2, and 3 are shown in Table 3, and are summarized in Supplementary Table S3. Among 134 enrolled patients, 115 (86%) reported ≥1 any grade TRAE, most commonly (≥10% incidence) thrombocytopenia (n = 43; 32%), nausea (n = 40; 30%), fatigue (n = 40; 30%), decreased appetite (n = 30; 22%), dysgeusia (n = 22; 16%), diarrhea (n = 21; 16%), vomiting (n = 21; 16%), anemia (n = 19; 14%), and hyperglycemia (n = 18; 13%). The most common grade ≥3 TRAEs (≥2% incidence) were thrombocytopenia (n = 24; 18%), anemia (n = 14; 10%), hyperglycemia (n = 4; 3%), dehydration (n = 3; 2%), and diarrhea, hyponatremia, INR increased, nausea, syncope, and vomiting (each n = 2; 2%). Seven patients reported treatmentrelated INR increased events, 2 of whom had grade 3 events. One of the patients with grade 3 INR increased experienced bleeding from umbilical hernia with concurrent grade 4 thrombocytopenia. Nineteen patients (14%) experienced serious TRAEs, the most common was thrombocytopenia (n = 7).
Overall, 15 patients (11%) discontinued treatment due to TRAEs, most commonly anemia and thrombocytopenia (each n = 3); 46 patients (34%) required dose interruptions, most commonly thrombocytopenia (n = 16); 15 patients (11%) required dose reductions, most commonly thrombocytopenia (n = 7; Supplementary Table S3).
Among 17 deaths occurring during the study, 2 were deemed related to treatment: The first due to acute kidney injury, infection (bacterial pneumonia), and clinical progression in a patient who had experienced treatment-related grade 3 thrombocytopenia and anemia; the second due to gastrointestinal (GI) hemorrhage as an outcome of a grade 4 GI hemorrhage TRAE in a patient with worsening pain/worsening performance status beginning 4 days after this event. This patient did not experience thrombocytopenia and the INR was unknown at the time of this event.
Overall treatment-emergent AEs across TGs in Parts 1, 2, and 3 are shown in Supplementary Table S4.
Overall efficacy
INCB054329
Among 69 patients receiving INCB054329, none had a confirmed clinical response (Supplementary Table S5). Twenty-one patients (all with solid tumors) had stable disease (SD) as best responses, 4 of whom maintained SD for ≥6 months (1 patient with renal cell carcinoma maintained SD for 1 year; another patient with breast cancer maintained SD for 2 years, both ongoing as of the data cutoff date). Thirty patients (43%) had progressive disease as best response.
INCB057643
Of 134 patients receiving INC057643, 6 patients had objective responses. Two patients achieved a confirmed complete response (CR; Supplementary Table S5) as best response, including one in a patient receiving 8 mg QD for heavily pretreated (3 prior therapies) follicular lymphoma (FL) that was ongoing >2 years as of the data cutoff date, and another in a patient receiving 12 mg QD for relapsed AML, which lasted for 57 days (2.7 cycles) before disease progression occurred. Four patients had confirmed PRs as best response, including 1 patient receiving 16 mg QD for FL, 2 patients each receiving 12 mg QD for FL (all 3 ongoing >1 year), and 1 patient receiving INCB057643 8 mg QD + paclitaxel 80 mg/m2 for breast cancer at the first disease assessment, but experienced clinical progression 6 days later. Two patients with MF receiving INCB057643 8 mg QD + ruxolitinib, and 12 mg QD INCB057643 monotherapy, respectively, experienced a 77% and 92.5% reduction in spleen size by manual palpation. Twenty-seven patients had SD as best response, 6 of whom (all with solid tumors) maintained SD for ≥6 months. Forty-one patients had progressive disease as best response. None of the 6 patients who responded had gene alterations postulated to be particularly susceptible to BET inhibition as reported by the investigators (Supplementary Table S2).
Discussion
BET inhibitors continue to garner interest as potential anticancer therapies, despite limited peer-reviewed evidence of favorable PK/PD characteristics, safety, tolerability, and efficacy. To this end, we evaluated 2 BET inhibitors, which were expected to have differentiated clinical PK profiles based on their distinct preclinical PK profiles (8, 11), in 2 separate phase 1/2 studies to test the hypothesis that such differentiated PK profiles would lead to differentiated safety profiles. On the basis of previous preclinical PK studies, INCB054329 (8) was predicted to have a shorter half-life, whereas INCB057643 was predicted to have a longer half-life and a flatter PK profile. However, both drugs demonstrated similar preclinical biologic and antitumor activities in vitro and in vivo (8, 12, 13). A BET inhibitor such as INCB054329, attaining a high peak serum concentration with a correspondingly short half-life, may have the advantage of providing a period of high-level target inhibition, followed by a relatively extended drug holiday period that could facilitate recovery from potential toxicities, including decreased platelets. Conversely, a BET inhibitor such as INCB057643, associated with a relatively flat PK profile and a longer half-life, may provide sustained inhibition of the oncogenic pathway, but with lower levels of target inhibition at Cmax (e.g., serum concentrations >IC50 for longer period of time, but <IC90 for target inhibition), which may offer a differentiated benefit/risk profile. The differentiated PK profiles demonstrated in preclinical pharmacology studies (8, 11) were confirmed in these two phase 1/2 clinical studies; however, the phase 1 data showed generally similar safety profiles between the 2 compounds, that is, no apparent advantage with either a short or a long terminal elimination half-life.
INCB054329 was confirmed to have a short half-life (<5 hours) in patients enrolled in study INCB 54329–101, which resulted in preferential exploration of a BID dosing schedule. However, the interpatient PK variability among patients receiving the same dose of INCB054329 was higher than expected, reflecting a higher than expected interpatient variability in drug clearance rate. Separate in vitro metabolism studies (results not shown) indicated that INCB054329 is metabolized by both CYP3A4 and CYP2C19 and it is possible that polymorphic expression of CYP2C19, along with variability in the expression of CYP3A4, contributed to the observed interpatient variability in PK. Furthermore, the interpatient PK variability in INCB054329 exposure was not bimodal, but rather had a broad distribution profile. Consequently, analyses of PK, PD, and safety of INCB054329 based strictly on dose levels were not considered clinically meaningful, and thus the RP2D based on dose level could not be determined. To circumvent this, intrapatient dose titration was attempted to define an optimal and tolerable INCB054329 dose on an individual basis. However, the starting dose (20 mg BID) was not tolerated and up-titration was only successful in 1 of 11 patients enrolled in this cohort.
INCB057643 demonstrated a longer half-life (~10 hours), and much lower interpatient PK variability than INCB054329 (CV% for CLss/F, 45% vs. 145%); therefore, INCB057643 was judged to have a more favorable PK profile. In addition, the observed small effect of receiving a high-fat meal on the PK of INCB057643 indicates that INCB057643 can be administered without regard to meals. For both INCB054329 and INCB057643, reduction in MYC levels, an indicator of BET target inhibition, correlated with the respective plasma concentration profiles, with effects of INCB054329 on MYC levels being more transient compared with INCB057643.
Despite their differentiated PK profiles, both INCB054329 and INCB057643 were associated with a similar incidence of decreased platelets/thrombocytopenia. Thrombocytopenia has also been observed in clinical trials of other BET inhibitors such as GSK525762, OTX015/MK-8628, BI-894999, and CPI-0610 (14), and is considered to be an on target toxicity (10). Here, thrombocytopenia was the dominant toxicity associated with INCB054329 and was the most common DLT and most frequent TRAE leading to dose interruption. Thrombocytopenia was also one of the major toxicities associated with INCB057643. There was an apparent correlation between drug exposure (AUC) and the magnitude of platelet decrease; however, it is difficult to delineate the effects of Cmax vs. AUC, because these parameters are themselves highly correlated. On the basis of the exposure versus platelet counts analyses in both studies (Fig. 3A), it appears that AUC or average inhibition certainly played a role. However, a greater magnitude of decrease in platelet counts as a function of exposure was observed with INCB054329 compared with INCB057643, suggesting that INCB054329 plasma concentrations (Cmax) could be contributing incrementally to thrombocytopenia, over and above the effect of AUC. Various intermittent dosing schedules have been explored to manage thrombocytopenia associated with BET inhibitors, including INCB054329 (5-days on/2-days off, 4-days on/3-days off, 7-days on/7-days off), OTX-015/MK-8628 (14-days on/7-days off; refs. 15, 16), and CPI-0610 (14-days on/7-days off; ref. 17). However, thrombocytopenia has remained as one of the most frequently encountered toxicities associated with BET inhibitor treatment resulting in dose interruption and reduction, and therefore maximal target inhibition that can be maintained in patients is limited by thrombocytopenia.
Seven patients receiving INCB057643 experienced TRAEs of increased INR, 2 of whom were grade 3 (1 event identified as a DLT in the INCB057643 16 mg QD dose escalation cohort), both of whom were receiving warfarin at the time. Increased INR was also observed in clinical trials of other BET inhibitors, including a phase 1/2 study of PLX51107 in 36 patients with advanced solid tumors, reporting an INR increase in 17% of patients (18). A decrease in Factor VII was observed in the INCB 57643–101 study through routine safety monitoring using a Factor VII assay (data not shown) and through the Olink assay (Fig. 3B). Decreases in Factor VII levels were also observed in clinical trials of other BET inhibitors, including OTX015/MK-8628 (15, 16, 19). It was hypothesized that INCB057643 may directly reduce Factor VII gene transcription, which resulted in Factor VII decreasing and INR increasing. In addition to Factor VII, other protein levels were altered following INCB057643 treatment; however, the biological and clinical significance of these effects are currently unknown, and further studies are required to investigate potential biological and clinical utilities such as PD and predictive biomarkers.
Both INCB054329 and INCB057643 were associated with limited antitumor activity in these 2 studies with an unselected patient population. For INCB054329, no confirmed clinical responses were observed; this might in part be due to the relatively short half-life of INCB054329 and the need for dose interruptions due to TRAEs, which would further lower drug exposure. For INCB057643, six confirmed responses were reported, including 2 CRs (FL, n = 1; AML, n = 1), and 4 PRs (FL, n = 3; breast cancer, n = 1). Because these two phase 1 studies enrolled patients with different disease types in both solid tumors and hematologic malignancies, retrospective comparison of antitumor activity or clinical response between the two drugs was not possible. Other BET inhibitors had also reported limited efficacies in their respective phase 1 studies (15, 16, 20).
The limited number of patients with clinical responses and the lack of patients with genomic and/or proteomic data in these studies prevents elucidation of correlations between clinical response and molecular signatures (i.e., delineation of predictive biomarkers). In study INCB 57643–101, 1 patient with breast cancer who had a MYC amplification experienced an SD for ≥6 months. However, none of the patients with known MYC and/or BET alterations, including those with double- or triple-hit DLBCL, and Nut-Midline carcinoma achieved a clinical response (Supplementary Table S2). Further investigations are required to generate hypotheses for predictive biomarkers among the six patients who experienced clinical responses.
In summary, we evaluated 2 BET inhibitors that, despite having divergent PK profiles, demonstrated similar safety outcomes (primarily thrombocytopenia as an overlapping toxicity). On the basis of the observed shorter than expected half-life and higher than expected interpatient PK variability, INCB054329 was judged to have an unfavorable clinical PK profile. By contrast, INCB057643 exhibited a more favorable PK profile with a longer half-life and much reduced interpatient PK variability. Thrombocytopenia was the major TRAE leading to dose interruption and reduction for both INCB054329 and INCB057643. Other TRAEs associated with BET inhibitors in clinical trials include increased INR and decreased Factor VII, and hyperglycemia, which are emerging as possible class-related or on-target AEs. Taken together, further efforts are required to identify predictive biomarkers for patient selection and to explore rational combinations to maximize efficacy.
Supplementary Material
Translational Relevance.
Bromodomain and extraterminal (BET) proteins are central in the regulation of genes that promote cell-cycle progression, survival, and inflammation, and are therefore garnering interest as potential therapeutic targets. INCB054329 and INCB057643 are 2 structurally distinct BET inhibitors that possess potent antitumor activities in preclinical models of hematologic malignancies and solid tumors. We report results from 2 independent first-in-human phase 1/2 studies (INCB 54329–101 and INCB 57643–101) in patients with advanced malignancies, wherein the 2 BET inhibitors under investigation demonstrated differentiated clinical pharmacokinetic profiles, yet resulted in generally similar safety profiles. A decrease in platelet count was identified as a key pharmacodynamic marker as well as an adverse effect, that correlated well with overall exposure. Other exploratory biomarkers were identified and further efforts are required to apply those findings in defining the appropriate patient populations for optimal efficacy and safety.
Acknowledgments
The authors wish to thank the patients and their families, the investigators, the site personnel who participated in these studies. The authors would also like to thank Dr. Ping Jiang (Incyte Research Institute, Incyte Corporation, Wilmington, DE) for biomarker analyses. Incyte Corporation (Wilmington, DE) sponsored these studies. Medical writing assistance was provided by Sneha DSilva, MD, and Simon J. Slater, PhD (Envision Pharma Group, Philadelphia, PA), and funded by Incyte Corporation.
Disclosure of Potential Conflicts of Interest
G. Falchook is an employee/paid consultant for EMD Serono; reports receiving other commercial research support through his institution from 3-V Biosciences, AbbVie, ADC Therapeutics, Aileron, American Society of Clinical Oncology, Amgen, ARMO, AstraZeneca, BeiGene, BioAtla, Biothera, Celldex, Celgene, Ciclomed, Curegenix, Curis, Delmar, eFFECTOR, Eli Lilly, EMD Serono, Exelixis, Fujifilm, Genmab, GlaxoSmithKline, Hutchison MediPharma, Ignyta, Incyte, Jacobio, Jounce, Koltan, Loxo, MedImmune, Millennium, Merck, miRNA Therapeutics, National Institutes of Health, Novartis, OncoMed, Oncothyreon, Precision Oncology, Regeneron, Rgenix, Strategia, Syndax, Takeda, Tesaro, Tocagen, U.T. MD Anderson Cancer Center, and Vegenics; reports receiving speakers’ bureau honoraria from Total Health Conferencing; is an unpaid consultant/advisory board member for Fujifilm; and reports receiving other remuneration from Wolters Kluwer, Bristol-Myers Squibb, EMD Serono, Fujifilm, and Millennium. P. LoRusso is an unpaid consultant/advisory board member for AbbVie, Agios, Five Prime, Halozyme, Roche-Genentech imCore Alliance, Genentech, CytomX, Takeda, SOTIO, Cybrexa, Agenus, Tyme, IQVIA, TRIGER, Pfizer, I-MAB, ImmunoMet, Black Diamond, Sartarius, and GlaxoSmithKline. J. Watts is an employee/paid consultant for and reports receiving speakers’ bureau honoraria from Jazz Pharma; reports receiving commercial research grants from Takeda; and is an unpaid consultant/advisory board member for Celgene and Pfizer. C.C. Coombs is an employee/paid consultant for AbbVie, and is an unpaid consultant/advisory board member for Octapharma, Loxo, Pharmacyclics, H3 Biomedicine, AbbVie, and Medscape. R. Kurzrock reports receiving commercial research grants to her institution from Incyte, Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health, Grifols, Konica Minolta, DeBiopharm, Boehringer Ingelheim, and OmniSeq; reports receiving speakers’ bureau honoraria from Roche; holds ownership interest (including patents) in IDbyDNA, CureMatch, and Soluventis; is an unpaid consultant/advisory board member for Gaido, LOXO, X-Biotech, Actuate Therapeutics, Roche, NeoMed, Soluventis, and Pfizer; and reports receiving other remuneration from CureMatch, IDbyDNA, and Soluventis. R. Cassaday is an employee/paid consultant for Seattle Genetics, Amgen, Pfizer, and Adaptive Biotechnologies; reports receiving commercial research grants from Amgen, Pfizer, Merck, Vanda Pharmaceuticals, and Incyte; and has immediate family members who hold ownership interest (including patents) in Seattle Genetics. D.C. Smith reports receiving commercial research grants from Incyte, Agensys, Eli Lilly, Bayer, MedImmune, Novartis, OncoMed, Seattle Genetics, Bristol-Myers Squibb/Medarex, Genentech, Medivation/Astellas, Merck, and F. Hoffman La Roche. S.A. Piha-Paul reports receiving other commercial research support through her institution from AbbVie, Aminex Therapeutics, BioMarin Pharmaceutical, Boehringer Ingelheim, Bristol-Myers Squibb, Cerulean Pharma, Chugai Pharmaceutical, Curis, Five Prime Therapeutics, Genmab A/S, GlaxoSmithKline, Helix BioPharma, Incyte, Jacobio Pharmaceuticals, MedImmune, Medivation, Merck Sharp and Dohme, NewLink Genetics/Blue Link Pharmaceuticals, Novartis, Pieris Pharmaceuticals, Pfizer, Pume Biotechnology, Rapt Therapeutics, Seattle Genetics, Taiho Oncology, Tesaro, Trans Thera Bio, XuanZhu Biopharma. M.S. Noel is an employee/paid consultant for Celgene. S. Yeleswaram is an employee/paid consultant for and holds ownership interest (including patents) in Incyte. P. Liu is an employee/paid consultant for and holds ownership interest (including patents) in Incyte. J. Switzky is an employee/paid consultant for Sponsor and holds ownership interest (including patents) in Company. G. Zhou is an employee/paid consultant for Incyte. F. Zheng is an employee/paid consultant for and holds ownership interest (including patents) in Incyte. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Filippakopoulos P, Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat Rev Drug Discovery 2014;13:337–56. [DOI] [PubMed] [Google Scholar]
- 2.Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem 2007;282:13141–5. [DOI] [PubMed] [Google Scholar]
- 3.Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 2011;478:529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu LL, Tian M, Li X, Li JJ, Huang J, Ouyang L, et al. Inhibition of BET bromodomains as a therapeutic strategy for cancer drug discovery. Oncotarget 2015;6:5501–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniguchi Y The bromodomain and extra-terminal domain (BET) family: functional anatomy of BET paralogous proteins. Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci U S A 2011;108:16669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011;146: 904–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stubbs MC, Burn TC, Sparks RB, Maduskuie T, Diamond S, Rupar M, et al. The novel bromodomain and extraterminal domain inhibitor INCB054329 induces vulnerabilities in myeloma cells that inform rational combination strategies. Clin Cancer Res 2019;25:300–11. [DOI] [PubMed] [Google Scholar]
- 9.Doroshow DB, Eder JP, LoRusso PM. BET inhibitors: a novel epigenetic approach. Ann Oncol 2017;28:1776–87. [DOI] [PubMed] [Google Scholar]
- 10.Gamsjaeger R, Webb SR, Lamonica JM, Billin A, Blobel GA, Mackay JP. Structural basis and specificity of acetylated transcription factor GATA1 recognition by BET family bromodomain protein BRD3. Mol Cell Biol 2011; 31:2632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Data on file. Incyte Corporation; Wilmington DE: 2018. [Google Scholar]
- 12.Stubbs M, Maduskuie T, Burn T, Diamond-Fosbenner S, Falahatpisheh N, Volgina A, et al. Preclinical characterization of the potent and selective BET inhibitor INCB057643 in models of hematologic malignancies. AACR Cancer Res 2017;77:Abstract 5071. [Google Scholar]
- 13.Vázquez R, Civenni G, Marchetti M, Zadic S, Liu P, Ruggeri B, et al. BET inhibitors INCB054329 and INCB057643 display significant activity in androgen-independent prostate cancer models. AACR Cancer Res 2017;77: Abstract 5080. [Google Scholar]
- 14.Bhattacharya S, Piya S, Borthakur G. Bromodomain inhibitors: what does the future hold? Clin Adv Hematol Oncol 2018;16:504–15. [PubMed] [Google Scholar]
- 15.Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, Leleu X, et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol 2016;3:e196–204. [DOI] [PubMed] [Google Scholar]
- 16.Berthon C, Raffoux E, Thomas X, Vey N, Gomez-Roca C, Yee K, et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol 2016;3:e186–95. [DOI] [PubMed] [Google Scholar]
- 17.Blum KA, Abramson J, Maris M, Flinn I, Goy A, Mertz J, et al. 41OA phase I study of CPI-0610, a bromodomain and extra terminal protein (BET) inhibitor in patients with relapsed or refractory lymphoma. Ann Oncol 2018;29:mdy048–mdy. [Google Scholar]
- 18.Patnaik A, Carvajal RD, Komatsubara KM, Britten CD, Wesolowski R, Michelson G, et al. Phase ib/2a study of PLX51107, a small molecule BET inhibitor, in subjects with advanced hematological malignancies and solid tumors. J Clin Oncol 2018;36:2550. [Google Scholar]
- 19.Dombret H, Preudhomme C, Berthon C, Raffoux E, Thomas X, Vey N, et al. A phase 1 study of the BET-bromodomain inhibitor OTX015 in patients with advanced acute leukemia. Blood 2014;124:117. [Google Scholar]
- 20.Dawson M, Stein EM, Huntly BJP, Karadimitris A, Kamdar M, Fernandez de Larrea C, et al. A Phase I study of GSK525762, a selective bromodomain (BRD) and extra terminal protein (BET) inhibitor: Results from Part 1 of Phase I/II open label single agent study in patients with acute myeloid leukemia (AML). Blood 2017;130:1377.28667012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.