Abstract
Vitamin D insufficiency during pregnancy is widespread. The effects of active vitamin D on the human placenta in vivo are unknown. We test the hypotheses that 25(OH)D sufficiency (arbitrarily defined as 25(OH)D ≥32 ng/mL) modulates placental structure and function in vivo in a population of women whose offspring are at risk for childhood asthma, and that placental pathology is more common in offspring that evolve asthma at age 3. Pregnant volunteers in the St. Louis, MO, cohort of the Vitamin D Antenatal Asthma Reduction Trial (VDAART, NIH grant #HL091528) participated in a nested case–control study and consented for the study of placentas after delivery. Maternal concentrations of 25(OH)D were measured at trial entry and in the third trimester. The histopathology of the placentas from women with sufficient 25(OH)D, versus insufficient, showed no clinically significant differences, but morphometry revealed villi of women with sufficient third-trimester 25(OH)D had a higher villous surface density. Notably, analyses of transcripts, extracted from formalin-fixed paraffin-embedded specimens, revealed higher expression of INTS9, vWF, MACC1, and ARMS2, and diminished expression of the CNTN5 genes in the insufficient group. A larger proportion of placentas showed chronic chorioamnionitis in offspring with versus without asthma at age 3. These findings suggest that maternal 25(OH)D insufficiency has a limited effect on human placental villous histopathology and morphometry, but attenuates a small number of placental gene expression profiles in this selected population. The association of placental chronic chorioamnionitis and offspring asthma is worthy of further study.
Keywords: placenta, vitamin D, morphometrics, histopathology, gene expression, asthma
Vitamin D and its metabolites are a family of steroid hormones, derived from the diet and by de novo synthesis from 7-hydroxy-cholesterol. The end products from this conversion are converted to 25-hydroxcycholecalciferol 25(OH)D in the liver, and then further hydroxylated to the most biologically active form, 1,25(OH)D (calcitriol), in the kidney and human placenta by the enzyme 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). The 1,25(OH)D binds the intracellular vitamin D receptor (VDR), eliciting both rapid, nongenomic responses and slower, genomic responses. Circulating concentrations of 25(OH)D reflect vitamin D status, and arbitrarily the normal range is between ~32 ng/mL and ~80 ng/mL, with values below ~32 ng/mL considered insufficient and values <20 ng/mL deficient (1). The endocrine activity of 1,25(OH)D generated by the kidney is responsible for the classic genomic actions of vitamin D in calcium metabolism and bone health. Nonclassical actions of vitamin D in the human placenta have recently been described, and these yield autocrine and paracrine responses that regulate trophoblast invasion and that modulate innate and adaptive immunity (1-3). These latter actions are dependent on tissue levels of vitamin D and derivatives of hormone.
The human placenta expresses all components for vitamin D signaling (4). Vitamin D intake is essential for maternal health and prevention of adverse outcomes (2), but vitamin D insufficiency during pregnancy is widespread, present in ~20% to 85% of women depending on the population studied (5,6). Placental transfer of 25(OH)D is the major source of vitamin D for the developing fetus, and important questions about the impact of maternal vitamin D status on fetal outcome persist (7). Vitamin D insufficiency predisposes to pre-eclampsia (PE), gestational diabetes, and infections in the mother and predisposes offspring to bone weakening, Type I diabetes and asthma (1).
The Vitamin D Antenatal Asthma Reduction Trial (VDAART) found that in pregnant women at risk of having an offspring with childhood asthma, supplementation with 4400 IU/days of vitamin D3 (cholecalciferol), compared with 400 IU/days, significantly increased 25(OH)D levels in women during pregnancy. Importantly, they also found fewer children developed asthma or recurrent wheezes in the 4400-IU group (24%) versus the 400-IU group (30%) by age 3 (8). There was no effect of maternal vitamin D supplementation on asthma and recurrent wheezes by age of 6 years (9).
Notably, a previous meta-analysis of 4 randomized controlled trials of vitamin D supplementation during pregnancy, conducted between 1937 and 2011, concluded that vitamin D supplementation reduced the risk of PE (10). The VDAART study showed women with 25(OH)D levels of ≥32 ng/mL at entry to the trial between 10 and 18 weeks’ gestation and, also, in the third trimester of pregnancy were associated with a lower risk of PE than women with insufficient 25(HO)D levels (11). Palacios and colleagues (12) reviewed randomized and quasi-randomized vitamin D trials and reported that supplementation with vitamin D alone during pregnancy likely reduces the risk of PE, gestational diabetes, and of having a baby with low birthweight (at ≤2500 g) compared with women who receive placebo or no intervention.
The root cause of most pre-eclamptic syndromes is the placenta (13). The effects of vitamin D on the above pregnancy complications could result from vitamin D’s modulation of placental structure or function. The effects of active vitamin D on the human placenta in vivo are unknown. We took advantage of the VDAART study design to harvest placentas from this cohort, who had well-characterized vitamin D status during pregnancy, in order to understand the effects of vitamin D on the placenta. We test the overall hypothesis that adequate 25(OH)D, versus insufficient or deficient, modulates placental structure and function in vivo. Kumar and colleagues suggested a potential link between preterm birth, placental chorioamnionitis, or both to the risk of offspring asthma (14). We thus also tested the hypothesis that placental pathology was more common in offspring that evolved asthma at age 3 years than in those who did not develop asthma.
Materials and Methods
Institutional Review Board consents and participants
The VDAART study was funded by NIH grant #HL091528 (registration identification number of NCT00902621 on ClinicalTrials.gov) as a 2-arm, randomized, double-blind, placebo-controlled trial to determine whether vitamin D supplementation daily at 400 IU versus 4400 IU to pregnant patients prevented asthma and allergy in offspring at age 3. The details of the trial population and outcome are published (8), but also summarized in the following: the offspring from the pregnancies studied had either a maternal or paternal history of asthma, eczema, or allergic rhinitis. Pregnant mothers admitted to the VDAART trial were between 18 and 39 years old, were enrolled between 10 and 18 weeks’ gestation, and participated until their child was age 3. The 3 clinical centers for the study were Boston Medical Center, Boston, MA, Washington University School of Medicine, St. Louis, MO, and Kaiser Permanente Southern California Region, San Diego, CA. Women were randomized to receive daily 4000 IU of vitamin plus a prenatal vitamin containing 400 IU of vitamin D (total 4400 IU) in the study group or randomized to receive a placebo plus a prenatal vitamin containing 400 IU of vitamin D in the control group. The coprimary outcomes included (1) parental report of physician-diagnosed asthma or recurrent wheezing through 3 years of age and (2) third-trimester maternal 25(OH)D levels.
We received approval from the VDAART oversight committee to harvest placentas in the St. Louis cohort and, thus, Institutional Review Board (IRB) consents for placental studies were included for patients at this site. The parent grant provided clinical data for the women, offspring, and maternal concentrations of 25(HO)D at trial entry and again in the third trimester. The trial began in October 2009 and completed follow-up in January 2015.
All participants were nonsmokers, spoke English or Spanish, and agreed to participate in the trial for 4 years, that is up to the third birthday of the offspring. The study protocol was approved by the IRB at each of the 3 participating institutions and the women in the St. Louis study center were offered the opportunity to participate in the placenta studies. Volunteers provided written informed consent for the use of delivered placentas for the nested case–control studies described here.
Measurement of vitamin D
In VDAART, samples for maternal vitamin D (25(OH)D) assessment were collected at 10-18 and 32-38 weeks of gestational age and levels were measured per VDAART protocol. 25(OH)D levels in all baseline and late pregnancy samples were obtained using the DiaSorin LIAISON® at Channing Division of Network Medicine, Brigham and Women’s Hospital, Boston, MA. The assay method for quantitative determination of 25(OH)D is a Food and Drug Administration-approved direct, competitive chemiluminescence immunoassay (15). The assay is cospecific for 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2, and total 25(OH)D values were used for the analysis. The inter- and intra-assay coefficients of variation (CVs) for this assay are 11.2% and 8.1% respectively. For quality control, samples of US National Institute of Standards and Technology level 1 standard reference material 972 Vitamin D in human serum were included in each run. All maternal sample 25(OH)D measurements were run using this method. Interassay CVs for 25(OH)D2 and 25(OH)D3 were all ≤6.8% over the range of concentrations in the samples processed (8). Total 25(OH)D values, the sum of 25(OH)D2 and 25(OH)D3, was used for the VDAART analyses, including this secondary analysis.
Placental sampling
Forty-seven placentas, as a convenience sample, were collected immediately after delivery; the cord and fetal membranes were removed, placental volume was determined by water displacement, and 5 villous specimens of about 0.5 g each were excised through systematic random sampling from cotyledons in the inner and intermediate perimeters, excluding the placental periphery. Villous tissues were immediately fixed for 24 hours in formalin, embedded in paraffin, and 4-µm sections were stained with hematoxylin and eosin.
Placental histopathology
Placentas were classified by trimmed weight percentiles for gestational age, with weights <10th percentile small for gestational age, weights between the 10th and 90th percentile average for gestational age, and weights >90th percentile large for gestational age were compared with nondiabetic control pregnancies (16). All specimens were evaluated by a board-certified pathologist with specialty training in placental histopathology (M.H.), and histopathology was categorized without knowledge of the vitamin D status of the pregnancy, using the nomenclature for placental histopathology defined elsewhere (17,18).
Stereological analysis of placental sections
Morphometric analysis was performed as previously described (19). Hematoxylin and eosin-stained sections of placental tissues were examined in the x–y direction using 1 corner of the coverslip as a random start point. Random digital color images were collected by an examiner blinded to vitamin D status, using a Nikon Eclipse E800 microscope equipped with 10×, 20×, and 40× objective and an Olympus DP71 camera. Digital images were overlain with a quadratic test grid using Image J software (http://rsbweb.nih.gov/ij/). The intersection of the horizontal and vertical lines of the test grids constituted a test point. An 80 μm × 80 μm grid was used for the analysis of villous types (Fig. 1A) and a 20 μm × 20 μm grid (Fig. 1B) was used for analysis of capillaries, due to the lesser frequency of encountering capillaries by test points or lines. Villi were classified by minimal diameter and the presence of tunica media. Stem villi were identified as minimal diameter >80 μm with tunica media; intermediate villi as minimal diameter >80 μm without tunica media, and terminal villi as villi minimal diameter <80 μm. Parameters for morphometry data included volume (cm3), surface (m2), and length (km) for each type of villous and the capillaries therein. The number of points falling on stem, intermediate, and terminal villi, fetal capillaries, intervillous space, and fibrin deposits were counted and expressed as a fraction of the total number of points falling on the sections. The number of intersections of test lines made with the villous surface and with the capillary luminal margins was also compiled, and the respective surface densities were estimated. Finally, the numbers of villous and capillary profiles were recorded, with 2 sides of the test grid acting as forbidden lines, and villous and capillary length densities calculated. Points, intersections, and profiles were converted to volume, surface area, and length using standard methods (20).
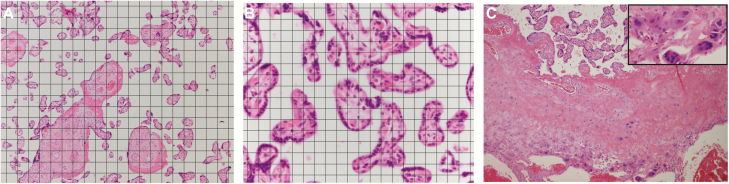
Figure 1.
Morphometrics and histopathological study of the effects of vitamin D on human placenta. Hematoxylin and eosin stained sections of placental chorionic villi. (A) A 80 μm × 80 μm grid was overlain on photomicrographs for analysis of villous types. (B) A 20 μm × 20 μm grid was overlain for analysis of capillaries, due to the lesser frequency of encountering capillaries by test points or lines with grid in A. (C) Villous structure at the basal plate shows persistent trophoblastic multinucleated giant cells, from a 39 weeks’ gestation placenta with third-trimester 25(OH)D of 63.7 ng/mL (>32 ng/mL, the sufficiency group). 100×. Inset: Trophoblastic multinucleated giant cells, 400×.
We chose our windows of sufficient and insufficient for the selected analyses based on the VDAART trial finding that women with >40 ng/mL 25(OH)D levels did not develop PE in a secondary analysis of the data (11). Since the definition of vitamin D insufficiency is arbitrary, we chose to study specimens with clearly sufficient and insufficient vitamin D levels.
RNA extraction
Ribonucleic acid (RNA) was extracted from formalin-fixed paraffin-embedded (FFPE) tissues using the Allprep kit (Qiagen, Germantown, MD) following kit instructions. The total RNA integrity was determined using Agilent Bioanalyzer or 4200 Tapestation.
RNA sequencing and gene analysis
RNA sequencing was performed in the Genome Technology Access Center, Washington University School of Medicine, St. Louis, MO. Library preparation was performed with 50 ng of total RNA. Double-stranded complementary deoxyribonucleic acid (cDNA) was prepared using the SeqPlex RNA Amplification Kit (Millipore Sigma, St. Louis, MO) per manufacturer’s protocol. cDNA was blunt-ended, had an A base added to the 3′ ends, and then had Illumina sequencing adapters ligated to the ends. Ligated fragments were then amplified for 12 cycles using primers incorporating unique index tags. Fragments were sequenced on a HiSeq-3000 using single reads extending 50 bases.
Analysis of RNA-sequencing data
Gene transcripts were quantified per samples using the DESeq2 package (version 1.26) in Bioconductor (version 3.1) with the analysis of raw RNA-seq read count data (21). Prefiltering was applied to keep only transcripts that had at least 10 reads total across samples. The plotPCA function was used to rule out any individual sample outlier. Differentially expressed genes adjusted for gestational age at delivery were identified based on the negative binomial (γ-Poisson) distribution across study conditions (25(OH)D <20 ng/mL vs 25(OH)D >40 ng/mL) as implemented in DESeq function. P values were adjusted for multiple testing using false-discovery rate correction. Transcripts with a base mean count >10 were included in the results. Analyses were conducted using R statistical software (version 3.6, R Foundation).
Statistical analysis
The Shapiro–Wilks test was used to check normality of the distribution of the placental morphometric variables. The mean and standard deviation of each placental morphometric variable were obtained and mean comparisons between groups were performed using a 2-sample t-test or Mann–Whitney U test, as appropriate. Chi-square or Fisher’s exact test was used as appropriate for categorical variables. Adjusted P values of less than 0.05 were determined to be significant. All the statistical tests were 2-sided and performed with SAS 9.4 (SAS Inc, Cary, NC).
Results
Demographics
Among the 47 singleton deliveries providing placentas as frozen tissue (Table 1), 40 women had baseline (between 10 and 18 weeks’ gestation) maternal serum 25(OH)D of <32 ng/mL (insufficient), and 7 had levels >32 ng/mL (sufficient). Moreover, 27 women had third-trimester maternal serum 25(OH)D <32 ng/mL and 20 women had levels >32 ng/mL. Six women had both baseline and the third-trimester serum 25(OH)D >32 ng/mL. No significant differences were found in the demographics between patients grouped by insufficient versus sufficient 25(OH)D levels at baseline (data not shown), in the third trimester (Table 1), or at both baseline and third trimester (data not shown). Among these 47 women, 5 had PE and 14 had a history of asthma. Most (43/47, 91.5%) deliveries occurred after 37 weeks of gestational age, with 4 deliveries during the 36th week.
Table 1.
Demographics of study groups defined by third-trimester maternal serum 25 hydroxycholecalciferol status at cutoff 32 ng/mL
| Variables | ≤32 ng/mL (total = 27) N (%) | >32 ng/mL (total = 20)N (%) | P value |
|---|---|---|---|
| Fetal sex | |||
| Female | 12 (44.4) | 9 (45) | NS |
| Male | 15 (55.6) | 11 (55) | NS |
| Mean gram birth weight (SD) | 3049 (546) | 3313 (594) | NS |
| Birth weight < 10th | 6 (22.2) | 3 (15) | NS |
| Birth weight > 90th | 1 (3.7) | 2 (10) | NS |
| Mean placental grams (SD) | 454 (74) | 490 (128) | NS |
| Placental weight < 10th | 3 (11.11) | 3 (15) | NS |
| Placental weight > 90th | 2 (7.4) | 2 (10) | NS |
| F/P ratio mean (SD) | 6.7 (1.4) | 7.1 (0.9) | NS |
| F/P ratio < 10th | 2 (7.4) | 2 (10) | NS |
| F/P ratio > 90th | 1 (3.7) | 0 (0) | NS |
| Birth < 37 weeks | 3 (11.1) | 1 (5) | NS |
| Mean delivery week (SD) | 38.3 (1.5) | 39.2 (1.5) | NS |
| Cesarean delivery Vaginal delivery | 16 (59.3) 11 (40.7) | 5 (25.0) 15 (75.0) | NS NS |
| Asthma diagnosis by age three | 6 (22.2) | 1 (5.0) | NS |
P > .05.
Abbreviations: NS, not significant, F/P ratio, fetoplacental weight ratio.
Histopathology
FFPE placental tissues were available from 27 deliveries. Table 2 shows the frequency of placental histopathology and compares the histopathology of placental specimens grouped by the third-trimester maternal 25(OH)D levels. For histopathology related to maternal vascular malperfusion, 11.1% placentas (3/27) harbored decidual vasculopathy, and 7.4% (2/27) had evidence of abruption. For features related to fetal vascular malperfusion, 7.4% (2/27) had avascular villi. The frequency of infectious/inflammatory pathology ranged from 18.5% (5/27) for chronic deciduitis to 7.4% (2/27) for acute chorioamnionitis.
Table 2.
Placental histopathology of study groups defined by third-trimester maternal serum 25 hydroxycholecalciferol status at cutoff of 32 ng/mL
| Pathology | ≤32 ng/mLn/Na (%) | >32 ng/mLn/N (%) | P value |
|---|---|---|---|
| Maternal vascular malperfusion | |||
| Decidual vasculopathy | 1/16 (6.25) | 2/11 (18.2) | NS |
| Accelerated villous maturation | 0/16 (0) | 0/11 (0) | NS |
| Basal plate trophoblastic multinucleated giant cells | 7/16 (43.8) | 9/11 (81.8) | .047 |
| Retroplacental hematoma (abruptio) | 0/14 (0) | 2/11 (18.2) | NS |
| Distal villous hypoplasia | 1/16 (6.25) | 1/11 (9.1) | NS |
| Intervillous thrombi | 0/16 (0) | 1/11 (9.1) | NS |
| Villous agglutination | 2/16 (12.5) | 1/11 (9.1) | NS |
| Fetal vascular malperfusion | |||
| Avascular villi | 2/16 (12.5) | 0/11 (0) | NS |
| Infectious or inflammatory processes | |||
| Acute chorioamnionitis | 2/15 (13.3) | 0/11 | NS |
| Sub-acute chorioamnionitis | 1/15 (6.25) | 0/11 | NS |
| Chronic chorioamnionitis | 2/16 (12.5) | 0/11 (0) | NS |
| Chronic deciduitis | 4/16 (25) | 1/11 (9.1) | NS |
| Villitis of unknown etiology | 2/16 (12.5) | 1/11 (9.1) | NS |
| Others | |||
| Delayed villous maturation | 3/16 (18.8) | 0/11 (0) | NS |
| Villous edema | 8/16 (50) | 4/11 (36.4) | NS |
aN, total number of cases evaluated.
A higher proportion of placentas with persistent trophoblastic multinucleated giant cells (P = .048; Fig. 1C) were observed in the sufficient group (9 of 11 patient specimens, 81.8%) compared with the insufficient group (7 of 16 patient specimens, 43.8%) by third-trimester maternal 25(OH)D levels. This finding was not seen in specimens classified by baseline, or by combined baseline and third-trimester maternal levels of 25(OH)D when compared (data not shown). There were no differences between these 2 groups related to histopathological features of maternal or fetal vascular malperfusion, or those for infectious or inflammatory processes by baseline, third-trimester, or both baseline and third-trimester levels of 25(OH)D.
Stereological analysis of placental sections by 25(OH)D
A planned, nested morphometric analysis was performed comparing placentas whose mothers had third-trimester maternal serum 25(OH)D levels at the opposite ends of the spectrum, from deficiency (<20 ng/mL) to marked sufficiency (>40 ng/mL). Morphometric analyses were conducted on 9 placentas from women with mean 25(OH)D levels deficient at 11.43 ng/mL (standard deviation [SD] ± 4.37 ng/mL) and 9 placentas from women with mean 25(OH)D levels clearly sufficient at 53.53 ng/mL (SD ± 12.56 ng/mL; Table 3). There was a significantly higher surface density of chorionic villi in the group with the highest 25(OH)D levels than in the group with the deficient levels (P = .043; Table 3). The other morphometric parameters were not different between the groups. Morphometric parameters of these 18 placentas were compared by baseline maternal serum 25(OH)D levels <32 (n = 15) versus ≥32 ng/mL (n = 3). There were no significant differences.
Table 3.
Nested case–control placental morphometry by third-trimester maternal serum 25 hydroxycholecalciferol levels.
| Variables | < 20 ng/mLN = 9 | >40 ng/mLN = 9 | P value |
|---|---|---|---|
| 25(OH)D 32-38 weeks’ gestation (ng/mL) | 11.43 (4.37) | 53.53 (12.56) | <.05 |
| Chorionic villi (surface density) | 0.056 (0.015) | 0.070 (0.0060) | .043 |
| Intermediate villi (volume density) | 0.14 (0.059) | 0.13 (0.059) | NS |
| Intervillous fibrinoid (volume density) | 0.045 (0.037) | 0.031 (0.014) | NS |
| Intervillous space (volume density) | 0.40 (0.059) | 0.38 (0.063) | NS |
| Stem villi (volume density) | 0.13 (0.034) | 0.12 (0.031) | NS |
| Terminal villi (volume density) | 0.28 (0.047) | 0.31 (0.037) | NS |
| Total villi (volume density) | 0.55 (0.066) | 0.58 (0.082) | NS |
| Villous fibrin (volume density) | 0.044 (0.037) | 0.045 (0.018) | NS |
| Villous trophoblast (volume density) | 0.070 (0.012) | 0.066 (0.0089) | NS |
| Villous capillaries (volume density) | 0.054 (0.028) | 0.052 (0.022) | NS |
| Villous capillaries (surface density) | 0.031 (0.013) | 0.035 (0.011) | NS |
| Villous stroma (volume density) | 0.37 (0.044) | 0.40 (0.089) | NS |
| Placental volume (mL) | 395 (53) | 501 (184) | NS |
| Placental weight (g) | 425 | 545 (145) | NS |
NS, not significant.
Gene expression and maternal 25(OH)D concentrations
Gene expression studies comparing sequenced RNA from 5 randomly selected placentas whose mothers had third-trimester maternal serum 25(OH)D <20 ng/mL, and 6 placentas with maternal 25(OH)D >40 ng/mL showed 5 genes differentially expressed in the villous sections (Table 4). The expression of 4 genes, including INTS9, vWF, MACC1, and ARMS2, was lower in the specimens with the higher 25(OH)D serum concentration (>40 ng/mL). Higher expression of the CNTN5 gene was observed in the >40 ng/mL 25(OH)D group than in the < 20 ng/mL 25(OH)D.
Table 4.
Nested case–control study of gene expression by RNA sequencing using formalin-fixed paraffin-embedded sections of tissues from third-trimester maternal serum 25 hydroxycholecalciferol levels <20 ng/mL versus >40 ng/mL.
| hgnc_symbol | ensembl | entrez | baseMean | log2FoldChange | lfcSE | stat | P value | padj |
|---|---|---|---|---|---|---|---|---|
| INTS9 | ENSG00000104299 | 55756 | 58.27034 | –1.72452 | 0.373072 | –4.62249 | 3.79 × 10–6 | 0.001154 |
| VWF | ENSG00000110799 | 7450 | 692.8416 | –1.16509 | 0.258307 | –4.51049 | 6.47 × 10–6 | 0.001903 |
| CNTN5 | ENSG00000149972 | 53942 | 29.93927 | 2.951361 | 0.726966 | 4.059833 | 4.91 × 10–5 | 0.013988 |
| MACC1 | ENSG00000183742 | 346389 | 33.90672 | –2.38386 | 0.608752 | –3.91598 | 9.00 × 10–5 | 0.025378 |
| ARMS2 | ENSG00000254636 | 387715 | 49.94864 | –3.70011 | 0.818778 | -4.51906 | 6.21 × 10–6 | 0.001848 |
Gene expression studies comparing sequenced RNA from randomly selected 5 placentas whose mothers had third-trimester maternal serum 25(OH)D < 20 ng/mL group and 6 placentas with maternal 25(OH)D > 40 ng/mL showed 5 genes differentially expressed in the villous sections.
Placental histopathology, morphometrics, gene expression, and offspring asthma
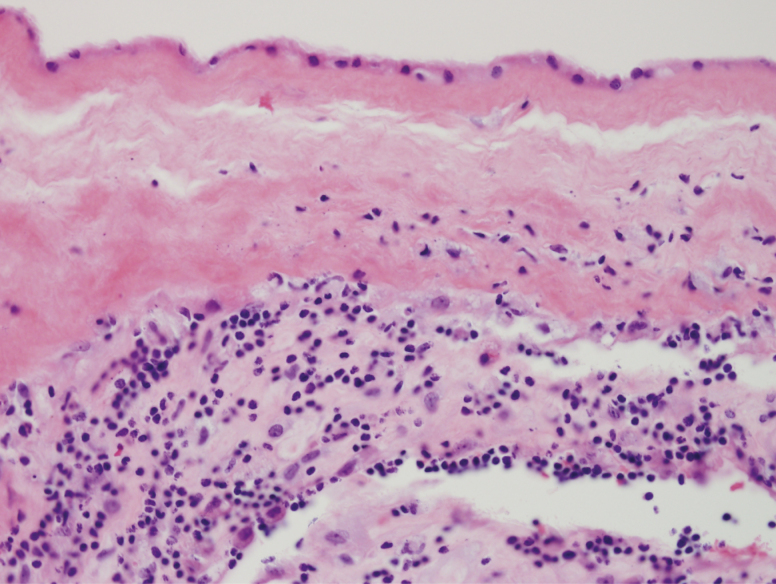
The VDAART cohort of women from whom the placentas were obtained was deemed at higher than baseline risk for offspring development of asthma by age 3, based on risk factors for asthma in either the mother or father of each progeny. We thus were able to conduct a nested, case–control study of a limited but random sampling of placental specimens in the patients whose offspring had asthma at 3 years of age compared with those that did not, independent of vitamin D levels. We tested the hypothesis that placental pathology was more common in offspring that evolved asthma at age 3, compared with those who did not develop asthma. None of the demographic parameters (as listed in Table 1 and data not shown) were different between mothers of offspring with (n = 6) versus without (n = 21) asthma at age 3 years. There was a significant difference (P < .043, Fisher’s exact test) in the proportion of placentas with chronic chorioamnionitis (Fig. 2) in the placentas of the offspring with asthma group (2/6, 33.3%) compared with the placentas of the offspring without asthma (0/21, 0.0%). There were no other differences between these 2 groups for other histopathology, including acute chorioamnionitis. There were no significant differences in placental morphometrics (data not shown).
Figure 2.
Chronic chorioamnionitis. Lymphocytes are present at the chorion and decidua of the placental membranes. Hematoxylin and eosin, 400×.
The 11 cases subjected to RNA sequencing were found to have a relatively equal number of cases between offspring with asthma versus without. Similar gene expression was observed when comparing 5 placentas whose offspring had an asthma diagnosis by age 3 and 6 placentas with offspring without asthma. The asthma group, versus no asthma group, placentas had lower expression of both HLA-DRB1 and HLA-DRB5.
Discussion
The data show that the demographics of the pregnancies providing placentas for this nested case–control study were similar among the patients with sufficient maternal 25(OH)D compared with those with insufficient levels. This finding helps validate that our “convenience sample” harvest of placentas for the study was unlikely to be biased by the sufficiency of vitamin D for the population studied in the cohort at Washington University School of Medicine in St. Louis, MO. With 1 exception, the histopathology of the placentas from women with sufficient 25(OH)D showed no significant histopathological differences compared with the placentas with 25(OH)D from the insufficient group. The exception to this statement was that the group of sufficient 25(OH)D in the third trimester showed a higher proportion of placentas with excess trophoblastic multinucleated giant cells at the basal plate. Moreover, the offspring identified with asthma at age 3 had a disproportionate number of placentas with histological evidence of chronic chorioamnionitis compared with a control group without offspring asthma. Morphometric analysis revealed women with clearly sufficient third-trimester 25(OH)D had placentas with a higher villous surface density. Notably, we identified 5 genes whose expression was associated with the presence compared with the absence of 25(OH)D sufficiency, 4 downregulated and only 1 expressed at higher levels. Collectively, the data show that sufficiency of maternal 25(OH)D level has limited effects on the placental structure as assessed by histopathology and morphometry. Whether or not CNTN5 (contactin), the only gene upregulated, or the 4 genes downregulated in vitamin D sufficiency, vWF, MACC1, ARMS2, and INTS9, modulate the function of placental villi remains to be determined. Notably, the offspring with and without asthma had differential placental gene expression for selected HLA isotypes, suggesting susceptibility to chronic chorioamnionitis and childhood asthma may be inherent to certain immune phenotypes.
Vitamin D and placental histopathology
Maternal serum vitamin D levels have rarely been related to human placental pathology (22), as previous studies have focused on primary human trophoblast cultures, trophoblast cell lines, or animal placenta studies. The exception is the Collaborative Perinatal Project, conducted between 1959 and 1966, which assessed 25(OH)D in 2048 patients with singleton pregnancies at ≤26 weeks. The pathologists correlated vitamin D levels, measured by older technology, with any of 12 pathologies, which included maternal or fetal vascular malperfusion by current nomenclature (17,18). The authors found that a maternal 25(OH)D concentration ≥80 nmol/L (32 ng/mL) compared with <50 nmol/L (20 ng/mL) was associated with a 50% lower risk of placental histopathology in male offspring. An influence of offspring gender on placental pathology, independent of vitamin D, has already been recognized (23). We saw no differences in pathology related to the sex of the offspring and the windows of vitamin D sufficiency used here.
We identified that sufficient third-trimester 25(OH)D correlated with higher numbers of placentas with multinucleated trophoblastic giant cells in the basal plate, also known as placental site giant cells. During placental development, the extravillous trophoblasts normally exit the trophoblast cell columns to form isolated invasive cells that infiltrate the myometrium. Once invasive extravillous trophoblasts penetrate to the inner third of the myometrium, they undergo terminal differentiation to form placental site giant cells. The presence of significant numbers of multinucleated trophoblastic (or placental site) giant cells more superficially in the decidua indicates truncation of the normal invasive process and is a sign of superficial implantation. Giant cells in this location are also associated with localized conditions of hypoxia in the human placenta. This trophoblast phenotype normally peaks in the early third trimester and declines as term approaches (24,25). We cannot predict how this histopathology affects placental function, and, thus, we speculate that this may be an epiphenomenon, statistically identified yet clinically insignificant.
25(OH)D and chorionic villous morphometry
We observed a significantly increased surface density of chorionic villi in the pregnancies with sufficient 25(OH)D compared with those with insufficient vitamin D levels. This finding indicates that there is an enhanced surface area of villi when 25(OH)D is sufficient, which functionally yields a higher transport capacity for nutrients to the fetus. Remarkably, there were no other morphometric findings that correlated with vitamin D sufficiency. The absence of changes in terminal villi, fibrin deposits, or villous capillaries, among other stereology components, argues that vitamin D influences the histology of the placenta and plays a small role in the development of the chorioallantoic placental structure. This stated, the increased surface of villi would present more area for maternal–fetal transport of nutrients and wastes (26,27).
25(OH)D modulates few placental genes
The sufficiency of 25(OH)D levels correlated with increased gene expression of CNTN5 and reduced expression of INTS9, vWF, MACC1, and ARMS2. CNTN5 (contactin 5) encodes a protein which is a member of the immunoglobulin superfamily (28). We do not know the role of CNTN5 in placenta, although the protein mediates cell surface interactions during nervous system development (27). Von Willebrand factor (VWF) and Factor VIII (FVIII) increase significantly during pregnancy (29), and levels of both are further increased in women with hypertensive disorders of pregnancy (30-33). These studies suggested that elevation in plasma VWF and FVIII might play a role in the pathogenesis of PE, while reduced gene expression in women with higher 25(OH)D is consistent with the fact that VDAART women with 25(OH)D levels >40 ng/mL showed no evidence of PE (11).
MACC1 (metastasis-associated in colon cancer 1) is a key regulator of the hepatocyte growth factor receptor pathway, which is involved in cellular growth, epithelial–mesenchymal transition, angiogenesis, cell motility, invasiveness, and metastasis (34,35). Downregulation of this gene would enhance hepatocyte growth factor activity in placentas, the effects of which remain to be determined. Age-Related Maculopathy Susceptibility 2 (ARMS2) gene is one of the 53 genes expressed differentially between the placental transcriptomes of pre-eclamptic versus nonpre-eclamptic control placentas (36). The role of ARMS2 also is unknown in normal and hypertensive pregnancy.
VDAART offspring asthma, placental histopathology, and gene expression
We tested the secondary hypothesis that placental pathology associated with an increased risk for asthma at age 3 in offspring. Our analysis revealed a higher incidence of chronic chorioamnionitis in the placentas of pregnancies with offspring asthma. Chronic chorioamnionitis results from lymphocytic infiltration that evolves in the chorioamnion membranes and suggests an autoimmune phenomenon; the histopathology differs from the acute inflammatory reaction that commonly associates with an infectious etiology (37). Whether or not offspring at increased risk for childhood asthma can be predicted by the presence or absence of chronic chorioamnionitis in their placental histopathology needs further exploration. Our gene expression comparisons in the offspring with and without asthma revealed reduced expression of HLA-DRB1 and HLA-DRB5 in the placentas associated with offspring asthma. Both genes have been recognized to be associated with asthma and our data indicate the placenta also reflects this differential gene expression (38,39).
Asthma is the most common chronic disease of childhood. Besides vitamin D deficiency as a risk factor investigated by the VDARRT study, parental asthma, prenatal environmental tobacco smoke, and prematurity are risk factors for childhood asthma (40). With infants enrolled in the Boston Birth Cohort, Kumar and colleagues found that the highest risk of wheezing (odds ratio [OR] 4.0; 95% confidence interval [CI] 2.0-8.0) and physician-diagnosed asthma (OR 4.4; 95% CI 2.2-8.7) was present in the very preterm children with chorioamnionitis. They did not find a significant association between offspring asthma in term infants with chorioamnionitis (P = .67) (14). Compared with their findings, most study subjects in the current study (43/47, 91.5%) were term births, and the current study specifically identified chronic chorioamnionitis to be associated with offspring asthma.
At present, there are growing concerns and attention to the prenatal modulation of cellular and molecular effectors that modulate airway function (41). We postulate that the placenta, and the maternal–fetal interface within the placenta, could play such a role, suggest that this possibility is worthy of further study.
Strengths and weaknesses of our study
The strengths of our study include the fact that the vitamin D status was known based on 25(OH)D levels for the patients whose placentas were studied after delivery, as opposed to comparing pregnancies based on presumed ingestion of pills containing a lower or higher amount of vitamin D. We, thus, were able to investigate the relationship of placental histopathology, morphometry, and gene expression with the 25(OH)D sufficiency status in the study participants. Our follow-up of the newborns from our cohort allowed us to identify a higher incidence of chronic chorioamnionitis as a potential marker for asthma in the offspring of an at-risk population. Another strength of our study is the examination of multiple dependent variables, including histopathology, morphometry, and gene expression, to identify potential targets of vitamin D action in pregnancy. Despite the multiple analyses, the data show vitamin D sufficiency has a remarkably limited effect on the placenta of the population studied.
The weaknesses of our study are several. In general, they included the limited relevance of the observed findings, the small sample size, and the observational study design. Offspring in the VDAART study were born to women with a history of asthma or atopy. This raises the question of whether the placental phenotypes of an unselected population of pregnant women will be similar to those in this study. We were unable to harvest all study placentas or to examine all retrieved placentas for all analyses. Indeed, the ones collected were from daytime deliveries, and one could argue that this biases the results. This stated, we are unaware of circadian rhythm for vitamin D effects. We chose 1 of our windows of vitamin D sufficiency and insufficiency for the selected analyses based on the VDAART trial finding that women with >40 ng/mL 25(OH)D levels did not develop PE in a secondary analysis of the data (11). We acknowledge that the definition of vitamin D insufficiency is arbitrary and contested. Sufficiency is defined as >32 ng/mL and this would be an alternative to study, yet we chose to study specimens with clearly sufficient and insufficient vitamin D levels. Although most specimens were used for histopathology and stereology, we were limited in the specimens that provided adequate tissue for the gene expression analyses. We did not analyze placental protein secretion or gestational diabetes, both of which are reported to be less likely with vitamin D sufficiency. Vitamin D insufficiency associates with high levels of diabetogenic hormones secreted by the placenta, including tumor necrosis factor-α, human growth hormone variant, and human placental lactogen. Notably, the material for gene expression studies necessarily was the FFPE sections and, although adequate, this source is not as optimal as fresh tissue analyses might be. With the above caveats, we believe the current study has yielded important information about how maternal vitamin D influences human placental structure and function.
In summary, VDAART provided an opportunity to study the in vivo effect of maternal 25(OH)D on placental structure and function. Maternal 25(OH)D insufficiency has a limited effect on human placental histopathology or morphometry, but vitamin D modulates the expression of a limited cassette of genes in this selected population. The association of placental chronic chorioamnionitis and offspring asthma is worthy of further study.
Acknowledgment
We acknowledge the contribution of Denae Larson, RN, BSN, lead research nurse coordinator for the VDAART study, Baosheng Chen, PhD, and Mark Longtine, PhD, for their assistance in harvesting tissues from delivered placentas, and Marina Platik, AMP core lab for her work on histopathological processing of placental specimens. We thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with the genomic analysis. The Center is partially supported by NCI Cancer Center Support Grant #P30 CA91842 to the Siteman Cancer Center and by ICTS/CTSA Grant# UL1TR002345 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. This publication is solely the responsibility of the authors and does not necessarily represent the official view of NCRR or NIH.
Financial Support: The Vitamin D Antenatal Asthma Reduction Trial (VDAART, NIH grant #HL091528) to STW and AAL; L30HL129467-01, 2L30HL129467 and 1K01HL146977-01A1 from NIH NHLBI to H.M. The Foundation for Barnes-Jewish Hospital to D.M.N. Faculty development fund: Department of Pathology & Immunology, Washington University, to M.H.
Clinical Trial Information: This study is an ancillary analysis from the VDAART with the registration identification number of NCT00902621 on ClinicalTrials.gov.
Glossary
Abbreviations
- 25 (OH)D
25-hydroxcycholecalciferol; calcitriol, 1,25(OH)D
- cDNA
complementary deoxyribonucleic acid
- FFPE
formalin-fixed paraffin-embedded
- PE
pre-eclampsia
- RNA
ribonucleic acid
- VDR
vitamin D receptor
Additional Information
Disclosure Summary: All authors have no financial conflict of interest to disclose.
Data Availability. The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31(12):1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colvin B, Longtine MS, Nelson DM. Ch 13. Vitamin D effects on pregnancy and trophoblast function limited data yet endless possibilities. In: Duttaroy AK, Basak S, eds. Human Placental Trophoblasts: Impact of Maternal Nutrition. 1st ed. Boca Raton, FL: CRC Press; 2015. [Google Scholar]
- 3. Ganguly A, Tamblyn JA, Finn-Sell S, et al. Vitamin D, the placenta and early pregnancy: effects on trophoblast function. J Endocrinol. 2018;236(2):R93-R103. [DOI] [PubMed] [Google Scholar]
- 4. Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab. 2012;303(7): E928-E935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(5):429.e1-429.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr 2007;137(2):447-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papadopoulou A, Bountouvi E, Papaevaggelou V, Priftis KN. Maternal vitamin D status and development of asthma and allergy in early childhood. Mini Rev Med Chem. 2015;15(11):900-912. [DOI] [PubMed] [Google Scholar]
- 8. Litonjua AA, Carey VJ, Laranjo N, et al. Effect of prenatal supplementation with vitamin d on asthma or recurrent wheezing in offspring by age 3 years: the VDAART Randomized Clinical Trial. JAMA. 2016;315(4):362-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382(6):525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hyppönen E, Cavadino A, Williams D, et al. Vitamin D and pre-eclampsia: original data, systematic review and meta-analysis. Ann Nutr Metab. 2013;63(4):331-340. [DOI] [PubMed] [Google Scholar]
- 11. Hooman M, Joseph L, Scott TW, et al. Early pregnancy vitamin D status and risk of preeclampsia. J Clin Invest. 2016;126(12):4702- 4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palacios C, Kostiuk LK, Peña‐Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev 2019;7:CD008873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The American College of Obstetricians and Gynecologists. Practice Bulletins 202 Gestational Hypertension and Preeclampsia, December 20, 2018. Obstet Gynecol 2019;133(1):e1-e25 [DOI] [PubMed] [Google Scholar]
- 14. Kumar R, Yu Y, Story RE, et al. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J Allergy Clin Immunol. 2008;121(4):878-84.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ersfeld DL, Rao DS, Body JJ, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37(10):867-874. [DOI] [PubMed] [Google Scholar]
- 16. Pinar H, Sung CJ, Oyer CE, Singer DB. Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med. 1996;16(6):901-907. [DOI] [PubMed] [Google Scholar]
- 17. Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop group consensus statement. Arch Pathol Lab Med. 2016;140(7):698-713. [DOI] [PubMed] [Google Scholar]
- 18. Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213(4 Suppl):S21-S28. [DOI] [PubMed] [Google Scholar]
- 19. Odibo AO, Zhong Y, Longtine M, et al. First-trimester serum analytes, biophysical tests and the association with pathological morphometry in the placenta of pregnancies with preeclampsia and fetal growth restriction. Placenta. 2011;32(4):333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mühlfeld C, Nyengaard JR, Mayhew TM. A review of state-of-the-art stereology for better quantitative 3D morphology in cardiac research. Cardiovasc Pathol. 2010;19(2):65-82. [DOI] [PubMed] [Google Scholar]
- 21. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gernand AD, Bodnar LM, Klebanoff MA, Parks WT, Simhan HN. Maternal serum 25-hydroxyvitamin D and placental vascular pathology in a multicenter US cohort. Am J Clin Nutr. 2013;98(2):383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naeye RL, Demers LM. Differing effects of fetal sex on pregnancy and its outcome. Am J Med Genet Suppl. 1987;3:67-74. [DOI] [PubMed] [Google Scholar]
- 24. Stanek J, Biesiada J. Sensitivity and specificity of finding of multinucleate trophoblastic giant cells in decidua in placentas from high-risk pregnancies. Hum Pathol. 2012;43(2):261-268. [DOI] [PubMed] [Google Scholar]
- 25. DaSilva-Arnold S, James JL, Al-Khan A, Zamudio S, Illsley NP. Differentiation of first trimester cytotrophoblast to extravillous trophoblast involves an epithelial-mesenchymal transition. Placenta. 2015;36(12):1412-1418. [DOI] [PubMed] [Google Scholar]
- 26. Teasdale F, Jean-Jacques G. Morphometry of the microvillous membrane of the human placenta in maternal diabetes mellitus. Placenta. 1986;7(1):81-88. [DOI] [PubMed] [Google Scholar]
- 27. Teasdale F, Jean-Jacques G. Intrauterine growth retardation: morphometry of the microvillous membrane of the human placenta. Placenta. 1988;9(1):47-55. [DOI] [PubMed] [Google Scholar]
- 28. CNTN5 contactin 5 [Homo sapiens (human)] https://www.ncbi.nlm.nih.gov/gene/53942. Accessed September 21, 2019.
- 29. Castaman G. Changes of von Willebrand factor during pregnancy in women with and without von Willebrand disease. Mediterr J Hematol Infect Dis. 2013;5(1):e2013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szpera-Goździewicz A, Majcherek M, Boruczkowski M, et al. Circulating endothelial cells, circulating endothelial progenitor cells, and von Willebrand factor in pregnancies complicated by hypertensive disorders [Published online ahead of print March 1, 2017]. Am J Reprod Immunol. 2017;77(3). doi:10.1111/aji.12625 [DOI] [PubMed] [Google Scholar]
- 31. Güngör ZB, Ekmekçi H, Tüten A, et al. Is there any relationship between adipocytokines and angiogenesis factors to address endothelial dysfunction and platelet aggregation in untreated patients with preeclampsia? Arch Gynecol Obstet. 2017;296(3):495-502. [DOI] [PubMed] [Google Scholar]
- 32. Aref S, Goda H. Increased VWF antigen levels and decreased ADAMTS13 activity in preeclampsia. Hematology. 2013;18(4):237-41. [DOI] [PubMed] [Google Scholar]
- 33. Hoppe KK, Drury-Stewart DN, Lannert KW, Gammill HS, Chung DW, Johnsen JM. Pregnancies with severe preeclampsia exhibit perturbed von Willebrand factor parameters. Reprod Sci. 2014;21(3):195A-196A. [Google Scholar]
- 34. Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15(1):59-67. [DOI] [PubMed] [Google Scholar]
- 35. Pachmayr E, Treese C, Stein U. Underlying mechanisms for distant metastasis – molecular biology. Visc Med. 2017;33(1):11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaartokallio T, Cervera A, Kyllönen A, Laivuori K, Kere J, Laivuori H; FINNPEC Core Investigator Group Gene expression profiling of pre-eclamptic placentae by RNA sequencing [Published online ahead of print September 21, 2015]. Sci Rep. 2015;5:14107. doi:10.1038/srep14107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gersell DJ, Phillips NJ, Beckerman K. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol. 1991;10(3):217-229. [PubMed] [Google Scholar]
- 38. Yao Y, Zhu L, Li J, Jin Y, He L. Association of HLA-DRB1 Gene polymorphism with risk of asthma: a meta-analysis [Published online ahead of print August 9, 2016]. Med Sci Monit Basic Res. 2016;22:80-86. doi: 10.12659/MSMBR.900193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242(1):10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, Celedón JC. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4(6):1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Piedimonte G, Harford TJ. Effects of maternal-fetal transmission of viruses and other environmental agents on lung development. Pediatr Res. 2020;87(2):420-426. [DOI] [PMC free article] [PubMed] [Google Scholar]