Abstract
Pelvic motion acting as a hinge between the spine and hips is essential to maintain proper balance during bipedalism. Pelvic rotation is recruited as a compensation mechanism when spinal malalignment occurs.
This pelvic rotation can affect functional acetabular orientation, and consequently functional cup positioning if a total hip arthroplasty (THA) is needed. Pelvic retroversion, frequently associated with degenerative spinal changes, implies an increase of acetabular version.
Patients with flexible lumbar spines (spine users) protect the hip joint. Patients with stiff, degenerated or fused lumbar spines (hip users) demand higher hip mobility, placing the THA at risk.
Pelvises in retroversion place the THA at risk for anterior dislocation when standing. In contrast, pelvises in anteversion or with low pelvic incidence (PI) can place THA at risk for posterior dislocation when sitting.
Try to set the cup in an anatomic position. However, bear in mind that low PI pelvises may need more acetabular ante-inclination, and high PI pelvises more acetabular retroversion.
If surgery is needed, start first by addressing the hip, except in patients with compensation (high pelvic retroversion), who may need spine surgery first to place the pelvis, and consequently the acetabulum, in a proper position.
Cite this article: EFORT Open Rev 2020;5:522-533. DOI: 10.1302/2058-5241.5.200032
Keywords: acetabular orientation, compensation mechanisms, pelvic incidence, pelvic retroversion, risk for dislocation, spine sagittal profile, total hip arthroplasty
Introduction
Humans evolved from primates to become bipeds, leaving their upper limbs free for handling. The morphology of the pelvis had to change to become thinner, wider and more horizontal, to be the base upon which the spine stands erect. The pelvic ring also acts as the hinge between the spine and the lower limbs, coupling lumbar lordosis (LL) with hip extension in the standing position with a minimal energy expenditure.1 Recent investigations have renewed interest in the pelvis as the key to study spine alignment and hip biomechanics. Surgical procedures for degenerative lumbar spine and total hip arthroplasty (THA) projections represent a significant burden, not only in terms of clinical implications but also for health resources.2 Understanding the interactions between the hip and spine can improve clinical assessment, surgical planning, and postoperative outcomes of both disciplines.
In this article, we try to explain and clarify the role of pelvic morphology and pelvic motion as the key bounding the spine and the hips.
Spine–pelvis–hip
Balance and alignment
Balance is a dynamic concept that reflects how the body can adapt its centre of mass and gravity while in motion. Agonist and antagonist forces act to maintain our body within the conus of energy economics.3 In a well-balanced individual, this line of gravity is projected onto the ground and delineated by the feet. If, for whatever reason, we fall out of the conus zone, the muscles will vigorously act and counteract in response. If this imbalance continues over time, the muscles of the spine and pelvis will eventually require more energy, leading to fatigue, discomfort and pain.4 Then, spinal surgery may be necessary to restore a more physiological sagittal profile and muscle balance, and improve the patient’s quality of life.5,6
Alignment is a static concept that refers to the positioning of the skeleton and the different skeletal elements when measured in a fixed image, e.g. as in a conventional radiograph. In the case of the spine, alignment evaluates the correct (malalignment being the incorrect) positioning of the spinal curves when maintaining the head over the pelvis.4,7 The classic parameter to measure alignment has been the sagittal vertical axis (SVA).8 The SVA is defined as the length of a horizontal line connecting the posterior superior sacral endplate to a vertical plumbline dropped from the centroid of C7. However, further studies have analysed other parameters added to the SVA that also include the pelvis in this evaluation to measure its contribution to compensation (spino-sacral angle [SSA],9 T1 pelvic angle [TPA],10 global tilt [GT]).11
Malalignment and compensation mechanisms
Spine malalignment is usually derived from spine degeneration.12 Secondary causes can also be fractures, tumours, or spine deformities. Ageing and osteoporosis lead to disc dehydration with loss of LL, and anterior spinal column collapse (due to vertebral fragility), which increases thoracic kyphosis (TK).9 This shifts the gravity line forward, pushing the body anteriorly,7,13 and a variety of compensatory mechanisms react gradually to maintain proper balance (Fig. 1).14 Compensation commonly starts with cranial adjacent segment retrolisthesis and thoracic hypokyphosis. If this is not effective, pelvic retroversion and subsequently hip extension is recruited. This is usually sufficient to compensate for disc degeneration. However, if these mechanisms become exhausted, then knee and ankle flexion are solicited; and finally, posterior trunk shifting can occur.15 To maintain the horizontal gaze a reactive cervical hyperlordosis also appears. All these compensation mechanisms try to shift our trunk posteriorly to keep our head on top of our pelvis and preserve balance. Throughout this whole process, pelvic motion (based on rotation also called version) is essential, demonstrating the crucial role of the pelvis in bipedalism and sagittal stability, and the importance of the pelvis acting as a hinge that connects the spine and the lower limbs.
Fig. 1.
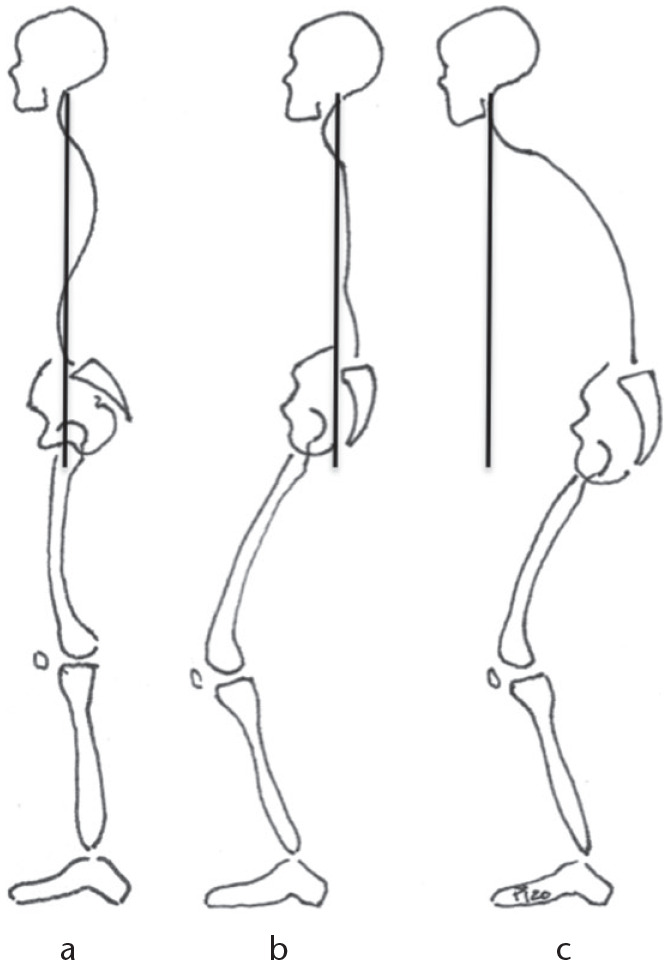
(A) Alignment is the correct positioning of the spinal curves when maintaining the head over the pelvis. (B) Malaligned but balanced due to compensation: cervical hyperlordosis, thoracic hypokyphosis, pelvic retroversion, hip extension, knee/ankle flexion, and posterior trunk shift. (C) Malaligned and unbalanced: compensation mechanisms are exhausted; malalignment appears shifting the gravity line forward, and pushing the body anteriorly.
Spino-pelvic interactions
Pelvic parameters
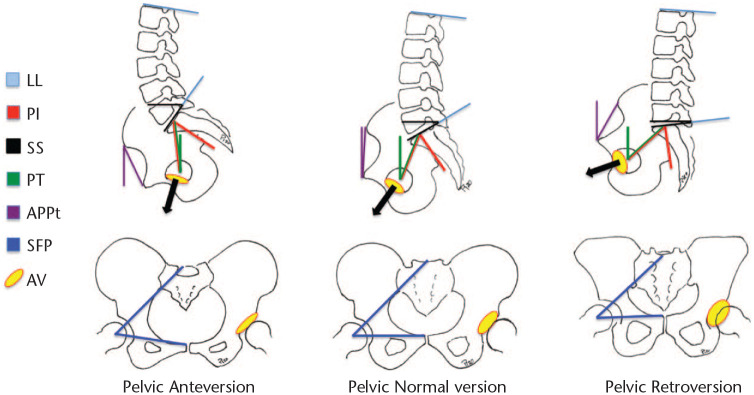
Pelvic involvement in these adjustments, both balance and alignment, is crucial. Pelvic morphology is assessed by the pelvic incidence (PI) angle, a concept that originated from Duval-Beaupère’s studies,16 which can be measured in a sagittal standing radiograph. PI is defined as the angle created by the intersection of the line drawn from the centre of the femoral heads to the middle of the sacral plate and the line running perpendicular to the middle of the sacral plate (Fig. 2, red lines). This angle slowly increases during growth until skeletal maturity is reached, remaining stable thereafter.17 Nevertheless, some changes can be found due to sacroiliac joint movement (nutation and contra-nutation) caused by ageing or surgical lumbar fusion, usually not exceeding 5°.18,19 PI is closely considered a fixed parameter, characteristic of each patient’s individuality, and clinically determines spino-pelvic interactions.20 Furthermore, LL angle (Fig. 2, light blue), in a healthy situation, is closely related to PI. Several formulae have been described in the literature to exactly calculate LL based on PI.21–24 However, it has been a simple equation that has stayed as a proxy to calculate LL (LL = PI ± 9°).13,15 Preoperative spine surgical planning should address this quantitative association to perform fusion in a correct position, matching PI and LL in every patient. However, to plan the ideal sagittal plane correction, several parameters need to be adjusted to the specific PI, the most important being: proper apex positioning, lumbar distribution (percentage of lordosis in the upper and lower lumbar arch), ideal pelvic version, and ideal global alignment. The Global Alignment and Proportion (GAP) score takes care of all these parameters, and is a useful tool for planning.23
Fig. 2.
Pelvic parameters. Pelvic anteversion decreases acetabulum version; pelvic retroversion increases acetabulum version; both can explain changes between standing and sitting.
Note. LL, lumbar lordosis; PI, pelvic incidence; SS, sacral slope; PT, pelvic tilt; APPt, anterior pelvic plane tilt; SFP, sacro-femoral pubic angle; AV, acetabulum version.
PI is also the sum of two other angles (PI = SS + PT): sacral inclination (sacral slope [SS]: the orientation of the sacral plate in relation to a horizontal line, depicted in Fig. 2, black lines); and pelvic version (pelvic tilt [PT]: the angle between the vertical line and the line drawn from the centre of the femoral heads to the centre of the upper sacral endplate, Fig. 2, green lines), which reflects the rotation of the pelvis around the femoral heads. These two parameters (SS and PT) are intrinsically related, so an increase in one of them will cause a decrease in the other.16 If sacral plateau inclination decreases pelvic retroversion (PT) increases, and the other way around (Fig. 2). Both SS and LL are highly correlated,16 so when lumbar degeneration begins, and LL decreases, the sacrum aquires a more horizontal position, SS decreases, thus PT increases.25 These physiological movements also occur during daily activities in a non-pathologic clinical situation, which is changing from a standing (increased lordosis, high SS, low PT) to a sitting position (decreased LL, low SS, high PT).26
Hip joint
Important changes are found in the hip joint as a consequence of these phenomena. First, pelvic sagittal rotation directly affects functional acetabular orientation. Pelvic retroversion (posterior pelvic tilt – increased PT) would increase the functional anteversion and the inclination of the acetabulum; whereas, pelvic anteversion (anterior pelvic tilt – decreased PT) would decrease functional acetabular anteversion and inclination (Fig. 2).27 This simple concept can be misleading due to nomenclature: retroversion, referring to the pelvis, is for spine surgeons what anteversion, referring acetabulum, is for hip surgeons. Lembeck et al reported that 1° of pelvic retroversion increase was associated with 0.7° of acetabular anteversion and 0.3° of acetabular inclination increase.28
Although less frequently, PT can also be measured with the anterior pelvic plane tilt (APPt) in a pelvic sagittal view.29 APPt is defined in a lateral view as the angle between the vertical and a line connecting the midpoint of the two superior iliac spines with the pubic symphysis (Fig. 2, purple lines).30 Less commonly, PT can also be evaluated by measuring the sacro-femoral-pubic angle (SFP).31 This is defined in a pelvis AP view as the midpoint of S1 endplate to the centroid of acetabuli to the superior border of the pubic symphysis (Fig. 2, dark blue lines).32
The sagittal rotation of the pelvis and, consequently, the changes in functional acetabular orientation between sitting and standing, have been well studied. When standing, the tilt of the pelvis is dictated by PI and the flexible lumbar spine adapts, accommodating the needed lordosis to SS, as the lower limbs remain extended. When sitting the pelvis tilts posteriorly (PT increase), while LL and SS decrease. It has been reported that when sitting, PT increases a mean of 20°, and a hip flexion of 55° to 70° is recruited; however, this amount of variation depends on pelvic morphology (PI). As pelvic retroversion increases during sitting, acetabular inclination and anteversion orientation increase (acetabulum opening) to allow motion of the femoral head and neck during hip flexion.33
The importance of lumbar lordosis
LL is identified as starting from the S1 plateau and ending cranially at the inflection point (which is the turning point where the orientation of the curves change from lordosis to kyphosis) regardless of its location (Fig. 3).20 Overall standing LL angle is determined by PI,34 and by SS as mentioned above.1
Fig. 3.
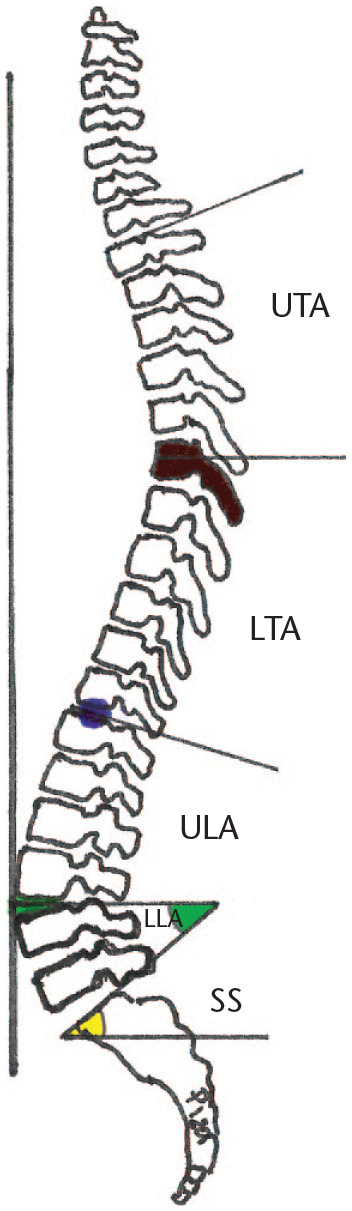
Sagittal curves distribution. Inflection point (blue dot), lumbar apex (green dot), lower lumbar arch angle (LLA, green), upper lumbar arch (ULA), lower thoracic arch (LTA), upper thoracic arch (UTA) thoracic apex (brown dark vertebra), sacral slope angle (SS, yellow).
LL is divided by the lumbar apex (Fig. 3) in a lower lumbar arch (LLA), and an upper lumbar arch (ULA). The lumbar apex is located at the most anterior point of the lumbar spine joining the vertical line.1 The ULA is symmetric and reciprocal to the lower thoracic arch (LTA), which expands from the thoracic apex down to the inflection point.35 The LLA has the same magnitude as the SS angle. This relation between arches (lumbar distribution) is dictated by SS and again by pelvic morphology (PI).1 Ideal surgical restoration of these parameters is important to try to avoid mechanical complications.36,37
Pelvic morphology and spine sagittal profile
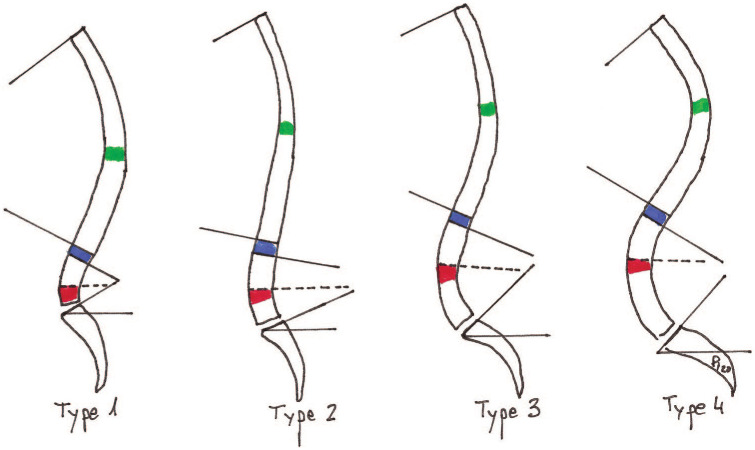
Four different types of sagittal profile have been described by Roussouly in a healthy population according to SS measurements (Fig. 4).38 As SS draws LL, patients with a low SS would show a straighter lumbar spine (static as named by Stagnara), whereas patients with a greater SS would show a more curved lumbar spine (dynamic as stated by Stagnara).4 In that way (Fig. 4), a wide pelvis will have a high PI, high SS and high LL, a more cranial lumbar apex, a longer lumbar lower arch and a higher inflection point, a more homogeneous lumbar distribution, and more vertebrae included in the lordosis. On the contrary, a narrow pelvis with low PI, low SS and low LL will have a more caudal lumbar apex and inflection point with a disproportion lumbar distribution, and fewer vertebrae in the lordosis.4,20
Fig. 4.
Roussouly sagittal shape classification in four types. Lumbar apex in red, inflection point in blue, thoracic apex in green. Global lumbar lordosis and global thoracic kyphosis are marked between lines. Doted line shows lower lumbar arch angle.
Degenerative lumbar changes and thoracolumbar coronal deformity can modify LL,9,39 and consequently SS. These effects alter the ideal Roussouly sagittal shape.12 SS then becomes an inadequate tool to classify sagittal types in pathologic patients, as it is not a constant parameter, being affected by these pathologic changes. PI, which is considered not to vary with pathology or compensation,16 now plays a critical role and has been incorporated as the basic parameter to found a renewed Roussouly sagittal profile classification.40 In this classification, low PI patients (< 45°) are classified as type 1 or type 2, intermediate PI (PI 45–60°) as type 3, while high PI patients (> 60°) have a type 4 profile.
This concept is important to realign patients after spine surgery. When planning sagittal surgical correction of a one-level disc degeneration41 up to an anterior malaligned deformity spine, restoring the ideal sagittal profile based on PI has been proven to be a key parameter to obtain a better result and lower rates of mechanical complications.23,36,42,43
Sagittal profile and spine pathology
It has also been reported that each pattern of the Roussouly sagittal profile classification predisposes to different spine pathologies, all originated from bipedalism.4 Type 1 patients concentrate all the lordosis between L4 and S1, the thoracolumbar junction runs deeply in kyphosis into L4. With degeneration, thoracolumbar kyphosis increases, retrolisthesis appears in the adjacent segments as well as hyperlordosis between L4 and S1, leading to distal facet arthritis and overload. In type 2 spines, upper lumbar arch disc orientation is very horizontal and has to withstand high-pressure forces, which can lead to central disc herniations. It is the pattern less adapted for weight-bearing or sports. Types 3 and 4 profiles support high mechanical forces in the lower lumbar segments which are more vertically orientated. The shear forces concentrated at the posterior aspect of L4/L5 can lead to L5 isthmic spondylolysis or L4–L5 spondylolisthesis by a sliding mechanism.4
Pelvic morphology and hip joints
This sagittal spine classification, and in the end pelvic morphology, also dictates hip range of motion. Narrower and more vertical pelvises, with low PI and low SS, have less ability to rotate (retrovert), creating a stiffer lumbosacral junction. Pelvic motion is then transferred to the hip joints. These patients are called ‘hip users’.44 The hip range of motion is very wide and mainly supported by femoral flexion-extension mobility. Because pelvic retroversion is limited, compensation quickly fails if recruited due to spinal malalignment.7 Anatomically, the pelvis compensates for greater acetabular inclination (45–50°) and anteversion (20–25°) to accommodate femoral head movement.44
On the other hand, wider and more horizontal pelvises have a higher PI and higher SS. In the absence of degenerative changes in the lumbar spine, there is a flexible situation, which increases the amplitude in the lumbosacral area, and, conversely, a wider range for pelvic sagittal rotation. However, there is a limited range of motion of the hip joints; both acetabular inclination (35–40°) and acetabular anteversion (12–20°) show lower anatomical values.44 These individuals are called ‘spine users’. When there is a spinal anterior malalignment, their pelvis allows these patients a lot of compensation before becoming exhausted; however, surgical difficulties can be found when restoring LL, as they usually require big posterior osteotomies or multiple anterior disc cages to recreate the amount of proper lordosis.7
The fate of total hip arthroplasty and lumbar spine disorders
Pelvic changes and acetabular component positioning
As mentioned above, the position of the pelvis varies between functional positions, and so does the acetabulum, and this can affect cup placement.27 Achieving ideal cup orientation is crucial in reducing edge-loading and articular impingement, which would otherwise lead to accelerated wear, squeaking, increased dislocation risk44 and prosthetic impingement (polyethylene liner fracture, neck notching and metallosis in case of ceramic liner).
Low PI pelvises have lower pelvic rotation ability (stiff lumbosacral movement) but hypermobile hip joints (leading to less protected THA). The excess of femoral flexion when sitting can produce anterior prosthetic impingement and leave an uncovered acetabular posterior space (posterior articular edge-loading) with an inherent risk of posterior dislocation.33,44
In high PI pelvises, there is a high potential for pelvic rotation, and less mobile hip joints (having better protection for THA). As femoral excursion is lower, the uncovered posterior acetabular space when sitting is low and the risk of posterior dislocation decreases. However, excessive pelvic retroversion leads to excessive acetabular anteversion, increased risk of posterior-superior edge-loading and posterior articular impingement. Thus, these patients are at higher risk for anterior dislocation while standing at maximum hip extension.44
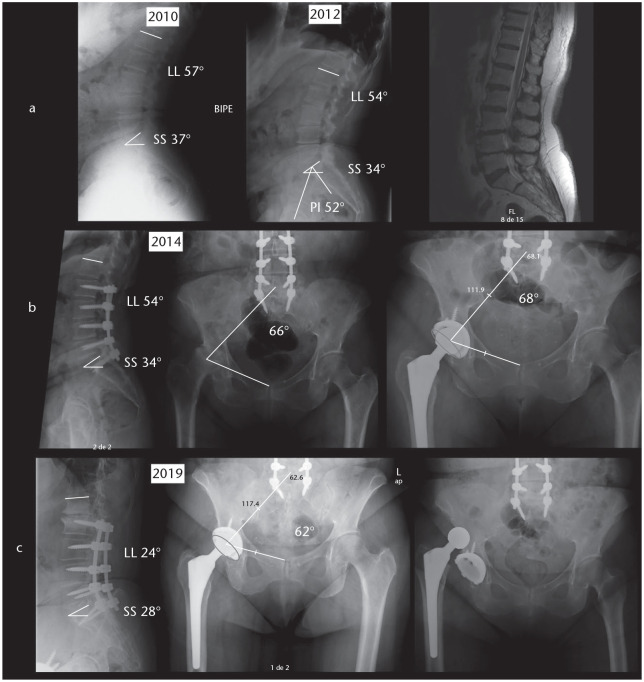
Finally, although pelvic tilt does not seem to significantly change after primary THA, at least in short-term follow-up and analysing average values,45 it can vary over time due to ageing and the possible appearance of sagittal spinal disorders, leading to anterior malalignment and pelvic compensation with acetabuli ante-inclination, thus functional cup positioning relative to the stem can be influenced with time due to these changes (Fig. 5).30,46
Fig. 5.
(A) Initially this patient had a lumbar lordosis of 57°, and SS of 37°, being PI 52°, her PT was 15°. Due to degenerative discopathy she underwent an L2–S1 posterior fusion and central decompression. (B) Two years after that she underwent a right-side THA. (C) After some years, an adjacent segment degeneration occurred, LL decreased to 24°, PT increased to 24° (pelvic retroversion) and SFP decreased to 62° (cup anteversion), and the patient suffered an anterior THA dislocation.
Note. SS, sacral slope; PI, pelvic incidence; PT, pelvic tilt; THA, total hip arthroplasty; LL, lumbar lordosis; SFP, sacro-femoral pubic angle.
Lumbar stiffness and acetabular component positioning
If one part of a mobile lumbar spine segment becomes stiff (due to degeneration changes or surgical lumbar fusion), other anatomical regions must accommodate to improve mobility, thus influencing THA (this is called spine hip syndrome). These phenomena have direct effects on complications, with a very large number of patients showing that THA dislocation rates are higher in patients undergoing lumbar spine fusion.47,48 On the contrary, hip surgery releases preoperative contractures increasing lumbo-sacral mobility, which endangers patients with previous lumbar instability.33
THA is probably the most cost-effective orthopaedic surgical procedure.49 However, there is an increasing number and projections for revision surgeries.2 Most revision cases are performed in the acetabular side due to wear/edge-loading, dislocation or impingement; factors determined by cup positioning.50–54 Classically, the acetabular component safe zone has been calculated according to Lewinnek’s criteria.55 However, further studies are changing this consensus,56 due to the spatial position of the hip influenced by functional pelvic and femur motion; the new concept of functional stability.57
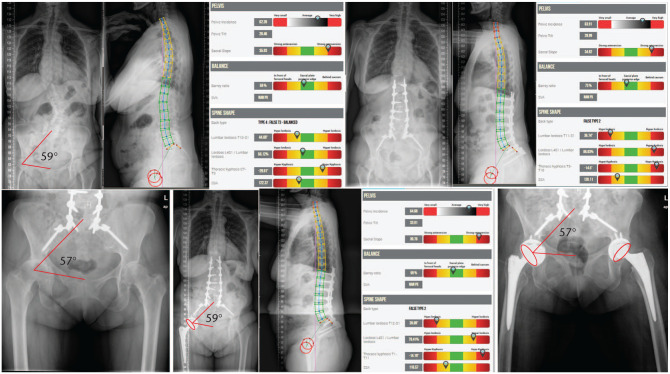
In patients with low spino-pelvic mobility, the acetabular component requires more coronal inclination and anteversion (except in patients with high compensation/retroversion).33 If the spine is fused with a fixed pelvic anteversion (uncommon situation), the functional cup version does not increase when sitting, leading to a higher risk for posterior dislocation; here, the cup may also need more anteversion. In the more common fused spine with a pelvic retroversion or a bad flat shape (flat back), or in pelvises forced to recruit more pelvic retroversion to compensate for malalignment (Fig. 6), there will be a higher risk of anterior dislocation with full standing; here the cup may need less anteversion (Fig. 7).44
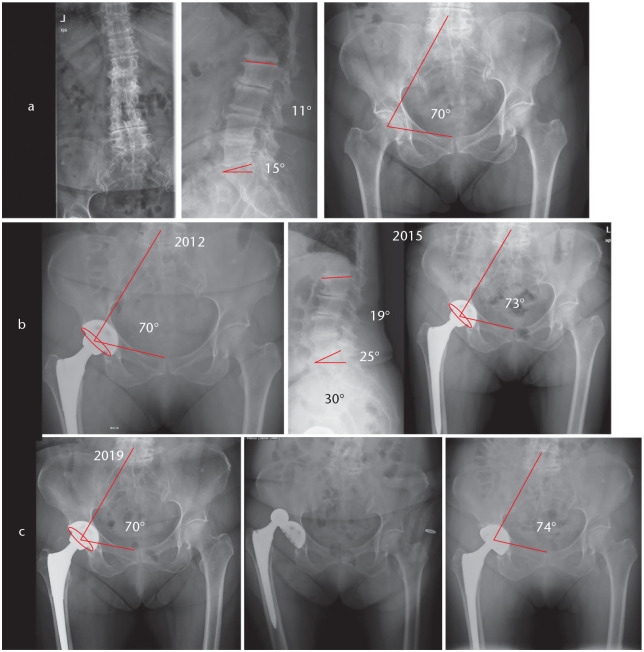
Fig. 6.
Patient with adult scoliosis, PI 62°. SS of 35° compensating with PT 26°, SFP 59°. She underwent long lumbar fusion to pelvis with insufficient lordosis correction and residual postoperative mismatch (PI–LL 26°), and still compensating SS 34° and PT 27°, SFP 57°. Fused in pelvic retroversion, care must be taken not to put the cup in anteversion as it would be at risk of anterior dislocation.
Note. PI, pelvic incidence; SS, sacral slope; PT, pelvic tilt; SFP, sacro-femoral pubic angle; LL, lumbar lordosis.
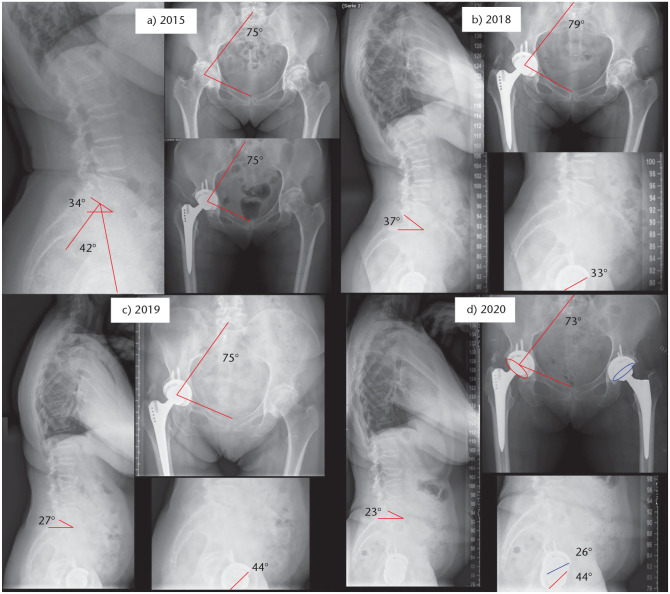
Fig. 7.
(A) A 62-year-old patient with 42° PI. Initially SS 34° and SFP 75°. (B) Three years after primary right THA, SS increased to 37° and SFP to 79°. Sagittal cup anteversion was 33°. (C) In 2019 she suffered an L3 fracture with loss of lordosis and consequently loss of SS (27°) and SFP 75° (pelvic retroversion), increasing sagittal cup anteversion (44°). (D) Left THA was performed after planning with previous associated pelvic retroversion; different cup anteversion can be found between both THA in the postoperative radiograph.
Note. PI, pelvic incidence; SS, sacral slope; SFP, sacro-femoral pubic angle; THA, total hip replacement.
Controversy still exists in terms of sacrificing an optimal acetabular component position according to these findings. Be aware that life is a kyphotic event, with associated retroversion of the pelvis expected with ageing, and, consequently, changes in the functional position of the cup increasing the risk for a late dislocation (Fig. 8) and poor prosthetic biomechanics (prosthetic impingement and edge-loading). Alternative acetabular component designs such as dual mobility cups may also be useful in patients with hip hypermobility and stiff degenerative lumbar spines, to lower dislocation rates.58 Other issues must be also analysed individually such as age, physical activity, and hip osteoarthritis aetiology, to find the best indication for each patient.59
Fig. 8.
(A) Female, 75 years old, with degenerative lumbar spine PI 30°, SS 15° and SFP 70° and primary osteoarthritis of the right hip; she underwent primary THA. (B) Three-year postoperative radiograph showing an SS increase of 10° and pelvic anteversion gaining. Clinical result in terms of pain, function and mobility was excellent. (C) Seven years after primary THA, SFP decreased due to degenerative lordosis loss with associated pelvic retroversion and two anterior hip dislocations occurred, requiring hip revision surgery with a dual mobility cup.
Note. PI, pelvic incidence; SS, sacral slope; SFP, sacro-femoral pubic angle; THA, total hip replacement.
Clinical outcome and instability: THA and lumbar pathology
It has been reported that patients with unilateral hip osteoarthritis and concomitant low back pain have a marked femoral neck anteversion in the arthritic hip and greater spino-pelvic malalignment. An improvement in alignment and relief from both hip and back pain is observed after THA.60
In a case-control-matched study, Grammatopoulos et al analysed acetabular and spinal parameters in patients undergoing THA with or without spinal fusion. They found a better clinical outcome (for hip and spine scores), lower dislocation rate, and fewer revision surgeries in patients without a lumbar fusion. Their observations were associated with a difference in spino-pelvic mobility between both groups due to a narrow zone of an optimum cup position. So they recommended preoperatively determining spinopelvic hypermobility.61 Knowing the role of the pelvis in spine-pelvis-hip biomechanics can help us improve our practice in the future.62
There are other factors associated with hip biomechanics than cup positioning. Edge-loading, soft-tissue balance (stiffness/laxity), femoral component positioning, leg length discrepancy, acetabular and femoral offsets, surgical approach, implant (femoral head size, bearings materials), and proper reconstruction of hip rotation centre are equally important.63–67 All these issues can affect both clinical outcomes and complication rates in THA. The kinematic alignment technique for THA aims to restore constitutional hip anatomy (unless caused by obvious developmental hip disease) and to adapt the cup position to the individual spine–hip relationship.68
Lastly, surgeons question whether hip or spine should be operated first when both are a cause of pain. If the patient has spine pathology leading to excessive pelvic retroversion, the spine should be addressed first (this is typical of ankylosing spondylitis), to implant the acetabular component in a correct position if THA is needed.69 Otherwise, THA should be performed first. In a very large Medicare study, both dislocation and revision rates were much higher in patients with THA after lumbar spine fusion than in patients with THA and a delayed spinal arthrodesis.70 In clinical and radiological analysis, Parilla et al emphasized the importance of the difficult decision of performing lumbar spine fusion due to PT changes in patients with THA.71 Despite this, they did not find significant differences in the order of procedures. The authors suggested that THA would be better performed one year after lumbar spine fusion, since PT changes are minimal and predictable. Moreover, severe spine deformities and lumbo-sacral fusion required further assessment.
Conclusions
The pelvis stands as the cornerstone between the spine and the hips. Pelvic motion is essential to maintain proper balance and sagittal alignment during bipedalism, and retroversion is recruited as a compensation mechanism when spinal malalignment occurs. This pelvic rotation can affect kinematic cup positioning, and consequently functional cup positioning if a total hip arthroplasty (THA) is needed. This is because pelvic retroversion, frequently associated with degenerative spinal changes and ageing, implies an increase of acetabular version.
Patients with flexible lumbar spines (spine users) protect the hip joint. Patients with stiff (degenerated or fused) lumbar spines (hip users) demand higher hip mobility, placing THA at risk. Pelvises in retroversion place the THA at risk of anterior dislocation when standing. In contrast, pelvises in anteversion or with low PI can place THA at risk for posterior dislocation when sitting. All of these concepts need to be taken into account when planning cup orientation. Although in the majority of cases the cup needs to be set in an anatomic position, patients with lumbo-pelvic stiffness and/or sagittal spinal malalignment (poor spine–hip relationship) may need adjustment (from the anatomical positioning) of the cup orientation and use of forgiving cup implant design. Low PI pelvises may need more acetabular ante-inclination, and high PI pelvises more acetabular retroversion.
If surgery is needed, it is recommend to start addressing the hip first, except in patients with compensation (high pelvic retroversion), who may need spine surgery first to place the pelvis, and, consequently the acetabulum, in a proper position.
Footnotes
ICMJE Conflict of interest statement: JP reports consultancy to Medtronic, outside the submitted work.
EGR declares no conflict of interest relevant to this work.
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Roussouly P, Pinheiro-Franco JL. Sagittal parameters of the spine: biomechanical approach. Eur Spine J 2011;20:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 2014;96:624–630. [DOI] [PubMed] [Google Scholar]
- 3. Dubousset J. Three-dimensional analysis of the scoliotic deformity. In: Weinstein SL, ed. The pediatric spine: principles and practice. Vol 1 New York: Raven Press, 1994:479–496. [Google Scholar]
- 4. Roussouly P, Pinheiro-Franco JL. Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur Spine J 2011;20:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lafage R, Schwab F, Challier V, et al. ; International Spine Study Group. Defining spino-pelvic alignment thresholds: should operative goals in adult spinal deformity surgery account for age? Spine (Phila Pa 1976) 2016;41:62–68. [DOI] [PubMed] [Google Scholar]
- 6. Takemoto M, Boissière L, Vital J-M, et al. Are sagittal spinopelvic radiographic parameters significantly associated with quality of life of adult spinal deformity patients? Multivariate linear regression analyses for pre-operative and short-term post-operative health-related quality of life. Eur Spine J 2017;26:2176–2186. [DOI] [PubMed] [Google Scholar]
- 7. Roussouly P, Nnadi C. Sagittal plane deformity: an overview of interpretation and management. Eur Spine J 2010;19:1824–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson RP, McManus AC. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size: a prospective controlled clinical study. Spine (Phila Pa 1976) 1994;19:1611–1618. [DOI] [PubMed] [Google Scholar]
- 9. Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases: a comparative study about 85 cases. Eur Spine J 2007;16:1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Protopsaltis T, Schwab F, Bronsard N, et al. ; International Spine Study Group. The T1 pelvic angle, a novel radiographic measure of global sagittal deformity, accounts for both spinal inclination and pelvic tilt and correlates with health-related quality of life. J Bone Joint Surg Am 2014;96:1631–1640. [DOI] [PubMed] [Google Scholar]
- 11. Boissière L, Takemoto M, Bourghli A, et al. ; European Spine Study Group (ESSG). Global tilt and lumbar lordosis index: two parameters correlating with health-related quality of life scores-but how do they truly impact disability? Spine J 2017;17:480–488. [DOI] [PubMed] [Google Scholar]
- 12. Sebaaly A, Grobost P, Mallam L, Roussouly P. Description of the sagittal alignment of the degenerative human spine. Eur Spine J 2018;27:489–496. [DOI] [PubMed] [Google Scholar]
- 13. Schwab F, Lafage V, Patel A, Farcy J-P. Sagittal plane considerations and the pelvis in the adult patient. Spine (Phila Pa 1976) 2009;34:1828–1833. [DOI] [PubMed] [Google Scholar]
- 14. Barrey C, Roussouly P, Le Huec J-C, D’Acunzi G, Perrin G. Compensatory mechanisms contributing to keep the sagittal balance of the spine. Eur Spine J 2013;22 Suppl 6:S834–S841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diebo BG, Ferrero E, Lafage R, et al. Recruitment of compensatory mechanisms in sagittal spinal malalignment is age and regional deformity dependent: a full-standing axis analysis of key radiographical parameters. Spine (Phila Pa 1976) 2015;40:642–649. [DOI] [PubMed] [Google Scholar]
- 16. Legaye J, Duval-Beaupère G, Hecquet J, Marty C. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J 1998;7:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mac-Thiong J-M, Labelle H, Berthonnaud E, Betz RR, Roussouly P. Sagittal spinopelvic balance in normal children and adolescents. Eur Spine J 2007;16:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cecchinato R, Redaelli A, Martini C, et al. Long fusions to S1 with or without pelvic fixation can induce relevant acute variations in pelvic incidence: a retrospective cohort study of adult spine deformity surgery. Eur Spine J 2017;26:436–441. [DOI] [PubMed] [Google Scholar]
- 19. Bao H, Liabaud B, Varghese J, et al. Lumbosacral stress and age may contribute to increased pelvic incidence: an analysis of 1625 adults. Eur Spine J 2018;27:482–488. [DOI] [PubMed] [Google Scholar]
- 20. Berthonnaud E, Dimnet J, Roussouly P, Labelle H. Analysis of the sagittal balance of the spine and pelvis using shape and orientation parameters. J Spinal Disord Tech 2005;18:40–47. [DOI] [PubMed] [Google Scholar]
- 21. Iyer S, Lenke LG, Nemani VM, et al. Variations in sagittal alignment parameters based on age: a prospective study of asymptomatic volunteers using full-body radiographs. Spine (Phila Pa 1976) 2016;41:1826–1836. [DOI] [PubMed] [Google Scholar]
- 22. Le Huec J-C, Hasegawa K. Normative values for the spine shape parameters using 3D standing analysis from a database of 268 asymptomatic Caucasian and Japanese subjects. Eur Spine J 2016;25:3630–3637. [DOI] [PubMed] [Google Scholar]
- 23. Yilgor C, Sogunmez N, Boissière L, et al. ; European Spine Study Group (ESSG). Global Alignment and Proportion (GAP) score: development and validation of a new method of analyzing spinopelvic alignment to predict mechanical complications after adult spinal deformity surgery. J Bone Joint Surg Am 2017;99:1661–1672. [DOI] [PubMed] [Google Scholar]
- 24. Legaye J, Duval-Beaupère G. Sagittal plane alignment of the spine and gravity: a radiological and clinical evaluation. Acta Orthop Belg 2005;71:213–220. [PubMed] [Google Scholar]
- 25. Boulay C, Tardieu C, Hecquet J, et al. Sagittal alignment of spine and pelvis regulated by pelvic incidence: standard values and prediction of lordosis. Eur Spine J 2006;15:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ochi H, Baba T, Homma Y, Matsumoto M, Nojiri H, Kaneko K. Importance of the spinopelvic factors on the pelvic inclination from standing to sitting before total hip arthroplasty. Eur Spine J 2016;25:3699–3706. [DOI] [PubMed] [Google Scholar]
- 27. Pierrepont J, Hawdon G, Miles BP, et al. Variation in functional pelvic tilt in patients undergoing total hip arthroplasty. Bone Joint J 2017;99-B:184–191. [DOI] [PubMed] [Google Scholar]
- 28. Lembeck B, Mueller O, Reize P, Wuelker N. Pelvic tilt makes acetabular cup navigation inaccurate. Acta Orthop 2005;76:517–523. [DOI] [PubMed] [Google Scholar]
- 29. Sautet P, Giorgi H, Chabrand P, et al. Is anatomic acetabular orientation related to pelvic morphology? CT analysis of 150 healthy pelvises. Orthop Traumatol Surg Res 2018;104:347–351. [DOI] [PubMed] [Google Scholar]
- 30. Buckland AJ, Vigdorchik J, Schwab FJ, et al. Acetabular anteversion changes due to spinal deformity correction: bridging the gap between hip and spine surgeons. J Bone Joint Surg Am 2015;97:1913–1920. [DOI] [PubMed] [Google Scholar]
- 31. Blondel B, Schwab F, Patel A, et al. Sacro-femoral-pubic angle: a coronal parameter to estimate pelvic tilt. Eur Spine J 2012;21:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ragsdale MI, Wong FS, Boutin RD, Meehan JP. Pelvic tilt evaluation from frontal radiographs: the validity, interobserver reliability and intraobserver reproducibility of the sacro-femoral-pubic parameter. J Arthroplasty 2017;32:1665–1669. [DOI] [PubMed] [Google Scholar]
- 33. Ike H, Dorr LD, Trasolini N, Stefl M, McKnight B, Heckmann N. Spine-pelvis-hip relationship in the functioning of a total hip replacement. J Bone Joint Surg Am 2018;100:1606–1615. [DOI] [PubMed] [Google Scholar]
- 34. Schwab F, Patel A, Ungar B, Farcy J-P, Lafage V. Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976) 2010;35:2224–2231. [DOI] [PubMed] [Google Scholar]
- 35. Clément J-L, Pelletier Y, Solla F, Rampal V. Surgical increase in thoracic kyphosis increases unfused lumbar lordosis in selective fusion for thoracic adolescent idiopathic scoliosis. Eur Spine J 2019;28:581–589. [DOI] [PubMed] [Google Scholar]
- 36. Pizones J, Moreno-Manzanaro L, Sánchez Pérez-Grueso FJ, et al. Restoring the ideal Roussouly sagittal profile in adult scoliosis surgery decreases the risk of mechanical complications. Eur Spine J 2020;29:54–62. [DOI] [PubMed] [Google Scholar]
- 37. Pizones J, Perez-Grueso FJS, Moreno-Manzanaro L, et al. ; ESSG (European Spine Study Group). Ideal sagittal profile restoration and ideal lumbar apex positioning play an important role in postoperative mechanical complications after a lumbar PSO. Spine Deform 2020;8:491–498. [DOI] [PubMed] [Google Scholar]
- 38. Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine (Phila Pa 1976) 2005;30:346–353. [DOI] [PubMed] [Google Scholar]
- 39. Pizones J, Martin MB, Perez-Grueso FJS, et al. ; ESSG European Spine Study Group. Impact of adult scoliosis on Roussouly sagittal shape classification. Spine (Phila Pa 1976) 2019;44:270–279. [DOI] [PubMed] [Google Scholar]
- 40. Laouissat F, Sebaaly A, Gehrchen M, Roussouly P. Classification of normal sagittal spine alignment: refounding the Roussouly classification. Eur Spine J 2018;27:2002–2011. [DOI] [PubMed] [Google Scholar]
- 41. Gille O, Bouloussa H, Mazas S, et al. A new classification system for degenerative spondylolisthesis of the lumbar spine. Eur Spine J 2017;26:3096–3105. [DOI] [PubMed] [Google Scholar]
- 42. Sebaaly A, Riouallon G, Obeid I, et al. Proximal junctional kyphosis in adult scoliosis: comparison of four radiological predictor models. Eur Spine J 2018;27:613–621. [DOI] [PubMed] [Google Scholar]
- 43. Barrey C, Darnis A. Current strategies for the restoration of adequate lordosis during lumbar fusion. World J Orthop 2015;6:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rivière C, Lazennec J-Y, Van Der Straeten C, Auvinet E, Cobb J, Muirhead-Allwood S. The influence of spine-hip relations on total hip replacement: a systematic review. Orthop Traumatol Surg Res 2017;103:559–568. [DOI] [PubMed] [Google Scholar]
- 45. Murphy WS, Klingenstein G, Murphy SB, Zheng G. Pelvic tilt is minimally changed by total hip arthroplasty. Clin Orthop Relat Res 2013;471:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taki N, Mitsugi N, Mochida Y, Akamatsu Y, Saito T. Change in pelvic tilt angle 2 to 4 years after total hip arthroplasty. J Arthroplasty 2012;27:940–944. [DOI] [PubMed] [Google Scholar]
- 47. Buckland AJ, Puvanesarajah V, Vigdorchik J, et al. Dislocation of a primary total hip arthroplasty is more common in patients with a lumbar spinal fusion. Bone Joint J 2017;99-B:585–591. [DOI] [PubMed] [Google Scholar]
- 48. Malkani AL, Garber AT, Ong KL, et al. Total hip arthroplasty in patients with previous lumbar fusion surgery: are there more dislocations and revisions? J Arthroplasty 2018;33:1189–1193. [DOI] [PubMed] [Google Scholar]
- 49. Lavernia CJ, Iacobelli DA, Brooks L, Villa JM. The cost-utility of total hip arthroplasty: earlier intervention, improved economics. J Arthroplasty 2015;30:945–949. [DOI] [PubMed] [Google Scholar]
- 50. Havelin LI, Fenstad AM, Salomonsson R, et al. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop 2009;80:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mäkelä KT, Furnes O, Hallan G, et al. The benefits of collaboration: the Nordic Arthroplasty Register Association. EFORT Open Rev 2019;4:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sugano N, Nishii T, Miki H, Yoshikawa H, Sato Y, Tamura S. Mid-term results of cementless total hip replacement using a ceramic-on-ceramic bearing with and without computer navigation. J Bone Joint Surg Br 2007;89:455–460. [DOI] [PubMed] [Google Scholar]
- 53. Wan Z, Boutary M, Dorr LD. The influence of acetabular component position on wear in total hip arthroplasty. J Arthroplasty 2008;23:51–56. [DOI] [PubMed] [Google Scholar]
- 54. García-Rey E, García-Cimbrelo E, Cordero-Ampuero J. Outcome of a hemispherical porous-coated acetabular component with a proximally hydroxyapatite-coated anatomical femoral component: a 12- to 15-year follow-up study. J Bone Joint Surg Br 2009;91:327–332. [DOI] [PubMed] [Google Scholar]
- 55. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am 1978;60:217–220. [PubMed] [Google Scholar]
- 56. Seagrave KG, Troelsen A, Malchau H, Husted H, Gromov K. Acetabular cup position and risk of dislocation in primary total hip arthroplasty. Acta Orthop 2017;88:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dorr LD, Callaghan JJ. Death of the Lewinnek ‘safe zone’. J Arthroplasty 2019;34:1–2. [DOI] [PubMed] [Google Scholar]
- 58. Dagneaux L, Marouby S, Maillot C, Canovas F, Rivière C. Dual mobility device reduces the risk of prosthetic hip instability for patients with degenerated spine: a case-control study. Orthop Traumatol Surg Res 2019;105:461–466. [DOI] [PubMed] [Google Scholar]
- 59. Pitto RP, Garland M, Sedel L. Are ceramic-on-ceramic bearings in total hip arthroplasty associated with reduced revision risk for late dislocation? Clin Orthop Relat Res 2015;473:3790–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Piazzolla A, Solarino G, Bizzoca D, et al. Spinopelvic parameter changes and low back pain improvement due to femoral neck anteversion in patients with severe unilateral primary hip osteoarthritis undergoing total hip replacement. Eur Spine J 2018;27:125–134. [DOI] [PubMed] [Google Scholar]
- 61. Grammatopoulos G, Gofton W, Jibri Z, et al. 2018 Frank Stinchfield Award: spinopelvic hypermobility is associated with an inferior outcome after THA: examining the effect of spinal arthrodesis. Clin Orthop Relat Res 2019;477:310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eftekhary N, Shimmin A, Lazennec J-Y, et al. A systematic approach to the hip–spine relationship and its applications to total hip arthroplasty. Bone Joint J 2019;101-B:808–816. [DOI] [PubMed] [Google Scholar]
- 63. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res 2011;469:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grammatopoulos G, Thomas GER, Pandit H, Beard DJ, Gill HS, Murray DW. The effect of orientation of the acetabular component on outcome following total hip arthroplasty with small diameter hard-on-soft bearings. Bone Joint J 2015;97-B:164–172. [DOI] [PubMed] [Google Scholar]
- 65. Esposito CI, Gladnick BP, Lee Y-Y, et al. Cup position alone does not predict risk of dislocation after hip arthroplasty. J Arthroplasty 2015;30:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. García-Rey E, García-Cimbrelo E. Abductor biomechanics clinically impact the total hip arthroplasty dislocation rate: a prospective long-term study. J Arthroplasty 2016;31:484–490. [DOI] [PubMed] [Google Scholar]
- 67. Timperley AJ, Biau D, Chew D, Whitehouse SL. Dislocation after total hip replacement: there is no such thing as a safe zone for socket placement with the posterior approach. Hip Int 2016;26:121–127. [DOI] [PubMed] [Google Scholar]
- 68. Rivière C, Harman C, Parsons T, Villet L, Cobb J, Maillot C. Kinematic alignment versus conventional techniques for total hip arthroplasty: a retrospective case control study. Orthop Traumatol Surg Res 2019;105:895–905. [DOI] [PubMed] [Google Scholar]
- 69. Hu J, Qian B-P, Qiu Y, et al. Can acetabular orientation be restored by lumbar pedicle subtraction osteotomy in ankylosing spondylitis patients with thoracolumbar kyphosis? Eur Spine J 2017;26:1826–1832. [DOI] [PubMed] [Google Scholar]
- 70. Malkani AL, Himschoot KJ, Ong KL, et al. Does timing of primary total hip arthroplasty prior to or after lumbar spine fusion have an effect on dislocation and revision rates? J Arthroplasty 2019;34:907–911. [DOI] [PubMed] [Google Scholar]
- 71. Parilla FW, Shah RR, Gordon AC, et al. Does it matter: total hip arthroplasty or lumbar spinal fusion first? Preoperative sagittal spinopelvic measurements guide patient-specific surgical strategies in patients requiring both. J Arthroplasty 2019;34:2652–2662. [DOI] [PubMed] [Google Scholar]