Abstract
Adverse knee pain occurs in 10–34% of all total knee replacements (TKR), and 20% of TKR patients experience more pain post-operatively than pre-operatively. Knee pain is amongst the top five reasons for knee replacement revision in the United Kingdom. The number of TKRs is predicted to continue increasing due to the ageing population.
A narrative literature review was performed on the different causes of pain following TKR. A database search on Scopus, PubMed, and Google Scholar was conducted to look for articles related to TKR, pain, and cause. Articles were selected based on relevance, publication date, quality of research and validation. Relevant sections were added to the review.
One hundred and fourteen articles were identified and potential causes of TKR pain included: arthrofibrosis, aseptic loosening, avascular necrosis, central sensitization, component malpositioning, infection, instability, nerve damage, overstuffing, patellar maltracking, polyethylene wear, psychological factors and unresurfaced patella.
It is important to tailor our approach to address the individual causes of pain. Certain controllable risk factors can be managed pre-operatively to minimize post-operative pain. Risk factors help to predict adverse pain outcomes and identify specific causes.
There are multiple causes of pain following TKR. Some factors will require further extensive studies, and as pain is a commonly attributed reason for TKR revision, its underlying aetiologies should be explored. Understanding these factors helps to develop effective methods for diagnosis, prevention and management of TKR pain, which help to improve patient outcomes.
Cite this article: EFORT Open Rev 2020;5:534-543. DOI: 10.1302/2058-5241.5.200031
Keywords: arthroplasty, knee, pain
Introduction
Total knee replacement (TKR) is a cost-effective surgical procedure.1 According to the national joint registry,2 274,495 total knee replacements were performed in England, Wales, Northern Ireland and the Isle of Man in 2016–2018. The majority of TKR in 2018 were cemented, unconstrained fixed TKR (60.8%); followed by cemented, posterior-stabilized fixed TKR (19.8%). By 2030, TKR demand is predicted to increase six-fold from its demand in 2005 in line with the increasingly ageing population.3
The most common indication for TKR is osteoarthritis (OA).1 In 2018, 96.2% of primary knee replacements were conducted solely due to osteoarthritis.2 TKR helps to improve quality of life and function in end-stage, symptomatic osteoarthritis patients.4–7 However, 10–34% of TKR patients receive adverse pain outcomes between three months and five years following surgery and around 20% of TKR patients experience more knee pain and swelling than before surgery.8–10 Despite advancements in surgical techniques, prostheses, pain control, and medical care, there is evidence to suggest worsening pain and functional scores of some patients over time following TKR surgery.11
In 2018, 6,357 revision knee joint operations were conducted compared to 4,417 revisions in 2008 in England, Wales, Northern Ireland and the Isle of Man. The most common causes for knee replacement revision in 2018 in descending order are aseptic loosening/lysis, infection, pain, progressive arthritis, and instability; knee pain continues to be one of the top five reasons for revision surgery.2 A retrospective study by Erivan R et al12 showed that in patients with unexplained chronic knee pain following TKR, 4.5% of cases were caused by infection, 2.7% were due to instability without real dislocation, 1.8% were due to placement error from rotational problems, 22.3% were due to loosening in tibia and femoral components, 8.0% were due to polyethylene wear, 33.9% were due to periarticular involvement with quadricep deficiency, iliotibial tendinitis, pes bursitis, stiffness or prepatellar bursitis, 18.8% were due to projected pain, 2.7% were due to complex regional pain syndrome and 6.3% had no explaining diagnosis. Following the report by Preston et al13 on the aetiologies of pain following TKR, newer evidence has surfaced to explain the process of pain development following TKR. This review aims to examine the causes of pain following TKR, assessing previous research in light of new evidence.
Materials and methods
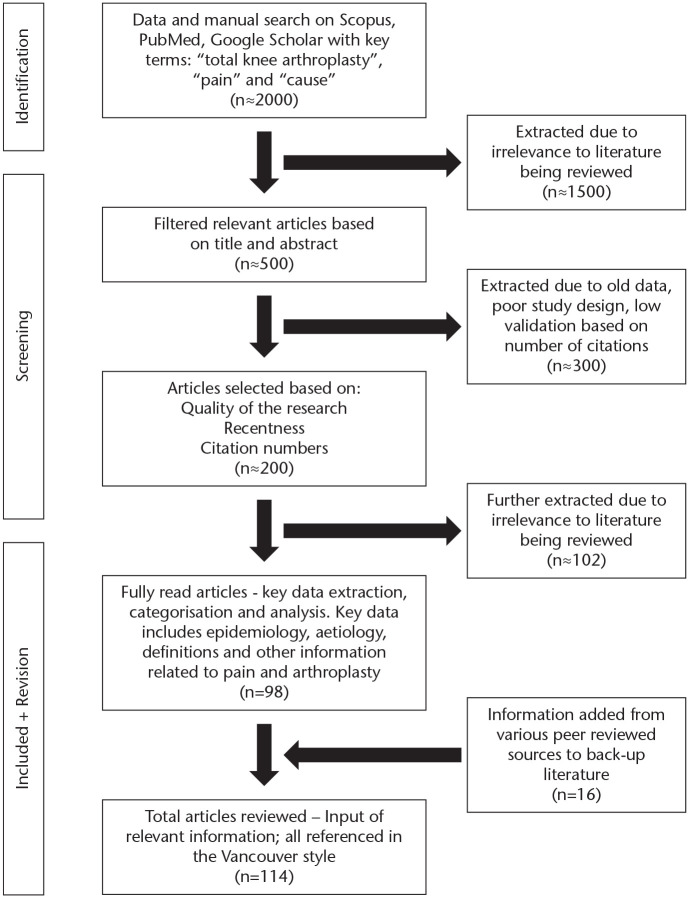
A literature review on the causes of pain following total knee replacements was performed based on research papers published up to 1 April 2020. A database search on Scopus, PubMed and Google Scholar was conducted to look for articles in English with the search terms “total knee replacement”, “pain”, and “cause”. The initial search yielded over 1,000 articles. One hundred and fourteen relevant articles were ultimately selected based on relevance, recentness, quality of research and citations. Fig. 1 summarizes the methods of research.
Fig. 1.
Flowchart showing how articles were selected and reviewed.
Arthrofibrosis
Arthrofibrosis is the excessive production of collagen and adhesions which contributes to pain and restrictive joint motion.14 Of TKR patients, 3–10% develop arthrofibrosis after TKR and report pain with activities from < 30% for light manual work to 78% for jumping and pivoting.15–18 Pathological development of fibrosis can also be seen in multiple organs following wound healing by fibroblasts.19–21 TGF-β1 is an important mediator causing fibrosis in multiple organs, and this has also been increased in arthrofibrosis.22 TGF-β1 has been known to increase expression of α-smooth muscle actin (α-SMA) which causes the activation of fibroblasts as well as other fibrotic associated proteins, e.g. collagen I/III, fibronectin.23 With increased fibrotic gene expression in fibroblasts caused by TGF-β1, they can also modulate their behaviours to changes in the microenvironment.23
Following TKR, receptors TLR3, TLR4, IL1-R1 are expressed in the infrapatellar fat and synovial membrane, where the strongest expression is by IL-1R1 and this is induced to produce an inflammatory process in response to IL-1α and IL-1β.23 Pain in a knee with arthrofibrosis can also be caused by femoropatellar joint impairment from increased pressure from peripatellar scar tissues. This in turn leads to joint overload and structural changes, e.g. cartilage destruction.24 Of patients with arthrofibrosis, 79% also develop osteoarthritis 5.7 years following surgery, which can lead to a painful joint.25 Pain in arthrofibrosis can also be attributed to reflex sympathetic dystrophy which is a spontaneous regional pain that happens in 15.2% of patients.24
Fibrous adhesions (abnormal tissue connections) are also correlated to intermittent pain, inflammation, loss of function and progressive joint degeneration.26,27 There has been evidence to show fibroblasts under inflammation cause monocyte recruitment through the production of the C-C motif chemokine ligand 2 (CCL2) and that levels of CCL2 are associated with reported pain. CCL2 expression (dependant on NFkB) is also stimulated by IL-1α which might explain persistent fibrosis that causes inflammation in response to IL-1α immune triggers. Sensory neurones contain C-C chemokine receptor type 2 (CCR2) and this engages with CCL2 to cause excitation of the nociceptive neurones that can lead to pain sensation.28–31 Following TKR, three anatomical parts of the knee have been found to express increased proinflammatory states. These are the infrapatellar fat pad, synovial membrane, and synovial fluid. Other inflammatory markers are increased in all these sites where IL-8 is the most induced.32
Instability
Thirty-two per cent of patients reported knee instability with activity limitation and pain six months following TKR.33 A study by Leichtenberg et al34 showed that 76% and 21% of patients reported instability before and at one year following TKR, respectively. Patients who retained instability also reported significantly more pain, poorer quality of life and increased activity limitation. Results show that 25% of patients with pre-operative instability retain the instability one year after TKR. This correlation between instability and knee pain has also been supported by other previous studies.33,35–37
Flexion instability occurs when there is an imbalance of flexion and extension, leading to hemarthrosis, swelling, knee pain and giving way.38 Flexion instability can be caused by tissue insufficiencies such as ligament injury or implant failure.39 Excessive release of the medial ligament, inadequate distal femoral resection, excessive tibial slope, internal rotation of components and an undersized femoral component can also lead to flexion instability.38–41 A study by Lewallen et al42 showed increased extracellular matrix (ECM) remodelling gene expression in TKR patients with flexion instability and also suggested a correlation between ECM degradation and exposure of oxidative stress during tissue remodelling and inflammation.
A retrospective study by Sharma43 defined mid-flexion instability as the restraint of the posterior knee capsule. The main contributors include the excessive release of the medial collateral ligament (a stabilizer between 30–60 degrees of motion) anteriorly, and malpositioning of the implant to epicondyles that causes malfunctioning of the tibial post-femoral geometry.
In a systematic review by Rouquette et al44 exploring the causes of tibiofemoral dislocation (a rare but serious form of instability) after TKR, key factors for tibiofemoral dislocation included comorbidities such as obesity and pre-operative deformity, and intra-operative iatrogenic destabilization. Higher rates of dislocation recurrence were associated with non-operative management such as splints.
A study by Slane et al45 demonstrated an increase in patellar tendon buckling in post-TKR patients compared to the control. These patients also exhibited larger buckling angle, magnitude, and amplitude. Lower distal buckling angles are also correlated with better Knee Society Scores. Buckling happens when the tendon ruffles back on itself when extended or moved passively, and many factors can contribute to this including patella positioning, infrapatellar fat pad resection (increasing joint space for buckling), alteration in patellar tracking following TKR and trauma from surgery. Greater increase in tendon buckling is known to increase knee instability and reduce the ability to reach full extension.46–48 Factors to consider are that patients with OA might already have differences in patellar tendon buckling as OA leads to weakness in the quadriceps due to the anatomical function of the patellar tendon as part of a muscle unit.45,49
Component malpositioning
TKR consists of the femoral and tibial components within the knee (a hinge joint). These articulate with each other and between the patellar and femoral surfaces within the patellofemoral joint. The femoral component articulates with the total polyethylene tibial component, tibial base plate and the polyethylene surface which allows flexion and extension with a slight lateral/medial motion.50
The causes of post-TKR pain can be attributed to modified kinematics, alteration of ligament tensions and increased retro patellar pressure which commonly leads to anterior knee pain.51–54 Poor component positioning affects the kinematic part of the knee, which also increases the risk of instability and the chance of polyethylene wear.55–57 Component positions also influence ligament tension in in vitro studies.58,59
Some clinical observation studies have shown that the internal rotation of the femoral implant component may contribute to patellofemoral pain syndrome.60–62 While internal rotation increases the stress that contributes to anterior knee pain, external rotation of the trochlear groove and femoral component reduces the retro patellar tension and therefore decreases pain.63
A study by Fottner et al64 has found that, using computer simulation, malpositioning of the tibial baseplate component mostly affects ligament tension (posterior cruciate and collateral ligaments) which influences the tibia and femur kinematics and their contact forces. This has its effect on poorer clinical outcomes following surgery, including pain, higher rate of early loosening, instability and reduced range of motion. Regarding kinematics, the greatest changes were observed in tibiofemoral rotation. Tibiofemoral rollback was also influenced by translation medially and laterally, with medialization having the greatest effect. A study by Nicoll et al65 showed that internal rotation errors of the tibial implant are associated with medial and anterior knee pain and suggested that the location happens either at the central part of the tibial tubercle or talus.
Aseptic loosening
According to studies by Dalury et al and Schroer et al,66,67 aseptic loosening is one of the leading causes for revision after TKR. It affects patients up to 20 years after surgery. It is, however, one of the least understood TKR failure mechanisms. Various factors can lead to aseptic loosening. These include instability, component malpositioning, and osteolysis secondary to polyethylene wear. Osteolysis involves the generation of debris from polyethylene wear and metal and cement particles which generate an immunological response upon access to the bone–implant interface. Loose locking mechanisms, backside wear and micromotion increase modularity and therefore increase wear particle generation.
Other studies from Crotti et al and Gehrke et al68,69 explained the immunological response is commenced by macrophages that phagocytose any small wear particles < 5 μm in diameter, and this induces the release of interleukin-1ß (IL-1ß) and tumour necrosis factor-α. These stimulate the recruitment and activation of osteoclasts via the RANK-L pathway and thus osteolysis occurs adjacent to the bone–implant interfaces. This may be followed by prosthesis micromotion and further debris particle dissemination. It is unclear whether these biological processes are solely responsible for the loosening of the joint.
Osteolysis is more common in the tibial compartment due to its relation to the polyethylene insert, gravity and the use of screws for fixation which facilitate wear particle migration into the bone.70 The access of the bone particles to the bone–implant surface is more frequent on, but not restricted to, cementless components. According to a study by Goodman,71 the wear rate of polyethylene is affected by multiple factors including manufacturing and sterilization methods, backside wear, alignment and stability of the TKR and patient activity level. Malalignment results in asymmetric loading and early loosening and is more common with varus formations.
A study by Math et al72 suggested aseptic loosening is usually painless in the early stages of the disease, but activity-related pain localized to the tissues surrounding the loose components may develop, particularly on weight-bearing. Tibial osteolysis is readily visible on anteroposterior (AP) radiographs with a radiolucent area around the implant or cement, varus or valgus subsidence of the tibial component, cement fragmentation and progressive widening of the cement–bone or bone–prosthesis interface.
Patellar maltracking
Patellar maltracking is the disproportional relationship in the trochlea and the patella which is associated with an abnormal anatomical change.73 The patellofemoral joint is high in complexity and involves the multidirectional (mostly cranial and caudal) articulations between the patellar and the femoral groove. It also consists of the muscles and ligaments which act on the patella for stability and tracking.50 The patella does not remain in the femoral groove during knee movement, which results in pain. Chronic maltracking causes pain by contributing to patellofemoral cartilage damage and OA.74 A study by Manghwani et al75 showed that anterior knee pain was significantly lower when there is a 100% contact between the femoral trochlea of the implant and the patella, suggesting that contact is an important factor in determining pain levels post-TKR.
Patellar maltracking can be caused by different risk factors: the surgical approach, implant or the patient. Factors related to patients are pre-operative patellar subluxation and valgus deformity which can cause the release of the lateral retinacula in the implant.76
Patellar maltracking can also be due to the lack of soft tissue balancing or malpositioning of different components. A dynamic valgus deformity from tibial and femoral internal rotation can be exacerbated by weak hip abductors causing more internal rotation of the femur.77 A cross-sectional study by Laubach et al78 demonstrated an association between anterior knee pain and the strength of the quadriceps muscles, as well as a lower patellar position and a thinner inlay. There was also a significantly lower tissue elasticity in the patellar and quadriceps tendons in subjects with anterior knee pain than those without.
In addition, pain normally depends on the size of the Q-angle, which is the angle formed by a line drawn from the anterior superior iliac spine to central patella, and a line drawn from tibial tubercle to central patella. This is normally 10–15 degrees.79 A large Q-angle due to excessive internal rotation of the implantation of a tibial tray can displace the tibial tuberosity laterally and pull on the patellar tendon.80 This in turn causes pain in the knee in addition to the tightness of the lateral retinaculum.81 Sanchis-Alfonso et al82 discovered that there is an increased density of nociceptors in patients with anterior knee pain at the lateral retinaculum. This could explain why patients with lateral patellar maltracking suffer more pain after TKR.
Overstuffing
Overstuffing occurs when there is an imbalance of the implant thickness compared to the femoral and patellar bone cuts following TKR, which involves a measured resection technique. It occurs in 80% of patients after TKR.83 The relationship between overstuffing and pain is unclear. A study by Marmor et al84 showed that overstuffing TKRs is associated with extensive osteophytes and intra-articular femoral valgus which may suggest an association with excessive joint tension due to implant protrusion contributing to knee pain and stiffness. A study by Kemp et al85 showed that there is no association between Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and combined patellofemoral overstuffing involving the patella and trochlea. The authors suggest that there is a significant association between knee pain/functional score and increasing anterior trochlear offset. However, a study by Beldman et al86 found no correlation between overstuffing and anterior knee pain after TKR and does not suggest any unexplained overstuffed knee joint revision surgery. An overstuffed patellofemoral compartment results in higher pressure and reaction forces in the joint which can contribute to periprosthetic fractures. Patellar maltracking has also been linked to post-operative patellar fractures.87
Polyethylene wear
Polyethylene wear of the arthroplasty articulating surfaces is a common mechanism of TKR failure. Gradual surface wear results in delamination and pitting of the polyethylene insert. Along with adhesive and abrasive wear, this results in the release of multiple debris particles. This is associated with osteolysis and painful synovitis. It most commonly affects the medial compartment and is demonstrated on radiographs as asymmetric joint space narrowing. Imaging may also aid bone loss monitoring, identification and pre-operative quantification of osteolysis.88 Wear-related failure is a complex phenomenon, arising from the inherent tribology of imperfectly congruent surfaces. Factors include the manufacturing and properties of polyethylene, implant design, surgical technique, and patient factors.89 Polyethylene properties, such as density and the degree of cross-linking, have been investigated in their contribution to wear, and in the generation wear-resistant highly cross-linked polyethylene.90 Whilst its introduction in hip arthroplasties has been successful, long-term clinical data on its efficacy in TKR are lacking. Implant factors include component design and polyethylene insert characteristics, e.g. thickness and structure. The surgical technique determines the implant alignment and joint axis which affects polyethylene wear. Patient factors include age, weight and activity level.91
Unresurfaced patella
Looking at patellar resurfacing rates from a global perspective, it appears that the practice varies with geographic location. Rates of resurfacing are highest in the United States at 82% and are far lower internationally, with Norway displaying the lowest rates at 4%. Fraser and Spangehl92 speculate that the reason for this variation hinges not solely on surgeons’ consideration for post-TKR anterior knee pain limitation, but rather on a multitude of factors such as past training methods, individual hospital protocols and personal preferences.
Currently, the literature is split when comparing which practice, patellar resurfacing or unresurfacing, limits post-TKR anterior knee pain the most. Several studies show evidence for and against the practice of patellar resurfacing by comparing rates of post-TKR anterior knee pain: some report that the resurfaced patella confers lower post-operative anterior knee pain rates,93–96 yet others find no difference.93,97,98 Two meta-analyses of 749 and 3,034 TKRs, the latter of which compiled the quantitative findings of 16 randomized controlled trials, found no evidence of a difference between post-operative anterior knee pain rates when comparing resurfaced and unresurfaced patellae.97 In contrast, another meta-analysis of 1,223 cases found that resurfaced patellae conferred a 13.8% absolute risk reduction in post-operative anterior knee pain.96 Overall, no one practice appears immediately favourable over the other.
Several authors have identified reasons patellar resurfacing may pose a risk to the structural integrity of the knee joint and therefore contribute to pain post-operatively. The practice, they argue, carries the underlying risks of patella fracture, patellar tendon injury, joint infection and instability, avascular necrosis, polyethylene wear of the patellar component, aseptic loosening and overstuffing.92–94 These factors can be considered throughout the TKR management pathway.
Infection
Pain is often associated with infections. The pain is thought to be secondary to inflammation and infection. The release of inflammatory cytokines during an infection contributes to the initiation and persistence of pain.99 Cytokines that cause pain primarily include IL-1β.100 In addition, there is also evidence to suggest direct activation of nociceptor neurons by pathogens themselves.101
A major cause for prosthetic joint infection is gram-positive cocci such as Staphylococcus aureus, coagulase-negative staphylococci species, and, increasingly, Corynebacterium species.102–104 Staphylococcus aureus produces pain through the release of α-hemolysin, which activates nociceptor neurones, while formyl peptide nociceptors are also stimulated by n-formyl peptides, which are by-products of all bacterial pathogens.103
Risk factors for infective prosthetic joint infection requiring revision include constrained condylar prostheses, use of posterior-stabilized fixed-bearing prostheses, the requirement of a tibial patellofemoral graft, inflammatory arthropathy, previous septic arthritis, surgery for trauma, peripheral vascular disease, connective tissue and rheumatic diseases, diabetes, chronic pulmonary disease, high body mass index (BMI), higher American Society of Anaesthesiologist (ASA) grade, young age and male gender.105
Other possible factors
A study by Bierke et al106 demonstrated that psychological factors such as somatization dysfunction and depressive symptoms can impact on post-TKR pain score, even with uncomplicated TKR, for up to five years post-operatively. This is consistent with the results from a review by Bonnin et al107 for patients with higher depressive and anxiety states in uncomplicated TKRs. This suggests that psychological variables may influence post-TKR pain. Damage to the saphenous infrapatellar nerve branch following a standard midline skin incision is common in TKRs and this causes painful neuroma that reduces the range of motion of the knee.108–110 In a study by Koh et al,111 central sensitization index (a measure of central sensitization for persistent pain and dissatisfaction following total knee arthroplasty due to the augmentation of the central nervous system signalling for pain) persists in individuals with high pre-operative central sensitization index following TKR compared to those with a lower level. Although with clinical improvement these patients also showed significantly worse pain scores, lower quality of life, functional disability and a correlation with dissatisfaction.
Discussion
In this review, we have identified supporting information for TKR pain, though conflicting results from meta-analyses on the practice of unresurfacing patellae and the findings from studies on overstuffing suggest further research is required. We gathered information on how arthrofibrosis causes pain, which can be explained by the cytokine pathways leading up to an inflammatory response or structural causes. Pain from instability can be explained by tissue insufficiency, buckling and other factors in relation to the implant and the surrounding tissues. Component malpositioning contributes to pain often due to kinematics, tensions and pressure which can be explained by the implant position involving both femoral and tibial components and their rotations. Malpositioning can also increase the risk of instability and polyethylene wear. Polyethylene wear causes pain via the release of debris particles initiating osteolysis. This release is affected by implant properties, patient factors and surgical techniques. Similar to polyethylene wear, pain from aseptic loosening can be explained by osteolysis and can initiate an immunological response. Aseptic loosening is often a result of aforementioned factors including instability, component malposition and polyethylene wear, making their relationship multifactorial and interlinked. In patellar maltracking, pain is often due to a lack of tissue balance or component malpositioning resulting in a poor relationship between the trochlea and the patella with ensuing cartilage damage and OA. In addition, this review has identified common pathogens for pain in TKR infections and discussed the biological explanation for pain. Other causes of post-TKR pain were explored involving the nervous system and psychological factors. Table 1 summarizes our findings.
Table 1.
A summary of the causes of pain identified in this review
| Causes of pain following total knee replacement identified |
|---|
| Arthrofibrosis |
| Aseptic loosening |
| Avascular necrosis |
| Central sensitization |
| Component malpositioning |
| Infection |
| Instability |
| Nerve damage |
| Overstuffing |
| Patellar maltracking |
| Polyethylene wear |
| Psychological factors |
| Unresurfaced patella |
The frequency of TKR surgery has increased over the past decade and its frequency is predicted to rise in the future.2,3 Although TKR surgery has a high satisfaction rate of 80–100%,112 10–34% of patients develop adverse pain as a complication.10 This highlights the importance of addressing pain expectations and managing pain post-operatively. It also emphasizes the importance of early detection and prompt management of the intra-articular causes of pain to improve patient satisfaction.
Being able to identify risk factors for adverse outcomes such as those highlighted in this review is important, as this can be used to predict the likelihood of adverse pain outcomes and to identify specific causes. Controllable risk factors, such as BMI,105 can be managed and considered pre- and post-operatively to minimize complications of pain.
It is likely that the causes of pain will become more evident in upcoming years with technological advancements and a greater understanding of pain aetiology. The consensus is that further studies are required to explore the different aetiologies of pain alongside the development of methods for diagnosis, prevention and management of each specific cause. Healthcare professionals should be prepared to make tailored changes to patient care as there is no ‘one size fits all’ solution.
Through developing effective prevention and management of post-TKR pain, fewer patients will suffer from pain or spend time receiving care as follow-ups, reducing both cost and time. Studies have suggested that insufficient management of acute TKR pain contributes to chronic pain, which also highlights the importance of early diagnosis and appropriate management following TKR.113,114 In addition, with reduced revision frequency, the risks patients are exposed to from invasive surgical procedures are minimized, improving patient outcomes. Resources in any publicly funded health system such as those in the United Kingdom and other European countries can therefore be redistributed.
Footnotes
ICMJE Conflict of interest statement: The author declares no conflict of interest relevant to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Varacallo M, Luo T, Johanson N. Total knee arthroplasty (TKA) techniques. National Center for Biotechnology Information, 2020. https://www.ncbi.nlm.nih.gov/books/NBK499896/ (date last accessed 1 April 2020). [Google Scholar]
- 2. National Joint Registry. NJR 16th Annual Report 2019. https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2016th%20Annual%20Report%202019.pdf (date last accessed 1 April 2020). [PubMed]
- 3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780–785. [DOI] [PubMed] [Google Scholar]
- 4. Rissanen P, Aro S, Slätis P, Sintonen H, Paavolainen P. Health and quality of life before and after hip or knee arthroplasty. J Arthroplasty 1995;10:169–175. [DOI] [PubMed] [Google Scholar]
- 5. Drewett RF, Minns RJ, Sibly TF. Measuring outcome of total knee replacement using quality of life indices. Ann R Coll Surg Engl 1992;74:286–289. [PMC free article] [PubMed] [Google Scholar]
- 6. Fitzgerald JD, Orav EJ, Lee TH, et al. Patient quality of life during the 12 months following joint replacement surgery. Arthritis Rheum 2004;51:100–109. [DOI] [PubMed] [Google Scholar]
- 7. Bachmeier CJ, March LM, Cross MJ, et al. ; Arthritis Cost and Outcome Project Group. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cartilage 2001;9:137–146. [DOI] [PubMed] [Google Scholar]
- 8. Bade MJ, Kohrt WM, Stevens-Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther 2010;40(9):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hofmann S, Seitlinger G, Djahani O, Pietsch M. The painful knee after TKA: a diagnostic algorithm for failure analysis. Knee Surg Sports Traumatol Arthrosc 2011;19:1442–1452. [DOI] [PubMed] [Google Scholar]
- 10. Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2:e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh JA, Lewallen DG. Are outcomes after total knee arthroplasty worsening over time? A time-trends study of activity limitation and pain outcomes. BMC Musculoskelet Disord 2014;15:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erivan R, Jacquet C, Villatte G, Ollivier M, Paprosky W. Epidemiology of painful knee after total knee arthroplasty in a tertiary care center: assessment by decision tree. Knee 2020;27:1049–1056. [DOI] [PubMed] [Google Scholar]
- 13. Preston S, Petrera M, Kim C, Zywiel MG, Gandhi R. Towards an understanding of the painful total knee: what is the role of patient biology? Curr Rev Musculoskelet Med 2016;9:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Usher KM, Zhu S, Mavropalias G, Carrino JA, Zhao J, Xu J. Pathological mechanisms and therapeutic outlooks for arthrofibrosis. Bone Res 2019;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayr H, Stöhr A. Arthroscopic treatment of arthrofibrosis after ACL reconstruction: local and generalized arthrofibrosis. Oper Orthop Traumatol 2014;26:7–18. [DOI] [PubMed] [Google Scholar]
- 16. Schiavone Panni A, Cerciello S, Vasso M, Tartarone M. Stiffness in total knee arthroplasty. J Orthop Traumatol 2009;10:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gandhi R, de Beer J, Leone J, Petruccelli D, Winemaker M, Adili A. Predictive risk factors for stiff knees in total knee arthroplasty. J Arthroplasty 2006;21:46–52. [DOI] [PubMed] [Google Scholar]
- 18. Gollwitzer H, Burgkart R, Diehl P, Gradinger R, Bühren V. [Therapy of arthrofibrosis after total knee arthroplasty]. Orthopade 2006;35:143–152. [DOI] [PubMed] [Google Scholar]
- 19. Phan SH. The myofibroblast in pulmonary fibrosis. Chest 2002;122:286S–289S. [DOI] [PubMed] [Google Scholar]
- 20. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 2007;170:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeichen J, Haeder L, Jagodzinski M, Lobenhoffer P, Bosch U, Brand J. [Localization of TGF-beta and PDGF and their relevance for the pathogenesis of arthrofibrosis]. Unfallchirurg 2008;111:79–84. [DOI] [PubMed] [Google Scholar]
- 23. Dixon D, Coates J, del Carpio Pons A, et al. A potential mode of action for Anakinra in patients with arthrofibrosis following total knee arthroplasty. Sci Rep 2015;5:16466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayr HO, Hochrein A. The stiff knee. Knee 2015;22:354–355. [DOI] [PubMed] [Google Scholar]
- 25. Mayr H, Tröger M, Hein W. Retropatellararthrose bei Arthrofibrose. Arthroskopie 2005;18:308–312. [Google Scholar]
- 26. Pujol N, Boisrenoult P, Beaufils P. Post-traumatic knee stiffness: surgical techniques. Orthop Traumatol Surg Res 2015;101:S179–S186. [DOI] [PubMed] [Google Scholar]
- 27. Kim YS, Youn HK, Kim BS, Choi YJ, Koh YG. Arthroscopic evaluation of persistent pain following supramalleolar osteotomy for varus ankle osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2016;24:1860–1807. [DOI] [PubMed] [Google Scholar]
- 28. Menetski J, Mistry S, Lu M, et al. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience 2007;149:706–714. [DOI] [PubMed] [Google Scholar]
- 29. White FA, Sun J, Waters SM, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A 2005;102:14092–14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov 2005;4:834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ji R-R, Xu Z-Z, Gao Y-J. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014;13:533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paish HL, Kalson NS, Smith GR, et al. Fibroblasts promote inflammation and pain via IL-1α induction of the monocyte chemoattractant chemokine (C-C Motif) ligand 2. Am J Pathol 2018;188:696–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleeton G, Harmer AR, Nairn L, et al. Self-reported knee instability before and after total knee replacement surgery. Arthritis Care Res (Hoboken) 2016;68:463–471. [DOI] [PubMed] [Google Scholar]
- 34. Leichtenberg CS, Vliet Vlieland TPM, Kroon HM, et al. ; LOAS Studygroup. Self-reported knee instability associated with pain, activity limitations, and poorer quality of life before and 1 year after total knee arthroplasty in patients with knee osteoarthritis. J Orthop Res 2018;36:2671–2678. [DOI] [PubMed] [Google Scholar]
- 35. van der Esch M, Knoop J, van der Leeden M, et al. Self-reported knee instability and activity limitations in patients with knee osteoarthritis: results of the Amsterdam osteoarthritis cohort. Clin Rheumatol 2012;31:1505–1510. [DOI] [PubMed] [Google Scholar]
- 36. Felson DT, Niu J, McClennan C, et al. Knee buckling: prevalence, risk factors, and associated limitations in function. Ann Intern Med 2007;147:534–540. [DOI] [PubMed] [Google Scholar]
- 37. Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum 2004;51:941–946. [DOI] [PubMed] [Google Scholar]
- 38. Abdel MP, Pulido L, Severson EP, Hanssen AD. Stepwise surgical correction of instability in flexion after total knee replacement. Bone Joint J 2014;96-B:1644–1648. [DOI] [PubMed] [Google Scholar]
- 39. Seil R, Pape D. Causes of failure and etiology of painful primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2011;19:1418–1432. [DOI] [PubMed] [Google Scholar]
- 40. Cottino U, Sculco PK, Sierra RJ, Abdel MP. Instability after total knee arthroplasty. Orthop Clin North Am 2016;47:311–316. [DOI] [PubMed] [Google Scholar]
- 41. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res 2001;392:315–318. [DOI] [PubMed] [Google Scholar]
- 42. Lewallen EA, Salib CG, Trousdale WH, et al. Molecular pathology of total knee arthroplasty instability defined by RNA-seq. Genomics 2018;110:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sharma RK. Mid flexion instability after primary total knee arthroplasty. Orthopaedic Proceedings 2013;95-B:326. [Google Scholar]
- 44. Rouquette L, Erivan R, Pereira B, Boisgard S, Descamps S, Villatte G. Tibiofemoral dislocation after primary total knee arthroplasty: a systematic review. Int Orthop 2019;43:1599–1609. [DOI] [PubMed] [Google Scholar]
- 45. Slane LC, Dandois F, Bogaerts S, Scheys L, Vandenneucker H. Patellar tendon buckling in post-operative total knee arthroplasty patients is more prominent than in healthy controls. Med Eng Phys 2019;69:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wilson CJ, Theodoulou A, Damarell RA, Krishnan J. Knee instability as the primary cause of failure following total knee arthroplasty (TKA): a systematic review on the patient, surgical and implant characteristics of revised TKA patients. Knee 2017;24:1271–1281. [DOI] [PubMed] [Google Scholar]
- 47. Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today: has anything changed after 10 years? J Arthroplasty 2014;29:1774–1778. [DOI] [PubMed] [Google Scholar]
- 48. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res 2002;404:7–13. [DOI] [PubMed] [Google Scholar]
- 49. Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi N-Y, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res 1994;12:796–803. [DOI] [PubMed] [Google Scholar]
- 50. Petersen W, Rembitzki IV, Brüggemann G-P, et al. Anterior knee pain after total knee arthroplasty: a narrative review. Int Orthop 2014;38:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lützner J, Kirschner S, Günther KP, Harman MK. Patients with no functional improvement after total knee arthroplasty show different kinematics. Int Orthop 2012;36:1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Kempen RW, Schimmel JJ, van Hellemondt GG, Vandenneucker H, Wymenga AB. Reason for revision TKA predicts clinical outcome: prospective evaluation of 150 consecutive patients with 2-years followup. Clin Orthop Relat Res 2013;471:2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech (Bristol, Avon) 2005;20:661–668. [DOI] [PubMed] [Google Scholar]
- 54. Vince KG. Why knees fail. J Arthroplasty 2003;18:39–44. [DOI] [PubMed] [Google Scholar]
- 55. Fuchs S, Skwara A, Tibesku CO, Rosenbaum D. Retropatellar contact characteristics before and after total knee arthroplasty. Knee 2005;12:9–12. [DOI] [PubMed] [Google Scholar]
- 56. Howell SM, Hodapp EE, Vernace JV, Hull ML, Meade TD. Are undesirable contact kinematics minimized after kinematically aligned total knee arthroplasty? An intersurgeon analysis of consecutive patients. Knee Surg Sports Traumatol Arthrosc 2013;21:2281–2287. [DOI] [PubMed] [Google Scholar]
- 57. Steinbruck A, Fottner A, Schroder C, Woiczinski M, Schmitt-Sody M, Muller T, et al. Influence of mediolateral tibial baseplate position in TKA on knee kinematics and retropatellar pressure. Knee Surg Sports Traumatol Arthrosc 2017;25:2602–2608. [DOI] [PubMed] [Google Scholar]
- 58. Kuriyama S, Ishikawa M, Furu M, Ito H, Matsuda S. Malrotated tibial component increases medial collateral ligament tension in total knee arthroplasty. J Orthop Res 2014;32:1658–1666. [DOI] [PubMed] [Google Scholar]
- 59. Thompson JA, Hast MW, Granger JF, Piazza SJ, Siston RA. Biomechanical effects of total knee arthroplasty component malrotation: a computational simulation. J Orthop Res 2011;29:969–975. [DOI] [PubMed] [Google Scholar]
- 60. Murakami AM, Hash TW, Hepinstall MS, Lyman S, Nestor BJ, Potter HG. MRI evaluation of rotational alignment and synovitis in patients with pain after total knee replacement. J Bone Joint Surg Br 2012;94:1209–1215. [DOI] [PubMed] [Google Scholar]
- 61. Berger RA, Crossett LS, Jacobs JJ, Rubash HE. Malrotation causing patellofemoral complications after total knee arthroplasty. Clin Orthop Relat Res 1998;356:144–153. [DOI] [PubMed] [Google Scholar]
- 62. Barrack RL, Schrader T, Bertot AJ, Wolfe MW, Myers L. Component rotation and anterior knee pain after total knee arthroplasty. Clin Orthop Relat Res 2001;392:46–55. [DOI] [PubMed] [Google Scholar]
- 63. Woiczinski M, Kistler M, Schröder C, et al. TKA design-integrated trochlea groove rotation reduces patellofemoral pressure. Knee Surg Sports Traumatol Arthrosc 2019;27:1680–1692. [DOI] [PubMed] [Google Scholar]
- 64. Fottner A, Woiczinski M, Schröder C, et al. Impact of tibial baseplate malposition on kinematics, contact forces and ligament tensions in TKA: a numerical analysis. J Mech Behav Biomed Mater 2020;103:103564. [DOI] [PubMed] [Google Scholar]
- 65. Nicoll D, Rowley DI. Internal rotational error of the tibial component is a major cause of pain after total knee replacement. J Bone Joint Surg Br 2010;92:1238–1244. [DOI] [PubMed] [Google Scholar]
- 66. Dalury DF, Pomeroy DL, Gorab RS, Adams MJ. Why are total knee arthroplasties being revised? J Arthroplasty 2013;28:120–121. [DOI] [PubMed] [Google Scholar]
- 67. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty 2013;28:116–119. [DOI] [PubMed] [Google Scholar]
- 68. Crotti TN, Smith MD, Findlay DM, et al. Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: expression of receptor activator NFkappaB, RANK ligand and osteoprotegerin. Biomaterials 2004;25:565–573. [DOI] [PubMed] [Google Scholar]
- 69. Gehrke T, Sers C, Morawietz L, et al. Receptor activator of nuclear factor kappaB ligand is expressed in resident and inflammatory cells in aseptic and septic prosthesis loosening. Scand J Rheumatol 2003;32:287–294. [DOI] [PubMed] [Google Scholar]
- 70. Higuera C, Parvizi J. 18 causes and diagnosis of aseptic loosening after total knee replacement. In: Hirschmann MT, Becker R, eds. The unhappy total knee replacement: a comprehensive review and management guide. Cham: Springer, 2015:225-237. [Google Scholar]
- 71. Goodman S. Wear particulate and osteolysis. Orthop Clin North Am 2005;36:41–48, vi. [DOI] [PubMed] [Google Scholar]
- 72. Math KR, Zaidi SF, Petchprapa C, Harwin SF. Imaging of total knee arthroplasty. Semin Musculoskelet Radiol 2006;10:47–63. [DOI] [PubMed] [Google Scholar]
- 73. Jibri Z, Jamieson P, Rakhra KS, Sampaio ML, Dervin G. Patellar maltracking: an update on the diagnosis and treatment strategies. Insights Imaging 2019;10:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tsavalas N, Katonis P, Karantanas AH. Knee joint anterior malalignment and patellofemoral osteoarthritis: an MRI study. Eur Radiol 2012;22:418–428. [DOI] [PubMed] [Google Scholar]
- 75. Manghwani J, Vaidya SV, Patel H, Vaidya CS. Does total contact of the patella with the femoral trochlea during no thumb test significantly reduce anterior knee pain? Knee 2019;26:1338–1347. [DOI] [PubMed] [Google Scholar]
- 76. Gasparini G, Familiari F, Ranuccio F. Patellar malalignment treatment in total knee arthroplasty. Joints 2013;1:10–17. [PMC free article] [PubMed] [Google Scholar]
- 77. MacIntyre NJ, Hill NA, Fellows RA, Ellis RE, Wilson DR. Patellofemoral joint kinematics in individuals with and without patellofemoral pain syndrome. J Bone Joint Surg Am 2006;88:2596–2605. [DOI] [PubMed] [Google Scholar]
- 78. Laubach M, Hellmann JT, Dirrichs T, et al. Anterior knee pain after total knee arthroplasty: a multifactorial analysis. J Orthop Surg (Hong Kong) 2020;28:2309499020918947. [DOI] [PubMed] [Google Scholar]
- 79. Horton MG, Hall TL. Quadriceps femoris muscle angle: normal values and relationships with gender and selected skeletal measures. Phys Ther 1989;69:897–901. [DOI] [PubMed] [Google Scholar]
- 80. McPherson EJ. Patellar tracking in primary total knee arthroplasty. Instr Course Lect 2006;55:439–448. [PubMed] [Google Scholar]
- 81. Mizuno Y, Kumagai M, Mattessich SM, et al. Q-angle influences tibiofemoral and patellofemoral kinematics. J Orthop Res 2001;19:834–840. [DOI] [PubMed] [Google Scholar]
- 82. Sanchis-Alfonso V, Roselló-Sastre E, Monteagudo-Castro C, Esquerdo J. Quantitative analysis of nerve changes in the lateral retinaculum in patients with isolated symptomatic patellofemoral malalignment: a preliminary study. Am J Sports Med 1998;26:703–709. [DOI] [PubMed] [Google Scholar]
- 83. Kandhari VK, Desai MM, Bava SS, Wade RN. Digging deeper into the patella-femoral joint: patello-femoral composite – a new dimension for overstuffing of patello-femoral joint. J Clin Diagn Res 2017;11:RC04–RC07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Marmor S, Renault E, Valluy J, Saffarini M. Over-voluming predicted by pre-operative planning in 24% of total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2019;27:1544–1551. [DOI] [PubMed] [Google Scholar]
- 85. Kemp MA, Metcalfe AJ, Sayers A, Wylde V, Eldridge JD, Blom AW. Does overstuffing of the patellofemoral joint in total knee arthroplasty have a significant effect on postoperative outcomes? Knee 2018;25:874–881. [DOI] [PubMed] [Google Scholar]
- 86. Beldman M, Breugem SJM, van Jonbergen HPW. Overstuffing in total knee replacement: no effect on clinical outcomes or anterior knee pain. Int Orthop 2015;39:887–891. [DOI] [PubMed] [Google Scholar]
- 87. Assiotis A, To K, Morgan-Jones R, Pengas IP, Khan W. Patellar complications following total knee arthroplasty: a review of the current literature. Eur J Orthop Surg Traumatol 2019;29:1605–1615. [DOI] [PubMed] [Google Scholar]
- 88. Fritz J, Lurie B, Miller TT. Imaging of hip arthroplasty. Semin Musculoskelet Radiol 2013;17:316–327. [DOI] [PubMed] [Google Scholar]
- 89. Chakrabarty G, Vashishtha M, Leeder D. Polyethylene in knee arthroplasty: a review. J Clin Orthop Trauma 2015;6:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lachiewicz PF, Geyer MR. The use of highly cross-linked polyethylene in total knee arthroplasty. J Am Acad Orthop Surg 2011;19:143–151. [DOI] [PubMed] [Google Scholar]
- 91. Naudie DD, Ammeen DJ, Engh GA, Rorabeck CH. Wear and osteolysis around total knee arthroplasty. J Am Acad Orthop Surg 2007;15:53–64. [DOI] [PubMed] [Google Scholar]
- 92. Fraser JF, Spangehl MJ. International rates of patellar resurfacing in primary total knee arthroplasty, 2004–2014. J Arthroplasty 2017;32:83–86. [DOI] [PubMed] [Google Scholar]
- 93. Meneghini RM. Should the patella be resurfaced in primary total knee arthroplasty? An evidence-based analysis. J Arthroplasty 2008;23:11–14. [DOI] [PubMed] [Google Scholar]
- 94. Migliorini F, Eschweiler J, Niewiera M, El Mansy Y, Tingart M, Rath B. Better outcomes with patellar resurfacing during primary total knee arthroplasty: a meta-analysis study. Arch Orthop Trauma Surg 2019;139:1445–1454. [DOI] [PubMed] [Google Scholar]
- 95. Nizard RS, Biau D, Porcher R, et al. A meta-analysis of patellar replacement in total knee arthroplasty. Clin Orthop Relat Res 2005;432:196–203. [DOI] [PubMed] [Google Scholar]
- 96. Pakos EE, Ntzani EE, Trikalinos TA. Patellar resurfacing in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am 2005;87:1438–1445. [DOI] [PubMed] [Google Scholar]
- 97. Fu Y, Wang G, Fu Q. Patellar resurfacing in total knee arthroplasty for osteoarthritis: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 2011;19:1460–1466. [DOI] [PubMed] [Google Scholar]
- 98. He JY, Jiang LS, Dai LY. Is patellar resurfacing superior than nonresurfacing in total knee arthroplasty? A meta-analysis of randomized trials. Knee 2011;18:137–144. [DOI] [PubMed] [Google Scholar]
- 99. Zhang J-M, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007;45:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Copray JC, Mantingh I, Brouwer N, et al. Expression of interleukin-1 beta in rat dorsal root ganglia. J Neuroimmunol 2001;118:203–211. [DOI] [PubMed] [Google Scholar]
- 101. Chiu IM, Pinho-Ribeiro FA, Woolf CJ. Pain and infection: pathogen detection by nociceptors. Pain 2016;157:1192–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev 2014;27:302–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chiu IM, Heesters BA, Ghasemlou N, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013;501:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hernandez NM, Buchanan MW, Cullen MM, et al. Corynebacterium total hip and knee arthroplasty prosthetic joint infections. Arthroplast Today 2020;6:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lenguerrand E, Whitehouse MR, Beswick AD, et al. ; National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lancet Infect Dis 2019;19:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bierke S, Häner M, Karpinski K, Hees T, Petersen W. Midterm effect of mental factors on pain, function, and patient satisfaction 5 years after uncomplicated total knee arthroplasty. J Arthroplasty 2020;35:105–111. [DOI] [PubMed] [Google Scholar]
- 107. Bonnin MP, Basiglini L, Archbold HAP. What are the factors of residual pain after uncomplicated TKA? Knee Surg Sports Traumatol Arthrosc 2011;19:1411–1417. [DOI] [PubMed] [Google Scholar]
- 108. Harris JD, Fazalare JJ, Griesser MJ, Flanigan DC. Infrapatellar branch of saphenous neurectomy for painful neuroma: a case report. Am J Orthop (Belle Mead NJ) 2012;41:37–40. [PubMed] [Google Scholar]
- 109. Kachar SM, Williams KM, Finn HA. Neuroma of the infrapatellar branch of the saphenous nerve a cause of reversible knee stiffness after total knee arthroplasty. J Arthroplasty 2008;23:927–930. [DOI] [PubMed] [Google Scholar]
- 110. Sundaram RO, Ramakrishnan M, Harvey RA, Parkinson RW. Comparison of scars and resulting hypoaesthesia between the medial parapatellar and midline skin incisions in total knee arthroplasty. Knee 2007;14:375–378. [DOI] [PubMed] [Google Scholar]
- 111. Koh IJ, Kang BM, Kim MS, Choi KY, Sohn S, In Y. How does preoperative central sensitization affect quality of life following total knee arthroplasty? J Arthroplasty 2020. 10.1016/j.arth.2020.04.004 [Epub ahead of print]. [DOI] [PubMed]
- 112. Kahlenberg CA, Nwachukwu BU, McLawhorn AS, Cross MB, Cornell CN, Padgett DE. Patient satisfaction after total knee replacement: a systematic review. HSS J 2018;14:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Perkins FM, Kehlet H. Chronic pain as an outcome of surgery: a review of predictive factors. Anesthesiology 2000;93:1123–1133. [DOI] [PubMed] [Google Scholar]
- 114. Puolakka PA, Rorarius MG, Roviola M, Puolakka TJ, Nordhausen K, Lindgren L. Persistent pain following knee arthroplasty. Eur J Anaesthesiol 2010;27:455–460. [DOI] [PubMed] [Google Scholar]