Abstract
The lung has a vital function in gas exchange between the blood and the external atmosphere. It also has a critical role in the immune defense against external pathogens and environmental factors. While the lung is classified as a relatively quiescent organ with little homeostatic turnover, it shows robust regenerative capacity in response to injury, mediated by the resident stem/progenitor cells. During regeneration, regionally distinct epithelial cell populations with specific functions are generated from several different types of stem/progenitor cells localized within four histologically distinguished regions: trachea, bronchi, bronchioles, and alveoli. WNT signaling is one of the key signaling pathways involved in regulating many types of stem/progenitor cells in various organs. In addition to its developmental role in the embryonic and fetal lung, WNT signaling is critical for lung homeostasis and regeneration. In this minireview, we summarize and discuss recent advances in the understanding of the role of WNT signaling in lung regeneration with an emphasis on stem/progenitor cells.
Keywords: β-catenin, lung homeostasis, lung regeneration, lung stem/progenitor cells, WNT signaling
INTRODUCTION
The lung is a structurally complex organ comprising at least 40 different types of cells with diverse functions. It can be sub-divided anatomically into four different regions—trachea, bronchi, bronchioles, and alveoli. To fulfill its different functions, the cellular composition and three-dimensional structure of the lung must be maintained throughout an organism’s lifetime. Under normal conditions, the lung shows a low rate of cellular turnover relative to other organs, such as the skin and intestine, which exhibit rapid kinetics of cellular replacement characteristics (Bowden et al., 1968; Kauffman, 1980; Wansleeben et al., 2013). However, after injury or damage caused by different agents, including infection, toxic compounds, and irradiation, the lung demonstrates a remarkable ability to regenerate the damaged tissue (Hogan et al., 2014; Lee and Rawlins, 2018; Wansleeben et al., 2013). If this regeneration process is not completed successfully, it leads to a disruption in proper lung function accompanied by chronic inflammation, pathologic remodeling, and fibrosis.
Normal cell turnover and regeneration after injury in the lung require regionally- specific epithelial stem/progenitor cells and unique microenvironment or neighboring cells that can crosstalk with each other (Lee and Rawlins, 2018; Rock and Hogan, 2011; Wansleeben et al., 2013). It has been challenging to distinguish the specific properties of lung stem/progenitor cells owing to the histological complexity of the lung and the multiplicity of cell types, besides the slow turnover of the respiratory epithelium. Each region comprises different cell types and the homeostasis in each region is guaranteed by regionally-specific stem/progenitor cells (Ardhanareeswaran and Mirotsou, 2013; Spurlin and Nelson, 2017; Wansleeben et al., 2013). In addition, the regenerative capacity of these lung stem/progenitor cells is not only determined by their intrinsic potential, but also by the microenvironment of their specific niche, which includes the extracellular matrix, stromal and other types of cells, as well as, the many signaling factors derived from them (Hogan et al., 2014; Lee and Rawlins, 2018; Stabler and Morrisey, 2017).
The WNT signaling pathway plays major roles during embryogenesis, tissue homeostasis, and regeneration in many organs (Kahn, 2018; Nusse and Clevers, 2017; Steinhart and Angers, 2018). The functional importance of WNT signaling in the lung is initially demonstrated during lung development, as early as at the stage of lung endoderm specification (Hussain et al., 2017; Majidinia et al., 2018). Recent studies have begun to reveal the roles of WNT signaling in lung homeostasis and regeneration. In this review, we discuss the recent findings on the role of WNT signaling in lung homeostasis and regeneration, while highlighting different lung stem/progenitor cells and the mechanisms that regulate their behavior and function.
OVERVIEW OF WNT SIGNALING PATHWAY
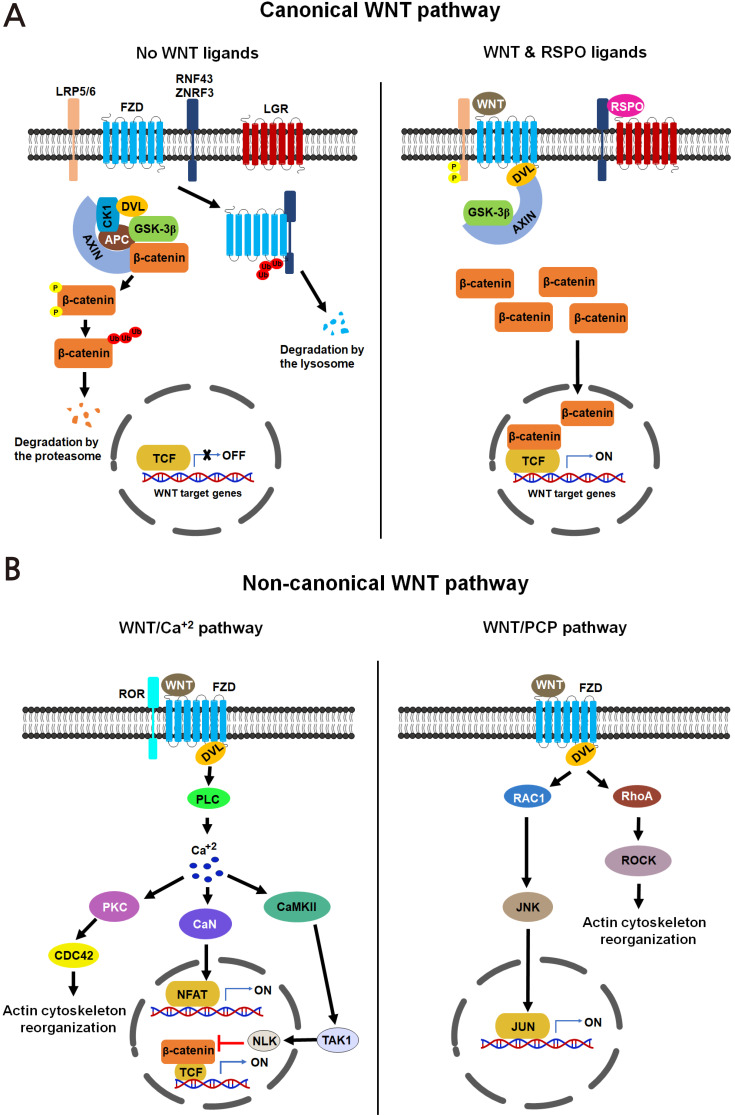
Secreted WNT ligands, comprising 19 members, are highly conserved signaling molecules that activate downstream signaling cascades involved in the regulation of gene expression, proliferation, differentiation, adhesion and migration, and polarity in signaling-receiving cells (Kahn, 2018; Nusse and Clevers, 2017; Steinhart and Angers, 2018). The two major WNT signaling pathways are canonical (WNT/β-catenin) and non-canonical pathways (Fig. 1). WNT1 (WNT2, WNT3, WNT3A, and WNT8A) and WNT5A (WNT4, WNT5A, WNT5B, WNT6, WNT7A, and WNT11) class ligands primarily determine the specificity of activation of the canonical and non-canonical pathways, respectively. In the canonical WNT signaling, binding of the WNT1 class ligands to the seven-pass trans-membrane Frizzled receptor family (FZD) and the low-density lipoprotein receptor-related protein family (LRP5/6) initiates a signaling cascade. A hallmark of this pathway is an accumulation of the normally unstable β-catenin protein within the cytoplasm through the inhibition of glycogen synthase kinase 3 beta (GSK-3β) and the subsequent translocation of β-catenin to the nucleus, where it engages in gene transcription (Nusse and Clevers, 2017; Steinhart and Angers, 2018). In contrast, non-canonical WNT signaling is activated by WNT5A class ligands via the FZD receptors, independent of LRP5/6 receptors (Chae and Bothwell, 2018; Steinhart and Angers, 2018). This ligand-receptor binding initiates the activation of WNT/PCP (planar cell polarity) and WNT/Ca2+ pathways. The WNT/PCP pathway activates JNK and Rho-kinase cascades, whereas the WNT/Ca2+ pathway increases intracellular calcium concentration and activates protein kinase C (PKC), calcineurin, and calcium/calmodulin-dependent protein kinase II cascades. In addition to the typical FZD and LRP5/6 receptors, receptor tyrosine kinases (RYK) and retinoic acid-related orphan receptors are also known to function in a cell type-specific manner as cognate receptors for canonical and non-canonical WNT signaling, respectively (Chae and Bothwell, 2018; Nusse and Clevers, 2017). Furthermore, while WNT signaling is positively regulated through secretory agonists, such as R-spondins (RSPOs) and norrin, it is negatively regulated through secretory antagonists, such as dickkopf-1 (DKK1), secreted FZD-related proteins, WNT inhibitory factor 1, and sclerostin (Chae and Bothwell, 2018; Nusse and Clevers, 2017; Raslan and Yoon, 2019).
Fig. 1. WNT signaling pathway.
Canonical (A) and non-canonical (B) WNT signaling pathways are shown. Both WNT and RSPO ligands synergistically act to generate a maximum activation in canonical WNT signaling. WNT, wingless-type MMTV integration site family; LRP5/6, low density lipoprotein receptor-related protein 5/6; FZD, frizzled; RNF43, ring finger protein 43; ZNRF3, zinc and ring finger 3; LGR, leucine-rich repeat containing G protein-coupled receptor; AXIN, axis inhibition protein; CK1, casein kinase 1; APC, adenomatous polyposis coli; DVL, dishevelled; GSK-3β, glycogen synthase kinase 3 beta; TCF, T-cell factor; RSPO, R-spondin; ROR, retinoic acid-related orphan receptors; RYK, receptor tyrosine kinases; PLC, phospholipase C; PKC, protein kinase C; CDC42, cell division cycle 42; CaN, calcineurin; NFAT, nuclear factor of activated T cells; CaMKII, calcium/calmodulin-dependent protein kinase II; TAK1, TGF-beta-activated kinase 1; NLK, Nemo-like kinase; RAC1, Rac family small GTPase 1; JNK, c-Jun N-terminal kinase; RhoA, ras homolog family member A; ROCK, Rho-associated coiled-coil containing protein kinase.
WNT SIGNALING IN LUNG REPAIR AND REGENERATION
The epithelial cells of the airway walls represent an effective physical barrier against the external atmosphere and are the first cell type that are damaged by exogenous microorganisms, airborne irritants, and allergens. The epithelial cells exhibit unique stereotypic patterns from the proximal airway axis to the distal airway axis. Multiple studies have shown that diverse types of cells, possessing stem/progenitor-like behavior in response to airway injury, are present throughout the airways (Lee and Rawlins, 2018; Rock et al., 2010; Stabler and Morrisey, 2017; Wansleeben et al., 2013). Both canonical WNT/β-catenin and non-canonical WNT signaling appear to play diverse roles in various stem/progenitor cells and niches cells in a region-dependent manner during regeneration.
Trachea
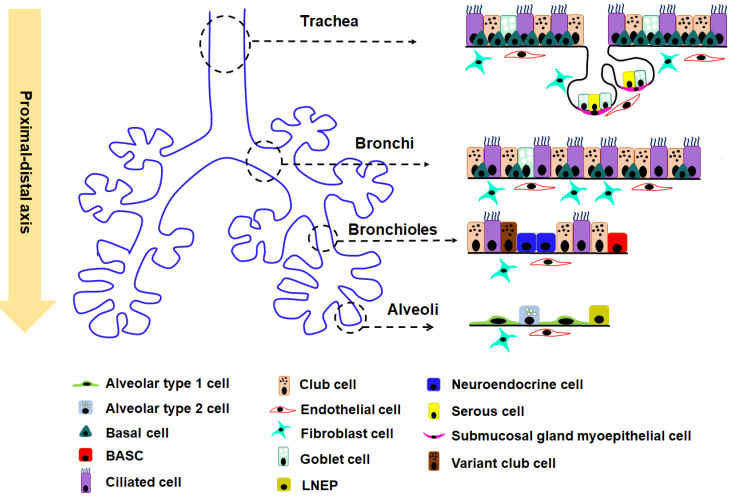
A pseudo-stratified columnar epithelium, consisting of basal, secretory (goblet and club [or Clara]), and ciliated cells, lines the trachea and bronchi (Fig. 2) (Hogan et al., 2014; Mercer et al., 1994; Wansleeben et al., 2013). In humans, a majority of the epithelial cells in the trachea are ciliated (Hogan and Tata, 2019; Meyerholz et al., 2018). In contrast, the majority of the epithelial cells in mouse trachea are non-ciliated (Hogan and Tata, 2019; Meyerholz et al., 2018). Moreover, few goblet cells and more club cells exist in mice trachea than in humans (Hogan and Tata, 2019; Meyerholz et al., 2018). Bronchioles contain simple columnar epithelial cells, including club, ciliated, and pulmonary neuroendocrine cells (NECs). NECs are more solitary and scattered in the airways in humans than in mice (Hogan and Tata, 2019; Meyerholz et al., 2018).
Fig. 2. Heterogeneity and distribution of epithelial cells in different regions of the mouse lung.
Cell distribution within four different regions—Trachea, Bronchi, Bronchioles, and Alveoli—are shown along the proximal-distal axis of mouse lung. BASC, bronchioalveolar stem cell; LNEP, lineage-negative epithelial stem/progenitor cells.
Two tracheal cell populations have been identified as stem/progenitor cells in response to injury. The first population is that of basal cells (BCs) that comprise about 30% of the total epithelial population in the trachea. BCs are pluripotent and express the transcription factor TRP63, cytokeratin 5 (KRT5), and the nerve growth factor receptor (NGFR) (Fig. 3, Table 1) (Hogan et al., 2014; Rock et al., 2009; Tata and Rajagopal, 2017). In steady-state and regeneration following damage, BCs can self-renew and differentiate into multiple cell lineages, including ciliated and secretory cells in vivo (Chen and Fine, 2016; Lee and Rawlins, 2018; Tata and Rajagopal, 2017). More recently, a population of basal-like cells (KRT5+cytokeratin14(KRT14+)actinα2+ (ACTA2), also called myoepithelial cells [MECs]) was identified in the submucosal gland of the trachea (only the proximal trachea in mice) (Lynch et al., 2018; Meyerholz et al., 2018). Following airway injury, these cells possess the ability to differentiate into basal-like stem cells and subsequently form ciliated, secretory, and non-ciliated columnar cells in vivo. It remains to be determined whether these cells are true tracheal stem cells.
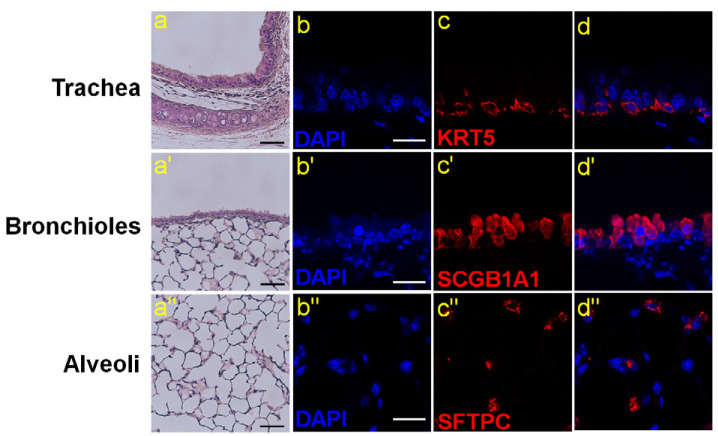
Fig. 3. Major stem cell populations in the lung.
(a, a’, a’’) Hematoxylin and eosin staining of the trachea, bronchioles and alveoli area in the adult lung. Scale bars = 50 μm. (b-d) Immunofluorescence staining of basal cells marker, cytokeratin 5 (KRT5) in the trachea. Scale bar = 20 μm. (b’-d’) Immunofluorescence staining of club cells marker, secretoglobin 1A1 (SCGB1A1) in bronchioles. Scale bar = 20 μm. (b’’-d’’) Immunofluorescence staining of AT2 cells marker, surfactant protein C (SFTPC) in alveoli. Scale bar = 20 μm.
Table 1.
WNT signaling in various epithelial stem/progenitor cells in mouse lung
| Stem cell type | Location | Markers | Contribution to regeneration | Differentiation outcome | WNT signaling role | Reference |
|---|---|---|---|---|---|---|
| Basal cells (BCs) | Trachea, bronchi (cartilaginous airways) | TRP63, KRT5 (and variably KRT14), NGFR, PDPN (T1α) | Major | Ciliated cells, secretory cells (club and goblet cells) | Increase cell expansion and direct differentiation | (Brechbuhl et al., 2011; Giangreco et al., 2012; Rock et al., 2009) |
| Club cells | Trachea, bronchi and bronchioles | SCGB1A1(also known as CCSP and CC10), UPK3A |
Major | Ciliated cells, secretory cells (club and goblet cells), basal cells, AT2 cells, AT1 |
Inhibit differentiation into goblet cells by RYK receptor | (Guha et al., 2017; Lee et al., 2017; Rawlins et al., 2009; Rock et al., 2009; Tata et al., 2013) |
| Submucosal gland duct cell (myoepithelial cells [MECs]) | Trachea, bronchi and bronchioles | TRP63, KRT5, KRT14, rich in ACTA2 | Minor | Trachea pithelium | Promotes the transition to basal cell phenotype | (Lynch et al., 2018) |
| Neuroendocrine cells (NECs) | Bronchioles | Calcitonin gene-related peptide | Minor | Ciliated cells, club cells | Not reported | (Song et al., 2012; Yao et al., 2018) |
| Bronchoalveolar stem cells (BASCs) | Bronchoalveolar duct junction | SCGB1A1, SFTPC | Major | Ciliated cells, club cells, AT1 cells, AT2 cells | Increase cell expansion | (Kim et al., 2005; Liu et al., 2019; Salwig et al., 2019; Zhang et al., 2008) |
| Lineage-negative epithelial stem/progenitor (LNEP) | Alveoli | TRP63, KRT5, H2-K1high | Minor | AT1 cells, AT2 cells | Enhance differentiation into AT2 | (Kathiriya et al., 2020; Kumar et al., 2011; Vaughan et al., 2015; Xi et al., 2017; Zuo et al., 2015) |
| Distal lung progenitors (SFTPC-ve AT2 cells) | Alveoli | Integrin α6β4 | Minor | Club cells, AT2 cells | Not reported | (Chapman et al., 2011) |
| Alveolar type 2 cells (AT2) | Alveoli | SFTPC, AQP3, ABCA3 | Major | AT1 cells | Increase cell expansion and proper differentiation during regeneration | (Barkauskas et al., 2013; Desai et al., 2014; Nabhan et al., 2018; Olajuyin et al., 2019; Tanjore et al., 2013; Zacharias et al., 2018) |
| Alveolar type 1 cells (AT1) | Alveoli | AQP5, PDPN (T1α), HOPX | Minor | AT2 cells | Not reported | (Jain et al., 2015) |
Independent of BCs, tracheal club cells expressing secretoglobin 1A1 (SCGB1A1, also known as CCSP, CC10, or uteroglobin) display a limited stem/progenitor cell property (Fig. 3, Table 1). While tracheal club cells exhibit a relatively slow rate of self-renewal (Rawlins et al., 2009), upon tracheal injury, they are able to give rise to ciliated cells, but not to other types of cells in vivo (Chen and Fine, 2016; Lee and Rawlins, 2018; Rawlins et al., 2009; Tata and Rajagopal, 2017). Interestingly, tracheal club cells show a plasticity to dedifferentiate into basal-like cells after genetic ablation of KRT5+ BCs (Tata et al., 2013). These basal-like cells are multipotent and following airway injury caused by sulfur dioxide or influenza virus infection, these cells are able to regenerate into the tracheal epithelium, including BCs, club, and ciliated cells.
In the trachea of adult mice, canonical WNT signaling is activated within BCs following tracheal damage (Brechbuhl et al., 2011; Giangreco et al., 2012) (Fig. 4). In a transgenic mouse model with dominant-negative (N-terminally truncated) lymphoid enhancer-binding factor1 (LEF1) expression driven under the Krt14 promoter, inhibition of β-catenin signaling decreases the proliferation of BCs and impedes the regeneration of lung airway epithelium (Giangreco et al., 2012). Furthermore, in BCs, the degradation-resistant, constitutively active β-catenin protein lacking GSK3 phosphorylation sites at the N-terminal drives the generation of ciliated cells and prevents the production of club-like cells in air-liquid interface culture (Brechbuhl et al., 2011). Recently, it has been reported that following injury, the MECs of submucosal glands in the trachea adopt a BC phenotype via activation of WNT/LEF1 signaling (Fig. 4) (Lynch et al., 2018). Lef1 induction in cultured MECs promotes the transition to a BC phenotype. In addition, conditional activation of Lef1 in MECs promotes the lineage commitment of these cells in the absence of injury.
Fig. 4. WNT signaling in lung repair and regeneration.
WNT signaling acting positively and negatively are shown in blue and red colors, respectively. Dotted lines with an arrow indicate the presumed interaction among different cell types. BC, basal cell; MEC, myoepithelial cell; ASMC, airway smooth muscle cell; BASC, bronchioalveolar stem cell; LNEP, lineage-negative epithelial stem/progenitor cells; AT2, alveolar type 2 cell; AT1, alveolar type 1 cell.
Bronchiole
Unlike trachea, the BC population decreases significantly in the main stem bronchi and is absent in the columnar epithelium of the distal bronchioles in the mouse lung. In contrast, BCs are present throughout all bronchi and bronchioles in the human lung. The goblet cells are very rare in mouse bronchioles airways (Hogan and Tata, 2019; Meyerholz et al., 2018). The epithelia of distal bronchioles comprise club, multi-ciliate, and NECs (Fig. 2) (Hogan and Tata, 2019; Meyerholz et al., 2018). Following naphthalene-induced injury in vivo in this part of the airways, variant club cells located near the neuroendocrine bodies showed the capability of self-renewal and differentiation into ciliated cells to regenerate the epithelium (Chen and Fine, 2016; Lee and Rawlins, 2018; Rawlins et al., 2009; Tata and Rajagopal, 2017) (Fig. 2, Table 1). In the terminal bronchioles, a specific population of club cells expressing Uroplakin3a (UPK3A) shows the ability to generate alveolar type 1 (AT1) and type 2 (AT2) cells and contribute to alveoli repair after bleomycin injury (Guha et al., 2017). Whether these club cells can directly differentiate into both AT1 and AT2 cells is still unclear and needs further investigation. Moreover, the NECs proliferated and a fraction of them gave rise to club and ciliated cells in naphthalene-induced injury in vivo (Song et al., 2012; Yao et al., 2018). Thus, variant club cells and a sub-population of NECs have the potency to become the stem/progenitor cells in bronchioles.
In the most distant region of the airways, bronchioalveolar stem cells (BASCs) that co-express SCGB1A1 (a club cell marker) and surfactant protein C, SFTPC (an AT2 cell marker) are identified in the bronchioalveolar duct junction. These cells are distinct from UPK3A+ club cells (Fig. 2) (Kim et al., 2005; Salwig et al., 2019). In response to airway injury by naphthalene or influenza virus infection, these BASCs yield diverse populations of differentiated airway cells, including club and ciliated cells in vivo (Kim et al., 2005; Liu et al., 2019; Salwig et al., 2019) (Table 1). These results indicate that BASCs are most likely the main stem/progenitor cells that maintain the airway epithelium of the distal lung.
After naphthalene-induced lung injury, WNT ligands (WMT3A, WNT5A, and WNT7B) are upregulated in club cells at an early stage of regeneration (Lee et al., 2017). This indicates the importance of WNT signaling in the regeneration of the bronchiolar epithelium. Surprisingly, club cell-specific deletion of the Ctnnb1 gene did not affect the proliferation or differentiation of the club cells following naphthalene-induced injury (Zemke et al., 2009). Thus, β-catenin appears to be dispensable for the function of club cells in the regeneration of the bronchiolar epithelium. Interestingly, upon naphthalene injury in the mouse lung depleted of club cells, activation of canonical WNT signaling is also detected in the niche containing BASCs within the distal bronchioles. Expansion of BASCs has been observed in these mice (Zhang et al., 2008). Thus, this suggests that unlike club cells, BASCs may be the major WNT-responsive stem/progenitor cells that engage in bronchiole regeneration (Fig. 4).
Several studies provide additional clues regarding the role of WNT signaling in bronchiole regeneration. Expression of leucine-rich repeat containing G protein-coupled receptor 6 (LGR6), a specific receptor for the WNT positive regulator, RSPOs, and a known marker for many types of stem cells (Barker et al., 2013; Raslan and Yoon, 2019; Schindler et al., 2018) is detected in airway smooth muscle cells (ASMCs), a stromal niche of club cells surrounding the airway epithelia (Lee et al., 2017). These LGR6+ ASMCs play an important role in the regeneration of the airway epithelia. Genetic depletion of LGR6+ ASMCs causes significant defects in airway repair following naphthalene-induced injury in the lung in vivo. In addition, the colony-forming efficiency of club cells co-cultured with mesenchymal cells depleted of the LGR6+ population is severely compromised in ex vivo organoid culture (Lee et al., 2017). It appears that the WNT ligands, expressed in the surviving club cells post injury, induce the expression of FGF10, a mitogenic and surviving factor for club cells, in ASMCs (Lee et al., 2017; Volckaert et al., 2011; 2013). In turn, FGF10 promotes expansion of WNT-independent club cells (Fig. 4). It is noteworthy that LGR6 is reported as a marker for epithelial progenitor cells (E-Cad/LGR6+) rather than mesenchymal cells in the human lung (Oeztuerk-Winder et al., 2012; Ruiz et al., 2014; Skronska-Wasek et al., 2018), suggesting that there may be a fundamental difference existing in the signaling between stem/progenitor cells and their niche in humans. Moreover, WNT-responsive (AXIN2+ PDGFRα–) mesenchymal cells surrounding airways act as myofibrogenic precursors and generate most of the myofibroblasts in the lung after naphthalene-induced injury (Zepp et al., 2017). It is still unknown whether FGF10 from ASMCs also promotes BASCs proliferation and differentiation. The collected evidence demonstrates the importance of the signaling crosstalk between stem/progenitor cells and their niche to ensure proper regeneration in response to injury in the bronchioles in mice.
According to a recent report, club cell-specific deletion of the gene for the canonical WNT receptor, Ryk, causes a cell number imbalance of goblet cells over club cells following naphthalene-induced injury (Fig. 4). In contrast, there are no discernable changes in the cell numbers of basal and ciliated cells (Kim et al., 2019). These results suggest that RYK may play an important role in maintaining the balance among airway epithelial cell populations during lung regeneration. Because β-catenin is dispensable in club cells, WNT signaling mediated by RYK may not be dependent on β-catenin. Alternatively, RYK function may be unrelated to WNT signaling and instead, be associated with another unknown signaling pathway.
Alveoli
Alveoli are populated by squamous, gas exchanging AT1 cells and cuboidal, surfactant secreting AT2 cells (Fig. 2). ATI cells are thin, flat cells that cover 95% of the alveolar surface (Fig. 2). They can be identified by the expression of markers such as aquaporin 5 (AQP5), podoplanin (PDPN), RAGE, and HOPX. It has long been considered that AT1 is the most vulnerable target for distal lung injury and aging. AT2 cells express markers such as SFTPC and a lipid transporter, ATP binding cassette A3 transporter (Fig. 3, Table 1). Similar to other epithelial cells in the lung, the alveolar epithelial cells also show a relatively slow rate of cellular turnover (about 28-35 days) under normal conditions. However, after injury by agents such as hyperoxia, NO2, ozone, and bleomycin, alveoli show robust regeneration capacity through extensive cell proliferation and differentiation (Barkauskas et al., 2013; Desai et al., 2014; Lee and Rawlins, 2018; Olajuyin et al., 2019).
Several distinct cell populations are identified as alveolar stem/progenitor cells. Firstly, in the alveolar-injury model induced by bleomycin or influenza virus infection, BASCs localized within the bronchioalveolar region expand and differentiate into AT1 and AT2 cells in vivo (Table 1) (Kim et al., 2005; Liu et al., 2019; Salwig et al., 2019). Secondly, AT2 cells act as stem/progenitor cells in the alveolar epithelium during development and injury. Both in vitro and in vivo pulse-labeling experiments have demonstrated that AT2 cells can proliferate and differentiate into AT1 cells (Barkauskas et al., 2013; Desai et al., 2014; Olajuyin et al., 2019) (Table 1). Moreover, a sub-population of AT2 cells (called distal lung progenitors) that specifically express the laminin receptor, integrin α6β4, but little or no SFTPC, also show alveolar stem cell-like characteristics (Chapman et al., 2011). Thirdly, lineage-negative epithelial stem/progenitor cells (LNEPs) expressing TRP63 have been identified in the distal mouse lung (Kumar et al., 2011; Vaughan et al., 2015; Zuo et al., 2015). They activate Krt5 expression after influenza virus infection or bleomycin injury in mice. These TRP63+ KRT5+ cells proliferate, migrate actively into the injured areas, and differentiate into mature alveolar epithelia (Table 1). More recently, a sub-population of LNEPs marked by H2-K1high, a major histocompatibility complex class I gene, has been identified using lineage tracing and single-cell transcriptome analysis (Kathiriya et al., 2020). These cells show high colony-forming efficiency in ex vivo three-dimensional organoid culture and following bleomycin-induced injury, these cells can robustly expand and differentiate into alveolar cell lineages in vivo. Finally, it has been demonstrated that a small number of HOPX+ AT1 cells can self-renew and differentiate into AT2 cells after partial pneumonectomy (Jain et al., 2015). In three-dimensional organoid culture, a single HOPX+ AT1 cell is able to generate organoids comprising both AT1 and AT2 cells, demonstrating the potency of these AT1 cells as stem/progenitor cells for alveolar regeneration.
Within the alveoli of the normal adult lung, most epithelial cells, including AT2 cells, do not exhibit active canonical WNT signaling. Canonical WNT signaling within AT2 cells gets activated only after bleomycin-induced injury (Flozak et al., 2010; Tanjore et al., 2013). Furthermore, in the bleomycin-injured alveoli, an increase in the number of β-catenin-positive nuclei, as well as, the expression of canonical Wnt3a and non-canonical Wnt5a genes has been observed in pericytes and endothelial niche cells (Andersson-Sjoland et al., 2016; Majidinia et al., 2018). In addition, LGR5+ stromal cells in the alveolar compartment express a high level of WNT ligands, particularly WNT3A and WNT5A (Lee et al., 2017). Consistent with these in vivo observations, the activity of canonical WNT signaling is very low, if at all, in freshly isolated AT2 cells; however, it increases in cultured AT2 cells. Recently, AXIN2+ AT2 (WNT-responsive) cells, a distinct AT2 sub-population, have been shown to represent evolutionarily conserved alveolar epithelial stem/progenitor cells (Nabhan et al., 2018; Zacharias et al., 2018). These cells are resident in the single fibroblast niches that provide paracrine or juxtacrine WNT signaling. WNT signaling is important for the maintenance of the stemness of these AXIN2+ AT2 cells (Nabhan et al., 2018). During alveoli regeneration after acute lung injury, active WNT signaling is important for the expansion of the AXIN2+ AT2 cells and the inhibition of differentiation of AT2 to AT1 (Frank et al., 2016; Nabhan et al., 2018; Zacharias et al., 2018). Similarly, in ex vivo organoid culture of AT2 cells with alveolar stromal cells, WNT3A treatment enhances organoid formation, further confirming the role of canonical WNT signaling in AT2 cell expansion (Lee et al., 2017). Alveolar epithelium-specific deletion of the Ctnnb1 gene further addresses the role of canonical WNT signaling during alveoli regeneration. Severe alveolar epithelial cell death is detected upon bleomycin-induced injury in Ctnnb1 gene knockout mice (Flozak et al., 2010; Tanjore et al., 2013).
In addition to the role in AT2 cell proliferation and survival, both canonical and non-canonical WNT signaling pathways have been shown to regulate AT2 cell differentiation. It is known that the interaction between p300 co-activator and β-catenin increases during the differentiation of AT2 cells into AT1 cells (Rieger et al., 2016). While a nuclear accumulation of β-catenin is induced by canonical WNT signaling, the regulation of p300/β-catenin interaction occurs through the non-canonical WNT5A/PKC signaling axis (Rieger et al., 2016). WNT5A regulates Ser-89 phosphorylation of p300 in a PKC-dependent manner, resulting in enhanced interaction with β-catenin. Inhibition of p300/β-catenin interaction by IQ-1, a specific small-molecule inhibitor, impairs the differentiation of primary AT2 cells. Unlike this study, Frank et al. (2016) showed that canonical WNT signaling inhibits AT2 cell differentiation. It is not yet defined why these contrasting results exist. Nonetheless, both canonical WNT3A and non-canonical WNT5A ligands are derived from the neighboring LGR5+ stromal cells to promote alveolar differentiation of both club and AT2 cells (Lee et al., 2017). Moreover, resident alveolar macrophages also produce WNT ligands, especially WNT4 and WNT16, to stimulate AT2 cell proliferation and improve alveoli regeneration after lung injury induced by Nippostrongylus brasiliensis or bleomycin (Basil et al., 2020; Hung et al., 2019).
A recent report showed that non-canonical WNT5A and WNT5B ligands repress the proliferation of alveolar epithelial stem/progenitor cells (Wu et al., 2019). It has been demonstrated in ex vivo lung organoid culture that WNT5A and WNT5B negatively regulate the canonical WNT signaling pathway in the alveolar epithelium. Furthermore, WNT5B preferentially restrains the differentiation of alveolar epithelial stem/progenitor cells. Interestingly, WNT5A could support the differentiation of mouse bone marrow-derived mesenchymal stem cell into AT2 cells in vitro (Liu et al., 2014), although the relevance of this activity in a native context remains to be determined. Similar to AT2 stem/progenitor cells, the up-regulation of canonical WNT signaling activity is also detected in LNEPs. WNT signaling activation in these cells blocks Notch signaling activation and Krt5 expression, resulting in their differentiation into AT2 cells and improved alveolar regeneration (Olajuyin et al., 2019; Xi et al., 2017). Therefore, it is obvious that interaction and imbalance between canonical and non-canonical WNT signaling are critical for alveolar stem/progenitor cells and their regenerative capacity.
In addition to the alveolar stem/progenitor cells, WNT signaling also plays a critical role in WNT-producing stromal cells, especially the AXIN2+ PDGFRα+ mesenchymal alveolar niche cells during regeneration (Basil et al., 2020; Zepp et al., 2017). These cells are demonstrated to promote the self-renewal and differentiation of AT2 cells during alveoli homeostasis and regeneration by providing different signals such as IL6, FGFs, and BMP antagonists likely induced by canonical WNT signaling. It still remains to be determined whether WNT-producing LGR5+ stromal cells and mesenchymal alveolar niche cells are the same population or not. Given that both Lgr5 and Axin2 genes are canonical WNT signaling targets, it is likely that both cell types are identical, or largely overlap.
CONCLUSION
The robust regenerative capacity of the lung after injury reflects the histological complexity of the lung. As the different regions of the lung contain unique populations of stem/progenitor cells and their microenvironment, an efficient and correct regeneration process appears to be imprinted within the identities and differentiation potentials of stem cells and their niche. Both canonical and non-canonical WNT signaling pathways play critical regulatory roles in the function and behavior of the different lung stem cell populations and their niche cells. Especially after lung injury, activated canonical WNT signaling is crucial for proliferation, survival, and differentiation of lung epithelial stem/progenitor cells. In contrast, the functional roles of non-canonical WNT signaling during regeneration are relatively limited and need to be further explored in the future.
Disruption of proper WNT signaling during regeneration results in inefficient regeneration. This insufficient repair and repetitive injury promote impairment of the epithelial-mesenchymal crosstalk for lung homeostasis, which plays a key role in the pathogenesis of chronic lung diseases such as idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD). Interestingly, in addition to its roles in the regeneration of the lung, WNT signaling is also involved in the pathology of these diseases. Either activated or repressed WNT signaling is observed in these diseases. As the regenerative capacity of the lung is severely destroyed in these diseases, a proper modulation of WNT signaling activity in these diseases will provide a way to reverse the disease state to normal by enhancing the intrinsic regenerative capacity of the lung.
ACKNOWLEDGMENTS
This work was supported by grants from the Global Research Development Center Program (2016K1A4A3914725) and the National Research Foundation of Korea (2016R1A2B4012956) to J.K.Y. Epifluorescence microscopy was performed using the equipment at the Soonchunhyang Biomedical Research Core Facility.
Footnotes
AUTHOR CONTRIBUTIONS
A.A.R. and J.K.Y. wrote the manuscript and J.K.Y. secured the funding.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Andersson-Sjoland A., Karlsson J.C., Rydell-Tormanen K. ROS-induced endothelial stress contributes to pulmonary fibrosis through pericytes and Wnt signaling. Lab. Invest. 2016;96:206–217. doi: 10.1038/labinvest.2015.100. [DOI] [PubMed] [Google Scholar]
- Ardhanareeswaran K., Mirotsou M. Lung stem and progenitor cells. Respiration. 2013;85:89–95. doi: 10.1159/000346500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., Randell S.H., Noble P.W., Hogan B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., Tan S., Clevers H. Lgr proteins in epithelial stem cell biology. Development. 2013;140:2484–2494. doi: 10.1242/dev.083113. [DOI] [PubMed] [Google Scholar]
- Basil M.C., Katzen J., Engler A.E., Guo M., Herriges M.J., Kathiriya J.J., Windmueller R., Ysasi A.B., Zacharias W.J., Chapman H.A., et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D.H., Davies E., Wyatt J.P. Cytodynamics of pulmonary alveolar cells in the mouse. Arch. Pathol. 1968;86:667–670. [PubMed] [Google Scholar]
- Brechbuhl H.M., Ghosh M., Smith M.K., Smith R.W., Li B., Hicks D.A., Cole B.B., Reynolds P.R., Reynolds S.D. Beta-catenin dosage is a critical determinant of tracheal basal cell fate determination. Am. J. Pathol. 2011;179:367–379. doi: 10.1016/j.ajpath.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae W.J., Bothwell A.L.M. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. 2018;39:830–847. doi: 10.1016/j.it.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.A., Li X., Alexander J.P., Brumwell A., Lorizio W., Tan K., Sonnenberg A., Wei Y., Vu T.H. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Fine A. Stem cells in lung injury and repair. Am. J. Pathol. 2016;186:2544–2550. doi: 10.1016/j.ajpath.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai T.J., Brownfield D.G., Krasnow M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flozak A.S., Lam A.P., Russell S., Jain M., Peled O.N., Sheppard K.A., Beri R., Mutlu G.M., Budinger G.R., Gottardi C.J. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J. Biol. Chem. 2010;285:3157–3167. doi: 10.1074/jbc.M109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.B., Peng T., Zepp J.A., Snitow M., Vincent T.L., Penkala I.J., Cui Z., Herriges M.J., Morley M.P., Zhou S., et al. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep. 2016;17:2312–2325. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A., Lu L., Vickers C., Teixeira V.H., Groot K.R., Butler C.R., Ilieva E.V., George P.J., Nicholson A.G., Sage E.K., et al. Beta-catenin determines upper airway progenitor cell fate and preinvasive squamous lung cancer progression by modulating epithelial-mesenchymal transition. J. Pathol. 2012;226:575–587. doi: 10.1002/path.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A., Deshpande A., Jain A., Sebastiani P., Cardoso W.V. Uroplakin 3a(+) cells are a distinctive population of epithelial progenitors that contribute to airway maintenance and post-injury repair. Cell Rep. 2017;19:246–254. doi: 10.1016/j.celrep.2017.03.051. [DOI] [PubMed] [Google Scholar]
- Hogan B., Tata P.R. Cellular organization and biology of the respiratory system. Nat. Cell Biol. 2019 doi: 10.1038/s41556-019-0357-7. 2019 Jul 25 [Epub]. https://doi.org/10.1038/s41556-019-0357-7. [DOI] [PubMed] [Google Scholar]
- Hogan B.L., Barkauskas C.E., Chapman H.A., Epstein J.A., Jain R., Hsia C.C., Niklason L., Calle E., Le A., Randell S.H., et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L.Y., Sen D., Oniskey T.K., Katzen J., Cohen N.A., Vaughan A.E., Nieves W., Urisman A., Beers M.F., Krummel M.F., et al. Macrophages promote epithelial proliferation following infectious and non-infectious lung injury through a Trefoil factor 2-dependent mechanism. Mucosal Immunol. 2019;12:64–76. doi: 10.1038/s41385-018-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Xu C., Lu M., Wu X., Tang L., Wu X. Wnt/beta-catenin signaling links embryonic lung development and asthmatic airway remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:3226–3242. doi: 10.1016/j.bbadis.2017.08.031. [DOI] [PubMed] [Google Scholar]
- Jain R., Barkauskas C.E., Takeda N., Bowie E.J., Aghajanian H., Wang Q., Padmanabhan A., Manderfield L.J., Gupta M., Li D., et al. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat. Commun. 2015;6:6727. doi: 10.1038/ncomms7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. Wnt signaling in stem cells and cancer stem cells: a tale of two coactivators. Prog. Mol. Biol. Transl. Sci. 2018;153:209–244. doi: 10.1016/bs.pmbts.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Kathiriya J.J., Brumwell A.N., Jackson J.R., Tang X., Chapman H.A. Distinct airway epithelial stem cells hide among club cells but mobilize to promote alveolar regeneration. Cell Stem Cell. 2020;26:346–358.:e4. doi: 10.1016/j.stem.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman S.L. Cell proliferation in the mammalian lung. Int. Rev. Exp. Pathol. 1980;22:131–191. [PubMed] [Google Scholar]
- Kim C.F., Jackson E.L., Woolfenden A.E., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R.T., Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kim H.T., Yin W., Nakamichi Y., Panza P., Grohmann B., Buettner C., Guenther S., Ruppert C., Kobayashi Y., Guenther A., et al. WNT/RYK signaling restricts goblet cell differentiation during lung development and repair. Proc. Natl. Acad. Sci. U. S. A. 2019;116:25697–25706. doi: 10.1073/pnas.1911071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.A., Hu Y., Yamamoto Y., Hoe N.B., Wei T.S., Mu D., Sun Y., Joo L.S., Dagher R., Zielonka E.M., et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Rawlins E.L. Developmental mechanisms and adult stem cells for therapeutic lung regeneration. Dev. Biol. 2018;433:166–176. doi: 10.1016/j.ydbio.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Tammela T., Hofree M., Choi J., Marjanovic N.D., Han S., Canner D., Wu K., Paschini M., Bhang D.H., et al. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell. 2017;170:1149–1163.:e12. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., Chen S., Cai S., Dong L., Liu L., Yang Y., Guo F., Lu X., He H., Chen Q., et al. Wnt5a through noncanonical Wnt/JNK or Wnt/PKC signaling contributes to the differentiation of mesenchymal stem cells into type II alveolar epithelial cells in vitro. PLoS One. 2014;9:e90229. doi: 10.1371/journal.pone.0090229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Liu K., Cui G., Huang X., Yao S., Guo W., Qin Z., Li Y., Yang R., Pu W., et al. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat. Genet. 2019;51:728–738. doi: 10.1038/s41588-019-0346-6. [DOI] [PubMed] [Google Scholar]
- Lynch T.J., Anderson P.J., Rotti P.G., Tyler S.R., Crooke A.K., Choi S.H., Montoro D.T., Silverman C.L., Shahin W., Zhao R., et al. Submucosal gland myoepithelial cells are reserve stem cells that can regenerate mouse tracheal epithelium. Cell Stem Cell. 2018;22:653–667.:e5. doi: 10.1016/j.stem.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidinia M., Aghazadeh J., Jahanban-Esfahlani R., Yousefi B. The roles of Wnt/beta-catenin pathway in tissue development and regenerative medicine. J. Cell. Physiol. 2018;233:5598–5612. doi: 10.1002/jcp.26265. [DOI] [PubMed] [Google Scholar]
- Mercer R.R., Russell M.L., Roggli V.L., Crapo J.D. Cell number and distribution in human and rat airways. Am. J. Respir. Cell Mol. Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- Meyerholz D.K., Suarez C.J., Dintzis S.M., Frevert C.W. Chapter 9-Respiratory system. In: Treuting P.M., Dintzis S.M., Montine K.S., editors. Comparative Anatomy and Histology. 2nd Edition. Academic Press; San Diego: 2018. pp. 147–162. [DOI] [Google Scholar]
- Nabhan A.N., Brownfield D.G., Harbury P.B., Krasnow M.A., Desai T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science (New York, NY) 2018;359:1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Oeztuerk-Winder F., Guinot A., Ochalek A., Ventura J.J. Regulation of human lung alveolar multipotent cells by a novel p38alpha MAPK/miR-17-92 axis. EMBO J. 2012;31:3431–3441. doi: 10.1038/emboj.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajuyin A.M., Zhang X., Ji H.L. Alveolar type 2 progenitor cells for lung injury repair. Cell Death Discov. 2019;5:63. doi: 10.1038/s41420-019-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raslan A.A., Yoon J.K. R-spondins: multi-mode WNT signaling regulators in adult stem cells. Int. J. Biochem. Cell Biol. 2019;106:26–34. doi: 10.1016/j.biocel.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Rawlins E.L., Okubo T., Xue Y., Brass D.M., Auten R.L., Hasegawa H., Wang F., Hogan B.L. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger M.E., Zhou B., Solomon N., Sunohara M., Li C., Nguyen C., Liu Y., Pan J.H., Minoo P., Crandall E.D., et al. p300/beta-catenin interactions regulate adult progenitor cell differentiation downstream of WNT5a/protein kinase C (PKC) J. Biol. Chem. 2016;291:6569–6582. doi: 10.1074/jbc.M115.706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Hogan B.L. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu. Rev. Cell Dev. Biol. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- Rock J.R., Onaitis M.W., Rawlins E.L., Lu Y., Clark C.P., Xue Y., Randell S.H., Hogan B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. U. S. A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Randell S.H., Hogan B.L. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz E.J., Oeztuerk-Winder F., Ventura J.J. A paracrine network regulates the cross-talk between human lung stem cells and the stroma. Nat. Commun. 2014;5:3175. doi: 10.1038/ncomms4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salwig I., Spitznagel B., Vazquez-Armendariz A.I., Khalooghi K., Guenther S., Herold S., Szibor M., Braun T. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019;38:e102099. doi: 10.15252/embj.2019102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler A.J., Watanabe A., Howell S.B. LGR5 and LGR6 in stem cell biology and ovarian cancer. Oncotarget. 2018;9:1346–1355. doi: 10.18632/oncotarget.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skronska-Wasek W., Gosens R., Konigshoff M., Baarsma H.A. WNT receptor signalling in lung physiology and pathology. Pharmacol. Ther. 2018;187:150–166. doi: 10.1016/j.pharmthera.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Song H., Yao E., Lin C., Gacayan R., Chen M.H., Chuang P.T. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17531–17536. doi: 10.1073/pnas.1207238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurlin J.W., Nelson C.M. Building branched tissue structures: from single cell guidance to coordinated construction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372:20150527. doi: 10.1098/rstb.2015.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler C.T., Morrisey E.E. Developmental pathways in lung regeneration. Cell Tissue Res. 2017;367:677–685. doi: 10.1007/s00441-016-2537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart Z., Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145:dev146589. doi: 10.1242/dev.146589. [DOI] [PubMed] [Google Scholar]
- Tanjore H., Degryse A.L., Crossno P.F., Xu X.C., McConaha M.E., Jones B.R., Polosukhin V.V., Bryant A.J., Cheng D.S., Newcomb D.C., et al. Beta-catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am. J. Respir. Crit. Care Med. 2013;187:630–639. doi: 10.1164/rccm.201205-0972OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata P.R., Mou H., Pardo-Saganta A., Zhao R., Prabhu M., Law B.M., Vinarsky V., Cho J.L., Breton S., Sahay A., et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata P.R., Rajagopal J. Plasticity in the lung: making and breaking cell identity. Development. 2017;144:755–766. doi: 10.1242/dev.143784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A.E., Brumwell A.N., Xi Y., Gotts J.E., Brownfield D.G., Treutlein B., Tan K., Tan V., Liu F.C., Looney M.R., et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T., Campbell A., De Langhe S. c-Myc regulates proliferation and Fgf10 expression in airway smooth muscle after airway epithelial injury in mouse. PLoS One. 2013;8:e71426. doi: 10.1371/journal.pone.0071426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T., Dill E., Campbell A., Tiozzo C., Majka S., Bellusci S., De Langhe S.P. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J. Clin. Invest. 2011;121:4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansleeben C., Barkauskas C.E., Rock J.R., Hogan B.L. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip. Rev. Dev. Biol. 2013;2:131–148. doi: 10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- Wu X., van Dijk E.M., Ng-Blichfeldt J.P., Bos I.S.T., Ciminieri C., Konigshoff M., Kistemaker L.E.M., Gosens R. Mesenchymal WNT-5A/5B signaling represses lung alveolar epithelial progenitors. Cells. 2019;8:1147. doi: 10.3390/cells8101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y., Kim T., Brumwell A.N., Driver I.H., Wei Y., Tan V., Jackson J.R., Xu J., Lee D.K., Gotts J.E., et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat. Cell Biol. 2017;19:904–914. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao E., Lin C., Wu Q., Zhang K., Song H., Chuang P.T. Notch signaling controls transdifferentiation of pulmonary neuroendocrine cells in response to lung injury. Stem Cells. 2018;36:377–391. doi: 10.1002/stem.2744. [DOI] [PubMed] [Google Scholar]
- Zacharias W.J., Frank D.B., Zepp J.A., Morley M.P., Alkhaleel F.A., Kong J., Zhou S., Cantu E., Morrisey E.E. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemke A.C., Teisanu R.M., Giangreco A., Drake J.A., Brockway B.L., Reynolds S.D., Stripp B.R. beta-Catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am. J. Respir. Cell Mol. Biol. 2009;41:535–543. doi: 10.1165/rcmb.2008-0407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp J.A., Zacharias W.J., Frank D.B., Cavanaugh C.A., Zhou S., Morley M.P., Morrisey E.E. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell. 2017;170:1134–1148.:e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Goss A.M., Cohen E.D., Kadzik R., Lepore J.J., Muthukumaraswamy K., Yang J., DeMayo F.J., Whitsett J.A., Parmacek M.S., et al. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat. Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W., Zhang T., Wu D.Z., Guan S.P., Liew A.A., Yamamoto Y., Wang X., Lim S.J., Vincent M., Lessard M., et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]