Abstract
Recently, tumor microenvironment (TME) and its stromal constituents have provided profound insights into understanding alterations in tumor behavior. After each identification regarding the unique roles of TME compartments, non-malignant stromal cells are found to provide a sufficient tumorigenic niche for cancer cells. Of these TME constituents, adipocytes represent a dynamic population mediating endocrine effects to facilitate the crosstalk between cancer cells and distant organs, as well as the interplay with nearby tumor cells. To date, the prevalence of obesity has emphasized the significance of metabolic homeostasis along with adipose tissue (AT) inflammation, cancer incidence, and multiple pathological disorders. In this review, we summarized distinct characteristics of hypertrophic adipocytes and cancer to highlight the importance of an individual's metabolic health during cancer therapy. As AT undergoes inflammatory alterations inducing tissue remodeling, immune cell infiltration, and vascularization, these features directly influence the TME by favoring tumor progression. A comparison between inflammatory AT and progressing cancer could potentially provide crucial insights into delineating the complex communication network between uncontrolled hyperplastic tumors and their microenvironmental components. In turn, the comparison will unravel the underlying properties of dynamic tumor behavior, advocating possible therapeutic targets within TME constituents.
Keywords: adipose tissue, cancer-associated adipocyte, inflammation, obesity, tumor microenvironment
INTRODUCTION
To date, heterogeneous features of tumor cells have emphasized the challenges to comprehensively treat cancer. Moreover, a lethal characteristic of cancer to develop multidrug resistance has contributed to the continuous modification of current treatment strategies in cancers (Tolios et al., 2020). Thus, therapeutic targeting has shifted from tumor cell-intrinsic pathways to the bidirectional communication between the tumor and its microenvironmental compartments (Hanahan and Coussens, 2012; Hanna et al., 2009; Roma-Rodrigues et al., 2019). Investigating these interactions has manifested extensive hallmarks for changes in tumor behavior; however, identifying substantial crosstalk between various cell types within TME remains complex (Hui and Chen, 2015).
The prevalence of obesity worldwide has tripled since 1975, increasing the incidence of chronic inflammation, insulin resistance, type 2 diabetes mellitus (T2DM), and cardiovascular diseases (CVD). Adipose tissue (AT) inflammation and metabolic dysfunction are proposed to influence tumorigenesis and cancer progression (Lengyel et al., 2018). This interaction between AT and cancer has been well documented, with severe effects observed among adipocyte-rich cancers such as breast, colon, prostate, ovarian, and pancreatic cancers (Table 1). Thus, the profound influence of systemic inflammation on the whole body emphasizes the importance of an individual’s metabolic health during cancer treatment.
| Tissue | Species | CAA status | Secretion | Cancer | Experiment | Reference |
|---|---|---|---|---|---|---|
| Pancreas | Human | Dedifferentiation Dysregulated metabolism Delipidation |
FFA, matrix remodeling factors, angiogenic factors | Enhanced migration Desmoplasia |
Co-culture, RNA analysis, trans-well, transcriptome analysis | (Cai et al., 2019) |
| Pancreas | Human | Overexpression of Wnt signaling pathway | EMT inducing factors, WNT paracrine factors | EMT induction Enhanced migration |
Wound healing assay, invasion assay, CM treatment, gene expression profile, RNA analysis | (Carbone et al., 2018) |
| Pancreas | Mouse | Dedifferentiation Lipolysis Fibrosis |
FFA, HGF | Wnt5a secretion Enhanced FFA uptake Enhanced migration and invasion Chemoresistance |
Organotypic fat invasion model, CM treatment, co-culture, FACS | (Okumura et al., 2017) |
| Breast | Mouse Human |
Delipidation Reduced lipid droplet size and number |
Protease, MMP11, IL-6, IL-1β, PAI1 | Enhanced invasive phenotype | Co-culture, metastasis assay | (Dirat et al., 2011) |
| Breast | Mouse Human |
Fibrosis Wnt/β-catenin activation FSP overexpression |
Fibronectin, collagen I | Enhanced migration and invasion Enhanced CAF marker Enhanced FSP-1 Wnt3a secretion |
Co-culture, fat pad injection, IHC, H&E staining, migration, invasion assay, glucose uptake assessment | (Bochet et al., 2013) |
| Breast | Human | IGFBP2 overexpression Delipidation |
FFA, IGFBP2, ANGPTL4, IL6sR, IL8, insulin, leptin, MIF, PDGF, TGF-β, TNF-α | Enhanced MMP2 Decreased E-cadherin Enhanced metastasis |
Co-culture, CM treatment, adipokine array, migration, invasion assay | (Wang et al., 2015) |
| Ovary | Mouse Human |
Lipolysis Homing and lipid supply to tumor |
FFA, IL-6, IL-8, MCP1, TIMP-1, adiponectin | Enhanced homing, migration, and invasion Overexpressed FABP4 Cytoplasmic lipid droplet accumulation |
Co-culture, protein array, H&E staining, xenograft | (Nieman et al., 2011) |
| Ovary | Mouse Human |
Lipid supply to tumor | IL8, IL6, FFA, leptin, adiponectin, prostaglandin, lipoxin, arachidonic acid | Enhanced chemoresistance Reduced cleaved PARP PI3K/Akt activation STAT3 activation |
CM treatment, FACS, lipidomic analysis, lipid extraction, protein fractionation | (Yang et al., 2019) |
| Ovary | Human | Downregulation of Acetyl-CoA carboxylase | FFA, IL8 | Enhanced metastasis Enhanced FFA uptake CD36 overexpression AMPK activation |
Co-culture, CD36 inhibition, xenograft, H&E staining, TUNEL assay | (Ladanyi et al., 2018) |
| Prostate | Mouse Human |
CCL7 and leptin overexpression Extraprostatic extension |
CCL7, CXCR2, CXCR4, CXCL12, IL6, MMPs | Enhanced migration Enhanced invasion CCR3 (master regulator) CCR4 |
CM treatment, IHC, proteomic analysis, orthotopic transplantation, FACS, Mass spec | (Laurent et al., 2016) |
| Colon | Mouse | Lipolysis Support cancer cell growth and survival | FFA, glycerol | Upregulated mitochondrial fatty acid oxidation Enhanced oxygen consumption AMPK/autophagy activation Dedifferentiation |
Co-culture, 3D organoids culture, xenograft, IHC, OCR assay, autophagy flux assay | (Wen et al., 2017) |
CAA, cancer-associated adipocytes; FFA, free fatty acid; EMT, epithelial-mesenchymal transition; CM, conditioned media; HGF, hepatocyte growth factor; FACS, fluorescence-activated cell sorting; MMP, metalloproteinase; IL, interleukin; PAI1, plasminogen activator inhibitor 1; CAF, cancer-associated fibroblast; FSP-1, fibroblast-specific protein-1; IHC, immunohistochemistry; IGFBP2, insulin growth factor-binding protein 2; ANGPTL4, angiopoietin-like 4; MIF, macrophage migration inhibitory factor; PDGF, platelet-derived growth factor receptor; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-α; MCP-1, monocyte chemoattractant protein-1; TIMP-1, tissue inhibitor of metalloproteinase 1; FABP4, fatty acid-binding protein 4; PARP, poly (ADP-ribose) polymerase; PI3K, phosphoinositide 3-kinase; Akt, protein kinase 8; STAT3, signaling transducer and activator of transcription 3; AMPK, AMP-activated protein kinase; CCL7, chemokine (C-C motif) ligand; CXCR, C-X-C motif chemokine receptor; CXCL, C-X-C motif chemokine ligand; CCR, C-C chemokine receptor; OCR, oxygen consumption rate.
A small number of approaches have attempted to list the commonalities between growing tumors and inflammatory AT. However, numerous factors such as cytokines, extracellular matrix (ECM), immune cells, hypoxic factors, and fibroblasts affect tumor growth and AT expansion in similar manners. During proliferation, similar arrays of cytokines, such as transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and other pro-inflammatory cytokines, are secreted from both tumor cells and adipocytes to their surroundings, reprogramming the ECM to favor growth (Fig. 1) (Berraondo et al., 2019). The reconstruction of the TME and AT is a comparable phenomenon since both conditions pursue adipogenic and tumorigenic niche via vascularization during expansion (Hinrichs and Rosenberg, 2014; Romagnani et al., 2001). Therefore, the classification of environmental changes in the AT provides profound insights into unraveling the mechanism underlying the dynamic tumor behavior, especially when the tumor is adjacent to a large population of adipocytes. Furthermore, this comparison may suggest effective therapeutic targets for TME, which could alleviate inflammatory stress from both tumorigenic regions and AT.
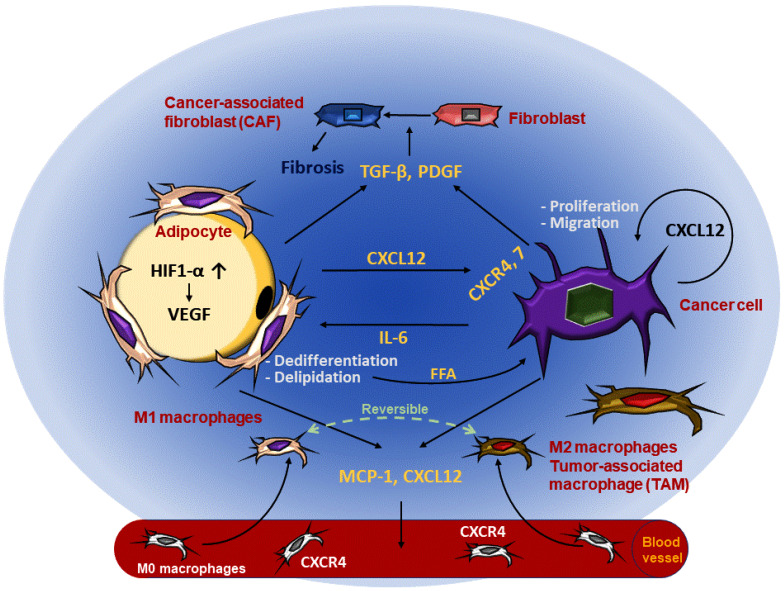
Fig. 1. Inflammatory AT and TME synergistically communicate to favor the growth of cancer cells.
Commonalities between propagating adipocytes and cancer cells reshaping ECM constituents and stiffness are illustrated above. The interplay between adipocytes and TME integrates multiple factors. Fibroblasts from both inflammatory AT and TME induce fibrosis which reorganizes ECM compartments. IL-6 secreted from cancer cells causes adipocyte dedifferentiation and delipidation to supply FFA to tumor cells. MCP-1 and CXCL12 cooperatively mediate macrophage infiltration into both AT and TME, where M1 and M2 polarization can be alternatively converted depending on the environmental conditions. Vascularization fulfills oxygen and nutritional demands for both adipocytes and cancer cells.
OVERVIEW OF ADIPOSE TISSUE INFLAMMATION
AT is mainly composed of adipocytes that play a pivotal role as energy storage. Additionally, AT senses the nutrient status to regulate energy mobilization depending on its nutritional accessibility (Zhang et al., 2003). As a crucial endocrine organ, AT relies on adipocytes that secrete hormones through coordinating with other adjacent and distant tissues (Reilly and Saltiel, 2017). However, under conditions of overnutrition, AT expansion occurs due to hypertrophic adipocytes. During nutritional excess, the enlarged adipocytes cause AT dysfunction, augmenting systemic inflammation that is highly implicated in insulin resistance and T2DM (Fischer-Posovszky et al., 2011). Numerous physiological outcomes are manifested during AT inflammation; for example, increased lipolysis resulting in hydrolysis of triglyceride (TG) to three free fatty acids (FFAs) and glycerol. In turn, the metabolic health of an individual is severely impaired, and numerous adipokines are altered.
Fibrosis, a phenomenon of dysfunctional AT, is characterized by excessive deposition of ECM. Here, myofibroblasts produce ECM components, namely collagen, fibronectin, and glycoproteins. TGF-β, platelet-derived growth factor-α (PDGF-α), and HIF1-α represent the activators of profibrotic myofibroblast differentiation (Buechler et al., 2015; Marcelin et al., 2019). Excessive deposition of ECM enhances its rigidity, resulting in angiogenic properties of AT. For instance, desmoplasia occurs with a process of disrupted tissue homeostasis during inflammation regulation mediated by myofibroblasts, which deposit stiff matrix components, including fibrillar collagens and fibronectins (Quail and Dannenberg, 2019). Moreover, TGF-β and PDGF-α control ECM dynamics by regulating metalloproteinases (MMPs) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) to mediate ECM reconstruction (Sun et al., 2013). Adjustment of ECM stiffness, rigidity, and composition is an essential procedure to accommodate hypertrophic adipocytes (Seo et al., 2015).
AT components, such as fibroblasts, preadipocytes, tissue-resident macrophages, and vascular constituents termed adipose tissue macrophages (ATMs), contribute to propagating pro-inflammatory responses under hypertrophic conditions (Huh et al., 2014; Ikeoka et al., 2010). ATMs represent the most abundant class of leukocytes, originating from blood monocytes (Russo and Lumeng, 2018). These macrophages are arranged around dead adipocytes, forming crown-like structures (CLS), which are hallmarks of the pro-inflammatory process in AT. Though the macrophage polarization status M1 (pro-inflammatory) and M2 (anti-inflammatory) could be an over-simplification, macrophages in inflammatory AT generally exhibit more M1 polarized phenotypes (Huh et al., 2014; Nguyen et al., 2007; Patsouris et al., 2008; Quail and Dannenberg, 2019).
The secretory pro-inflammatory response in AT involves TNF-α, IL-6, MCP-1, inducible nitric oxide synthase (iNOS), TGF-β, and HIF1-α (Greenberg and Obin, 2006; Hotamisligil et al., 1993). Indeed, macrophages infiltrating into AT and cancer are both responsible for local TNF-α and IL-6 secretion (Weisberg et al., 2003). This secretion mediates direct communication between adipocytes and nearby compartments, followed by induction of angiogenic factors. For instance, HIF1-α, expressed from adipocytes during AT inflammation, directly regulates the transcription of vascular endothelial growth factor (VEGF) (He et al., 2011). Reduced adiponectin secretion during obesity promotes TNF-α-induced monocyte adhesion and vascularization (Palanisamy et al., 2019). Conversely, leptin induces angiogenesis, vascular fenestration, and vascular remodeling (Buechler et al., 2015).
AN OVERVIEW OF THE TUMOR MICROENVIRONMENT
TME comprises a variety of cellular (fibroblasts, endothelial cells, pericytes, and adipocytes) and non-cellular (growth factors, cytokines, RNA, DNA, metabolites, and ECM) components exchanging a substantial network of signals. The vast networks of communication within the TME compartments include proliferative signals, resistance to apoptosis, uncontrolled multiplication, vascularization, and immune evasion (Petrova et al., 2018). Often followed by hypoxic conditions, the ECM of malignant cancers undergo dynamic alterations in its components, which are critical determinants of tumor behavior and differentiation (Walker et al., 2018).
The ECM comprises a complex mixture of macromolecules with major components providing structural support. Fibroblasts maintain the ECM homeostasis via collagen synthesis, which is implicated in the structural deposition that undergoes constant reconstruction during tumor progression (Eble and Niland, 2019; Fang et al., 2014). Fibroblasts are stromal tissue that forms a baseline for membrane compartments, regulate differentiation of epithelial cells, modulate immune responses, and mediate tissue homeostasis (Kalluri and Zeisberg, 2006; Quail and Joyce, 2013). Fibroblasts adjacent to malignant tumor cells are activated by growth factors, cell-cell communication, and reactive oxygen species (ROS), acquiring abnormal features to transform into cancer-associated fibroblasts (CAFs). Particularly, during tumor progression, TGF-β, fibroblast growth factor (FGF), and PDGF stimulate the transition from normal fibroblasts to CAFs. CAFs then produce MMPs, modulating Notch and p53 signaling pathways, releasing angiogenic factors, reprograming ECM plasticity and architecture (Bauer et al., 2010; Quail and Joyce, 2013; Xing et al., 2010).
Fibrillar collagen is one of the dominant components of ECM. Growing evidence has indicated that collagen undergoes qualitative and quantitative reorganization to provide a supportive structure for tumor progression (Sun et al., 2013; Zhou et al., 2017). Collagens directly interact with cancer cells through the discoidin domain receptor (DDR) to mediate the behavioral changes during cancer. Furthermore, cancer cells also reshape collagen to reinforce the cell-collagen loop, which gradually fosters cancer progression. Additionally, the direct binding of integrin and collagen leads to the activation of the Akt/PI3K, MAPK, Rho, and MEK/ERK signaling pathways, thereby inducing cell proliferation and evasion of apoptosis (Xu et al., 2019a).
The proliferating tumor inevitably develops a hypoxic environment that drives signal transduction via the upregulation of HIFs within the microenvironment. In the tumor, the most compelling consequence of excessive oxygen and nutrient demand is the secretion of angiogenic and lymphangiogenic factors (Ji, 2014). The correlation between the HIF pathway and pro-angiogenic genes to promote vascular permeability, endothelial cell proliferation, migration, adhesion, and tube formation is well elicited (Bos et al., 2005; Bryant et al., 2010). Additionally, hypoxia is involved in the regulation of multiple transcription factors, including Notch and Wnt signaling, to induce multiple gene expressions associated with EMT, cell cycle progression, and survival (Jung et al., 2015). Thus, hypoxia exerts a powerful impact on the surrounding hyperplastic tumor cells.
Based on epidemiological studies, the link between obesity and cancer progression has been proposed. In addition to the role of WAT to store energy, it also acts as an endocrine organ, secreting an extensive variety of adipokines communicating with both proximal and distant organs (Paz et al., 2011). Though overnutrition is accompanied by multiple systemic effects, the co-culture system suggests the direct effects of adipokines and FFA on tumor behavior. Interestingly, it has been reported that dysfunctional AT determines tumor malignancy, entailing massive contributions of adipocytes toward cancer progression and metastasis (Johnson et al., 2012).
INVESTIGATING THE INTERRELATIONSHIP BETWEEN ADIPOSE TISSUE INFLAMMATION AND TUMOR MICROENVIRONMENT
Cancer-associated adipocytes
The association between obesity and cancer prognosis has been well established, and epidemiological studies support a strong correlation between the prevalence of obesity and cancer. However, the direct mechanism remains elusive due to the complex network of TME compartments (Quail and Dannenberg, 2019). Thus, to inspect the impact of adipocytes on proliferating tumors, several studies have conducted a co-culture system to observe the direct interplay between cancer cells and adipocytes. Though yet to be further established, adipokines and cytokines exchanged between two compartments dramatically alter the morphology of both cell types. Common phenotypes exhibited by the co-culture system included adipocytes when grown along with cancer cells originating from adipocyte-rich environments, undergoing dedifferentiation, and delipidation. Along with the downregulation of mature adipocyte markers, these observations imply that the cancer-associated adipocytes (CAAs) supply the tumorigenic niche to nearby cancer cells followed by adipocyte death (Table 1).
Notably, CAAs from different tumor origins release important secretory factors. The chemokine ligand C-X-C motif chemokine ligand (CXCL) 12 and the receptors, C-X-C chemokine receptor (CXCR) 4 and 7, are overexpressed in tumors and implicated in cell proliferation, migration, and tumor metastasis (Zhou et al., 2019). The binding of CXCL12 to CXCR4 is now documented to activate divergent signals on multiple pathways, such as extracellular signal-regulated kinase1/2 (ERK1/2), p38, stress-activated protein kinase (SAPK)/c-Jun NH(2)-terminal kinase (JNK), Akt, mammalian target of rapamycin (mTOR), and Bruton tyrosine kinase. CXCL12/CXCR4 flux regulates intracellular calcium flux, chemotaxis, transcription, and cell survival (Scala, 2015). These cytokines are not only secreted by malignant tumors but also secreted from adipocytes when co-cultured with certain cancers (Laurent et al., 2016).
Interleukin 6 (IL-6), another major cytokine in the TME, is frequently deregulated in cancers. Interestingly, IL-6 secretion has been implicated in obese patients and in CAAs co-cultured with breast, ovary, and prostate cancer cells, indicating the correlation between IL-6 and AT inflammation (Table 1). IL-6 overexpression can promote tumorigenesis and evasion of immune surveillance via Janus kinases (JAK) and signal transducer and activator of transcription 3 (STAT3) activation (Fisher et al., 2014; Kang et al., 2019). This mediates anti-apoptotic signals such as B-cell lymphoma 2 (Bcl-2), survivin, and myeloid cell leukemia-1 (Mcl-1), and enhances proliferation by overexpression of c-Myc, Cyclin D1, and MMP (Chonov et al., 2019).
Extracellular matrix
ECM remodeling is essential for both AT inflammation and tumor growth. Similar to inflammatory AT, the TME experiences modification of ECM stiffness via CAF-mediated desmoplasia. Myofibroblasts in TME also deposit stiff matrix components, such as fibrillar collagens and fibronectins. As previously mentioned, transformation into CAFs is mediated via TGF-β, FGF, and PDGF expression from the cancerous region, stimulating angiogenesis, cell proliferation, invasion, and motility (Karagiannis et al., 2012). These outcomes are relatively common in TME with enriched ECM; for example, breast cancer becomes abundant with myofibroblasts during obesity to promote ECM stiffness (Seo et al., 2015). The transition of normal fibroblast to CAFs initiates cancer fibrosis by increasing the stiffness of the ECM in TME and AT. ECM isolated from inflammatory stromal cells promotes the invasive behavior of breast cancer cells by stimulating mechano-signal transduction, however, this could be reversed by weight loss (Seo et al., 2015). Nintedanib, a tyrosine kinase inhibitor targeting platelet-derived growth factor receptor (PDGFR) and vascular endothelial growth factor receptor (VEGFR), is a well-known chemotherapeutic drug and has been approved to treat idiopathic pulmonary fibrosis (IPF) (Rivera-Ortega et al., 2018). Similarly, drugs capable of simultaneously alleviating both inflammatory AT and TME are listed in Table 2.
Table 2.
Anti-inflammatory drugs can be applied to suppress adipocyte-rich cancer progression
| Drug name | Types of cancer | Inflammatory disease | Mechanism | Reference/trial identifier |
|---|---|---|---|---|
| Aspirin | Gastrointestinal cancer Colorectal cancer |
ARDS | COX inhibitor | (Huang et al., 2015; Panka et al., 2017; Tougeron et al., 2014) |
| Salsalate | Prostate cancer treated with radiotherapy Colorectal cancer treated with Curcumin |
Rheumatoid arthritis Liver fibrosis |
Inhibition of NF-κB pathway -AMPK activation |
(Bombardier et al., 1995; Broadfield et al., 2019; Hawley et al., 2012; Liang et al., 2015; Wu et al., 2019; Yuan et al., 2001) |
| Etanercept | Breast cancer Bone cancer Non-Hodgkin’s lymphoma Skin cancer |
Rheumatoid arthritis T2DM IPF |
Immunoneutralization of TNF receptors | (Aksu et al., 2011; Burge, 2003; Canestaro et al., 2016; Madhusudan et al., 2004; Pfeifer et al., 2017; Stanley et al., 2011; Yang et al., 2017; Zhao et al., 2018) |
| Rosiglitazone | Ovarian cancer treated with Olaparib Breast cancer Colorectal cancer treated with 5-fluorouracil |
T2DM BDL-induced liver fibrosis Obesity |
Agonist for PPARγ nuclear receptor | (Dang et al., 2018; Lau et al., 2019; Quintanilla Rodriguez and Correa, 2020; Wang et al., 2020; Wei et al., 2019; Xu et al., 2019b) |
| Metformin | Colorectal cancer Prostate cancer Breast cancer Urothelial cancer Kidney cancer |
T2DM IPF Obesity |
Not specified | (Coyle et al., 2016; Rangarajan et al., 2018; Rena et al., 2017) |
| Imatinib | Gastrointestinal stromal tumors CML |
Nephrogenic systemic fibrosis Myelofibrosis |
PDGFR antagonist | (Hantel and Larson, 2018; Hasselbalch et al., 2003; Joensuu et al., 2017; Madke and Khopkar, 2011) |
| Nintedanib | Pancreas cancer Breast cancer Colorectal cancer |
SSc IPF |
VEGFR/PDGFR antagonist | (Distler et al., 2019; Reguera-Nunez et al., 2019; Rivera-Ortega et al., 2018; Rossi et al., 2017; Varone et al., 2018) |
| 17-AAG (tanespimycin) | Breast cancer | SSc | HIF-1α inhibitor | (Sontake et al., 2017; Talamantez-Lyburn et al., 2019) |
ARDS, acute respiratory distress syndrome; COX, cyclooxygenase; NF-κB, nuclear factor-kappa B; AMPK, AMP-activated protein kinase; T2DM, type 2 diabetes mellitus; IPF, idiopathic pulmonary fibrosis; TNF, tumor necrosis factor; BDL, bile duct ligation; PPARγ, peroxisome proliferator-activated receptor γ; CML, chronic myelomonocytic leukemia; PDGFR, platelet-derived growth factor receptor; SSc, systemic sclerosis; VEGFR, vascular endothelial growth factor receptor.
Another major regulator of the ECM structure is collagen. Collagens are known to be the dominant components of ECM in both TME and AT. Specifically, collagen VI deposition plays a pivotal role in AT to induce epithelial hyperplasia (Quail and Dannenberg, 2019). During obesity, collagen VI is highly synthesized, which reflects a broad shift toward AT fibrosis (Khan et al., 2009). During inflammatory states, AT experiences robust changes in ECM, that coincide with the delivery of the pro-tumorigenic niche as they disrupt tissue homeostasis. Fibrillar collagens that reside in TME directly interact with tumor cells to modify the environment, resembling the function of collagens under inflammatory AT.
Angiogenesis
Hypoxic conditions within malignant tumor and inflammatory AT secrete HIF1-α mediated angiogenic factors into their adjacent environment. The most prominent factor involved in this condition is VEGF. Since oxygen and nutrient demands from both proliferating tumor cells and hypertrophic adipocytes are immense, they need to be highly vascularized. However, the vessel function is often dysfunctional or insufficient in both cases. In the case of inflammatory AT, CLS forming near the adipocytes are inaccessible to blood vessels. This phenomenon is exaggerated in obese patients due to vascular control, which is unable to meet the necessary demand (Quail and Dannenberg, 2019). Obese patients also demonstrate higher IL-6 and FGF-2 expression levels, which can potentially result in resistance to anti-VEGF therapy. Reportedly, a mouse model treated with IL-6 blockade abrogated resistance to this therapy. Additionally, the downregulation of the FGF-2 expression by metformin or FGF receptor inhibition sensitized obese mice to anti-VEGF therapy (Incio et al., 2018). Thus, both the inflammatory AT and TME communicate with nearby elements to facilitate vascularization and meet demands.
Macrophages
Complicated networks of TME involve notable players of unique classes and subclasses of the tumor immune microenvironment (TIME), including tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), natural killer cells (NKs), dendritic cells (DCs), and T cells (Binnewies et al., 2018). TAMs are responsible to maintain the host defense and tissue homeostasis; thus, they are well characterized in both inflammatory TME and AT (Erreni et al., 2011; Thomas and Apovian, 2017). Correspondingly, TAMs are considered major components of tumor-infiltrating immune cells, providing a favorable TME for cancer to develop uncontrolled growth, neovascularization, and resistance to apoptosis (Chanmee et al., 2014). TAMs are characterized by the polarization status, which alternates between M1 and M2 polarized macrophages. Frequently expressing M2-like phenotypes, TAMs promote tumor progression and contribute to chemoresistance. Thus, M2 macrophage density often correlates with poor prognosis in different types of cancer patients. While M1 macrophages counteract cancer progression and metastasis via pro-inflammatory and antimicrobial phenotypes, M2 macrophages facilitate anti-inflammatory responses, tissue repair and remodeling, and immune tolerance (Chanmee et al., 2014). M2 can be further classified into M2a, b, c, and d according to their phenotypes, indicating the diverse functions of M2 macrophages (Yao et al., 2019). M2d macrophages are the most prevalent TAMs to drive angiogenesis, immunosuppression, and tumor progression (Chanmee et al., 2014). Although the macrophage polarization status is contradictory between inflammatory AT and TME, both conditions are associated with high-grade macrophage infiltration (Pang et al., 2008). In obese mice, CXCL12 enhances macrophage recruitment and insulin resistance, which could be reversed via CXCL12 blockade (Kim et al., 2014). In parallel, CXCL12/CXCR4, 7 flux in the TME shapes monocyte differentiation to a distinct type of macrophages with pro-angiogenic and immunosuppressive phenotypes (Sanchez-Martin et al., 2011). MCP-1, the master regulator of monocyte/macrophage recruitment, has been implicated in multiple adipocyte-rich cancers, such as breast, prostate, ovarian, and non-small lung cancers (Table 1). Therefore, macrophages, demonstrating alternating behavior of M1 and M2 polarization, actively communicate with adjacent compartments to support the proliferative niche.
CONCLUSION
The worldwide prevalence of obesity has been dramatically increasing for the past decades. Both clinically and experimentally, pathological consequences of AT inflammation have been substantially demonstrated. In the presence of cancer, obese patients develop chemoresistance with high frequency, suggesting that distinct therapeutic approaches are required depending on an individual’s metabolic health. Body mass index (BMI) and waist circumference are the main assessments in assigning obesity; however, this fails to fully examine the metabolic health. As AT homeostasis is critical for TME, a qualitative analysis, including a systematic histological, biochemical, and gene expression analysis, of AT function is necessary when treating cancer.
Notably, there exist commonalities between growing tumors and dysfunctional AT, indicating that both conditions undergo environmental reconstruction to favor growth (Fig. 1). Interestingly, during simultaneous growth, tumors grow more rapidly and invasively (Table 1). Both tumor cells and adipocytes secrete cytokines, promote immune cell infiltrations, and reconstruct their microenvironment, supporting the transition of tumor behavior to be more malignant. Adipocytes and tumor cells experience hypoxic conditions during proliferation, that activates HIF1-α-mediated VEGF, PDGF, and FGF expression to further induce angiogenic factors. These features of cancer cells and adipocytes reconstruct ECM to favor the supply of both inflammatory and tumorigenic niche.
An intensive inflammation is a common feature among obese cancer patients. Especially those with adipocyte-rich cancers experience fatal growth of tumor fueled by chronic inflammation within the TME (Quail and Dannenberg, 2019). Hence, to counteract the TME which favors rapid progression, more attempts to reduce secretion of the inflammatory cytokines and chemokines should be made by administration of non-steroidal anti-inflammatory drugs (NSAIDs). Aspirin, for example, plays a critical role in suppressing inflammatory environment through inhibition of COX2, making it possible to be applied in cancer treatment. In addition to aspirin, we provide a potential therapeutic approach to target both inflammatory AT and TME with a single drug delivery (Table 2) (Cuzick et al., 2009). For instance, the inhibitory effect of metformin on AMPK/mTOR pathway is now implemented with numerous chemotherapeutic drugs (Saraei et al., 2019). Taking this into account, our rearrangement provides a broader application of anti-inflammatory drugs to inhibit the secretion of pro-inflammatory agents. Here, we not only suggest both direct and combined applications of anti-inflammatory drugs against tumor progression, but also emphasize the necessity to identify the patients’ metabolic health.
ACKNOWLEDGMENTS
This work was supported by the grant from the National Research Foundation of Korea (NRF) funded by the MEST (2018R1A2A1A05022746, 2017R1A4A1015328), and was supported in part by Brain Korea 21 (BK21) PLUS program.
Footnotes
AUTHOR CONTRIBUTIONS
Y.C.S. wrote the manuscript. S.E.L. wrote the manuscript. Y.J. designed the manuscript. H.W.P. oversaw the manuscript. K.H.C. oversaw the manuscript. H.W.L. oversaw the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Aksu K., Cagirgan S., Ozsan N., Keser G., Sahin F. Non-Hodgkin's lymphoma following treatment with etanercept in ankylosing spondylitis. Rheumatol. Int. 2011;31:1645–1647. doi: 10.1007/s00296-009-1265-0. [DOI] [PubMed] [Google Scholar]
- Bauer M., Su G., Casper C., He R., Rehrauer W., Friedl A. Heterogeneity of gene expression in stromal fibroblasts of human breast carcinomas and normal breast. Oncogene. 2010;29:1732–1740. doi: 10.1038/onc.2009.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berraondo P., Sanmamed M.F., Ochoa M.C., Etxeberria I., Aznar M.A., Perez-Gracia J.L., Rodriguez-Ruiz M.E., Ponz-Sarvise M., Castanon E., Melero I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochet L., Lehuede C., Dauvillier S., Wang Y.Y., Dirat B., Laurent V., Dray C., Guiet R., Maridonneau-Parini I., Le Gonidec S., et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–5668. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- Bombardier C., Peloso P.M., Goldsmith C.H. Salsalate, a nonacetylated salicylate, is as efficacious as diclofenac in patients with rheumatoid arthritis. Diclofenac Study Group. J. Rheumatol. 1995;22:617–624. [PubMed] [Google Scholar]
- Bos R., van Diest P.J., de Jong J.S., van der Groep P., van der Valk P., van der Wall E. Hypoxia-inducible factor-1alpha is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology. 2005;46:31–36. doi: 10.1111/j.1365-2559.2005.02045.x. [DOI] [PubMed] [Google Scholar]
- Broadfield L.A., Marcinko K., Tsakiridis E., Zacharidis P.G., Villani L., Lally J.S.V., Menjolian G., Maharaj D., Mathurin T., Smoke M., et al. Salicylate enhances the response of prostate cancer to radiotherapy. Prostate. 2019;79:489–497. doi: 10.1002/pros.23755. [DOI] [PubMed] [Google Scholar]
- Bryant C.S., Munkarah A.R., Kumar S., Batchu R.B., Shah J.P., Berman J., Morris R.T., Jiang Z.L., Saed G.M. Reduction of hypoxia-induced angiogenesis in ovarian cancer cells by inhibition of HIF-1 alpha gene expression. Arch. Gynecol. Obstet. 2010;282:677–683. doi: 10.1007/s00404-010-1381-9. [DOI] [PubMed] [Google Scholar]
- Buechler C., Krautbauer S., Eisinger K. Adipose tissue fibrosis. World J. Diabetes. 2015;6:548–553. doi: 10.4239/wjd.v6.i4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge D. Etanercept and squamous cell carcinoma. J. Am. Acad. Dermatol. 2003;49:358–359. doi: 10.1067/S0190-9622(03)00811-9. [DOI] [PubMed] [Google Scholar]
- Cai Z., Liang Y., Xing C., Wang H., Hu P., Li J., Huang H., Wang W., Jiang C. Cancer-associated adipocytes exhibit distinct phenotypes and facilitate tumor progression in pancreatic cancer. Oncol. Rep. 2019;42:2537–2549. doi: 10.3892/or.2019.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canestaro W.J., Forrester S.H., Raghu G., Ho L., Devine B.E. Drug treatment of idiopathic pulmonary fibrosis: systematic review and network meta-analysis. Chest. 2016;149:756–766. doi: 10.1016/j.chest.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Carbone C., Piro G., Gaianigo N., Ligorio F., Santoro R., Merz V., Simionato F., Zecchetto C., Falco G., Conti G., et al. Adipocytes sustain pancreatic cancer progression through a non-canonical WNT paracrine network inducing ROR2 nuclear shuttling. Int. J. Obes. (Lond.) 2018;42:334–343. doi: 10.1038/ijo.2017.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanmee T., Ontong P., Konno K., Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonov D.C., Ignatova M.M.K., Ananiev J.R., Gulubova M.V. IL-6 activities in the tumour microenvironment. Open Access Maced. J. Med. Sci. 2019;7:2391–2398. doi: 10.3889/oamjms.2019.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle C., Cafferty F.H., Vale C., Langley R.E. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann. Oncol. 2016;27:2184–2195. doi: 10.1093/annonc/mdw410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J., Otto F., Baron J.A., Brown P.H., Burn J., Greenwald P., Jankowski J., La Vecchia C., Meyskens F., Senn H.J., et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- Dang Y.F., Jiang X.N., Gong F.L., Guo X.L. New insights into molecular mechanisms of rosiglitazone in monotherapy or combination therapy against cancers. Chem. Biol. Interact. 2018;296:162–170. doi: 10.1016/j.cbi.2018.09.019. [DOI] [PubMed] [Google Scholar]
- Dirat B., Bochet L., Dabek M., Daviaud D., Dauvillier S., Majed B., Wang Y.Y., Meulle A., Salles B., Le Gonidec S., et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- Distler O., Gahlemann M., Maher T.M. Nintedanib for systemic sclerosis-associated interstitial lung disease. Reply. N. Engl. J. Med. 2019;381:1596–1597. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- Eble J.A., Niland S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis. 2019;36:171–198. doi: 10.1007/s10585-019-09966-1. [DOI] [PubMed] [Google Scholar]
- Erreni M., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron. 2011;4:141–154. doi: 10.1007/s12307-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., Yuan J., Peng C., Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 2014;35:2871–2882. doi: 10.1007/s13277-013-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Posovszky P., Wang Q.A., Asterholm I.W., Rutkowski J.M., Scherer P.E. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology. 2011;152:3074–3081. doi: 10.1210/en.2011-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.T., Appenheimer M.M., Evans S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014;26:38–47. doi: 10.1016/j.smim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A.S., Obin M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanna E., Quick J., Libutti S.K. The tumour microenvironment: a novel target for cancer therapy. Oral Dis. 2009;15:8–17. doi: 10.1111/j.1601-0825.2008.01471.x. [DOI] [PubMed] [Google Scholar]
- Hantel A., Larson R.A. Imatinib is still recommended for frontline therapy for CML. Blood Adv. 2018;2:3648–3652. doi: 10.1182/bloodadvances.2018018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbalch H.C., Bjerrum O.W., Jensen B.A., Clausen N.T., Hansen P.B., Birgens H., Therkildsen M.H., Ralfkiaer E. Imatinib mesylate in idiopathic and postpolycythemic myelofibrosis. Am. J. Hematol. 2003;74:238–242. doi: 10.1002/ajh.10431. [DOI] [PubMed] [Google Scholar]
- Hawley S.A., Fullerton M.D., Ross F.A., Schertzer J.D., Chevtzoff C., Walker K.J., Peggie M.W., Zibrova D., Green K.A., Mustard K.J., et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Gao Z., Yin J., Zhang J., Yun Z., Ye J. Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am. J. Physiol. Endocrinol. Metab. 2011;300:E877–E885. doi: 10.1152/ajpendo.00626.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs C.S., Rosenberg S.A. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol. Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Huang W.K., Tu H.T., See L.C. Aspirin use on incidence and mortality of gastrointestinal cancers: current state of epidemiological evidence. Curr. Pharm. Des. 2015;21:5108–5115. doi: 10.2174/1381612821666150915110450. [DOI] [PubMed] [Google Scholar]
- Huh J.Y., Park Y.J., Ham M., Kim J.B. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol. Cells. 2014;37:365–371. doi: 10.14348/molcells.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L., Chen Y. Tumor microenvironment: sanctuary of the devil. Cancer Lett. 2015;368:7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- Ikeoka D., Mader J.K., Pieber T.R. Adipose tissue, inflammation and cardiovascular disease. Rev. Assoc. Med. Bras. 2010;56:116–121. doi: 10.1590/S0104-42302010000100026. [DOI] [PubMed] [Google Scholar]
- Incio J., Ligibel J.A., McManus D.T., Suboj P., Jung K., Kawaguchi K., Pinter M., Babykutty S., Chin S.M., Vardam T.D., et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci. Transl. Med. 2018;10:eaag0945. doi: 10.1126/scitranslmed.aag0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R.C. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014;346:6–16. doi: 10.1016/j.canlet.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Joensuu H., Wardelmann E., Sihto H., Eriksson M., Sundby Hall K., Reichardt A., Hartmann J.T., Pink D., Cameron S., Hohenberger P., et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an exploratory analysis of a randomized clinical trial. JAMA Oncol. 2017;3:602–609. doi: 10.1001/jamaoncol.2016.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.R., Milner J.J., Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.Y., Fattet L., Yang J. Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin. Cancer Res. 2015;21:962–968. doi: 10.1158/1078-0432.CCR-13-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kang J.H., Jang Y.S., Lee H.J., Lee C.Y., Shin D.Y., Oh S.H. Inhibition of STAT3 signaling induces apoptosis and suppresses growth of lung cancer: good and bad. Lab. Anim. Res. 2019;35:30. doi: 10.1186/s42826-019-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis G.S., Poutahidis T., Erdman S.E., Kirsch R., Riddell R.H., Diamandis E.P. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol. Cancer Res. 2012;10:1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T., Muise E.S., Iyengar P., Wang Z.V., Chandalia M., Abate N., Zhang B.B., Bonaldo P., Chua S., Scherer P.E. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Kim J., Yoon J.H., Ghim J., Yea K., Song P., Park S., Lee A., Hong C.P., Jang M.S., et al. CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia. 2014;57:1456–1465. doi: 10.1007/s00125-014-3237-5. [DOI] [PubMed] [Google Scholar]
- Ladanyi A., Mukherjee A., Kenny H.A., Johnson A., Mitra A.K., Sundaresan S., Nieman K.M., Pascual G., Benitah S.A., Montag A., et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 2018;37:2285–2301. doi: 10.1038/s41388-017-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau M.F., Chua K.H., Sabaratnam V., Kuppusamy U.R. Rosiglitazone enhances the apoptotic effect of 5-fluorouracil in colorectal cancer cells with high-glucose-induced glutathione. Sci. Prog. 2019;103:36850419886448. doi: 10.1177/0036850419886448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V., Guerard A., Mazerolles C., Le Gonidec S., Toulet A., Nieto L., Zaidi F., Majed B., Garandeau D., Socrier Y., et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel E., Makowski L., DiGiovanni J., Kolonin M.G. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4:374–384. doi: 10.1016/j.trecan.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Verschuren L., Mulder P., van der Hoorn J.W., Verheij J., van Dam A.D., Boon M.R., Princen H.M., Havekes L.M., Kleemann R., et al. Salsalate attenuates diet induced non-alcoholic steatohepatitis in mice by decreasing lipogenic and inflammatory processes. Br. J. Pharmacol. 2015;172:5293–5305. doi: 10.1111/bph.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudan S., Foster M., Muthuramalingam S.R., Braybrooke J.P., Wilner S., Kaur K., Han C., Hoare S., Balkwill F., Talbot D.C., et al. A phase II study of etanercept (Enbrel), a tumor necrosis factor alpha inhibitor in patients with metastatic breast cancer. Clin. Cancer Res. 2004;10:6528–6534. doi: 10.1158/1078-0432.CCR-04-0730. [DOI] [PubMed] [Google Scholar]
- Madke B., Khopkar U. Nephrogenic systemic fibrosis. Indian Dermatol. Online J. 2011;2:51–56. doi: 10.4103/2229-5178.85990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin G., Silveira A.L.M., Martins L.B., Ferreira A.V.M., Clement K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Invest. 2019;129:4032–4040. doi: 10.1172/JCI129192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M.T., Favelyukis S., Nguyen A.K., Reichart D., Scott P.A., Jenn A., Liu-Bryan R., Glass C.K., Neels J.G., Olefsky J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., Romero I.L., Carey M.S., Mills G.B., Hotamisligil G.S., et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura T., Ohuchida K., Sada M., Abe T., Endo S., Koikawa K., Iwamoto C., Miura D., Mizuuchi Y., Moriyama T., et al. Extra-pancreatic invasion induces lipolytic and fibrotic changes in the adipose microenvironment, with released fatty acids enhancing the invasiveness of pancreatic cancer cells. Oncotarget. 2017;8:18280–18295. doi: 10.18632/oncotarget.15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy K., Nareshkumar R.N., Sivagurunathan S., Raman R., Sulochana K.N., Chidambaram S. Anti-angiogenic effect of adiponectin in human primary microvascular and macrovascular endothelial cells. Microvasc. Res. 2019;122:136–145. doi: 10.1016/j.mvr.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Pang C., Gao Z., Yin J., Zhang J., Jia W., Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am. J. Physiol. Endocrinol. Metab. 2008;295:E313–E322. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panka B.A., de Grooth H.J., Spoelstra-de Man A.M., Looney M.R., Tuinman P.R. Prevention or treatment of Ards with aspirin: a review of preclinical models and meta-analysis of clinical studies. Shock. 2017;47:13–21. doi: 10.1097/SHK.0000000000000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D., Li P.P., Thapar D., Chapman J., Olefsky J.M., Neels J.G. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz G., Lim E.L., Wong M.L., Licinio J. Associations between adipokines and obesity-related cancer. Front. Biosci. (Landmark Ed.) 2011;16:1634–1650. doi: 10.2741/3810. [DOI] [PubMed] [Google Scholar]
- Petrova V., Annicchiarico-Petruzzelli M., Melino G., Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. doi: 10.1038/s41389-017-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer E.C., Saxon D.R., Janson R.W. Etanercept-induced hypoglycemia in a patient with psoriatic arthritis and diabetes. J. Investig. Med. High Impact Case Rep. 2017;5:2324709617727760. doi: 10.1177/2324709617727760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail D.F., Dannenberg A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019;15:139–154. doi: 10.1038/s41574-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla Rodriguez B.S., Correa R. Rosiglitazone. In: Abai B., editor. StatPearls [Internet] StatPearls Publishing; Treasure Island: 2020. [DOI] [Google Scholar]
- Rangarajan S., Bone N.B., Zmijewska A.A., Jiang S., Park D.W., Bernard K., Locy M.L., Ravi S., Deshane J., Mannon R.B., et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med. 2018;24:1121–1127. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera-Nunez E., Xu P., Chow A., Man S., Hilberg F., Kerbel R.S. Therapeutic impact of Nintedanib with paclitaxel and/or a PD-L1 antibody in preclinical models of orthotopic primary or metastatic triple negative breast cancer. J. Exp. Clin. Cancer Res. 2019;38:16. doi: 10.1186/s13046-018-0999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S.M., Saltiel A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Ortega P., Hayton C., Blaikley J., Leonard C., Chaudhuri N. Nintedanib in the management of idiopathic pulmonary fibrosis: clinical trial evidence and real-world experience. Ther. Adv. Respir. Dis. 2018;12:1753466618800618. doi: 10.1177/1753466618800618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma-Rodrigues C., Mendes R., Baptista P.V., Fernandes A.R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019;20:840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani P., Annunziato F., Lasagni L., Lazzeri E., Beltrame C., Francalanci M., Uguccioni M., Galli G., Cosmi L., Maurenzig L., et al. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J. Clin. Invest. 2001;107:53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A., Latiano T.P., Parente P., Chiarazzo C., Limosani F., Di Maggio G., Maiello E. The potential role of nintedanib in treating colorectal cancer. Expert Opin. Pharmacother. 2017;18:1153–1162. doi: 10.1080/14656566.2017.1346086. [DOI] [PubMed] [Google Scholar]
- Russo L., Lumeng C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–417. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Martin L., Estecha A., Samaniego R., Sanchez-Ramon S., Vega M.A., Sanchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117:88–97. doi: 10.1182/blood-2009-12-258186. [DOI] [PubMed] [Google Scholar]
- Saraei P., Asadi I., Kakar M.A., Moradi-Kor N. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Manag. Res. 2019;11:3295–3313. doi: 10.2147/CMAR.S200059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala S. Molecular pathways: targeting the CXCR4-CXCL12 axis--untapped potential in the tumor microenvironment. Clin. Cancer Res. 2015;21:4278–4285. doi: 10.1158/1078-0432.CCR-14-0914. [DOI] [PubMed] [Google Scholar]
- Seo B.R., Bhardwaj P., Choi S., Gonzalez J., Andresen Eguiluz R.C., Wang K., Mohanan S., Morris P.G., Du B., Zhou X.K., et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl. Med. 2015;7:301ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontake V., Wang Y., Kasam R.K., Sinner D., Reddy G.B., Naren A.P., McCormack F.X., White E.S., Jegga A.G., Madala S.K. Hsp90 regulation of fibroblast activation in pulmonary fibrosis. JCI Insight. 2017;2:e91454. doi: 10.1172/jci.insight.91454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley T.L., Zanni M.V., Johnsen S., Rasheed S., Makimura H., Lee H., Khor V.K., Ahima R.S., Grinspoon S.K. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2011;96:E146–E150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Tordjman J., Clement K., Scherer P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamantez-Lyburn S., Brown P., Hondrogiannis N., Ratliff J., Wicks S.L., Nana N., Zheng Z., Rosenzweig Z., Hondrogiannis E., Devadas M.S., et al. Gold nanoparticles loaded with cullin-5 DNA increase sensitivity to 17-AAG in cullin-5 deficient breast cancer cells. Int. J. Pharm. 2019;564:281–292. doi: 10.1016/j.ijpharm.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Apovian C. Macrophage functions in lean and obese adipose tissue. Metabolism. 2017;72:120–143. doi: 10.1016/j.metabol.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolios A., De Las Rivas J., Hovig E., Trouillas P., Scorilas A., Mohr T. Computational approaches in cancer multidrug resistance research: Identification of potential biomarkers, drug targets and drug-target interactions. Drug Resist. Updat. 2020;48:100662. doi: 10.1016/j.drup.2019.100662. [DOI] [PubMed] [Google Scholar]
- Tougeron D., Sha D., Manthravadi S., Sinicrope F.A. Aspirin and colorectal cancer: back to the future. Clin. Cancer Res. 2014;20:1087–1094. doi: 10.1158/1078-0432.CCR-13-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varone F., Sgalla G., Iovene B., Bruni T., Richeldi L. Nintedanib for the treatment of idiopathic pulmonary fibrosis. Expert Opin. Pharmacother. 2018;19:167–175. doi: 10.1080/14656566.2018.1425681. [DOI] [PubMed] [Google Scholar]
- Walker C., Mojares E., Del Rio Hernandez A. Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci. 2018;19:3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Gao C., Meng K., Qiao H., Wang Y. Human adipocytes stimulate invasion of breast cancer MCF-7 cells by secreting IGFBP-2. PLoS One. 2015;10:e0119348. doi: 10.1371/journal.pone.0119348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Gao J., Ohno Y., Liu H., Xu C. Rosiglitazone ameliorates senescence and promotes apoptosis in ovarian cancer induced by olaparib. Cancer Chemother. Pharmacol. 2020;85:273–284. doi: 10.1007/s00280-019-04025-8. [DOI] [PubMed] [Google Scholar]
- Wei Z., Zhao D., Zhang Y., Chen Y., Zhang S., Li Q., Zeng P., Li X., Zhang W., Duan Y., et al. Rosiglitazone ameliorates bile duct ligation-induced liver fibrosis by down-regulating NF-kappaB-TNF-alpha signaling pathway in a PPARgamma-dependent manner. Biochem. Biophys. Res. Commun. 2019;519:854–860. doi: 10.1016/j.bbrc.2019.09.084. [DOI] [PubMed] [Google Scholar]
- Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y.A., Xing X., Harris J.W., Zaytseva Y.Y., Mitov M.I., Napier D.L., Weiss H.L., Mark Evers B., Gao T. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 2017;8:e2593. doi: 10.1038/cddis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Koh G.Y., Huang Y., Crott J.W., Bronson R.T., Mason J.B. The combination of curcumin and salsalate is superior to either agent alone in suppressing pro-cancerous molecular pathways and colorectal tumorigenesis in obese mice. Mol. Nutr. Food Res. 2019;63:e1801097. doi: 10.1002/mnfr.201801097. [DOI] [PubMed] [Google Scholar]
- Xing F., Saidou J., Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. (Landmark Ed.) 2010;15:166–179. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Xu H., Wang W., Li S., Li H., Li T., Zhang W., Yu X., Liu L. The role of collagen in cancer: from bench to bedside. J. Transl. Med. 2019a;17:309. doi: 10.1186/s12967-019-2058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.Y., Liu H., Su L., Xu N., Xu D.H., Liu H.Y., Spaner D., Bed-David Y., Li Y.J. PPARgamma inhibits breast cancer progression by upregulating PTPRF expression. Eur. Rev. Med. Pharmacol. Sci. 2019b;23:9965–9977. doi: 10.26355/eurrev_201911_19563. [DOI] [PubMed] [Google Scholar]
- Yang J., Zaman M.M., Vlasakov I., Roy R., Huang L., Martin C.R., Freedman S.D., Serhan C.N., Moses M.A. Adipocytes promote ovarian cancer chemoresistance. Sci. Rep. 2019;9:13316. doi: 10.1038/s41598-019-49649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhang J., Gao Q., Bo J., Ma Z. Etanercept attenuates thermal and mechanical hyperalgesia induced by bone cancer. Exp. Ther. Med. 2017;13:2565–2569. doi: 10.3892/etm.2017.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Xu X.H., Jin L. Macrophage polarization in physiological and pathological pregnancy. Front. Immunol. 2019;10:792. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Konstantopoulos N., Lee J., Hansen L., Li Z.W., Karin M., Shoelson S.E. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- Zhang H.H., Souza S.C., Muliro K.V., Kraemer F.B., Obin M.S., Greenberg A.S. Lipase-selective functional domains of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J. Biol. Chem. 2003;278:51535–51542. doi: 10.1074/jbc.M309591200. [DOI] [PubMed] [Google Scholar]
- Zhao S., Mysler E., Moots R.J. Etanercept for the treatment of rheumatoid arthritis. Immunotherapy. 2018;10:433–445. doi: 10.2217/imt-2017-0155. [DOI] [PubMed] [Google Scholar]
- Zhou W., Guo S., Liu M., Burow M.E., Wang G. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr. Med. Chem. 2019;26:3026–3041. doi: 10.2174/0929867324666170830111531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.H., Ji C.D., Xiao H.L., Zhao H.B., Cui Y.H., Bian X.W. Reorganized collagen in the tumor microenvironment of gastric cancer and its association with prognosis. J. Cancer. 2017;8:1466–1476. doi: 10.7150/jca.18466. [DOI] [PMC free article] [PubMed] [Google Scholar]