Abstract
NB4 cell, the human acute promyelocytic leukemia (APL) cell line, was treated with various concentrations of arsenic trioxide (ATO) to induce apoptosis, measured by staining with 7-amino-actinomycin D (7-AAD) by flow cytometry. 2’, 7’-dichlorodihydro-fluorescein-diacetate (DCF-DA) and MitoSOXTM Red mitochondrial superoxide indicator were used to detect intracellular and mitochondrial reactive oxygen species (ROS). The steady-state level of SO2 (Cysteine sulfinic acid, Cys-SO2H) form for peroxiredoxin 3 (PRX3) was measured by a western blot. To evaluate the effect of sulfiredoxin 1 depletion, NB4 cells were transfected with small interfering RNA and analyzed for their influence on ROS, redox enzymes, and apoptosis. The mitochondrial ROS of NB4 cells significantly increased after ATO treatment. NB4 cell apoptosis after ATO treatment increased in a time- dependent manner. Increased SO2 form and dimeric PRX3 were observed as a hyperoxidation reaction in NB4 cells post- ATO treatment, in concordance with mitochondrial ROS accumulation. Sulfiredoxin 1 expression is downregulated by small interfering RNA transfection, which potentiated mitochondrial ROS generation and cell growth arrest in ATO- treated NB4 cells. Our results indicate that ATO-induced ROS generation in APL cell mitochondria is attributable to PRX3 hyperoxidation as well as dimerized PRX3 accumulation, subsequently triggering apoptosis. The downregulation of sulfiredoxin 1 could amplify apoptosis in ATO-treated APL cells.
Keywords: acute promyelocytic leukemia, arsenic trioxide, peroxiredoxin 3
INTRODUCTION
Acute promyelocytic leukemia (APL) is characterized by the expression of promyelocytic leukemia-retinoic acid receptor alpha (PML-RARα), as a result of t(15;17). Despite excellent initial responses to all-trans retinoic acid (ATRA), relapsed APL patients are often resistant to ATRA if ATRA treatment is attempted again. Arsenic trioxide (As2O3; ATO) has been used to treat relapsed diseases, and has demonstrated efficacy on newly diagnosed APL patients with or without ATRA (Mathews et al., 2010; Sanz et al., 2005; Soignet et al., 1998; 2001). The therapeutic effects of ATO, which binds through its RBCC (RING, B Boxes, and coiled coil) PML moiety domains, induced apoptosis and leukemic cell differentiation to facilitate the clearance of the leukemia-initiating cells of APL (de Thé and Chen, 2010; Mi et al., 2012). ATO exerts its anti-leukemia effect by raising oxidative stress in the APL. ATO-induced apoptosis is modulated via the cellular glutathione redox system by increasing intracellular levels of reduced glutathione to inhibit APL cells. ATO inhibits glutathione peroxidase (GPX) activity and consequently increases cellular reactive oxygen species (ROS) in APL cells (Jing et al., 1999). Meanwhile, Lu et al. (2007) reported that thioredoxin (TRX) is a target for ATO-induced ROS generation, leading to death in breast cancer cells. ATO not only directly targets the mitochondrial electron transport chain, producing ROS in acute leukemia cells (Pelicano et al., 2003), but may also affect antioxidant expression within leukemic cells (Sumi et al., 2010; Vivas-Mejía et al., 2009).
2-Cys peroxiredoxins (PRXs), members of a novel peroxidase family that catalyzes H2O2 reduction, are homodimeric cytosolic proteins containing a conserved N-terminal cystein residue with TRX, thioredoxin reductase (TRX-R), and nicotinamide adenine dinucleotide phosphate (NADPH) (Kang et al., 2005; Rhee et al., 2005). Several studies have demonstrated that some subtype of PRX is involved in leukemogenesis, resistance to chemotherapy, and tumor cell survival (Agrawal-Singh et al., 2012; Vivas-Mejía et al., 2009; Wen and Van Etten, 1997; Zhang et al., 1997). The relationship between PRX and leukemia has not been fully evaluated, although it may be the key to oncogene-induced leukemia growth and to therapeutic responses. APL cells are resistant to ATO treatment, in some cases, although it is an effective therapeutic drug for APL. The mechanisms of ROS generation by ATO coupled with redox enzymes, including PRX and TRX during ATO-induced apoptosis, remain elusive.
This study aimed to identify ROS sources during ATO treatment in APL cells and what role anti-oxidant enzymes, especially PRXs, have in ROS generation with ATO. We also tried to determine how to potentiate the effect of ROS production for more APL cell apoptosis by blocking sulfiredoxin (SRX) induction after ATO treatment.
MATERIALS AND METHODS
Cell culture and reagents
The NB4 cell, a human APL cell line, and the A431 cell, a human epidermoid carcinoma cell line, were cultivated in RPMI media (Welgene, Korea) supplemented with 10% fetal bovine serum, 100 units/ml of penicillin, and 100 μg/ml of streptomycin (Gibco BRL, USA) in 5% CO2 at 37°C. Arsenic trioxide (ATO), 2’, 7’-dichlorodihydro-fluorescein-diacetate (DCF-DA), 7-amino-actinomycin D (7-AAD), methylthiazolyldiphenyl-tetrazolium bromide (MTT), and rotenone were purchased from Sigma (USA). We obtained MitoSOXTM Red mitochondrial superoxide indicator from Molecular Probes (USA) and Wright’s stain solution (Wright’s Eosin Methylene Blue Solution) from Muto Pure Chemicals (Japan). Antibodies specific for PRX1-3 and TRX-R2 have been previously described (Kang et al., 1998; Lee et al., 1999). Antibodies specific for caspase 3 and caspase 9 were purchased from Cell Signaling Technology (USA), and antibody for alpha-tubulin was from Calbiochem (USA). Antibodies for superoxide dismutase 2 (SOD2), GPX, PRXs-SO2, and TRX2 were purchased from AbFrontier (Korea). SRX1 antibody was purchased from Thermo Scientific (USA). Anti-mouse and anti-rabbit horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Ctuz Biotechnology (USA). We purchased Complete Mini protease inhibitor cocktail from Roche (Germany), a PierceTM BCA protein assay kit from Thermo Scientific, and a small interfering RNA (siRNA) against SRX1, AllStars negative control siRNA and HiperFect siRNA transfection reagent from Qiagen (USA). Dr. Murphy, M.P. (MRC Mitochondrial Biology Unit, UK) gifted us the mitoquinone (mitoQ).
Intracellular ROS and mitochondria-specific superoxide levels
After NB4 cells were treated with ATO, 10 μM of DCF-DA or 5 μM of MitoSOXTM Red mitochondrial superoxide indicator in dimethyl sulfoxide was mixed with the cells and incubated for 20 min at room temperature. The cells were washed, resuspended with phosphate buffered saline (PBS), and detected with the FL1 or FL2 channel of flow cytometry to measure intracellular ROS or mitochondrial superoxide, respectively (FACSCalibur; BD Biosciences, USA).
Wright’s staining and 7-AAD staining
After NB4 cells were treated with 2 μM of ATO for the indicated times, apoptotic cell death was examined by staining with Wright’s stain solution. The procedure was as follows: 2 × 104 cells were washed and suspended in 100 μl of PBS and collected onto a clean slide glass using cytospin. The slide glass was dried and stained with Wright’s stain solution, then observed under light microscopy (original magnification, ×100). Quantitative analysis of apoptotic cell death was assessed by 7-AAD staining. 7-AAD was dissolved in methanol and diluted in PBS at a concentration of 200 μg/ml. The diluted 7-AAD solution was mixed with the cells and incubated for 20 min at room temperature. The cells were washed, resuspended with PBS, and analyzed using Cell Quest software of flow cytometry (FACSCalibur).
MTT assay
We cultured 5 × 104 cells/well in a 96 well-plate containing 2 μM of ATO for the indicated times. MTT solution (5 mg/ml) was added into each well, and the mixture was incubated for 4 h. Dark purple formazan crystals formed and they were solubilized (10% SDS in 0.01 N HCl) overnight. The optical density at 560 nm was read with an iMarkTM microplate reader (BioRad, USA).
Western blot
NB4 cells were exposed to the drugs at the indicated concentrations and times. These cells were rinsed with ice-cold PBS and lysed in lysis buffer (50 mM Tris (pH 8.0), 0.15 M NaCl, 1% NP-40 with protease inhibitor cocktail). A431 cells were treated with 1 mM of H2O2 for 10 min and used as a positive marker for PRXs-SO2. The total protein concentration was determined with a PierceTM BCA protein assay kit according to the manufacturer’s protocol (Thermo Scientific). Equal amounts of total proteins in loading buffer were boiled for five min, separated using SDS gel electrophoresis, and electrotransferred onto a polyvinylidene difluoride (PVDF) membrane (GE Healthcare Life Sciences, USA) using miniVE system (GE Healthcare Bio-sciences AB, USA). The PVDF membrane was blocked with 5% skim milk in Tris-buffered saline (TBS) for 1 h and washed three times (five min/time) with TBS containing 0.1% Tween 20 (TBS-T) at room temperature. The membrane and a primary antibody were incubated in TBS containing 5% bovine serum albumine for 2 h at room temperature and washed three times with TBS-T. The membrane was then incubated with a horseradish peroxidase-conjugated secondary antibody (in TBS containing 5% skim milk) for 1 h and washed three times with TBS-T. The membrane was incubated with picoEPD (ELPIS Biotech, Korea). The specific bands were visualized by ImageQuant LAS 3000 and analyzed using MutiGuage software (ver. 3.0; Fujifilm, Japan).
SRX1 knockdown
siRNA against SRX1 and Allstars negative control siRNA were transfected into NB4 cells to knockdown the SRX1 gene using a HiPerFect transfection reagent according to the manufacturer’s protocol. The target sequence of siRNA against SRX1 was 5’-CAGATGTACCATGGTGATGTA-3’. After siRNA transfection, 2 μM of ATO was treated for 24 h to study sulfinic PRX3 expression, cell growth arrest, and mitochondrial ROS generation in NB4 cells.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (ver. 23; IBM, USA). The quantitative data were presented as mean ± SD and were analyzed with the two-tailed Student’s t-test. The asterisks in the figures indicate statistical significance (*P < 0.05).
RESULTS
ATO-induced intracellular and mitochondrial ROS levels in NB4 cells
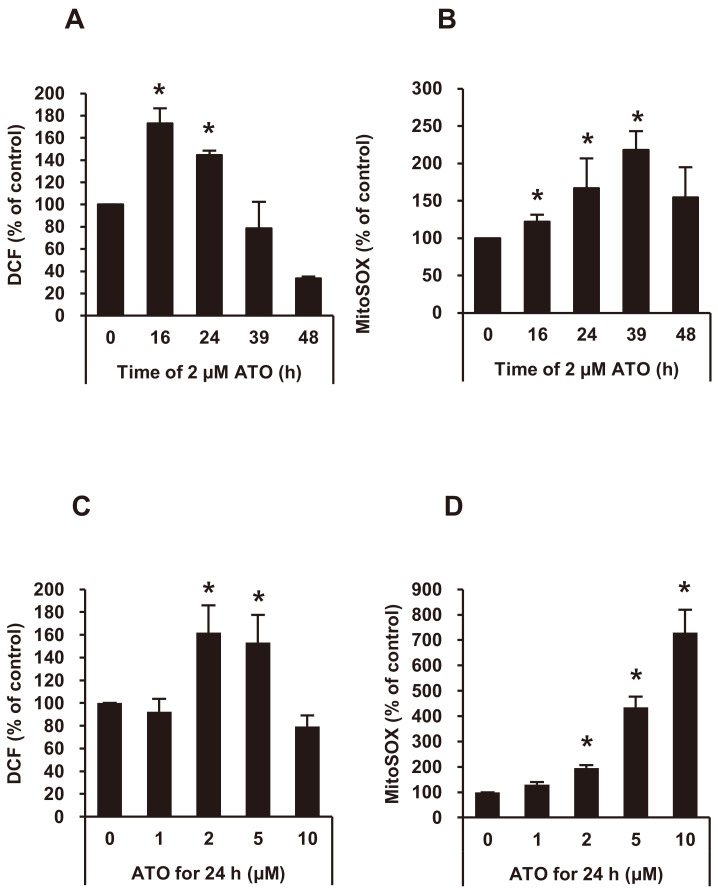
We measured intracellular and mitochondrial ROS after ATO treatment in NB4 cells. A flow cytometric analysis demonstrated that intracellular ROS of NB4 cells was significantly increased after 16-24 h of 2 μM of ATO, but decreased after 39 h (Fig. 1A). The mitochondrial ROS of NB4 cells was significantly increased after 16-39 h of 2 μM of ATO (Fig. 1B). Intracellular ROS level increased with DCF-DA staining at 2-5 μM of ATO concentrations (Fig. 1C), and the mitochondrial ROS level increased with MitoSOXTM Red mitochondrial superoxide indicator staining at 2-10 μM of ATO (Fig. 1D).
Fig. 1. The level of ROS of NB4 cells after ATO treatment.
(A and B) Intracellular ROS and mitochondrial superoxide (mitoSOX) levels of NB4 cells were measured by staining with 2’, 7’-dichlorodihydro-fluorescein-diacetate (DCF-DA) and MitoSOXTM Red mitochondrial superoxide indicator after 2 μM of ATO treatment for the indicated times, and were analyzed by flow cytometry. (C and D) Intracellular ROS and mitoSOX levels of NB4 cells were measured by staining with DCF-DA and MitoSOXTM Red mitochondrial superoxide indicator after ATO treatment at the indicated concentrations for 24 h, and were analyzed by flow cytometry. Experiments were presented as mean ± SD. *P < 0.05 vs ATO non-treated baseline.
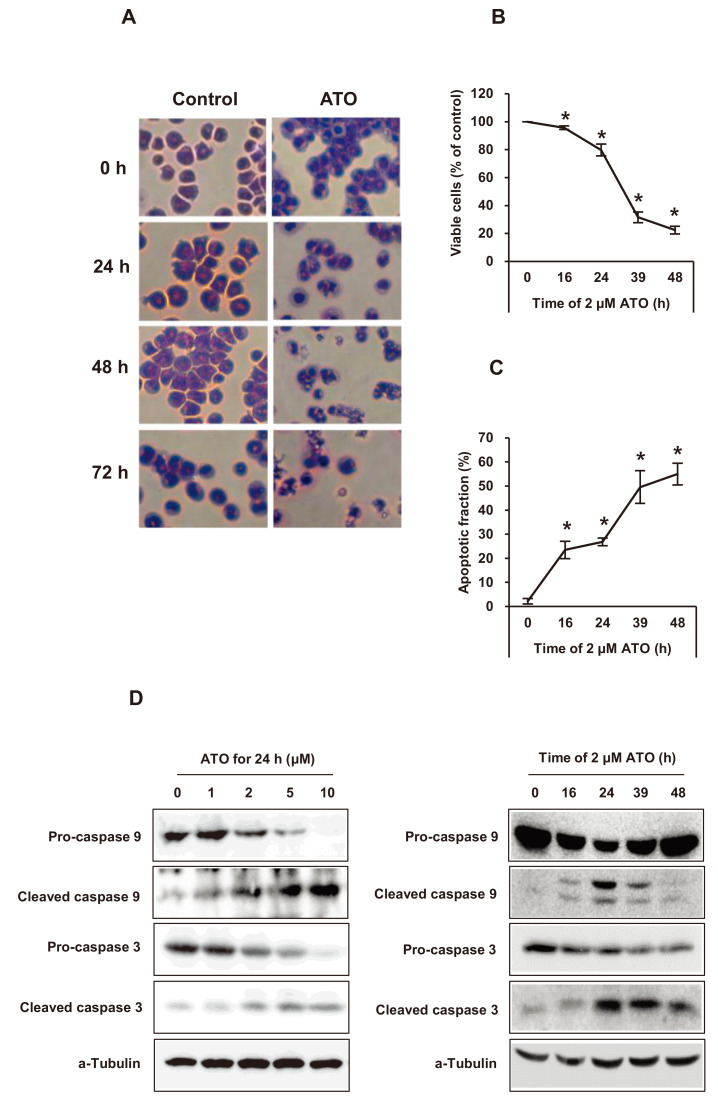
Apoptosis of NB4 cells by microscopy, MTT assay, and 7-AAD staining and the expression of cleaved caspase 9 and cleaved caspase 3 after ATO treatment
After ATO treatment in NB4 cells, apoptotic changes were studied by Wright’s staining, MTT assay, and flow cytometric analysis using 7-AAD staining. Viable cells were decreased and apoptotic cells increased with increasing ATO treatment time (Figs. 2A-2C). Cleaved caspase 3 and caspase 9 were also observed with ATO treatment, suggesting their role in the intrinsic pathway of apoptotsis (Fig. 2D).
Fig. 2. Apoptotic cell death of NB4 cells after ATO treatment.
(A) Microscopic findings of apoptosis by Wright’s staining after 2 μM of ATO treatment for the indicated times. The original magnification was ×100. (B and C) Cell viability by methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay and apoptosis by flow cytometry with 7-amino-actinomycin D staining after 2 μM of ATO treatment for the indicated times. Experiments were presented as mean ± SD. *P < 0.05 vs ATO non-treated baseline. (D) Pro- and cleaved caspase 3 or pro- and cleaved caspase 9 expression on western blot analysis after ATO treatment at the indicated concentrations and times. α-Tubulin was used as a loading control.
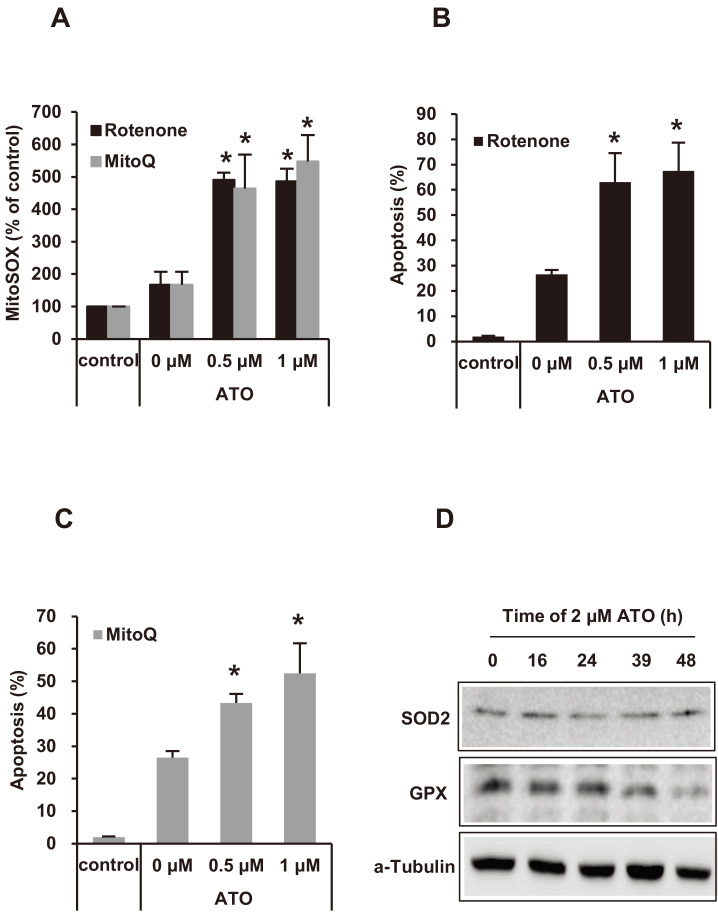
Mitochondrial superoxide activities and apoptosis induced by rotenone or mitoQ with ATO of NB4 cells
Rotenone or mitoQ increased the production of ATO-induced mitochondrial superoxide (mitoSOX) (Fig. 3A). ATO-induced apoptosis of the NB4 cells was potentiated with rotenone or mitoQ co-treatment according to the mitoSOX level, suggesting that mitoSOX is important for ATO-induced APL cell death (Figs. 3B and 3C). Our study showed that the intrinsic apoptosis pathway was activated with an increase in mitochondrial ROS in NB4 cells after ATO treatment. Therefore, we measured the expression of SOD2, a mitochondria matrix enzyme, and GPX, which is associated with apoptosis, in NB4 cells after ATO treatment. Expression of SOD2 did not change with ATO treatment, but there were slight decreases in GPX detected after 48 h of ATO treatment (Fig. 3D).
Fig. 3. The incremental level of mitochondrial superoxide (mitoSOX) and the apoptotic fraction of NB4 cells treated with ATO.
(A) MitoSOX level of NB4 cells was increased after 2 μM of ATO co-treatment with the indicated concentrations of rotenone or mitoQ for 24 h. (B and C) Apoptotic cell fraction was increased after 2 μM of ATO co-treatment with the indicated concentraions of rotenone or mitoQ for 24 h. Experiments were presented as mean ± SD. *P < 0.05 vs rotenone or mitoQ non-treated baseline in ATO treatment. (D) The expression levels of SOD2 and GPX were detected by western blot assay after 2 μM of ATO treatment for the indicated times. α-Tubulin was used as a loading control.
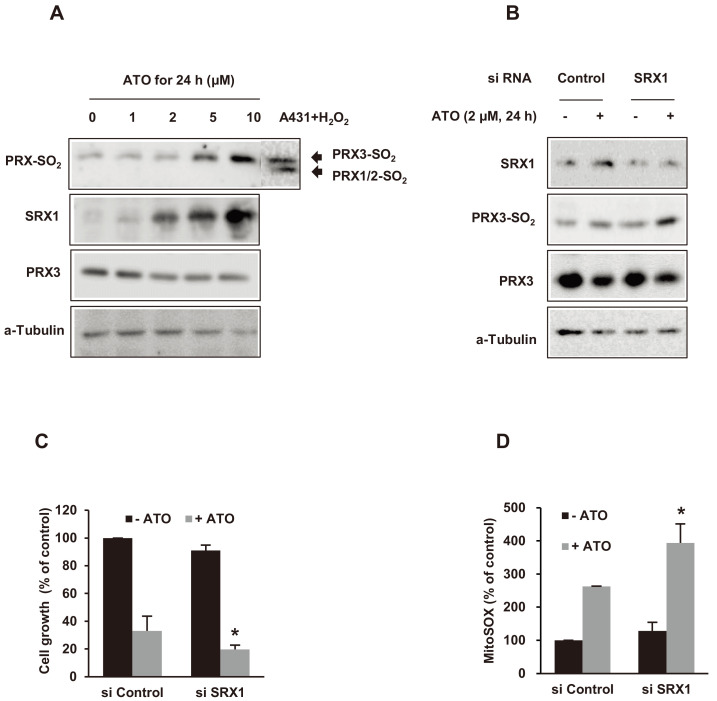
PRX3 hyperoxidation and the impact of SRX1 downregulation by siRNA in ATO-treated NB4 cells
After NB4 cells were exposed to 2 μM of ATO for 24 h, we analyzed the total levels of PRX1, PRX2, and PRX3, and the change of cysteine sulfinic acid (Cys–SO2H), and the dimeric form of PRXs by western blot analysis. Despite the ATO treatment, the total expression levels of PRX1 and PRX2 in NB4 cells remained unchanged (data not shown). We detected slight decreases in the total PRX3 expression level in ATO-treated NB4 cells. We found the upper band, overoxidized PRX3, and the lower band, overoxidized PRX1/2 in Cys–SO2H PRXs, in our previous western blot assays for PRX-SO2 (Noh et al., 2009). In this study, the intensity of the cysteine sulfinic acid form of PRX3 increased as a hyperoxidation reaction after ATO treatment in concordance with the mitochondrial ROS. In contrast, Cys–SO2H PRX1/2 was not detected during ATO exposure. We also evaluated SRX1 expression and found SRX1 upregulation in NB4 cells after ATO treatment (Fig. 4A). To evaluate the effect of SRX1 downregulation, NB4 cells were transfected with a small interfering RNA (siRNA) against SRX1 and exposed to 2 μM of ATO for 24 h. As we expected, SRX1 expression decreased with siRNA. Cys–SO2H PRX3 expression increased simultaneously by ATO in a werstern blot assay (Fig. 4B). As a result, NB4 cell growth was inhibited, and mitochondrial ROS increased along with the downregulation of SRX1 by siRNA transfection (Figs. 4C and 4D).
Fig. 4. Effects of sulfiredoinx 1 (SRX1) downregulation by siRNA transfection on hyperoxidized PRX3, cell growth, and mitochondrial siperoxide (mitoSOX) generation in NB4 cells after ATO treatment.
(A) The levels of peroxiredoxin 3 hyperoxidation (PRX3-SO2) and SRX1 expression were increased after the indicated concentrations of ATO treatment for 24 h. A431 cells, treated with 1 mM of H2O2 for 10 min, were used as a positive control for the hyperoxidized forms (-SO2) of PRX1/2 and PRX3. α-Tubulin was used as a loading control. (B) Hyperoxidized PRX3 (PRX3-SO2) was increased, despite SRX1 downregulation by siRNA transfection against SRX1. α-Tubulin was used as a loading control. (C and D) Cell growth arrest and mitoSOX generation were increased after SRX1 downregulation by siRNA transfection against SRX1 and 2 μM of ATO treatment for 24 h. Experiments were presented as mean ± SD. *P < 0.05 vs control siRNA transfection in ATO treatment.
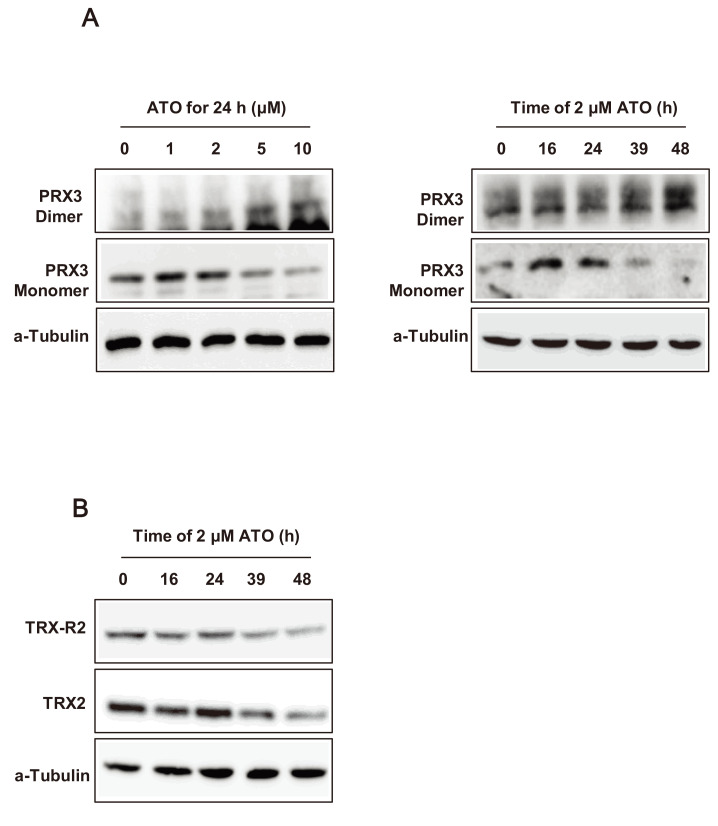
Dimerization of PRX3 and downregulation of TRX2 and TRX-R2 in NB4 cells post-ATO treatment
Dimeric PRX3 (oxidized form) increased and monomeric PRX3 (reduced form) decreased in NB4 cells after increasing in ATO concentration and expousure time (Fig. 5A). In contrast, changes to the dimeric or monomeric form of PRX1/2 in NB4 cells were not noticeable after ATO treatment (data not shown). These results suggest that the change of PRX3 oxidation status is important for ATO-induced APL cell death and that these changes correlate with the level of mitochondrial ROS after ATO treatment. To evaluate the change of TRX2 and TRX-R2 after ATO-induced APL cell apoptosis, the total expression levels of TRX2 and TRX-R2 were measured after ATO treatment. The results show that the intensity of TRX2 and TRX-R2 decreased after ATO treatment over time passed (Fig. 5B), and an inverse correlation with the intensity of oxidative PRX3. These results suggest that TRX2 and TRX-R2 expression was suppressed by ATO treatment in NB4 cells so that mitochondrial ROS increased through inhibition of oxidized PRX3 reduction.
Fig. 5. Western blot assay for PRX3 dimerization and thioredoxin 2 (TRX2)/thioredoxin reductase 2 (TRX-R2) downregulation in NB4 cells after ATO treatment.
(A) Dimeric and monomeric PRX3 in NB4 cells after ATO treatment. In the left panel, NB4 cells were treated with the indicated concentrations of ATO for 24 h, and in the right panel, NB4 cells were treated with 2 μM of ATO for the indicated times. (B) The expression levels of TRX2 and TRX-R2 in NB4 cells after 2 μM of ATO treatment for the indicated times. α-Tubulin was used as a loading control.
DISCUSSION
APL is characterized by reciprocal chromosomal translocation, t(15;17), which results in the production of PML–RARα fusion protein that causes a defect in the differentiation from myeloid precursors. The use of single-agent ATO or ATO combined with ATRA instead of chemotherapy is a promising APL treatment, resulting in durable remissions and minimal toxicity (George et al., 2004; Ghavamzadeh et al., 2006; Hu et al., 2009; Mathews et al., 2010; Ravandi et al., 2009). Recent evidence has suggested that oncogenes, such as BCR-ABL, Flt3-ITD, Ras, and c-Kit, directly manipulate leukemia signaling but also alter the redox homeostasis of leukemia cells elevating ROS production (Hole et al., 2011; Irwin et al., 2013; Sallmyr et al., 2008). APL leukemogenesis and redox are important, as: the downregulation of antioxidants can result in a net increase in the total ROS levels by decreased clearance (Irwin et al., 2013). Also, there are ROS-producing therapeutics that employ oxidative stress to affect the balance of growth, survival, and cell death (Hole et al., 2011; Irwin et al., 2013; Maraldi et al., 2009). In addition, standard leukemia therapeutics, including anthracyclines, cytarabine, vincristine, and ATO, have produced ROS in some capacity (Gewirtz, 1999; Groninger et al., 2002; Iacobini et al., 2001; Kanno et al., 2004; Lu et al., 2007; Potmesil et al., 1984; Vivas-Mejía et al., 2009). Last, upregulation of various antioxidant enzyme systems in leukemic cells after chemotherapy can negatively affect their anti-leukemic activities (Coe and Schimmer, 2008; Kearns et al., 2001; Maung et al., 1994).
While the therapeutic effect of ATO was demonstrated by the induction of apoptosis and differentiation in leukemic cells, ATO’s anti-leukemic effect is mainly induced by increasing oxidative stress in the leukemic cells (de Thé and Chen, 2010; Jing et al., 1999; Mi et al., 2012; Sumi et al., 2010). Studies indicated that ATO’s mitochondrial ROS production associated with the activation and propagation of apoptotic and necrotic cell death (Liu et al., 2005; Partridge et al., 2007). ATO reportedly activates NADPH oxidase and inhibits antioxidant enzymes such as GPX and TRX-R, thereby inducing ROS-dependent apoptosis (Chou et al., 2004; Jing et al., 1999; Lu et al., 2007). There is a tightly regulated balance between mitochondrial ROS production and the mitochondrial antioxidant defense systems, in which PRX3 is a major antioxidant enzyme that eliminates approximately 90% of H2O2 in the mitochondria (Cox et al., 2010). In our study, we observed an increase in mitochondrial ROS and apoptosis in NB4 cells post-ATO treatment in a time-dependent manner, although NOX-derived ROS also increased. Also, ATO-induced apoptosis of NB4 cells was potentiated with rotenone or mitoQ treatment by the level of mitochondrial superoxide (Fig. 3). Rotenone and mitoQ were known to play a role in inhibiting mitochondria-derived ROS production, but they also increase mitochondrial ROS production, as our experiment demonstrated. Li et al. (2003) reported that rotenone can induce apoptosis by enhancing the amount of mitochondrial ROS in the HL60 cell. Huang et al. (2015) reported that mitoQ treatment in a taurolithocholic acid 3-sulphate (TLCS)-induced acute pancreatitis model was not protective and caused a biphasic effect on ROS production, including elevating inflammatory markers. Our findings are consistent with previous reports in which mitochondrial ROS production is an important mechanism of ATO-induced death (Maraldi et al., 2009; Pelicano et al., 2003). In the catalytic cycle of 2-Cys PRXs, the conserved N-terminal Cys-SH is first oxidized by peroxides to cysteine sulfenic acid (Cys-SOH), which then reacts with the conserved COOH-terminal Cys-SH of the other homodimer subunit to form a disulfide bond. This disulfide is reduced by TRX, whose oxidized form is then regenerated by TRX-R, using NADPH-reducing equivalents (Rhee et al., 2012). Our results demonstrated that Cys–SO2H PRX3 increased as a hyperoxidation reaction in NB4 cells after ATO treatment incrementally by mitochondrial over cytosolic ROS. In contrast, Cys–SO2H PRX1/2 and dimeric PRX1/2, located in the cytosol, did not change with ATO exposure (data not shown). These findings suggest that PRX3 inactivation by hyperoxidation and multimerization is the main mechanism of ATO-induced ROS accumulation in mitochondria of APL cells, resulting in apoptosis in APL cells.
Research on the fate of the sulfinylated PRX enzyme has shown that its sulfinylation is reversible in mammalian cells. SRX regenerates inactive 2-Cys PRXs, returning it to the catalytic cycle and preventing its permanent oxidative inactivation by strong oxidative stress (Woo et al., 2005). Evidence suggests that SRX, in cytosol, makes a redox-balance by reducing PRX1 and PRX2 hyperoxidation. Noh et al. (2009) suggested that a reduction of PRX3 hyperoxidation, a mitochondrial antioxidant, is the result of translocating SRX1 to the mitochondria. In the murine model of pyrazole liver injury, removing mitochondrial oxidative stress results in apoptosis inhibition by translocation of SRX1 in the cytosol to the mitochondria that are reducing the hyperoxidized PRX3 (Bae et al., 2012). ATO-treated NB4 cells have increased Cys-SO2H PRX3 and mitochondrial ROS, which increases NB4 cell apoptosis. However, an increase in SRX1 in ATO-treated NB4 cells induces downregulation of mitochondrial ROS increase preventing sufficient anti-leukemic activity. SRX1 suppression through siRNA lessened the reduction of Cys-SO2H and increased its expression, resulting in an accumulation of mitochondral ROS that inhibits NB4 cell proliferation. These results suggest that suppressing increases of SRX1 by siRNA while ATO treating NB4 cells may maximize ATO-induced oxidative stress and induce sufficient anti-leukemic activity (Fig. 4). Despite the SRX1 elevation, PRX3 hyperoxidation did not decrease, which suggests that the rate of PRX3 hyperoxidation in the mitochondria exceeded the capacity of SRX molecules to reduce the hyperoxidation. Our co-workers made similar observations in mice with pyrazole-induced oxidative liver injury (Bae et al., 2012). Our observations suggest that improving the anti-leukemic effects of ATO by concomitant inhibition of SRX1 induction in leukemic cells could be enhance ATO’s anti-leukemia effects. We evaluated the ATO-induced apoptosis in NB4 cells transfected with human SRX siRNA. ATO-induced apoptosis increased due to the downregulation of the decrease in oxidized PRX3, resulting in ROS accumulation in leukemic cells (Figs. 4B-4D).
In conclusion, our results demonstrated that active PRX3 downregulation is the main mechanism of ATO-induced ROS generation in APL cell mitochondria, subsequently inducing apoptosis. ATO-induced ROS generation in APL cell mitochondria is attributable to PRX3 hyperoxidation and dimerized PRX3 accumulation. Interestingly, SRX1 inhibition induced apoptosis upregulation by inducing ROS accumulation with ATO. Our findings may support the development of novel therapies that increase ROS to potentiate ATO-induced apoptosis in APL cells.
ACKNOWLEDGMENTS
We wish to thank Dr. Murphy, M.P. (MRC Mitochondrial Biology Unit) for providing the mitoquinone. This research was supported by grants from YUHAN (2015), SK Plasma (2015), Boryung Co., Ltd. (2014), and Kyowa Kirin Korea Co., Ltd. (2015).
Footnotes
AUTHOR CONTRIBUTIONS
Y.C.M. and C.M.S. were conceptualized, designed, and analyzed the study. Y.C.M. wrote the initial draft of the manuscript. E.S.Y., K.E.L., E.M.N., J.H., H.A.W., and S.G.R. substantially revised the manuscript. J.Y.A. contributed to the experiment and analyzed the data.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Agrawal-Singh S., Isken F., Agelopoulos K., Klein H.U., Thoennissen N.H., Koehler G., Hascher A., Bäumer N., Berdel W.E., Thiede C., et al. Genome-wide analysis of histone H3 acetylation patterns in AML identifies PRDX2 as an epigenetically silenced tumor suppressor gene. Blood. 2012;119:2346–2357. doi: 10.1182/blood-2011-06-358705. [DOI] [PubMed] [Google Scholar]
- Bae S.H., Sung S.H., Lee H.E., Kang H.T., Lee S.K., Oh S.Y., Woo H.A., Kil I.S., Rhee S.G. Peroxiredoxin III and sulfiredoxin together protect mice from pyrazole-induced oxidative liver injury. Antioxid. Redox Signal. 2012;17:1351–1361. doi: 10.1089/ars.2011.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou W.C., Jie C., Kenedy A.A., Jones R.J., Trush M.A., Dang C.V. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe E., Schimmer A.D. Catalase activity and arsenic sensitivity in acute leukemia. Leuk. Lymphoma. 2008;49:1976–1981. doi: 10.1080/10428190802353617. [DOI] [PubMed] [Google Scholar]
- Cox A.G., Winterbourn C.C., Hampton M.B. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 2010;425:313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- de Thé H., Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat. Rev. Cancer. 2010;10:775–783. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- George B., Mathews V., Poonkuzhali B., Shaji R.V., Srivastava A., Chandy M. Treatment of children with newly diagnosed acute promyelocytic leukemia with arsenic trioxide: a single center experience. Leukemia. 2004;18:1587–1590. doi: 10.1038/sj.leu.2403480. [DOI] [PubMed] [Google Scholar]
- Gewirtz D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999;57:727–741. doi: 10.1016/S0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Ghavamzadeh A., Alimoghaddam K., Ghaffari S.H., Rostami S., Jahani M., Hosseini R., Mossavi A., Baybordi E., Iravani M., et al. Treatment of acute promyelocytic leukemia with arsenic trioxide without ATRA and/or chemotherapy. Ann. Oncol. 2006;17:131–134. doi: 10.1093/annonc/mdj019. [DOI] [PubMed] [Google Scholar]
- Groninger E., Meeuwsen-De Boer G.J., De Graaf S.S., Kamps W.A., De Bont E.S. Vincristine induced apoptosis in acute lymphoblastic leukaemia cells: a mitochondrial controlled pathway regulated by reactive oxygen species? Int. J. Oncol. 2002;21:1339–1345. doi: 10.3892/ijo.21.6.1339. [DOI] [PubMed] [Google Scholar]
- Hole P.S., Darley R.L., Tonks A. Do reactive oxygen species play a role in myeloid leukemias? Blood 117, 5816-5826. 2011 doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- Hu J., Liu Y.F., Wu C.F., Xu F., Shen Z.X., Zhu Y.M., Li J.M., Zhao W.L., Wu W., et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3342–3347. doi: 10.1073/pnas.0813280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Cash N., Wen L., Szatmary P., Mukherjee R., Armstrong J., Chvanov M., Tepikin A.V., Murphy M.P., Sutton R., et al. Effects of the mitochondria-targeted antioxidant mitoquinone in murine acute pancreatitis. Mediators Inflamm. 2015;2015:1–13. doi: 10.1155/2015/901780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobini M., Menichelli A., Palumbo G., Multari G., Werner B., Del Principe D. Involvement of oxygen radicals in cytarabine-induced apoptosis in human polymorphonuclear cells. Biochem. Pharmacol. 2001;61:1033–1040. doi: 10.1016/S0006-2952(01)00548-2. [DOI] [PubMed] [Google Scholar]
- Irwin M.E., Rivera-Del Valle N., Chandra J. Redox control of leukemia: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2013;18:1349–1383. doi: 10.1089/ars.2011.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y., Dai J., Chalmers-Redman R.M., Tatton W.G., Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. doi: 10.1182/blood.V94.6.2102. [DOI] [PubMed] [Google Scholar]
- Kang S.W., Chae H.Z., Seo M.S., Kim K., Baines I.C., Rhee S.G. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-α. J. Biol. Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- Kang S.W., Rhee S.G., Chang T.S., Jeong W., Choi M.H. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol. Med. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno S., Higurashi A., Watanabe Y., Shouji A., Asou K., Ishikawa M. Susceptibility to cytosine arabinoside (Ara-C)-induced cytotoxicity in human leukemia cell lines. Toxicol. Lett. 2004;152:149–158. doi: 10.1016/j.toxlet.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Kearns P.R., Pieters R., Rottier M.M., Pearson A.D., Hall A.G. Raised blast glutathione levels are associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood. 2001;97:393–398. doi: 10.1182/blood.V97.2.393. [DOI] [PubMed] [Google Scholar]
- Lee S.R., Kim J.R., Kwon K.S., Yoon H.W., Levie R.L., Ginsburg A., Rhee S.G. Molecular cloning and characterization of a mitochondrial selenocysteine-containing thioredoxin reductase from rat liver. J. Biol. Chem. 1999;274:4722–4734. doi: 10.1074/jbc.274.8.4722. [DOI] [PubMed] [Google Scholar]
- Li N., Ragheb K., Lawler G., Sturgis J., Rajwa B., Melendez J.A., Robinson J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- Liu S.X., Davidson M.M., Tang X., Walker W.F., Athar M., Ivanov V., Hei T.K. Mitochondrial damage mediates genotoxicity of arsenic in mammalian cells. Cancer Res. 2005;65:3236–3242. doi: 10.1158/0008-5472.CAN-05-0424. [DOI] [PubMed] [Google Scholar]
- Lu J., Chew E.H., Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12288–12293. doi: 10.1073/pnas.0701549104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraldi T., Prata C., Fiorentini D., Zambonin L., Landi L., Hakim G. Induction of apoptosis in a human leukemic cell line via reactive oxygen species modulation by antioxidants. Free Radic. Biol. Med. 2009;46:244–252. doi: 10.1016/j.freeradbiomed.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Mathews V., George B., Chendamarai E., Lakshmi K.M., Desire S., Balasubramanian P., Viswabandya A., Thirugnanam R., Abraham A., Shaji R.V., et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J. Clin. Oncol. 2010;28:3866–3871. doi: 10.1200/JCO.2010.28.5031. [DOI] [PubMed] [Google Scholar]
- Maung Z.T., Hogarth L., Reid M.M., Proctor S.J., Hamilton P.J., Hall A.G. Raised intracellular glutathione levels correlate with in vitro resistance to cytotoxic drugs in leukaemic cells from patients with acute lymphoblastic leukemia. Leukemia. 1994;8:1487–1491. [PubMed] [Google Scholar]
- Mi J.Q., Li J.M., Shen Z.X., Chen S.J., Chen Z. How to manage acute promyelocytic leukemia. Leukemia. 2012;26:1743–1751. doi: 10.1038/leu.2012.57. [DOI] [PubMed] [Google Scholar]
- Noh Y.H., Baek J.Y., Jeong W., Rhee S.G., Chang T.S. Sulfiredoxin translocation into mitochondria plays a crucial role in reducing hyperoxidized peroxiredoxin III. J. Biol. Chem. 2009;284:8470–8477. doi: 10.1074/jbc.M808981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge M.A., Huang S.X., Hernandez-Rosa E., Davidson M.M., Hei T.K. Arsenic induced mitochondrial DNA damage and altered mitochondrial oxidative function: implications for genotoxic mechanisms in mammalian cells. Cancer Res. 2007;67:5239–5247. doi: 10.1158/0008-5472.CAN-07-0074. [DOI] [PubMed] [Google Scholar]
- Pelicano H., Feng L., Zhou Y., Carew J.S., Hileman E.O., Plunkett W., Keating M.J., Huang P. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J. Biol. Chem. 2003;278:37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- Potmesil M., Israel M., Silber R. Two mechanisms of adriamycin-DNA interaction in L1210 cells. Biochem. Pharmacol. 1984;33:3137–3142. doi: 10.1016/0006-2952(84)90069-8. [DOI] [PubMed] [Google Scholar]
- Ravandi F., Estey E., Jones D., Faderl S., O'Brien S., Fiorentino J., Pierce S., Blamble D., Estrov Z., Wierda W., et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J. Clin. Oncol. 2009;27:504–510. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.G., Kang S.W., Jeong W., Chang T.S., Yang K.S., Woo H.A. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Rhee S.G., Woo H.A., Kil I.S., Bae S.H. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J. Biol. Chem. 2012;287:4403–4410. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallmyr A., Fan J., Rassool F.V. Genomic instability in myeloid malignancies: increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 2008;270:1–9. doi: 10.1016/j.canlet.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Sanz M.A., Fenaux P., Lo Coco F. Arsenic trioxide in the treatment of acute promyelocytic leukemia. A review of current evidence. Haematologica. 2005;90:1231–1235. [PubMed] [Google Scholar]
- Soignet S.L., Frankel S.R., Douer D., Tallman M.S., Kantarjian H., Calleja E., Stone R.M., Kalaycio M., Scheinberg D.A., Steinherz P., et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J. Clin. Oncol. 2001;19:3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- Soignet S.L., Maslak P., Wang Z.G., Jhanwar S., Calleja E., Darnashti L.J., Corso D., DeBlasio A., Gabrilove J., Scheinberg D.A., et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- Sumi D., Shinkai Y., Kumagai Y. Signal transduction pathways and transcription factors triggered by arsenic trioxide in leukemia cells. Toxicol. Appl. Pharmacol. 2010;244:385–392. doi: 10.1016/j.taap.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Vivas-Mejía P.E., Ozpolat B., Chen X., Lopez-Berestein G. Downregulation of the c-MYC target gene, peroxiredoxin III, contributes to arsenic trioxide-induced apoptosis in acute promyelocytic leukemia. Int. J. Cancer. 2009;125:264–275. doi: 10.1002/ijc.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S.T., Van Etten R.A. The PAG gene product, a stress-induced protein with antioxidant properties, is an Abl SH3-binding protein and a physiological inhibitor of c-Abl tyrosine kinase activity. Genes Dev. 1997;11:2456–2467. doi: 10.1101/gad.11.19.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H.A., Jeong W., Chang T.S., Park K.J., Park S.J., Yang J.S., Rhee S.G. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. J. Biol. Chem. 2005;280:3125–3128. doi: 10.1074/jbc.C400496200. [DOI] [PubMed] [Google Scholar]
- Zhang P., Liu B., Kang S.W., Seo M.S., Rhee S.G., Obeid L.M. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J. Biol. Chem. 1997;272:30615–30618. doi: 10.1074/jbc.272.49.30615. [DOI] [PubMed] [Google Scholar]