Abstract
Background:
Subjective cognitive decline (SCD) may represent a low-burden indicator of dementia risk. The value of SCD as a proxy marker, however, depends on the consistency of associations between subjective and objective cognitive measures across sociodemographic and psychological factors.
Methods:
We evaluated baseline data from the Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) study (n=1,615). SCD was measured using the 12-item Everyday Cognition (ECog) scale. Using linear regression models with interaction terms, we evaluated six potential modifiers (age, gender, race/ethnicity, educational attainment, family history of dementia, and depressive symptoms) of the association between cognitive performance (episodic memory, executive function) with SCD.
Results:
Lower episodic memory and executive function scores were associated with higher log(ECog scores) (more SCD). Older age and elevated depressive symptoms were associated with higher log(ECog scores). Age (interaction p-value=0.002) and education (interaction p-value=0.01) modified the association between executive function and log(ECog scores). Specifically, associations between executive function and log(ECog scores) were stronger among participants with more education and less pronounced among older participants.
Conclusions:
The association between cognitive performance and log(ECog scores) differed little across sociodemographic and psychological factors. SCD as measured by the ECog may be a valuable proxy for cognitive performance in diverse older adults.
Keywords: Subjective cognitive concerns, Assessment of cognitive disorders/dementia, Cohort studies, Neuropsychological tests, Cognitive dysfunction, Prodromal symptoms, Psychosocial factors
INTRODUCTION
More than one in ten U.S. adults age 65 years or older report subjective cognitive decline (SCD),1 defined by self-perceived decline in cognitive capabilities.2 SCD may be a sensitive measure of cognitive change, evidenced by the fact that dementia incidence is more than doubled among persons who report SCD compared to those who do not.3 SCD can be quickly assessed with standardized questionnaires and may serve as a low-burden indicator of risk of future cognitive decline compared to more resource- and time-intensive neuropsychological tests or clinical exams.4
The value of SCD as a proxy for risk of future cognitive impairment among older adults without dementia depends on the consistency of the association between subjective and objective cognitive measures across sociodemographic and psychological factors. While the correspondence between SCD and objective cognitive performance is limited in persons with dementia,5 many studies support the correspondence between the two measures in the absence of severe cognitive degradation.3,6–8 Regardless, the correspondence between SCD and objective test performance has been shown to be influenced by depressive symptoms and personality traits,9–11 and may be affected by sociodemographic factors.12 Nearly all evidence is from predominantly non-Latino white populations, and some findings suggest that the correspondence between SCD and cognitive test scores may differ by race/ethnicity.13–15 Prior studies have found that SCD in older adults may be exacerbated by life experiences such as a family history of dementia or may be influenced by cultural expectations of cognitive aging, which may differ by race/ethnicity.16 There is currently a lack of knowledge regarding how SCD reflects the corresponding cognitive test performance in diverse cohorts with differing sociodemographic characteristics.
Our objective was to evaluate the extent to which the correspondence between cognitive test performance and SCD, as measured by self-rated decline in cognitively-based everyday activities, was modified by sociodemographic and psychological factors in a diverse cohort of older adults without dementia.
METHODS
Study Population
We used baseline data from the Kaiser Healthy Aging and Diverse Life Experiences (KHANDLE) cohort which comprises community-dwelling older adults residing in the San Francisco Bay and Sacramento areas of California. KHANDLE aims to evaluate how race/ethnicity and life course health and sociocultural factors influence late-life brain health and cognitive decline. Individuals eligible for KHANDLE were long-term members of Kaiser Permanente Northern California, an integrated healthcare delivery system, were age 65 years or older on January 1, 2017, spoke English or Spanish, and had previously participated in Kaiser Permanente multiphasic health checkup exams between 1964–1985. Stratified random sampling by race/ethnicity and educational attainment was used with the goal of recruiting approximately equal proportions of Asian, Black, Latino, and White participants and diversity in educational attainment. Exclusion criteria included: electronic medical record diagnosis of dementia or other neurodegenerative disease (frontotemporal dementia, Lewy body disease, Pick’s disease, Parkinson’s disease with dementia, Huntington’s disease); and presence of health conditions that would impede participation in study interviews, defined by hospice activity in the past 12 months, history of severe chronic obstructive pulmonary disease in the past 6 months, congestive heart failure hospitalizations in the past 6 months, and history of end stage renal disease or dialysis in the past 12 months. At baseline, 1,712 individuals were enrolled. The present analysis excluded participants missing more than half the items on the Everyday Cognition scale (n=41 missing more than half the items), those missing cognitive assessments (n=16), educational attainment (n=5), or depressive symptoms (n=34). The final analytic sample included 1,615 participants.
KHANDLE was approved by the Institutional Review Boards at Kaiser Permanente Division of Research and University of California, Davis. All participants provided written informed consent. Because the current study is an analysis of de-identified KHANDLE data, it was certified as exempt from review by the University of California, Los Angeles Institutional Review Board.
Everyday Cognition scale (subjective cognitive decline)
Subjective cognitive decline was measured using the short version of the Everyday Cognition (ECog) scale.17,18 The ECog was designed to be sensitive to functional limitations associated with cognitive impairment that precede loss of independence. The short version of the scale comprises 12 items assessing perceived change in everyday functioning compared to the participant’s level of functioning 10 years prior (Supplementary Table 1, ). For each item, participants rated the amount of change on a four-point scale: 1 = “There has been no change in my ability compared to 10 years ago”; 2 = “I occasionally perform the task worse but not all of the time”; 3 = “I consistently perform the task a little worse than 10 years ago”; or 4 = “I perform the task much worse than 10 years ago”; or “I don’t know.” Consistent with prior studies,17–20 we calculated a total ECog score for each participant by summing the participant’s ratings and dividing by the total number of completed items. The distribution of total ECog scores was right skewed (i.e. fewer limitations); we applied a natural log transformation of the total ECog score to reduce skewness Supplementary Figure 1). Our analyses were performed using log-transformed ECog scores.
Neuropsychological measures of cognitive performance
Two cognitive domains best matching the functional domains investigated by the ECog scale (verbal episodic memory and executive functioning) were derived from the Spanish and English Neuropsychological Assessment Scales (SENAS). The SENAS was administered in either English or Spanish with language of administration determined by an algorithm that considered preferred language and everyday language usage in a variety of settings. The SENAS is a battery of cognitive tests that has previously undergone extensive development for valid comparisons of cognitive change across racially/ethnically and linguistically diverse groups. A verbal episodic memory score was derived from a multi-trial word-list-learning test. Executive function was measured using a composite score derived from component tasks of category fluency, phonemic (letter) fluency, and working memory (digit-span backward, visual-span backward, list sorting). SENAS measures were developed using item response methodology so that floor and ceiling effects were eliminated, and psychometric characteristics were matched both across different measures and across different racial/ethnic and linguistic groups. Details of the administration procedures, development, and psychometric characteristics have been extensively described in previous publications.21,22 The KHANDLE sample is drawn from the population of long-term Kaiser Permanente Northern California members ages 65 and older. Each domain was z-standardized using the full baseline sample mean and standard deviation; thus, a score of 0 represents the mean and a score of 1.0 is one standard deviation above the mean.
Potential modifiers
The sociodemographic and psychological factors we considered as potential modifiers of the effect of cognitive test performance measures on SCD included age (in decades, centered at age 75), gender (female, male), race/ethnicity, educational attainment, family history of dementia, and depressive symptoms. Participants self-reported race/ethnicity (Asian, Black, Latino, and White). Self-reported categories of educational attainment were converted to years as follows: no college = 0–12 years; some college, but no degree = 13 years; associate’s degree = 14 years; bachelor’s degree = 16 years; master’s degree = 18 years; doctoral or equivalent degree = 20 years. For participants with less than an associate’s degree, vocational degrees and certificates (of ≥6 months of formal training) were counted as an additional year of education. Education was centered at 12 years for analyses.
Family history of dementia (yes/no) was based on participant report of any living or deceased first-degree relative (parent or sibling) with a dementia diagnosis. Depressive symptoms were assessed using the computerized adaptive tests version of the Patient Reported Outcome Measurement Information System (PROMIS) 23 for depressive symptoms. PROMIS measures are provided as a standardized value based on the normative data of adults aged 18 and older from the US population centered on fifty with a standard deviation of ten. We rescaled it to be centered on zero with a standard deviation of one.
Statistical analysis
We first compared distributions of log(ECog scores) and ECog scores across strata (65 to <75, 75 to <85, ≥85 years), gender, race/ethnicity, education (0 to 12, 13 to 16, >16 years), family history of dementia, and depressive symptoms (<0, ≥0). Next, we estimated linear regression models with each cognitive performance measure as the independent variable and log(ECog score) as the dependent variable, including interaction terms to evaluate the extent to which sociodemographic and psychological factors modified the associations between verbal episodic memory and log(ECog scores) and between executive function and log(ECog scores). Preliminary analyses suggested the association between episodic memory and log(ECog scores) was approximately linear throughout the range of memory scores. For executive function, preliminary analyses suggested that the relationship with log(ECog scores) values differed for values of executive function scores less than versus greater than one standardized unit (Supplementary Figure 2). Final models using executive function as an independent variable therefore included a linear spline with a knot at one standardized unit.
Base models included age and an interaction term between age and cognitive test performance (episodic memory or executive function). Subsequent models expanded this base model by including one potential modifier (gender, race/ethnicity, years of education, family history of dementia, or depressive symptoms) and an interaction term between that modifier and cognitive test performance. Because educational attainment is patterned by race/ethnicity in the U.S.,24 the model evaluating effect modification by educational attainment additionally included race/ethnicity. In addition, prior studies have reported gender differences in depressive symptoms;25 we additionally included gender in the model evaluating effect modification by depressive symptoms.
RESULTS
Mean age was 75.9 years and 59.2% of participants were women (Table 1). There was approximately balanced representation of Asian, Black, Latino, and White racial/ethnic groups. The average duration of education was 14.7 years (range 0–20 years). The mean level of depressive symptoms was slightly lower than the general US population of adults. One third of participants reported having at least one first-degree relative with dementia. On the ECog scale, 39.3% of participants reported “I consistently perform the task a little worse than 10 years ago” on at least one of the twelve ECog items, and 12.8% reported “I perform the task much worse than 10 years ago” on at least one ECog item. ECog scores ranged from 1 to 3.45.
Table 1.
Characteristics of KHANDLE study participants (n = 1,615).
| Variable | n or mean | % or SD |
|---|---|---|
| Age (years) | 75.9 | 6.7 |
| Female gender | 956 | 59.2 |
| Race/ethnicity | ||
| Asian | 400 | 24.8 |
| Black | 409 | 25.3 |
| Latino | 329 | 20.4 |
| Non-Latino White | 477 | 29.5 |
| Years of Education | 14.7 | 3.1 |
| Family history of dementia | 539 | 33.4 |
| Depressive symptoms a | −0.1 | 0.7 |
| ECog score b | 1.4 | 0.4 |
| log(ECog score) | 0.3 | 0.3 |
Depressive symptoms standardized to a normative sample from the U.S. population (population mean = 0, standard deviation = 1.0).
ECog: Everyday Cognition scale; in the current work we used the natural logarithm transformed scores log(ECog scores)
Average log(ECog scores) were higher (i.e., more SCD) among persons of older age, those with fewer years of education, and those with higher levels of depressive symptoms (Table 2). We did not observe notable differences in average log(ECog scores) by gender, race/ethnicity, or family history of dementia.
Table 2.
Summary of log(ECog scores) and ECog scores by sociodemographic and psychological factors.
| Variable | n | log(ECog score) mean (SD) | ECog median [Q1, Q3] | |
|---|---|---|---|---|
| Age (years) | 65 to <75 | 840 | 0.28 (0.24) | 1.25 [1.08, 1.50] |
| 75 to <85 | 577 | 0.32 (0.26) | 1.33 [1.09, 1.58] | |
| ≥85 | 198 | 0.39 (0.30) | 1.44 [1.17, 1.79] | |
| Gender | Male | 659 | 0.30 (0.26) | 1.25 [1.08, 1.58] |
| Female | 956 | 0.31 (0.25) | 1.33 [1.10, 1.58] | |
| Race/ethnicity | Asian | 400 | 0.33 (0.27) | 1.33 [1.09, 1.67] |
| Black | 409 | 0.33 (0.26) | 1.33 [1.17, 1.60] | |
| Latino | 329 | 0.29 (0.23) | 1.25 [1.09, 1.50] | |
| Non-Latino-White | 477 | 0.29 (0.25) | 1.25 [1.08, 1.50] | |
| Education (years) | 0 to 12 | 287 | 0.35 (0.26) | 1.33 [1.17, 1.65] |
| 13 to 16 | 951 | 0.31 (0.25) | 1.33 [1.09, 1.58] | |
| >16 | 377 | 0.28 (0.26) | 1.25 [1.08, 1.50] | |
| Depressive symptoms | ≤0 | 920 | 0.26 (0.22) | 1.25 [1.08, 1.50] |
| >0 | 695 | 0.37 (0.28) | 1.42 [1.17, 1.75] | |
| Family history of dementia | No | 1076 | 0.31 (0.26) | 1.27 [1.09, 1.58] |
| Yes | 539 | 0.31 (0.25) | 1.33 [1.08, 1.58] | |
Abbreviations: SD = standard deviation; Q1 = first quartile; Q3 = third quartile.
In linear regression models, lower episodic memory scores and executive function scores were associated with higher log(ECog scores) (i.e., more SCD). Overall, older participants reported slightly higher log(ECog scores) (Tables 3 and 4, Figure 1 A-B). The association between episodic memory scores and log(ECog scores) was not modified by age. The association between executive function scores and log(ECog scores) was modified by age such that for individuals with executive function scores less than one standardized unit, the slope was steeper among younger participants than older participants. Translating regression model results from the log(ECog score) to the original ECog score scale, at an executive function score one standardized unit below average, the difference in predicted ECog scores is just 0.03 for a 65-year-old (predicted ECog score: 1.44) versus an 85-year-old (predicted ECog score: 1.47). However, at an executive function score one standardized unit above average, the difference in predicted ECog scores is 0.19 for a 65-year-old (predicted ECog score: 1.17) versus an 85-year-old (predicted ECog score: 1.36).”
Table 3.
Estimated regression coefficients (95% confidence intervals) using episodic memory scores to predict log(ECog scores) from linear regression models including interactions with potential modifiers.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|
|---|---|---|---|---|---|---|
| Modifier: Age | Modifier: Sex | Modifier: Race/Ethnicity | Modifier: Education | Modifier: Family history of dementia | Modifier: Depressive symptoms |
|
| Intercept | 0.31 (0.29, 0.32) | 0.29 (0.27, 0.31) | 0.29 (0.27, 0.31) | 0.30 (0.28, 0.33) | 0.30 (0.29, 0.32) | 0.31 (0.29, 0.33) |
| Episodic memory | −0.03 (−0.05, −0.02) | −0.03 (−0.06, −0.01) | −0.04 (−0.07, −0.02) | −0.02 (−0.04, −0.01) | −0.03 (−0.05, −0.01) | −0.03 (−0.05, −0.02) |
| Age (decades, centered at 75) | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.06) | 0.05 (0.03, 0.07) | 0.04 (0.02, 0.06) | 0.05 (0.03, 0.07) | 0.05 (0.03, 0.07) |
| Episodic memory*Age | 0.01 (−0.01, 0.03) | 0.01 (−0.01, 0.03) | 0.01 (−0.01, 0.03) | 0.00 (−0.01, 0.02) | 0.01 (−0.01, 0.03) | 0.01 (−0.01, 0.03) |
| Female (vs. Male) | 0.03 (0.01, 0.06) | 0.01 (−0.01, 0.04) | ||||
| Episodic memory*Female | −0.01 (−0.03, 0.02) | |||||
| Race/Ethnicity (vs. Non-Latino White) | ||||||
| Asian | 0.04 (0.01, 0.08) | 0.05 (0.01, 0.08) | ||||
| Episodic memory*Asian | 0.02 (−0.02, 0.05) | |||||
| Black | 0.04 (0.00, 0.07) | 0.03 (0.00, 0.07) | ||||
| Episodic memory*Black | 0.02 (−0.01, 0.05) | |||||
| Latino | −0.01 (−0.04, 0.03) | −0.01 (−0.05, 0.02) | ||||
| Episodic memory*Latino | 0.01 (−0.03, 0.04) | |||||
| Education (years, centered at 12) | −0.01 (−0.01, 0.00) | |||||
| Episodic memory*Education | 0.00 (−0.01, 0.00) | |||||
| Family history of dementia | 0.01 (−0.01, 0.04) | |||||
| Episodic memory*Family history of dementia | −0.01 (−0.04, 0.01) | |||||
| Depressive symptoms | 0.10 (0.08, 0.11) | |||||
| Episodic memory*Depressive symptoms | −0.01 (−0.02, 0.01) |
Table 4.
Estimated regression coefficients (95% confidence intervals) using executive function scores to predict log(ECog scores) from linear regression models including interactions with potential modifiers.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| Modifier: Age | Modifier: Sex | Modifier: Race/Ethnicity | Modifier: Education | Modifier: Family history of dementia | Modifier: Depressive symptoms | |
| Intercept | 0.30 (0.29, 0.32) | 0.29 (0.27, 0.31) | 0.30 (0.28, 0.33) | 0.32 (0.29, 0.35) | 0.30 (0.28, 0.31) | 0.31 (0.29, 0.33) |
| Executive function(<1)a | −0.07 (−0.09, −0.05) | −0.07 (−0.09, −0.04) | −0.07 (−0.11, −0.04) | −0.05 (−0.07, −0.03) | −0.07 (−0.09, −0.05) | −0.06 (−0.08, −0.05) |
| Executive function(≥1) | 0.02 (−0.03, 0.07) | −0.01 (−0.11, 0.09) | 0.02 (−0.05, 0.08) | −0.07 (−0.18, 0.03) | 0.05 (−0.02, 0.13) | 0.01 (−0.04, 0.06) |
| Age (decades, centered at 75) | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.07) | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.07) | 0.05 (0.03, 0.07) |
| Executive function(<1)*Age | 0.03 (0.01, 0.06) | 0.03 (0.01, 0.06) | 0.03 (0.01, 0.06) | 0.03 (0.00, 0.05) | 0.03 (0.01, 0.06) | 0.03 (0.01, 0.05) |
| Executive function(≥1)*Age | −0.03 (−0.12, 0.07) | −0.03 (−0.12, 0.07) | −0.03 (−0.12, 0.07) | 0.00 (−0.10, 0.10) | −0.03 (−0.12, 0.07) | −0.03 (−0.12, 0.06) |
| Female (vs. Male) | 0.02 (0.00, 0.05) | 0.01 (−0.01, 0.03) | ||||
| Executive function(<1)*Female | 0.00 (−0.04, 0.03) | |||||
| Executive function(≥1)*Female | 0.04 (−0.07, 0.15) | |||||
| Race/Ethnicity (vs. Non-Latino White) | ||||||
| Asian | 0.01 (−0.03, 0.05) | 0.02 (−0.02, 0.05) | ||||
| Executive function(<1)*Asian | 0.00 (−0.05, 0.05) | |||||
| Executive function(≥1)*Asian | 0.11 (−0.09, 0.31) | |||||
| Black | 0.01 (−0.03, 0.05) | 0.01 (−0.02, 0.05) | ||||
| Executive function(<1)*Black | 0.00 (−0.04, 0.05) | |||||
| Executive function(≥1)*Black | 0.03 (−0.16, 0.22) | |||||
| Latino | −0.03 (−0.07, 0.01) | −0.03 (−0.07, 0.01) | ||||
| Executive function(<1)*Latino | 0.01 (−0.04, 0.05) | |||||
| Executive function(≥1)*Latino | 0.00 (−0.22, 0.22) | |||||
| Education (years, centered at 12) | −0.01 (−0.01, 0.00) | |||||
| Executive function(<1)*Education | −0.01 (−0.01, 0.00) | |||||
| Executive function(≥1)*Education | 0.02 (0.00, 0.05) | |||||
| Family history of dementia | 0.02 (−0.01, 0.05) | |||||
| Executive function(<1)*Family history of dementia | 0.00 (−0.04, 0.03) | |||||
| Executive function(≥1)*Family history of dementia | −0.06 (−0.15, 0.04) | |||||
| Depressive symptoms | 0.10 (0.08, 0.12) | |||||
| Executive function(<1)*Depressive symptoms | 0.00 (−0.02, 0.02) | |||||
| Executive function(≥1)*Depressive symptoms | −0.03 (−0.10, 0.04) |
Model allows the slope of log(ECog score) to differ for values of executive function above and below 1 standardized unit.
Figure 1.
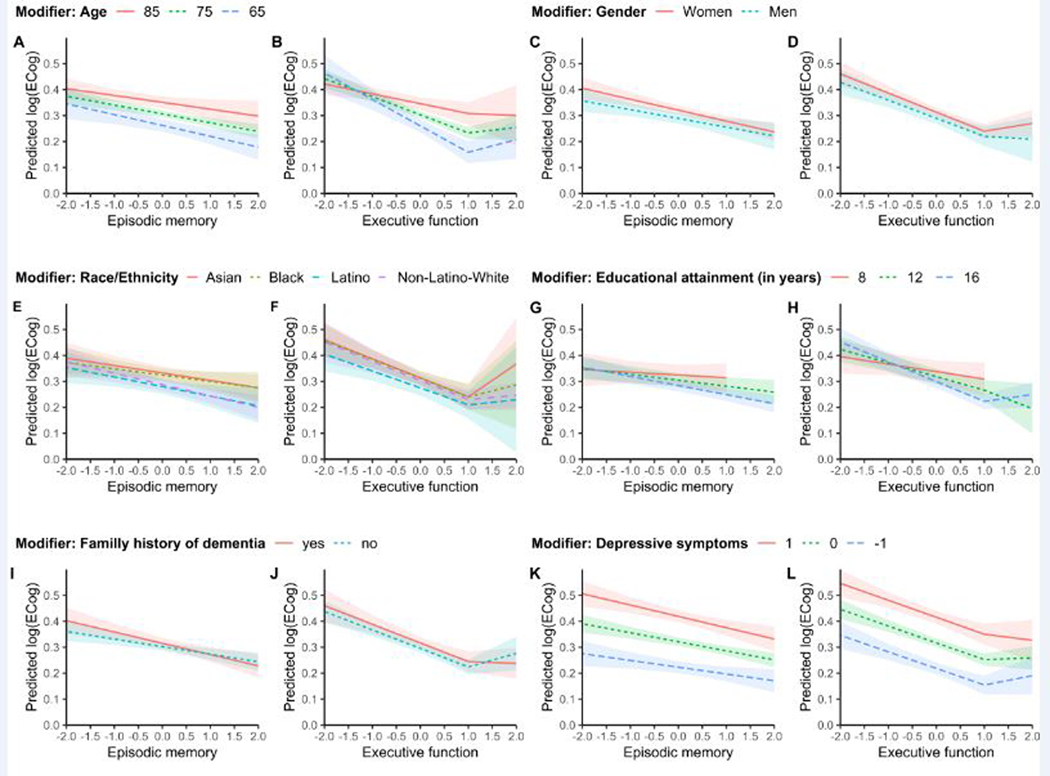
Predicted values of log(ECog score) by neuropsychological measures for each potential modifier. For each modifier the pair of plots shows the predicted values of log(ECog scores) for each value of episodic memory (left) and executive function (right) from linear regression models by age (A, B), gender (C, D), race/ethnicity (E, F), years of educational attainment (G, H), family history of dementia (I, J), and depressive symptoms (K, L). All models controlled for age and predictions shown are for a 75-year-old participant. Models for educational attainment (G, H) additionally controlled for race/ethnicity and predictions are shown for a non-Latino white participant. Models for depressive symptoms (K, L) additionally controlled for gender and predictions shown are for a male participant. We suppressed the predictions for ranges of the predictor variables with very few observations (G,H).
In models controlling for race/ethnicity, the association between episodic memory scores and log(ECog scores) did not differ by years of education, but the slope of the association between executive function scores less than one standardized unit and log(ECog scores) was slightly steeper for participants with more years of formal education (change in slope: −0.03 standardized units for every five years of education; 95% CI: −0.05, −0.01) (Tables 3–4, Figure 1 G-H). Translated to the original ECog score scale, at an executive function score one standardized unit below average, the difference in predicted ECog scores is 0.02 between a person with 8 years of education (predicted ECog score: 1.44) versus a person with 16 years of education (predicted ECog score: 1.46). However, at an executive function score one standardized unit above average, the difference in predicted ECog scores is 0.11 for a person with 8 years of education (predicted ECog score: 1.36) versus 16 years of education (predicted ECog score: 1.25).
Depressive symptoms were associated with higher log(ECog scores) across all levels of measured memory performance Tables 3–4, Figure K-L). For example, at an average executive function score, the predicted ECog score for a person with depressive symptoms one standardized unit below the national average is 1.24 and the predicted ECog score of a person with depressive symptoms one standardized unit above the national average is 1.52. There were no major differences in the correspondence between episodic memory or executive function scores and log(ECog scores) by gender, race/ethnicity, or family history of dementia, although some estimates were imprecise (Tables 3–4, Figure 1 C-F, I-J).
DISCUSSION
To assess the performance of SCD as an indicator of cognitive performance in a diverse sample, we investigated whether the associations between cognitive test performance in two domains (episodic memory and executive function) and SCD differed by sociodemographic and psychological factors. As expected, higher episodic memory and executive function scores were associated with less SCD. Associations between episodic memory and SCD were approximately linear throughout the range of episodic memory scores. Although higher executive function predicted lower SCD for most of the range of executive function scores, at high levels of executive function, the association was approximately null. In other words, above a certain level of executive function, higher cognitive test scores did not predict lower SCD. SCD as measured by log(ECog score) differed by age, educational attainment, and depressive symptoms, with participants of older ages, with less formal education, and with higher depressive symptoms reporting more SCD. However, the correspondence between cognitive test performance and SCD only differed by age and educational attainment. Family history of dementia was not associated with SCD and the correspondence between SCD and cognitive test scores did not differ by family history of dementia..
Overall, our findings are in agreement with previous reports of higher SCD prevalence in older individuals and in persons with elevated depressive symptoms1,9,26,27 and of more pronounced correspondence between SCD and objective cognitive performance in persons with higher levels of education.28 However, our findings differ from prior studies that report a stronger correspondence between SCD and objective memory performance in older versus younger participants.12,29 This could be due to differences in the measures of SCD, as these prior studies specifically focused on subjective memory decline, while the ECog captures broader cognitive domains. Despite prior literature reporting higher SCD among women compared to men,9,30 we did not observe gender differences in SCD in our study. Prior studies have found either no difference in SCD in persons with versus without a family history of dementia8 or moderately higher reporting of SCD mostly explained by differences in depressive symptoms.31 In our study, age and education modified the association between executive function and SCD, but not the association between episodic memory and SCD. Older age and lower educational attainment attenuated the association between executive function and level of SCD. In other terms, a one standardized unit difference in executive function scores would correspond to more SCD in younger versus older participants and in participants with more versus fewer years of education.
The link between SCD and current or future objective cognitive performance among older adults without dementia is supported by multiple studies.6,27,32–34 SCD is also commonly considered a risk factor for incident dementia 2,35,36 However, the impact of diverse sociodemographic factors on this correspondence had not been previously evaluated. Nearly all extant evidence is from predominantly non-Latino White individuals, and there is limited evidence on the correspondence between SCD and objective cognitive test performance by race/ethnicity. Several bi-racial studies have reported more discrepancies between cognitive test performance and subjective memory ratings in Black compared to White participants,13,14,37 while another study found no difference in overall prevalence of SCD, cross-sectional correspondence between SCD and cognitive test performance, or odds of developing dementia.38 The discrepancy in the extant literature may be due to differing sample characteristics, measures of SCD, neuropsychological assessments used, or analytic approaches. To our knowledge, the only study to estimate the prevalence of SCD in a sample representative of the US population found differences in SCD prevalence by race/ethnicity, with the lowest prevalence in Asian Americans. However, this study did not examine correspondence between SCD and objective cognitive performance and assessed SCD with one binary question.1 The current work is the first large study evaluating SCD and objective cognitive performance to include multiple racial/ethnic groups.
One strength of our study is the use of the ECog scale to assess SCD, which, unlike binary measures of SCD, reflects severity of SCD. A comparative study found good congruence between the ECog and several other multi-item SCD scales,39 suggesting the findings of our study may extend to other multi-item SCD measures.
Our motivation in this study was different from many prior studies evaluating the link between objective versus subjective measures of cognition, in which authors often view SCD as the exposure and operationalize analyses with the intent of predicting cognitive test performance. The goal of such studies is generally to assess whether respondents have insight into cognitive degradation before it becomes manifest in objectively measured cognitive testing.8 In contrast, we hypothesized that preexisting cognitive decline causes an individual to experience and report subjective impairments through their daily activities. Thus, we evaluated cognitive test performance as a predictor of SCD and evaluated whether these associations were consistent across sociodemographic or psychological factors. We found that associations were, for the most part, consistent, but that most of the sociodemographic and psychological factors independently predicted SCD. These associations are not surprising and imply that, for example, if a person with elevated depressive symptoms has worse SCD than a person with average levels of depressive symptoms, this does not necessarily signify that they have worse cognitive test performance than a person with average levels of depressive symptoms. Future investigations should evaluate whether changes in an individual’s own SCD over time may give the best indicator of changes in that individual’s cognitive test performance regardless of gender, race/ethnicity, depressive symptoms, or family history of dementia.
Limitations of our study include sparse data for unusual combinations of covariates. For example, there were very few individuals with the highest levels of executive function who were also in the oldest age band, highest depressive symptom category, or lowest education category (Supplementary Table 2). Our findings should therefore not be extrapolated to these relatively rare groups. Our finding that the association between executive function and log(ECog scores) was approximately null for individuals with executive function scores more than one standardized unit above average suggests limited sensitivity of the ECog scale to detect SCD among people with high objective cognitive performance. Future studies should also evaluate SCD measures to help identify questions sensitive to the earliest cognitive concerns for high-functioning individuals. We excluded persons with incomplete data from our statistical analysis, and though the percent excluded was fairly small, this introduces possible bias since more impaired participants may be more likely to have incomplete data. Individuals with very poor cognitive test performance were underrepresented in our sample due to exclusion of diagnosed dementia in the KHANDLE study. The lower functional range of cognitive performance will be studied in more detail in the longitudinal follow-up phases of the study. Finally, cognitive test performance was measured cross-sectionally; when longitudinal data are available, associations between SCD and change in cognitive test scores over time could be examined.
We aimed to evaluate the impact of sociodemographic and psychological factors on the association between SCD and in neuropsychological test performance. To our knowledge, this study is the first to evaluate this relationship in a diverse cohort that includes multiple racial/ethnic groups. Corroborating prior research, we found that older age, and higher depressive symptoms corresponded to more SCD. Persons with elevated depressive symptoms reported higher SCD throughout the range of episodic memory and executive function scores. Only age and educational attainment modified the association between executive function scores and SCD. These findings overall imply relatively limited influence of sociodemographic factors on the correspondence between cognitive test performance and subjective cognitive measures. This suggests that in future studies, SCD may represent a proxy for objective cognitive performance in the normal to prodromal (MCI) range of cognitive performance. Because some sociodemographic and psychological factors predict SCD independently of cognitive test performance measures, SCD may be most relevant when based on within-person comparisons, i.e., when individuals report SCD repeatedly over time.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health grant numbers R00AG053410 and RF1AG052132.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Taylor CA, Bouldin ED, Mcguire LC. Morbidity and Mortality Weekly Report Subjective Cognitive Decline Among Adults Aged ≥45 Years-United States, 2015–2016 [Internet]. 2018[cited 2019 Jul 26] Available from: https://www.cdc.gov/mmwr/cme/conted_info.html#weekly. [DOI] [PMC free article] [PubMed]
- 2.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease [Internet] Alzheimer’s Dement. 2014;10(6):844–852.[cited 2019 Sep 26] Available from: https://www.sciencedirect.com/science/article/pii/S1552526014000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell AJ, Beaumont H, Ferguson D, et al. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis [Internet]. Acta Psychiatr. Scand 2014;130(6):439–451.[cited 2019 Jul 23] Available from: http://doi.wiley.com/10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- 4.Jessen F, Wiese B, Bickel H, et al. Prediction of dementia in primary care patients. PLoS One 2011;6(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmonds EC, Weigand AJ, Thomas KR, et al. Increasing Inaccuracy of Self-Reported Subjective Cognitive Complaints Over 24 Months in Empirically Derived Subtypes of Mild Cognitive Impairment. [Internet]. J. Int. Neuropsychol. Soc 2018;24(8):842–853.[cited 2019 Jul 3] Available from: http://www.ncbi.nlm.nih.gov/pubmed/30278855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid LM, Maclullich AMJ, Maclullich R/. Subjective Memory Complaints and Cognitive Impairment in Older People [Internet]. Dementia 2006;(22):471–485.[cited 2019 Jul 22] Available from: www.karger.com [DOI] [PubMed] [Google Scholar]
- 7.Singh-Manoux A, Dugravot A, Ankri J, et al. Subjective cognitive complaints and mortality: Does the type of complaint matter? [Internet]. J. Psychiatr. Res 2014;48(1):73–78.[cited 2019 Jul 23] Available from: https://www.sciencedirect.com/science/article/pii/S0022395613003142 [DOI] [PubMed] [Google Scholar]
- 8.Kielb S, Rogalski E, Weintraub S, Rademaker A. Objective features of subjective cognitive decline in a United States national database. Alzheimer’s Dement. 2017;13(12):1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmand B, Jonker C, Geerlings MI, Lindeboom J. Subjective memory complaints in the elderly: Depressive symptoms and future dementia [Internet]. Br. J. Psychiatry 1997;171(4):373–376.[cited 2019 Jul 22] Available from: https://www.cambridge.org/core/product/identifier/S0007125000148287/type/journal_article [DOI] [PubMed] [Google Scholar]
- 10.Cargin JW, Collie A, Masters C, Maruff P. The nature of cognitive complaints in healthy older adults with and without objective memory decline [Internet]. J. Clin. Exp. Neuropsychol 2008;30(2):245–257.[cited 2020 Feb 4] Available from: https://www.tandfonline.com/doi/full/10.1080/13803390701377829 [DOI] [PubMed] [Google Scholar]
- 11.Amariglio RE, Townsend MK, Grodstein F, et al. Specific Subjective Memory Complaints in Older Persons May Indicate Poor Cognitive Function [Internet]. J. Am. Geriatr. Soc 2011;59(9):1612–1617.[cited 2019 Nov 8] Available from: http://doi.wiley.com/10.1111/j.1532-5415.2011.03543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crumley JJ, Stetler CA, Horhota M. Examining the relationship between subjective and objective memory performance in older adults: A meta-analysis [Internet]. Psychol. Aging 2014;29(2):250–263.[cited 2020 Feb 3] Available from: https://www.researchgate.net/publication/263356339 [DOI] [PubMed] [Google Scholar]
- 13.Jackson JD, Rentz DM, Aghjayan SL, et al. Subjective cognitive concerns are associated with objective memory performance in Caucasian but not African-American persons [Internet]. Age Ageing 2017;46(6):988–993.[cited 2019 Sep 27] Available from: https://academic.oup.com/ageing/article/46/6/988/3811071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blazer DG, Hays JC, Fillenbaum GG, Gold DT. Memory complaint as a predictor of cognitive decline: a comparison of African American and White elders. [Internet]. J. Aging Health 1997;9(2):171–84.[cited 2020 Feb 5] Available from: http://www.ncbi.nlm.nih.gov/pubmed/10182402 [DOI] [PubMed] [Google Scholar]
- 15.Sims RC, Whitfield KE, Ayotte BJ, et al. Subjective memory in older African Americans. Exp. Aging Res 2011;37(2):220–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LADITKA JN, LADITKA SB, LIU R, et al. Older adults’ concerns about cognitive health: commonalities and differences among six United States ethnic groups [Internet]. Ageing Soc. 2011;31(7):1202–1228.[cited 2019 Sep 27] Available from: https://www.cambridge.org/core/product/identifier/S0144686X10001273/type/journal_article [Google Scholar]
- 17.Farias ST, Mungas D, Reed BR, et al. The Measurement of Everyday Cognition (ECog): Scale Development and Psychometric Properties [Internet]. Neuropsychology 2008;22(4):531[cited 2019 Jul 1] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18590364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomaszewski Farias S, Mungas D, Harvey DJ, et al. The measurement of everyday cognition: Development and validation of a short form of the Everyday Cognition scales [Internet]. Alzheimer’s Dement. 2011;7(6):593–601.[cited 2019 Jul 9] Available from: https://www.sciencedirect.com/science/article/pii/S1552526011000896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farias ST, Chou E, Harvey DJ, et al. Longitudinal trajectories of everyday function by diagnostic status. [Internet]. Psychol. Aging 2013;28(4):1070–5.[cited 2019 Jul 23] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24364409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farias ST, Park LQ, Harvey DJ, et al. Everyday cognition in older adults: associations with neuropsychological performance and structural brain imaging. [Internet]. J. Int. Neuropsychol. Soc 2013;19(4):430–41.[cited 2019 Jul 1] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23369894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mungas D, Reed BR, Crane PK, et al. Spanish and English Neuropsychological Assessment Scales (SENAS): Further development and psychometric characteristics. Psychol. Assess 2004; [DOI] [PubMed] [Google Scholar]
- 22.Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales: Relationship to demographics, language, cognition, and independent function. Neuropsychology 2005; [DOI] [PubMed] [Google Scholar]
- 23.Bevans M, Ross A, Cella D. Patient-Reported Outcomes Measurement Information System (PROMIS): Efficient, standardized tools to measure self-reported health and quality of life [Internet]. Nurs. Outlook 2014;62(5):339–345.[cited 2019 Sep 16] Available from: https://www.sciencedirect.com/science/article/abs/pii/S0029655414001067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol. Rev 2008;18(3 SPEC. ISS.):223–254. [DOI] [PubMed] [Google Scholar]
- 25.Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: The Cache County study. Arch. Gen. Psychiatry 2000;57(6):601–607. [DOI] [PubMed] [Google Scholar]
- 26.Hänninen T, Reinikainen KJ, Helkala E-L, et al. Subjective Memory Complaints and Personality Traits in Normal Elderly Subjects [Internet]. J. Am. Geriatr. Soc 1994;42(1):1–4.[cited 2019 Jul 22] Available from: http://doi.wiley.com/10.1111/j.1532-5415.1994.tb06064.x [DOI] [PubMed] [Google Scholar]
- 27.Weber MT, Maki PM. Subjective memory complaints and objective memory performance. In: Biological Measures of Human Experience across the Lifespan: Making Visible the Invisible. 2016. p. 275–299. [Google Scholar]
- 28.Zelinski EM, Burnight KP, Lane CJ. The relationship between subjective and objective memory in the oldest old: comparisons of findings from a representative and a convenience sample. [Internet]. J. Aging Health 2001;13(2):248–66.[cited 2020 Feb 7] Available from: http://www.ncbi.nlm.nih.gov/pubmed/11787514 [DOI] [PubMed] [Google Scholar]
- 29.Parisi JM, Gross AL, Rebok GW, et al. Modeling Change in Memory Performance and Memory Perceptions: Findings from the ACTIVE Study. Psychol. Aging 2011;26(3):518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies [Internet]. Int. J. Geriatr. Psychiatry 2000;15(11):983–991.[cited 2019 Jul 25] Available from: http://doi.wiley.com/10.1002/1099-1166%28200011%2915%3A11%3C983%3A%3AAID-GPS238%3E3.0.CO%3B2-5 [DOI] [PubMed] [Google Scholar]
- 31.La Rue A, Small G, McPherson S, et al. Subjective memory loss in age-associated memory impairment: Family history and neuropsychological correlates. Aging, Neuropsychol. Cogn 1996;3(2):132–140. [Google Scholar]
- 32.Dufouil C, Fuhrer R, Alpérovitch A. Subjective Cognitive Complaints and Cognitive Decline: Consequence or Predictor? The Epidemiology of Vascular Aging Study [Internet]. J. Am. Geriatr. Soc 2005;53(4):616–621.[cited 2019 Sep 16] Available from: http://doi.wiley.com/10.1111/j.1532-5415.2005.53209.x [DOI] [PubMed] [Google Scholar]
- 33.Peters R, Beckett N, Antikainen R, et al. Subjective memory complaints and incident dementia in a high risk older adult hypertensive population [Internet]. Age Ageing 2019;48(2):253–259.[cited 2019 Jun 10] Available from: https://academic.oup.com/ageing/article/48/2/253/5274651 [DOI] [PubMed] [Google Scholar]
- 34.Jungwirth S, Fischer P, Weissgram S, et al. Subjective Memory Complaints and Objective Memory Impairment in the Vienna-Transdanube Aging Community [Internet]. J. Am. Geriatr. Soc 2004;52(2):263–268.[cited 2019 Jul 22] Available from: http://doi.wiley.com/10.1111/j.1532-5415.2004.52066.x [DOI] [PubMed] [Google Scholar]
- 35.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a … [Lancet Neurol. 2010] - PubMed result [Internet]. Lancet Neurol. 2010;9(11):1118–27.[cited 2019 Nov 6] Available from: http://www.ncbi.nlm.nih.gov/pubmed/20934914 [DOI] [PubMed] [Google Scholar]
- 36.Visser PJ, Verhey F, Knol DL, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study [Internet]. Lancet Neurol. 2009;8(7):619–627.[cited 2019 Sep 26] Available from: https://www.sciencedirect.com/science/article/pii/S1474442209701395 [DOI] [PubMed] [Google Scholar]
- 37.Sims RC, Whitfield KE, Ayotte BJ, et al. Subjective Memory in Older African Americans [Internet]. Exp. Aging Res 2011;37(2):220–240.[cited 2019 Jul 25] Available from: http://www.tandfonline.com/doi/abs/10.1080/0361073X.2011.555640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arvanitakis Z, Leurgans SE, Fleischman DA, et al. Memory complaints, dementia, and neuropathology in older blacks and whites [Internet]. Ann. Neurol 2018;83(4):718–729.[cited 2019 Sep 27] Available from: http://doi.wiley.com/10.1002/ana.25189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 2012;50(12):2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


