Abstract
Dyspnea is an uncomfortable sensation with the potential to cause psychological trauma. Patients presenting with acute respiratory failure, particularly when tidal volume is restricted during mechanical ventilation, may experience the most distressing form of dyspnea known as air hunger. Air hunger activates brain pathways known to be involved in posttraumatic stress disorder (PTSD), anxiety, and depression. These conditions are considered part of the post-intensive care syndrome. These sequelae may be even more prevalent among patients with ARDS. Low tidal volume, a mainstay of modern therapy for ARDS, is difficult to avoid and is likely to cause air hunger despite sedation. Adjunctive neuromuscular blockade does not prevent or relieve air hunger, but it does prevent the patient from communicating discomfort to caregivers. Consequently, paralysis may also contribute to the development of PTSD. Although research has identified post-ARDS PTSD as a cause for concern, and investigators have taken steps to quantify the burden of disease, there is little information to guide mechanical ventilation strategies designed to reduce its occurrence. We suggest such efforts will be more successful if they are directed at the known mechanisms of air hunger. Investigation of the antidyspnea effects of sedative and analgesic drugs commonly used in the ICU and their impact on post-ARDS PTSD symptoms is a logical next step. Although in practice we often accept negative consequences of life-saving therapies as unavoidable, we must understand the negative sequelae of our therapies and work to minimize them under our primary directive to “first, do no harm” to patients.
Key Words: ARDS, dyspnea, mechanical ventilation, posttraumatic stress disorder, respiratory failure
Imagine you have developed ARDS and cannot breathe for yourself. A mechanical ventilator supports you, giving small breaths. Hypercapnia resulting from reduced tidal volumes has increased your drive to breathe, and stretch receptors in your lungs are telling you my breaths are too small. The sensation of wanting—needing—to take in more air is a form of dyspnea known as “air hunger,” a very unpleasant symptom that is singular in its ability to evoke acute anxiety, panic, frustration, and fear.1 You see your care team around your bed and think, do these people know I’m not getting enough air? Because you are sedated and unable to follow what is happening to you, you start to wonder are these people trying to kill me?
Of course, your physicians are doing their best to protect your lungs with smaller breaths, but you may not understand that. They are concerned about your comfort and want to improve the situation. To improve the situation, the team gives you more sedation, but you still have trouble breathing comfortably on the ventilator, so they institute neuromuscular blockade to paralyze your breathing muscles. Someone says, “The patient looks more comfortable now,” as they walk out of your room. You now have no way to communicate your discomfort.
Some physicians still believe that contraction of respiratory muscles is the primary cause of dyspnea,2 although that theory was disproven years ago.3 , 4 Current evidence shows that air hunger arises from an increase in respiratory drive projecting to the cerebral cortex, and it is normally offset by increased tidal inflation of the lungs. Paralysis does not reduce your air hunger because air hunger does not arise from your respiratory muscles.4 Your care team may not even be considering the possibility of persistent dyspnea after starting the paralytic.5
We hypothesize that some patients suffer unnecessary but long-lasting psychological sequelae, such as depression, anxiety, and posttraumatic stress disorder (PTSD), from dyspnea that persists despite pharmacologic sedation during severe acute respiratory failure. Dyspnea, in particular air hunger, is a potentially psychologically traumatizing sensation6 that is not necessarily relieved by mechanical ventilation, sedation, and other routine therapies for respiratory failure, which can also serve to worsen these symptoms. We fear that administration of neuromuscular blocking agents (paralytics), which do not relieve dyspnea, may exacerbate this problem because both patient communication and observable signs of dyspnea are obliterated.3 , 4 Efforts to better understand this problem can lead to development of treatment protocols that retain the benefits of therapies for refractory respiratory failure while minimizing psychological trauma.
Why Might ARDS Be Linked to Adverse Psychiatric Outcomes?
Critical Illness, Dyspnea, and Air Hunger Can Be Psychologically Traumatizing
Air hunger is uncomfortable and induces anxiety and fear,1 , 7 presumably as an evolutionary adaptation to ensure a prompt behavioral response to threatened homeostasis of blood gases. This primal sensation can be so distressing that the emotional response has been exploited in methods of torture.6 There is evidence that air hunger is the most common form of dyspnea in non-ICU patients8 and in ICU patients being mechanically ventilated.9
The hypothesis that air hunger during mechanical ventilation for respiratory failure is an important causative factor in post-ICU psychological sequelae is driven by: the potential for psychological trauma; post-ICU patient recall of frightening air hunger; and the observation that PTSD has been correlated with recall of dyspnea in ICU and ARDS survivors.10 , 11
As clinicians, we aim to relieve distress; nonetheless, clinically significant symptoms of PTSD, including nightmares and flashbacks of dyspnea, are estimated to occur in about 22% of general ICU survivors and are considered part of the post-intensive care syndrome.12, 13, 14, 15, 16 These symptoms can coexist with depression, anxiety, and other behavioral disorders, and are correlated with recalled respiratory distress during the ICU stay and with duration of mechanical ventilation.10 , 17 They may be even more common among survivors of ARDS.11 , 18, 19, 20 Individual patient factors also contribute to the likelihood of developing PTSD. Patients with preexisting common psychiatric diseases seem to be at higher risk of developing posttraumatic stress following critical illness, mechanical ventilation, and ARDS.15 , 19
Air Hunger Stimulates Brain Regions Involved in the Development of PTSD
The Diagnostic and Statistical Manual of Mental Disorders defines PTSD as a collection of intrusive distressing symptoms in response to an extreme stressor or traumatic event, characterized by its capacity to provoke fear, helplessness, or horror in response to the threat of injury or death. Symptoms include frightening thoughts, images, perceptions, and dreams of the stressor or event, which can be associated with re-living of the experience (flashbacks, nightmares), avoidance of reminders of the event, and a negative view of the future.21
Air hunger activates the principal cortical regions comprising the “salience network”22: the anterior insula, anterior cingulate cortex, and amygdala.23 , 24 These are regions of the brain that integrate external stimuli and emotions in a manner necessary to sustain homeostasis and survival.25 The salience network is known to be involved in the development of PTSD.26 , 27
Post-ICU PTSD symptoms are strongly linked with memories of “in-ICU frightening experiences.”10 , 13 Qualitative studies of recalled experiences suggest that air hunger plays a central role. In a study of 30 patients who experienced dyspnea during mechanical ventilation following cardiac surgery, 83% reported having a “mortal fear of death” during and following an acute episode of dyspnea.28 In another study of 126 ICU survivors, 39% recalled feeling at risk of imminent death, and 55% recalled feeling they were being suffocated.29
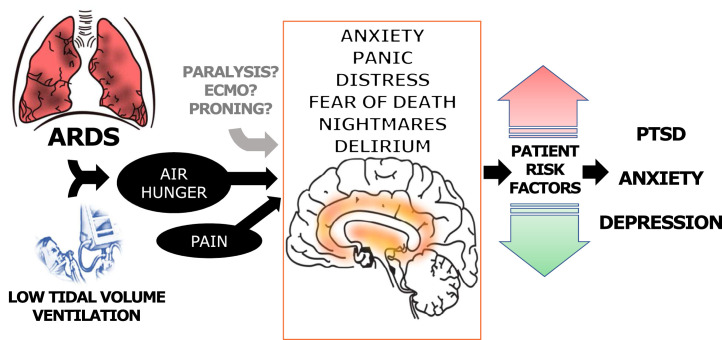
A schema illustrating the hypothesized connections between mechanical ventilation and psychological trauma is shown in Figure 1 .
Figure 1.
Proposed pathways from ARDS to adverse psychiatric outcomes among survivors. Respiratory failure due to ARDS symptomatically presents with dyspnea, a symptom known to cause psychological trauma. Mechanical ventilation at low tidal volumes, a therapy which can itself lead to dyspnea, is a mainstay of treatment for ARDS. It is unknown whether adjunctive therapies for ARDS such as paralytics, prone positioning, or ECMO may lead to psychological trauma either through increased dyspnea or other pathways. Black arrows indicates known pathways; gray arrow indicates unknown possible pathways. ECMO = extracorporeal membrane oxygenation.
Dyspnea Is Associated With PTSD Symptoms
Dyspnea and, inconsistently, duration of mechanical ventilation, have previously been associated with development of PTSD symptoms.6 , 10 , 13 , 17 , 28 A study of 80 ARDS survivors showed a correlation between recollection of traumatic experiences, including dyspnea or respiratory distress, and subsequent PTSD symptoms.11 The conscious and subconscious psychological responses to dyspnea, including memories of frightening ICU experiences, may also contribute to the associated long-term psychiatric sequelae of ICU care that have been described in post-intensive care syndrome and are common following ICU stay and ARDS.14 , 19 , 20 , 29, 30, 31, 32, 33
One study following up 196 survivors of critical illness for a median of about 3 years found that 52% had prolonged psychiatric symptoms: 38% had symptoms of generalized anxiety, 32% had symptoms of depression, and 23% had symptoms of PTSD.19 Another study, specifically of ARDS survivors, found that of 102 patients who underwent neuropsychiatric evaluation 1 year following hospitalization, 36% had depression, 62% had anxiety, and 39% had PTSD.31
“Low” Tidal Volume Is Relative
A healthy adult at rest may comfortably take tidal volumes of 6 to 8 mL/kg of ideal body weight and will increase tidal volume as part of a normal physiological response to conditions requiring increased oxygen delivery and minute ventilation, such as exercise or systemic illness. When lung tissue is injured or inflamed and gas exchange is impaired, as in ARDS, neural input from chemo-, lung, and chest wall receptors can increase respiratory drive and give rise to the sensation of dyspnea,34 reflexively stimulating further increases in tidal volume.9 Patients receiving mechanical ventilation via an endotracheal tube will not have control over inspiratory flow rate, and airflow will not stimulate “cool” receptors in the upper airway; both factors can contribute to the feeling of inadequate tidal volume.35 , 36 This combination of underperceived tidal volume and increased drive can provoke air hunger at the tidal volumes typically used in lung protective ventilation, which may be 6 mL/kg ideal body weight or less.
Relief From Dyspnea Can Conflict With Lung-Protective Ventilation
To the best of our knowledge, no studies have examined the relationship of low tidal volume ventilation strategies to adverse psychiatric outcomes; however, there are numerous reasons to think a link may exist. Low tidal volume ventilation strategies conflict with the most straightforward form of symptomatic relief from dyspnea: a bigger breath.37 Patients with ARDS who are receiving low-tidal volume breaths are more likely to experience dyspnea and more likely to be mechanically ventilated for a prolonged period of time, which may put them at increased risk for development of PTSD symptoms.25
Acute hypercapnia can also be a powerful provocation for breathing discomfort. In a large international cohort,38 Pco 2 > 60 mm Hg was present at the time of ARDS diagnosis in 13.3% of patients. Healthy patients prevented from taking bigger breaths characterize the experience of hypercapnia as “like suffocation,” feeling “starved for air,” and “air hunger.”7 Hypercapnia can often be corrected by increasing the respiratory rate; nevertheless, permissive hypercapnia to maintain low tidal volumes is still occasionally necessary.38 Although patients may eventually adapt to higher Pco 2,39 adaptation requires several days, making acute rises in Pco 2 especially problematic.
Sedatives May Not Be Adequately Protective
Patients who are mechanically ventilated are routinely sedated in hopes of avoiding discomfort, but sedation and amnesia may be incomplete. For example, some patients treated with paralytics remember aspects of their care despite the accompanying heavy sedation,40 , 41 and they may experience a loss of a sense of reality, a sense of victimization and loss of control over their own bodies, and blurring of the line between life and death. Benzodiazepines, which are frequently used for sedation in the ICU, have a negligible effect in treating discomfort from dyspnea.42
We must also remember that in critically ill patients, excessive sedation may be harmful and increase the risk of psychological sequelae.43 , 44 In a trial of general ICU patients who were mechanically ventilated, those receiving no sedation had significantly reduced duration of mechanical ventilation and ICU length of stay.45 As patients with ARDS shift from acute illness toward recovery, daily spontaneous awakening and breathing trials, which allow patients to relieve dyspnea symptoms by consciously or subconsciously taking larger tidal volumes, can reduce the duration of mechanical ventilation, symptoms of PTSD, and length of stay.46 , 47
Drugs With Antidyspnea Effects May Hold Promise
It is important to make the distinction between sedation, pain relief, dyspnea relief, and relief from traumatizing stress. Because different drugs provide varying levels of dyspnea relief, the drugs selected for sedation during mechanical ventilation may modify risk of PTSD. Unlike benzodiazepines, opiates are more likely to provide relief from dyspnea and have not been independently linked to the development of post-ICU PTSD. Low doses of opioids have been shown to reduce symptoms of air hunger and breathlessness in both acute and chronic states of dyspnea,48 , 49 and an animal model of physiological and emotional stress has shown behavioral and structural neurologic benefits associated with use of morphine.50
Propofol, a highly effective amnestic sedative, has been shown to be relatively ineffective at suppressing activation of the amygdala; that is, patients may still develop maladaptive fear responses to stimuli in the absence of conscious memories.51 , 52 Although its effect on dyspnea has not been studied, deep sedation with propofol fails to diminish pain-related activations in the cerebral cortex.53 Further study of our commonly used sedatives and analgesics, many with complex and poorly understood pharmacodynamic parameters, may thus reveal ways to prevent adverse psychological sequelae without sacrificing necessary aspects of critical care. For example, the effects of sedatives such as propofol, ketamine, or dexmedetomidine on post-ICU psychiatric disease are largely unknown.54
Delirium and Corticosteroids May Mitigate Risk of PTSD
Although delirium has not been independently associated with development of PTSD or other psychiatric outcomes, it commonly complicates critical illness and mechanical ventilation and has been associated with longer ICU stays and prolonged duration of mechanical ventilation.14 , 55 Delirium has been associated with cognitive impairment and functional disability following ICU stays,55 , 56 which may predispose to psychiatric disease. Patients have reported bizarre, persecutorial, or traumatic memories of mechanical ventilation40 that may be secondary to delirium and could plausibly serve as anchor memories for the intrusive symptoms described in PTSD. Use of benzodiazepines has been independently associated with both the development of delirium57 and post-ICU PTSD,13 although no causal link between the two has been established.
Stimulation of the hypothalamic-pituitary-adrenal axis and the release of cortisol is part of the brain’s response to extreme stress.58 Some studies suggest that corticosteroids protect against post-ICU PTSD, whereas other studies have found no effect.19 , 30 , 59 Administration of corticosteroids in the ICU have been associated with delirium in acute lung injury.60 Further research is clearly needed.
Research on the Psychological Effects of Neuromuscular Blockade in ARDS Is Limited
Neuromuscular blockade is estimated to be used in 21.7% of all ARDS cases and 37.8% of severe cases worldwide38; little is known about its possible effect on post-ICU psychiatric disease. A confounding factor is that receipt of paralytics may be a marker of disease severity. One small observational study of 24 patients showed no association between disease severity and psychiatric outcome; however, it did show an association between psychiatric sequelae and both higher sedative doses and longer duration of neuromuscular blockade.61
The Systemic Early Neuromuscular Blockade (ROSE) study18 was the first major clinical trial to examine psychological outcomes following mechanical ventilation in ARDS, and despite relatively small sample sizes at 6- and 12-month follow-up (total of 267 and 197 patients, respectively), the trial provided more post-ARDS psychological data than previous studies. Patients in both the paralytic arm and the control arm remembered feeling their breathing tube, feeling pain, having disturbing dreams, and having anxiety; patients in the control arm were slightly more likely to remember their ICU stay and have disturbing dreams compared with the intervention group. At 6-month follow-up, scores on the Post-Traumatic Stress Syndrome (PTSS) 14-question screening tool62 were high in both groups, with 31 of 122 (25.4%) control patients and 38 of 145 (26.2%) intervention patients having high PTSS scores > 45, indicating a higher likelihood of PTSD-like symptoms. At 12 months, there was substantial loss to follow-up, but high PTSS scores remained common in both groups: 38 of 94 (40.4%) control patients had high scores compared with 21 of 103 (20.4%) intervention patients (95% CI for the difference, 7.4-32.6).
An important takeaway from the ROSE study supplementary material18 is that the incidence of significant psychological trauma was high in both groups of patients with ARDS who were mechanically ventilated. However, it is curious that the prevalence of PTSD symptoms rose in the control group at 12 months. One possible explanation is that patients not experiencing PTSD were more likely to drop out; another possible explanation is that paralyzed patients were more heavily sedated or received more opiates, as evidenced by lower scores on the Richmond Agitation-Sedation scale prior to the initiation of paralysis.63 It is possible that more drugs with antidyspnea effects (namely opiates) were used to achieve deeper sedation in the intervention group. Deeper sedation may also have provided more effective amnesia, reducing conscious recollection of events, although trauma may have still been experienced subconsciously.
Anticipated Future Advances
Psychological Outcomes Must Be Measured in Clinical Trials
Until the ROSE trial,18 most investigations of ARDS interventions had not examined long-term psychiatric outcomes. Causal links between development of post-ARDS psychological sequelae, disease symptoms, and interventions such as low tidal volume ventilation, neuromuscular blockade, prone positioning, or extracorporeal membrane oxygenation, have not been well examined but should be a focus in future studies. (One study from Italy,64 however, did report high rates of PTSD, depression, and anxiety symptoms among both ARDS survivors who received veno-venous extracorporeal membrane oxygenation and their caregivers.) Interventions that may plausibly reduce the risk of PTSD, such as the use of opiates with known antidyspnea effects and/or drugs such as dexmedetomidine or ketamine, are equally in need of rigorous investigation.
Clinical Education Must Focus On Dyspnea Management
Educational interventions for ICU clinicians, including physicians, nurses, respiratory therapists, and pharmacists, can target the challenging aspects of dyspnea management. Such interventions may help with awareness of situations that could lead to air hunger, such as low tidal volume ventilation and neuromuscular blockade, and improve recognition of often-missed65 dyspnea in patients who are ventilated. Recognizing that treatment of ARDS should include symptom management in addition to improved oxygenation through lung-protective therapies may help clinicians consider antidyspnea as well as sedative and analgesic effects when choosing drugs.
Other educational interventions targeting factors that could plausibly mitigate risk of post-ICU psychological sequelae and are otherwise beneficial, such as prevention of delirium and enhanced communication (even with sedated patients), should be seriously considered as well.
Observational Data Are More Powerful Than Ever
Technological advancements have allowed the collection of massive amounts of clinical and physiological data obtained in the ICU that can be linked to patient-centered outcomes in the postacute care period. Wider availability of large, multi-site datasets containing clinical data and waveforms from computerized ventilators can allow investigators to retrospectively link specific breathing patterns, ventilator settings, drugs, and other treatments to symptoms and other outcomes such as PTSD, depression, or anxiety. These data can inform prospective randomized trials looking at effects of different sedation, analgesia, and multifaceted antidyspnea strategies (including drugs and ventilator settings) on subsequent mental health outcomes in patients with ARDS. For example, large clinical datasets collected in the delivery of routine critical care could be used to further investigate the possible PTSD-sparing effects of corticosteroids reported in small trials66 , 67 and the poorly understood mechanisms of sedative and analgesic drugs.
Large physiological datasets, such as from ventilators, could be used to determine whether changing ventilator parameters such as inspiratory flow36 can reduce dyspnea and long-term sequelae while remaining within the pressure and volume constraints imposed to minimize lung damage. Study of the neuro-physiological basis of ventilator asynchrony and phenomena such as entrainment, characterized as respiratory efforts that are subconsciously matched to rhythms experienced by the body,68 may also provide new avenues for investigation. For example, music therapy, which introduces an external auditory rhythm, has been associated with reduced anxiety in patients who are mechanically ventilated.69
We Must Learn What We Can From Coronavirus Disease 2019
Coronavirus disease 2019 will unfortunately lead to tens of thousands around the world requiring mechanical ventilation for ARDS from viral pneumonia, creating massive potential for psychological trauma and mental health sequelae.70 Early studies from Italy suggest that psychiatric sequelae following coronavirus disease 2019 infection are common.71
However, the pandemic creates opportunity for research (eg, through clinical trials or retrospective analyses permitting causal inference) into the pathophysiology of post-ICU mental illness, risk factors, and disparities.72 Long-term data collected on these patients, whether as part of research studies or in the delivery of clinical care, must be used wisely so that the death and suffering brought on by the global pandemic are not in vain.
Improving Mental Health After the ICU
Given the inherent severity of disease in ARDS, preventing traumatic experiences altogether may be impossible, and we thus must recognize that post-ICU mental illness will occur. Identifying ARDS survivors at risk for post-ICU psychiatric disease and evaluating them appropriately with validated screening and diagnosis tools are essential in both clinical care and research. Post-ICU clinics73 with multidisciplinary efforts to manage post-intensive care syndrome12 are considered beneficial and should be encouraged, although further study is needed in both ARDS and general ICU survivors to determine how to best improve post-ICU outcomes. Symptom measurement, including dyspnea in the ICU, traumatic memories, and symptoms of PTSD, depression, and anxiety among survivors, is also critical for both research and clinical care in both the ICU and the post-ICU setting.
Secondary prevention efforts to address PTSD symptoms in the general post-ICU population, such as trials of counseling 74 and diary programs,75 have, at best, seen limited success. Support of ongoing research in the post-intensive care setting will be critical to our understanding of psychological trauma in the ICU and ways to prevent psychological sequelae from it.
Conclusions
Minimizing physical harm and mortality are fundamental goals of critical care interventions, but the risk of psychiatric morbidity in the survivors should not be forgotten. Financial and logistical constraints of clinical trials make studying long-term mental health sequelae of our treatments difficult, but we believe this must be an important consideration in the design of trials moving forward. Given the particularly high potential for psychological trauma among ARDS survivors following ICU care, we must be thoughtful in recommending low tidal volumes, paralysis, or other uncomfortable therapies in the presence of preexisting dyspnea and be attentive to the best approaches to mitigate potential complications. Antidyspnea interventions, such as opioids, must be considered along with sedation and analgesia in patients with severe respiratory failure. Furthermore, as we recall medicine’s first principle to “do no harm,” it is essential that studies of the management of these patients include prospective examination of the long-term psychological effects that may be associated with various approaches to sedation, mechanical ventilation, and adjunctive therapies for the treatment of severe ARDS.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: C. M. W. reports receiving consulting fees unrelated to this work from Analysis Group. None declared (R. B. B., R. M. S.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: R. B. Banzett receives support from National Institutes of Health [Grant R01 NR10006].
References
- 1.Banzett R.B., Pedersen S.H., Schwartzstein R.M., Lansing R.W. The affective dimension of laboratory dyspnea. Am J Resp Crit Care. 2008;177(12):1384–1390. doi: 10.1164/rccm.200711-1675OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell E.J., Howell J.B. The sensation of breathlessness. Br Med Bull. 1963;19:36–40. doi: 10.1093/oxfordjournals.bmb.a070002. [DOI] [PubMed] [Google Scholar]
- 3.Gandevia S.C., Killian K., McKenzie D.K., et al. Respiratory sensations, cardiovascular control, kinaesthesia and transcranial stimulation during paralysis in humans. J Physiol. 1993;470:85–107. doi: 10.1113/jphysiol.1993.sp019849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banzett R.B., Lansing R.W., Brown R., et al. ‘Air hunger’ from increased PCO2 persists after complete neuromuscular block in humans. Respir Physiol. 1990;81(1):1–17. doi: 10.1016/0034-5687(90)90065-7. [DOI] [PubMed] [Google Scholar]
- 5.Torbic H., Bauer S.R., Personett H.A., et al. Perceived safety and efficacy of neuromuscular blockers for acute respiratory distress syndrome among medical intensive care unit practitioners: a multicenter survey. J Crit Care. 2017;38:278–283. doi: 10.1016/j.jcrc.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 6.Başoğlu M. Effective management of breathlessness: a review of potential human rights issues. Eur Respir J. 2017;49(5):1602099. doi: 10.1183/13993003.02099-2016. [DOI] [PubMed] [Google Scholar]
- 7.Banzett R.B., Lansing R.W., Evans K.C., Shea S.A. Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Respir Physiol. 1996;103(1):19–31. doi: 10.1016/0034-5687(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 8.Stevens J.P., Sheridan A., Bernstein H., et al. A multidimensional profile of dyspnea in hospitalized patients. Chest. 2019;156(3):507–517. doi: 10.1016/j.chest.2019.04.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M., Demoule A., Polito A., et al. Dyspnea in mechanically ventilated critically ill patients. Crit Care Med. 2011;39(9):2059–2065. doi: 10.1097/CCM.0b013e31821e8779. [DOI] [PubMed] [Google Scholar]
- 10.Schelling G. Effects of stress hormones on traumatic memory formation and the development of posttraumatic stress disorder in critically ill patients. Neurobiol Learning Memory. 2002;78(3):596–609. doi: 10.1006/nlme.2002.4083. [DOI] [PubMed] [Google Scholar]
- 11.Schelling G., Stoll C., Haller M., et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med. 1998;26(4):651–659. doi: 10.1097/00003246-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Needham D.M., Davidson J., Cohen H., et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 13.Davydow D.S., Gifford J.M., Desai S.V., Needham D.M., Bienvenu J.O. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatr. 2008;30(5):421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson J.C., Pandharipande P.P., Girard T.D., et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel M.B., Jackson J.C., Morandi A., et al. Incidence and risk factors for intensive care unit-related post-traumatic stress disorder in veterans and civilians. Am J Respir Crit Care Med. 2016;193(12):1373–1381. doi: 10.1164/rccm.201506-1158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapfhammer H.P., Rothenhäusler H.B., Krauseneck T., Stoll C., Schelling G. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004;161(1):45–52. doi: 10.1176/appi.ajp.161.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Cuthbertson B.H., Hull A., Strachan M., Scott J. Post-traumatic stress disorder after critical illness requiring general intensive care. Intensive Care Med. 2004;30(3):450–455. doi: 10.1007/s00134-003-2004-8. [DOI] [PubMed] [Google Scholar]
- 18.National Heart, Lung, and Blood Institute PETAL Clinical Trials Network Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bienvenu O.J., Friedman L.A., Colantuoni E., et al. Psychiatric symptoms after acute respiratory distress syndrome: a 5-year longitudinal study. Intensive Care Med. 2018;44(1):38–47. doi: 10.1007/s00134-017-5009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davydow D.S., Desai S.V., Needham D.M., Bienvenu J.O. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosomatic Med. 2008;70(4):512. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Psychiatric Association . American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [Google Scholar]
- 22.Oort Jv, Tendolkar I., Hermans E.J., et al. How the brain connects in response to acute stress: a review at the human brain systems level. Neurosci Biobehav Rev. 2017;83:281–297. doi: 10.1016/j.neubiorev.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Evans K.C., Banzett R.B., Adams L., McKay L., Frackowiak R.S., Corfield D.R. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88(3):1500–1511. doi: 10.1152/jn.2002.88.3.1500. [DOI] [PubMed] [Google Scholar]
- 24.Evans K.E., Banzett R.B. In: Dyspnea: Mechanisms, Measurement, and Management. 3rd ed. Mahler D.A., O’Donnell D.E., editors. CRC Press; Boca Raton, FL: 2014. Neuroimaging of dyspnea. [Google Scholar]
- 25.Banzett R.B., Similowski T., Brown R. In: Principles and Practice of Mechanical Ventilation. 3rd ed. Tobin M.J., editor. The McGraw-Hill Companies; New York, NY: 2013. Addressing respiratory discomfort in the ventilated patient. [Google Scholar]
- 26.Kunimatsu A., Yasaka K., Akai H., Kunimatsu N., Abe O. MRI findings in posttraumatic stress disorder. J Magn Reson Imaging. 2020;52(2):380–396. doi: 10.1002/jmri.26929. [DOI] [PubMed] [Google Scholar]
- 27.Abdallah C.G., Averill C.L., Ramage A.E., et al. Salience network disruption in US Army soldiers with posttraumatic stress disorder. Chronic Stress. 2019;3 doi: 10.1177/2470547019850467. 2470547019850467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih F.J., Chu S.H. Comparisons of American-Chinese and Taiwanese patients’ perceptions of dyspnea and helpful nursing actions during the intensive care unit transition from cardiac surgery. Heart Lung. 1999;28(1):41–54. doi: 10.1016/s0147-9563(99)70042-7. [DOI] [PubMed] [Google Scholar]
- 29.de Miranda S., Pochard F., Chaize M., et al. Postintensive care unit psychological burden in patients with chronic obstructive pulmonary disease and informal caregivers: a multicenter study. Crit Care Med. 2011;39(1):112–118. doi: 10.1097/CCM.0b013e3181feb824. [DOI] [PubMed] [Google Scholar]
- 30.Parker A.M., Sricharoenchai T., Raparla S., Schneck K.W., Bienvenu J.O., Needham D.M. Posttraumatic stress disorder in critical illness survivors. Crit Care Med. 2015;43(5):1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 31.Mikkelsen M.E., Christie J.D., Lanken P.N., et al. The Adult Respiratory Distress Syndrome Cognitive Outcomes Study. Am J Resp Crit Care. 2012;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angus D.C., Musthafa A.A., Clermont G., et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1389–1394. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 33.Ann M.P., Thiti S., Sandeep R., Kyle W.S., Bienvenu O.J., Dale M.N. Posttraumatic stress disorder in critical illness survivors. Crit Care Med. 2015;43(5):1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 34.Manning H.L., Schwartzstein R.M. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547–1553. doi: 10.1056/NEJM199512073332307. [DOI] [PubMed] [Google Scholar]
- 35.Schwartzstein R.M., Lahive K., Pope A., Weinberger S.E., Weiss J.W. Cold facial stimulation reduces breathlessness induced in normal subjects. Am Rev Respir Dis. 1987;136(1):58–61. doi: 10.1164/ajrccm/136.1.58. [DOI] [PubMed] [Google Scholar]
- 36.Manning H.L., Molinary E.J., Leiter J.C. Effect of inspiratory flow rate on respiratory sensation and pattern of breathing. Am J Resp Crit Care. 1995;151(3):751–757. doi: 10.1164/ajrccm/151.3_Pt_1.751. [DOI] [PubMed] [Google Scholar]
- 37.Manning H.L., Shea S.A., Schwartzstein R.M., Lansing R.W., Brown R., Banzett R.B. Reduced tidal volume increases ‘air hunger’ at fixed PCO2 in ventilated quadriplegics. Respir Physiol. 1992;90(1):19–30. doi: 10.1016/0034-5687(92)90131-f. [DOI] [PubMed] [Google Scholar]
- 38.Bellani G., Laffey J.G., Pham T., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 39.Bloch-Salisbury E., Shea S.A., Brown R., Evans K., Banzett R.B. Air hunger induced by acute increase in PCO2 adapts to chronic elevation of PCO2 in ventilated humans. J Appl Physiol (1985) 1996;81(2):949–956. doi: 10.1152/jappl.1996.81.2.949. [DOI] [PubMed] [Google Scholar]
- 40.Ballard N., Robley L., Barrett D., Fraser D., Mendoza I. Patients' recollections of therapeutic paralysis in the intensive care unit. Am J Crit Care. 2006;15(1):86–94. [PubMed] [Google Scholar]
- 41.Wagner B.K., Zavotsky K.E., Sweeney J.B., Palmeri B.A., Hammond J.S. Patient recall of therapeutic paralysis in a surgical critical care unit. Pharmacotherapy. 1998;18(2):358–363. [PubMed] [Google Scholar]
- 42.Simon S.T., Higginson I.J., Booth S., Harding R., Weingärtner V., Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev. 2016;10(10):CD007354. doi: 10.1002/14651858.CD007354.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooper M.H., Girard T.D. Sedation and weaning from mechanical ventilation: linking spontaneous awakening trials and spontaneous breathing trials to improve patient outcomes. Crit Care Clin. 2009;25(3):515–525. doi: 10.1016/j.ccc.2009.04.002. viii. [DOI] [PubMed] [Google Scholar]
- 44.Treggiari M.M., Romand J.A., Yanez N.D., et al. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med. 2009;37(9):2527–2534. doi: 10.1097/CCM.0b013e3181a5689f. [DOI] [PubMed] [Google Scholar]
- 45.Strøm T., Martinussen T., Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375(9713):475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 46.Girard T.D., Kress J.P., Fuchs B.D., et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 47.Kress J.P., Gehlbach B., Lacy M., Pliskin N., Pohlman A.S., Hall J.B. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Resp Crit Care. 2003;168(12):1457–1461. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 48.Banzett R.B., Adams L., O'Donnell C.R., Gilman S.A., Lansing R.W., Schwartzstein R.M. Using laboratory models to test treatment: morphine reduces dyspnea and hypercapnic ventilatory response. Am J Resp Crit Care. 2011;184(8):920–927. doi: 10.1164/rccm.201101-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekström M., Bajwah S., Bland J.M., Currow D.C., Hussain J., Johnson M.J. One evidence base; three stories: do opioids relieve chronic breathlessness? Thorax. 2018;73(1):88–90. doi: 10.1136/thoraxjnl-2016-209868. [DOI] [PubMed] [Google Scholar]
- 50.Abdullahi P., Vafaei A., Ghanbari A., Dadkhah M., Rashidy-Pour A. Time-dependent protective effects of morphine against behavioral and morphological deficits in an animal model of posttraumatic stress disorder. Behavioural Brain Res. 2019;364:19–28. doi: 10.1016/j.bbr.2019.01.058. [DOI] [PubMed] [Google Scholar]
- 51.Ma P., Liu J., Xi X., et al. Practice of sedation and the perception of discomfort during mechanical ventilation in Chinese intensive care units. J Crit Care. 2010;25(3):451–457. doi: 10.1016/j.jcrc.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Pryor K.O., Root J.C., Mehta M., et al. Effect of propofol on the medial temporal lobe emotional memory system: a functional magnetic resonance imaging study in human subjects. Br J Anaesth. 2015;115(suppl 1):i104–i113. doi: 10.1093/bja/aev038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hofbauer R.K., Fiset P., Plourde G., Backman S.B., Bushnell C.M. Dose-dependent effects of propofol on the central processing of thermal pain. Anesthesiology. 2004;100(2):386–394. doi: 10.1097/00000542-200402000-00031. [DOI] [PubMed] [Google Scholar]
- 54.Morena M., Berardi A., Peloso A., et al. Effects of ketamine, dexmedetomidine and propofol anesthesia on emotional memory consolidation in rats: consequences for the development of post-traumatic stress disorder. Behavioural Brain Res. 2017;329:215–220. doi: 10.1016/j.bbr.2017.04.048. [DOI] [PubMed] [Google Scholar]
- 55.Brummel N.E., Jackson J.C., Pandharipande P.P., et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ely E.W., Shintani A., Truman B., et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 57.Clegg A., Young J.B. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40(1):23–29. doi: 10.1093/ageing/afq140. [DOI] [PubMed] [Google Scholar]
- 58.Charney D.S. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 59.Bienvenu O.J., Gellar J., Althouse B.M., et al. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med. 2013;43(12):2657–2671. doi: 10.1017/S0033291713000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schreiber M.P., Colantuoni E., Bienvenu O.J., et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480–1486. doi: 10.1097/CCM.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson B.J., Weinert C.R., Bury C.L., Marinelli W.A., Gross C.R. Intensive care unit drug use and subsequent quality of life in acute lung injury patients. Crit Care Med. 2000;28(11):3626–3630. doi: 10.1097/00003246-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 62.Twigg E., Huris G., Jones C., Bramwell R., Griffiths R.D. Use of a screening questionnaire for post-traumatic stress disorder (PTSD) on a sample of UK ICU patients. Acta Anaesth Scand. 2008;52(2):202–208. doi: 10.1111/j.1399-6576.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 63.Sessler C.N., Gosnell M.S., Grap M., et al. The Richmond Agitation–Sedation Scale. Am J Resp Crit Care. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 64.Sanfilippo F., Ippolito M., Santonocito C., et al. Long-term functional and psychological recovery in a population of acute respiratory distress syndrome patients treated with VV-ECMO and in their caregivers. Minerva Anestesiol. 2019;85(9):971–980. doi: 10.23736/S0375-9393.19.13095-7. [DOI] [PubMed] [Google Scholar]
- 65.Haugdahl H.S., Storli S.L., Meland B., Dybwik K., Romild U., Klepstad P. Underestimation of patient breathlessness by nurses and physicians during a spontaneous breathing trial. Am J Resp Crit Care. 2015;192(12):1440–1448. doi: 10.1164/rccm.201503-0419OC. [DOI] [PubMed] [Google Scholar]
- 66.Schelling G., Kilger E., Roozendaal B., et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biol Psychiatry. 2004;55(6):627–633. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 67.Schelling G., Briegel J., Roozendaal B., Stoll C., Rothenhausler H.B., Kapfhammer H.P. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50(12):978–985. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- 68.Graves C., Glass L., Laporta D., Meloche R., Grassino A. Respiratory phase locking during mechanical ventilation in anesthetized human subjects. Am J Physiol. 1986;250(5):R902–R909. doi: 10.1152/ajpregu.1986.250.5.R902. [DOI] [PubMed] [Google Scholar]
- 69.Hetland B., Lindquist R., Chlan L.L. The influence of music during mechanical ventilation and weaning from mechanical ventilation: a review. Hear Lung J Acute Critical Care. 2015;44(5):416–425. doi: 10.1016/j.hrtlng.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Worsham C.M., Banzett R.B., Schwartzstein R.M. Air hunger and psychological trauma in ventilated COVID-19 patients: an urgent problem. Ann Am Thorac Soc. 2020;17(8):926–927. doi: 10.1513/AnnalsATS.202004-322VP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mazza M.G., De Lorenzo R., Conte C., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson S.F., Tiako M.J.N., Flash M.J., Lamas D.J., Alba G.A. Disparities in the recovery from critical illness due to COVID-19. Lancet Psychiatry. 2020;7(8):e54–e55. doi: 10.1016/S2215-0366(20)30292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown S.M., Bose S., Banner-Goodspeed V., et al. Approaches to addressing post–intensive care syndrome among intensive care unit survivors. a narrative review. Ann Am Thoracic Soc. 2019;16(8):947–956. doi: 10.1513/AnnalsATS.201812-913FR. [DOI] [PubMed] [Google Scholar]
- 74.Cox C.E., Hough C.L., Carson S.S., et al. Effects of a telephone- and web-based coping skills training program compared with an education program for survivors of critical illness and their family members. A randomized clinical trial. Am J Resp Crit Care. 2017;197(1):66–78. doi: 10.1164/rccm.201704-0720OC. [DOI] [PubMed] [Google Scholar]
- 75.Jones C., Bäckman C., Capuzzo M., et al. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Crit Care. 2010;14(5):1–10. doi: 10.1186/cc9260. [DOI] [PMC free article] [PubMed] [Google Scholar]