Abstract
Patients with cancer have been disproportionately affected by the COVID-19 pandemic. This effect has included the adverse outcomes in patients with cancer who develop COVID-19, the impact of the COVID-19 pandemic on the delivery of cancer care, and the severe disruption to cancer research. However, patients with cancer are a heterogeneous population, and recent studies have now documented factors that allow risk stratification of patients with cancer in order to optimize care. In this review, we highlight data at the intersection of COVID-19 and cancer, including the biological interplay between the two diseases and practical recommendations for the treatment of patients with cancer during the pandemic. We additionally discuss the potential long-lasting impact of the pandemic on cancer care due to its deleterious effect on cancer research, as well as biological insights from the cancer research community that could help develop novel therapies for all patients with COVID-19.
Keywords: COVID-19, cancer, pandemic, cancer research
Patients with cancer have been disproportionately affected by the COVID-19 pandemic. This effect has included the adverse outcomes in patients with cancer who develop COVID-19, the impact of the COVID-19 pandemic on the delivery of cancer care, and the severe disruption to cancer research. However, patients with cancer are a heterogeneous population, and recent studies have now documented factors that allow risk stratification of patients with cancer in order to optimize care. In this review, we highlight data at the intersection of COVID-19 and cancer, including the biological interplay between the two diseases and practical recommendations for the treatment of patients with cancer during the pandemic. We additionally discuss the potential long-lasting impact of the pandemic on cancer care due to its deleterious effect on cancer research, as well as biological insights from the cancer research community that could help develop novel therapies for all patients with COVID-19.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has resulted in an ongoing pandemic; at the time of writing (September 2020) over 30 million people have developed the resulting illness, COVID-19, causing more than 970,000 deaths worldwide (New York Times, 2020). Although the pandemic has, either directly or indirectly, had an impact on the entire world, certain subgroups of patients have been disproportionately affected. In particular, the Centers for Disease Control and Prevention (CDC) in the United States identify patients with certain medical conditions as being at particularly high risk of developing severe COVID-19 illness; among these are patients with cancer (CDC, 2020).
Patients with cancer have been found to have particularly adverse outcomes with COVID-19 (Garassino et al., 2020; Giannakoulis et al., 2020; Kuderer et al., 2020; Pinato et al., 2020; Rivera et al., 2020; Saini et al., 2020; Westblade et al., 2020). Moreover, care delivery for chronic conditions, including cancer, has been at least partially disrupted during the peaks of the pandemic due to prioritization and limitations on resources. Health care providers and patients have also had to continually reassess the balance between the risks and the benefits of cancer-directed interventions within the context of the added risk of infection by SARS-CoV-2 (Schrag et al., 2020). In addition to this disruption in cancer care delivery, cancer research has also been upended during the pandemic, including basic, translational, and clinical cancer research.
This review aims to comprehensively detail the relationship between COVID-19 and cancer, from biological interactions to specific clinical characteristics of patients with cancer who develop COVID-19, in the context of the reduced cancer care that the pandemic has brought on. The review additionally highlights the potential lasting effect of COVID-19 on cancer care attributable to the significant interruption of cancer research.
Interplay of SARS-CoV-2 and Cancer Biology: Risks and Opportunities
Multiple pieces of evidence have indicated an interplay between the biology of SARS-CoV-2 and cancer (Box 1 ). Evidence suggests that patients with cancer are more likely to be infected by SARS-CoV-2 (Liang et al., 2020; Rogado et al., 2020), more likely develop a severe COVID-19 infection (Garassino et al., 2020; Kuderer et al., 2020; Westblade et al., 2020), and more likely to die as a result of COVID-19 (Garassino et al., 2020; Kuderer et al., 2020; Saini et al., 2020; Westblade et al., 2020; Williamson et al., 2020). In contrast, certain anti-neoplastic hormonal treatments have been suggested to potentially play a protective role in patients with SARS-CoV-2 infections (Stopsack et al., 2020). This clinical interaction between COVID-19 and cancer therefore warrants discussion of the potential underlying biological mechanisms.
Box 1. Interplay of SARS-CoV-2 and Cancer Biology.
-
•
SARS-CoV-2 is internalized through binding to ACE2 and cleavage by TMPRSS2.
-
•
ACE2 gene expression in the lungs correlates with smoking, which is frequent in patients with cancer.
-
•
TMPRSS2 gene expression in the prostate is androgen receptor dependent, but there are conflicting data on whether the androgen receptor regulates TMPRSS2 in lung tissue.
-
•
Androgen-targeted therapies, which are used in the treatment of prostate cancer, could limit SARS-CoV-2 infection by downregulating TMPRSS2 and/or stimulating an anti-SARS-CoV-2 immune response.
SARS-CoV-2 Structure and Internalization
The Structure of the SARS-CoV-2 Virus
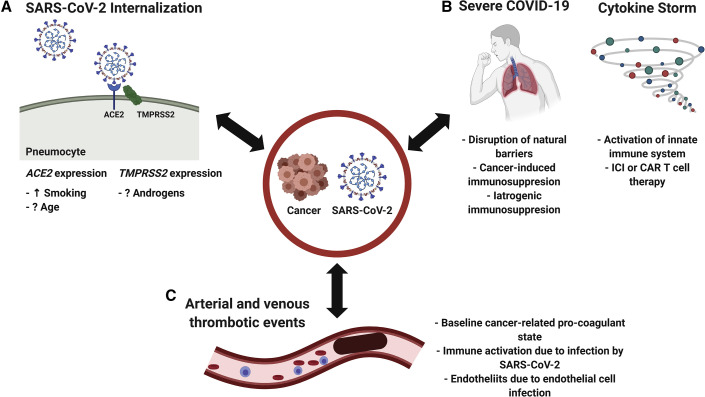
SARS-CoV-2 is an enveloped, positive-sense, single-strand RNA virus and is part of the Coronaviridae family (Andersen et al., 2020; Oberfeld et al., 2020). Other viruses of the same family have been implicated in both mild seasonal illnesses and severe outbreaks, namely severe acute respiratory syndrome coronavirus (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS-CoV), which caused the severe acute respiratory syndrome (SARS) in 2002 and Middle East respiratory syndrome (MERS) in 2013 (Andersen et al., 2020; Oberfeld et al., 2020), respectively. SARS-CoV-2's envelope is made up of the Matrix (M) glycoprotein and Envelope (E) protein and is coated by the Spike (S) protein (Andersen et al., 2020; Oberfeld et al., 2020). SARS-CoV-2's S protein plays a key role in viral binding to host cells and internalization: it has been shown to bind to the ACE2 surface receptor of epithelial cells in the respiratory tree and is subsequently cleaved by a host serine protease, Transmembrane Serine Protease 2 (TMPRSS2), to be internalized (Andersen et al., 2020; Hoffmann et al., 2020; Oberfeld et al., 2020). Once the virus is within an endocytic vesicle, other proteases, such as the host cathepsin L (CTSL) protein, are thought to play a role in cleaving the S protein to release the single-stranded RNA into the host cell's cytoplasm (Liu et al., 2020; Ou et al., 2020). Given that multiple host proteins have been suggested to play a key role in SARS-CoV-2 infection, their expression patterns and the modulators of their expression have been recently studied (Figure 1 A).
Figure 1.
The Interplay between SARS-CoV-2 and Cancer Biology
(A) SARS-CoV-2 internalization.
(B) Immune interactions.
(C) Arterial and venous thrombotic events.
Increased ACE2 Expression and Cancer
ACE2, or Angiotensin-Converting Enzyme 2, is a membrane-bound enzyme that physiologically plays a role in maintaining blood pressure homeostasis by cleaving angiotensin II (Keidar et al., 2007). ACE2 expression in lung tissue, where it is thought to play a key role in SARS-CoV-2 infection, has been suggested to increase with age (Chen et al., 2020; Muus et al., 2020). Since patients with cancer are, on average, older than the general population (Siegel et al., 2020), this has been proposed to be one of the mechanisms mediating the particularly adverse clinical outcomes observed in older patients. However, the relationship between age and ACE2 expression in lung tissue is disputed by some other studies that have found either no relationship (Smith et al., 2020a) or an inverse relationship (Booeshaghi and Pachter, 2020) between age and ACE2 expression in lung tissue. In addition, multiple studies have recently found that ACE2 expression in lung tissue is increased in patients who smoke or who suffer from smoking-related lung diseases such as chronic obstructive pulmonary disease (Baratchian et al., 2020; Pinto et al., 2020; Smith et al., 2020a). Moreover, there appears to be a dose-response relationship between number of pack-years of smoking and ACE2 expression (Smith et al., 2020a). Smokers are at increased risk of various cancers, and especially lung cancer (Siegel et al., 2020), and both lung cancer (Garassino et al., 2020) and smoking (Kuderer et al., 2020) have been found to especially correlate with adverse outcomes in patients with COVID-19. It is therefore possible that these clinical findings are, in part, mediated by the increased expression of ACE2 in the lung tissue of smokers. The use of some anti-hypertensive therapies, such as angiotensin II receptor blockers or angiotensin-converting enzyme (ACE) inhibitors, has also been found to correlate with increased expression of ACE2 (Chung et al., 2020). However, no relationship between the use of these agents and the clinical outcomes of patients with COVID-19 has been found (Chung et al., 2020).
TMPRSS2 Expression: Insights from the Field of Prostate Cancer Research
TMPRSS2 is a membrane-bound serine protease that has been studied in the context of prostate cancer, where it has been shown to be highly expressed, and has been found to be implicated in gene fusions with members of the ETS family of transcription factors (particularly ERG) in up to 50% of primary prostate cancers (Stopsack et al., 2020). TMPRSS2 expression is modulated by the androgen receptor (AR) in normal prostate tissue and prostate cancer (Lucas et al., 2014). Beyond prostate tissue, it has been recently noted that TMPRSS2 is expressed in lung tissue at the mRNA and protein levels (Stopsack et al., 2020).
However, it is still unclear whether the regulation of TMPRSS2 expression in normal human lung cells is androgen dependent (as it has been shown to be in prostate tissue). TMPRSS2 gene expression has been found to be similar between males and females in normal lung tissue (Baratchian et al., 2020; Stopsack et al., 2020), arguing against androgen-dependent regulation of TMPRSS2 in lung tissue. Moreover, in mouse models, TMPRSS2 gene and protein expression in lung tissue did not decrease upon exposure to a potent AR antagonist, enzalutamide (Baratchian et al., 2020). These findings contrasted with those of other studies that found TMPRSS2 to be upregulated by testosterone treatment in a lung adenocarcinoma cell line (A549) and that AR is a regulator of TMPRSS2 in these cell lines based on chromatin immunoprecipitation assays (Mikkonen et al., 2010). In addition, TMPRSS2 expression in lung tissue has been found to correlate with the expression of androgen and estrogen-related pathways ( Wang et al., 2020b), further suggesting that TMPRSS2 may be regulated by AR.
Clinically, a retrospective population-based study from the Veneto region in Italy reported that, although males generally had worse outcomes when they developed COVID-19, patients with prostate cancer who were treated by androgen deprivation therapy (ADT) were less likely to be infected by SARS-CoV-2, compared with patients with prostate cancer not on ADT and with patients with other tumor types (Montopoli et al., 2020). In parallel, another retrospective study from a cancer center in the northeastern United States found that patients with prostate cancer treated with ADT were less likely to develop adverse clinical outcomes (hospitalization and oxygen supplementation), compared with those not treated with ADT (Patel et al., 2020).
Randomized clinical trials that aim to evaluate the role of androgen-targeted therapies for the treatment of COVID-19 are currently ongoing. These clinical trials include those assessing agents that are currently part of the standard of care of management of prostate cancer, including AR antagonists such as bicalutamide (NCT04509999 and NCT04374279) and enzalutamide (NCT04475601) as well as gonadotropin-releasing hormone antagonists such as degarelix (NCT04397718).
Cancer, COVID-19, and the Immune System
There is evidence to suggest that the immune response to SARS-CoV-2 can play differing roles (Figure 1B). Patients who have certain forms of pre-existing primary or secondary immune suppression appear to be at increased risk of severe outcomes when they develop COVID-19 (Fung and Babik, 2020), highlighting the importance of an intact immune system in limiting and responding to SARS-CoV-2 infection. Conversely, the severe COVID-19 clinical syndrome, which develops in a subset of patients and is characterized by respiratory failure or death, has been attributed to a “cytokine storm” (Mangalmurti and Hunter, 2020). Although not yet strictly defined by clinical or laboratory criteria, this term has been used to refer to a hyperinflammatory state, biologically characterized by increased cytokine levels and clinically by an acute respiratory distress syndrome-like syndrome (Ackermann et al., 2020; Mangalmurti and Hunter, 2020). Further evidence supporting the cytokine storm theory as a cause for adverse outcomes in patients with COVID-19 comes from the observation that patients with adverse outcomes of COVID-19 appear to have increased levels of pro-inflammatory cytokines, such as interleukin-6 (IL-6) (Cummings et al., 2020; Lucas et al., 2020; Mo et al., 2020; Qin et al., 2020; Robilotti et al., 2020; Wu et al., 2020), and the observation of a similar phenomenon with previous coronavirus epidemics, SARS (Huang et al., 2005) and MERS (Min et al., 2016). Of note, an alternative hypothesis of the mechanism underlying the severe COVID-19 clinical syndrome has been termed a “bradykinin storm.” This hypothesis is mainly based on analysis of RNA-sequencing data from bronchoalveolar lavage samples showing that ACE is downregulated while ACE2 is upregulated in patients with COVID-19, compared with non-infected control patient samples. Since ACE physiologically reduces bradykinin and ACE2 increases bradykinin receptor-sensitizing peptides (angiotensin 1-9), these changes have been proposed to induce vasodilation, vascular hyperpermeability, hyaluronic acid accumulation, and, consequently, many of the clinical manifestations of severe COVID-19 (Garvin et al., 2020). The “bradykinin storm” and “cytokine storm” hypotheses are also not entirely mutually exclusive, since IL-2 has been found to be upregulated by bradykinin (Garvin et al., 2020).
In patients with cancer, immune system function can be either suppressed or hyperactivated, because of the disease itself or therapies used to treat it. Immune suppression is inherent to many cancer types and the development of cancer itself is thought to be, in part, due to a deficit in immune surveillance (Swann and Smyth, 2007). Reversal of mechanisms of tumor immune evasion has led to effective anti-cancer therapies, namely immune checkpoint inhibitors (ICIs) (Sharma and Allison, 2015). Moreover, hematologic and solid tumors can increase the risk of various infections due to neutropenia, lymphopenia, disruption of anatomical surfaces (such as the nasal mucosa), splenic and humoral defects, and the administration of cytotoxic or other immune-suppressive therapies (Rolston, 2017; Safdar and Armstrong, 2011). This immune suppression could be one of the factors implicated in the increased risk of infection by SARS-CoV-2 of patients with cancer (Liang et al., 2020; Rogado et al., 2020). Of particular interest to physicians is whether cytotoxic chemotherapy agents and targeted therapies could potentiate these SARS-CoV-2-related risks and whether certain anti-cancer therapies need to be withheld. The biological interactions between the wide range of cancer-directed cytotoxic and targeted therapy agents and the immune system are very diverse. These effects include immune suppression and stimulation (such as through depletion of immune-regulatory cells) and depend on the specific mechanism of action of each agent, as previously reviewed (Galluzzi et al., 2015). Therefore, as discussed in detail from a clinical standpoint below, physician choices should be guided by the emerging evidence of the safety of specific agents, patient-specific treatment goals, and contemporaneous assessment of the risk of SARS-CoV-2 infection (Schrag et al., 2020).
Cancer is also associated with a chronic pro-inflammatory state, however, with activation of the innate immune system (Coussens and Werb, 2002), and anti-cancer immunotherapies have become part of the standard of care treatment of multiple cancer types. These therapies, including ICIs, bispecific T cell engagers (BiTEs), cytokine-based therapies, tumor-infiltrating lymphocyte or chimeric antigen receptor T (CAR-T) cell therapy, and allogeneic stem cell transplantation, lead to anti-cancer immune responses but also stimulate a general immune response that can affect normal healthy tissues, leading to immune-related adverse events (Postow et al., 2018; Waldman et al., 2020). These immune-related adverse events can include pneumonitis, an often severe inflammation of the lungs (Postow et al., 2018). Beyond the decrease in respiratory reserve that pneumonitis entails, it is also the overlapping immune mechanisms of ICI-induced pneumonitis and COVID-19 pneumonia that could potentially increase the risk of immune-mediated lung damage in patients treated by ICI and who develop COVID-19 (Ackermann et al., 2020; Reuss et al., 2020). In addition to the risk of lung-specific immune-related adverse events, immunotherapies pose the risk of systemic immune overactivation, which could hypothetically potentiate cytokine release, given that many of the same cytokines involved in ICI effectiveness also mediate the cytokine storm, such as interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), granulocyte-macrophage colony stimulating factor, and IL-2 (Berraondo et al., 2019; Chen and Mellman, 2013; Mangalmurti and Hunter, 2020). This is especially true for CAR-T cell and BiTE therapy, a severe complication of which is cytokine release syndrome, a syndrome that shares many similarities with the cytokine storm associated with COVID-19 (Shimabukuro-Vornhagen et al., 2018). The potential for interaction between immune therapies and SARS-CoV-2 infection has been further suggested by the recent finding that the expression of the receptor of this virus, ACE2, is upregulated by IFN-γ, a downstream effector of immune therapies (Ziegler et al., 2020). Clinically, receipt of ICI therapy for cancer has been associated with worse outcomes in patients with cancer who develop COVID-19 in one cohort (Robilotti et al., 2020), although the same relationship was not found in another study (Lee et al., 2020a, 2020c).
The parallels between the mechanisms of immunotherapy-related toxicities and the COVID-19 cytokine storm have led investigators to evaluate cancer immunotherapy-inspired therapeutic agents. In particular, tocilizumab, an anti-IL-6-receptor antibody used in the treatment of BiTE- and CAR-T-induced cytokine release syndrome (Le et al., 2018; Teachey et al., 2013), has been evaluated for the management of COVID-19 (Guaraldi et al., 2020; Ip, 2020; Jordan et al., 2020; Price et al., 2020; Somers et al., 2020), including in patients with cancer (Rivera et al., 2020). Although some of these retrospective studies had suggested that a potential benefit was associated with tocilizumab in patients with COVID-19 (Guaraldi et al., 2020; Ip, 2020; Jordan et al., 2020), a recent randomized placebo-controlled clinical trial evaluating tocilizumab, COVACTA (NCT04320615), did not meet its primary or secondary endpoints (based on a press release) (Genentech Press Release, 2020). Another anti-IL-6-receptor antibody, sarilumab, has also been evaluated in a randomized placebo-controlled clinical trial (NCT04315298), with a press release similarly reporting that it did not meet its primary or secondary endpoints and with the trial being discontinued in the United States (Regeneron Press Release, 2020). Despite these disappointing results, IL-6 inhibitors continue to be evaluated in clinical trials for the treatment of COVID-19 (NCT04412772; NCT04409262). Other therapies inspired by the care of patients with cancer have also been evaluated in patients with COVID-19. A single-arm, off-label, non-randomized prospective study of 19 patients with severe COVID-19 treated with acalabrutinib, a selective Bruton tyrosine kinase (BTK) that is used in the treatment of lymphoid malignancies, resulted in rapid improvement in oxygen requirements in most patients and precipitous reduction in inflammatory markers (including IL-6) (Roschewski et al., 2020). Randomized clinical trials evaluating the treatment of patients with COVID-19 with BTK inhibitors are currently ongoing (NCT04439006, NCT04528667, NCT04346199). In a phase II randomized placebo-controlled clinical trial of 43 patients with severe COVID-19, treatment with ruxolitinib, a JAK1/2 inhibitor used in the treatment of myelofibrosis and polycythemia vera, tended to be associated with more rapid improvement of clinical, biologic (lymphopenia), and radiographic features of COVID-19 (Cao et al., 2020). The treatment of patients with COVID-19 with JAK inhibitors is currently being evaluated in multiple randomized clinical trials (NCT04477993, NCT04377620, NCT04390061, NCT04424056, NCT04366232). Corticosteroids have wide-ranging immune-suppressive functions that are believed to decrease the host immune-mediated lung damage following SARS-CoV-2 infection. These proposed benefits could be counterbalanced by the risk that this immune suppression may diminish antiviral immune response, as has been previously suggested with MERS-CoV (Arabi et al., 2018) and SARS-CoV (Lee et al., 2004), and more recently with SARS-CoV-2 (Westblade et al., 2020). Clinically, dexamethasone, a corticosteroid widely used in many medical applications, including in the treatment of cancer, was recently found to decrease the mortality of patients with COVID-19 who require respiratory support in a large randomized controlled trial (RECOVERY, 2020a), establishing its role in this setting. However, the question of whether this benefit applies to patients with cancer, a population of patients with specific immune features as outlined above, is not fully settled, because the RECOVERY trial, which had established this benefit, did not specifically report on the outcomes of patients with cancer. Given the immune specificities of patients with cancer and their particularly high risk of developing adverse outcomes with COVID-19, future clinical trials should aim to specifically enroll and report on the outcomes of patients with cancer.
SARS-CoV-2 and Cancer: A Perfect Storm for Thrombosis?
COVID-19 has been found to be associated with significant venous and thrombotic events (Figure 1C), with the relationship termed COVID-19-associated coagulopathy (Connors and Levy, 2020). Moreover, although the rate of symptomatic thrombotic events has been found to vary between 9% and 21% (Klok et al., 2020; Moll et al., 2020; Poissy et al., 2020), one study that screened critically ill patients with COVID-19 using complete duplex ultrasound found that 100% of those on prophylactic anti-coagulation had venous thromboembolic events (VTEs) compared with 56% of those on therapeutic anti-coagulation (Llitjos et al., 2020). This propensity to develop thrombotic events (including venous or, more rarely, arterial thromboses) appears to be, at least in part, related to inflammation and the activation of the innate immune system, which can activate systemic coagulation pathways (Connors and Levy, 2020; Engelmann and Massberg, 2013). In addition, endothelial cells also express the ACE2 receptor (Ferrario et al., 2005), and viral inclusions have been demonstrated in endothelial cells of patients with COVID-19 (Varga et al., 2020), suggesting that viral endotheliopathy and microcirculatory dysfunction can further contribute to the thrombotic complications seen in patients with COVID-19.
Patients with cancer are particularly at risk of thrombotic complications, since they have been found to have a 4- to 7-fold increased risk of having VTEs compared with the general population (Lee, 2003). This risk appears to vary by cancer type or stage, receipt of local or systemic therapy, and the presence of comorbidities (Falanga et al., 2013; Lee, 2003). The inherent pro-coagulant state associated with cancer has been attributed to the expression by cancer cells of coagulation factors implicated in the extrinsic pathway of coagulation (such as tissue factor) and of pro-inflammatory factors (such as TNF-α and IL-1β) (Falanga et al., 2013). Clinically, a single-center study evaluated venous thromboembolisms in patients with cancer who developed COVID-19. Patients who developed VTEs had all been admitted to the intensive care unit (ICU), with a cumulative incidence of events of 9.3% of symptomatic VTE in ICU patients. Most patients with symptomatic VTEs had developed these events early in their disease course, suggesting that the ICU stay was not a causative factor in these events but rather an indicator of severe COVID-19 disease (Moll et al., 2020). In another study comparing patients with active cancer with those without cancer who developed COVID-19, patients with active cancer did not have an increased risk of thrombotic events (arterial or venous) (Patell et al., 2020). Despite the lack of clinical evidence thus far of increased thrombotic events in patients with cancer in small observational studies, the occurrence of occult thrombotic events cannot be discounted as being one of the potential factors in the increased rate of mortality due to COVID-19 in patients with cancer (Garassino et al., 2020; Giannakoulis et al., 2020; Kuderer et al., 2020; Williamson et al., 2020), especially given the strong prior evidence of a pro-coagulable state in patients with cancer.
Defining and Identifying COVID-19 in Patients with Cancer
Incidence and Prevalence
The incidence and prevalence of COVID-19 in patients with cancer continue to fluctuate as the pandemic unfolds, partially related to differences in numbers of patients tested over time, in line with distinct and changing local policies.
Early reports from China suggested that patients with cancer might be at increased risk for infection (Liang et al., 2020; Yu et al., 2020). For instance, data reported in January 2020 from Wuhan, China, showed that, among patients hospitalized with COVID-19 symptoms, 1%–2% had concomitant cancer diagnoses. This was higher than the overall incidence of cancer in the general Chinese population (0.29%) (Liang et al., 2020). As the pandemic spread, data from Italy reported in April 2020 showed that, among patients with COVID-19 admitted to ICUs, 81 of 1,591 (8%) patients had active or prior malignancy (Grasselli et al., 2020). Similarly, reports of patients with COVID-19 at two large academic health care systems in New York showed that 6%–7% of patients hospitalized with COVID-19 had active cancer (Argenzian et al., 2020; Richardson et al., 2020).
Reasons for the documented elevated prevalence of COVID-19 among patients with cancer may be due to biologic and immunologic factors, discussed above, that predispose patients to COVID-19. However, it is also possible that non-biologic factors may contribute to the relative increase in prevalence, for example, the increased frequency of interfacing with the health care system due to cancer treatment and surveillance, as well as the close relationships that cancer patients have with health care staff, perhaps leading to expedited testing in cancer patients with any symptoms.
Presentation and Outcomes
Based on symptomatology alone, the clinical presentation of COVID-19 in patients with cancer does not differ compared with patients without cancer. Patients generally experience fever, chills, myalgias, respiratory symptoms, sore throat, and/or loss of taste or smell (Kuderer et al., 2020; Luo et al., 2020a, 2020b). Although the clinical spectrum of COVID-19 is heterogeneous, patients with active malignancy experience more severe disease and higher observed death rates from COVID-19, which have ranged between 5% and 61%, with a recent meta-analysis-based estimate of 25.6% (Dai et al., 2020; Giannakoulis et al., 2020; Kuderer et al., 2020; Mehta et al., 2020; Saini et al., 2020).
Given the significant risk COVID-19 poses to patients with cancer, pro-active strategies to improve early detection and reduce the likelihood of infection have become top priorities for health care institutions providing oncologic care. Several professional societies, including the American Society of Clinical Oncology (ASCO) (ASCO, 2020a), the European Society for Medical Oncology (ESMO) (ESMO, 2020), the Infectious Diseases Society of America (IDSA, 2020), and the American College of Surgeons (ACS, 2020a), among others, are pro-actively engaged in issuing guidelines and practice recommendations to help clinical practice.
Testing Procedures
The principle tenets involved in caring for and protecting vulnerable patients with cancer during the COVID-19 pandemic include implementation of robust screening and testing algorithms for both patients and health care providers. In addition, a focus on the physical environment, proper distancing, and personal protective equipment is a key issue in developing institutional guidelines.
The two most widely used laboratory tests for SARS-CoV-2 include nucleic acid amplification tests (RT-PCR) and antibody (serology) tests. The preferred testing method for screening and diagnosis is the RT-PCR test, while the antibody-based tests serve as supplemental tools for tracking immune response (Tang et al., 2020). During the early days of the outbreak in China, both oropharyngeal (OP) and nasopharyngeal (NP) swabs were used for testing and diagnosis. However, the NP swab became preferred after data showed that SARS-CoV-2 RNA detection was 32% in OP swabs compared with 63% in NP swabs (Wang et al., 2020a). The NP swab is the primary test currently used in screening algorithms for patients and health care employees. Although the NP RT-PCR test is sensitive, specific, and able to be processed in large batches of samples, the turnaround time can be on the order of weeks. A point-of-care test, with results available in minutes, could change the nature of the pandemic with respect to clinical care and political mandates (Wolters et al., 2020).
The ASCO guidance on cancer care delivery during the COVID-19 pandemic, discussed below, is based on recommendations from the IDSA and is particularly helpful regarding the procedure for implementing a pre-screening and screening program in the ambulatory setting (ASCO, 2020b). In all cases of symptomatic patients, whether in hospital or in an ambulatory setting, the recommendation is to test (Box 2 ). Similarly, all patients who are initiating treatment with cytotoxic chemotherapy, stem cell transplantation, long-acting biologic therapy, cellular immunotherapy, or high-dose corticosteroids for the first time should be considered for testing 48–72 h before initiation of therapy, although this is not an international recommendation. Patients should be pre-screened 48–72 h prior to subsequent cycles using a standardized questionnaire addressing symptoms and potential exposure. If patients have history of COVID-19 exposure, respiratory symptoms, or two other symptoms (cough, shortness of breath, fever, chills, myalgias, sore throat, new loss of taste or smell, or other flu-like symptoms), they should be triaged to testing. It is recommended to have testing performed at a site other than the cancer care facility, if able, to avoid bringing positive cases into cancer treatment facilities. If patients have a negative pre-screen, defined by lack of symptoms and no close or proximate contact with anyone who tests positive for COVID-19, they should present for care on the planned treatment day. Upon arrival at the health care facility, patients should again be screened to determine if there has been a change in status and/or need for testing or retesting.
Box 2. Defining and Identifying COVID-19 in Patients with Cancer.
-
•
Patients initiating some anti-cancer therapy for the first time may be considered for testing for SARS-CoV-2 using nucleic acid amplification for viral RNA (PCR).
-
•
All patients undergoing cancer-directed therapy should be pre-screened using a standard questionnaire 48–72 h prior to subsequent treatment cycles.
-
•
If patients are positive on the pre-screen questionnaire, they should be referred for PCR testing prior to treatment.
-
•
If the pre-screen is negative, they should be rescreened on the day of treatment and if not symptomatic and without known exposure to COVID-19, they may be appropriate for treatment.
In a few cases, asymptomatic chronic carriers have been identified. In this situation, data suggest that it is best to observe for a period of time and monitor for COVID-19 symptomatology prior to administering treatment. Recent analysis of test results from asymptomatic patients (1,988 tests from 1,226 patients) undergoing chemotherapy from the UK Birmingham Chemotherapy Cancer COVID-19 cohort revealed that the asymptomatic prevalence is 0.6% (Lee et al., 2020b). Follow-up data on rates of symptomatic disease are not currently available. Although a smaller analysis, data from a universal pre-chemotherapy testing program at a hospital in Dubai, United Arab Emirates, revealed that 7 of 85 asymptomatic patients with cancer were positive by PCR screening (8.2%; 95% CI 2.4%–14.1%), all of whom subsequently developed symptomatic disease (Al-Shamsi et al., 2020). It should also be noted that the rates of asymptomatic carriage are directly linked to the rate of infection where studies are performed. These data highlight the principle of early detection of pre-symptomatic patients and risk mitigation with COVID-19 in the context of cytotoxic and myelosuppressive or immunomodulatory treatments.
If a patient has a positive test result, an immediate treatment break or delay is warranted. However, some patients may still be able to receive treatment if they are on low-risk regimens, if disease burden mandates undertaking such a risk, and provided there is a designated COVID-19 treatment area or linear accelerator with dedicated staff in place. When or if to restart treatment after a positive test result is not clearly defined by published guidelines. However, expert consensus in line with ASCO guidelines is anchored on consideration of the context of medical necessity. Some providers may retest to ensure two successive negative PCR tests a minimum of 24 h apart, unless testing is unavailable or not required by local guidelines; however, long-term shedding does occur and highlights the necessity of using clinical context and judgment. Indeed, in addition, and often exclusively, both criteria of a complete clinical recovery of at least 10–14 days since symptom onset, without fever or significant symptoms for at least 48–72 h, are mandated. Available data suggest that, once symptoms have resolved, persistent viral shedding can be observed for up to 66 days, leading to positive PCR tests after recovery that may not represent infectiousness; but this is an area of ongoing investigation (Agarwal et al., 2020; Bullard et al., 2020).
Physical Environment and Personal Protective Equipment
In the COVID-19 era, the physical spaces of health care centers have been transformed by intentional focus on crowd control and flow of people. It is quite common for institutions to have a single point of entry equipped with triage stations and testing capabilities. Physical distancing (6 ft, 2 m) is encouraged and maintained, appointment times are staggered, and hours of operation are extended to decrease the number of patients in waiting rooms. Most elevators now have “dots” on the floor indicating where people are supposed to stand to prevent overcrowding. All employees and patients are required to wear medical-grade face masks, when in facilities, or N95 respirators, if patients are confirmed positive or suspected of having COVID-19. Early on during the pandemic, health care centers often limited caregivers and other family members from accompanying patients on visits, which brought particular challenges to patients with cancer diagnoses. These policies are often dependent on each state's mandates, phase of recovery, and local and regional case load.
Planning for and ensuring a robust supply chain and inventory of personal protective equipment (PPE) are essential to all health care centers as the pandemic continues. Data from a survey of 343 oncologists from 28 countries with a self-reported COVID-19 outbreak (99.7%) reported that PPE was available to all participants (Ürün et al., 2020). It should be noted that the type of PPE used by the participating oncologists varied, and while 89.8% reported using surgical face masks, only 39.7% and 32.4% reported using eye goggles/glasses and N95 masks, respectively. Moreover, the responses to the survey were recorded between April 1 and April 29, 2020, during which time the incidence of COVID-19 infection varied widely across the geographically diverse respondents to the survey. Therefore, although this survey is somewhat reassuring in that it shows that oncologists had some degree of PPE available during the pandemic, its results should be noted with caution given the diversity in the type of PPE that was used by the responding oncologists and the fact that the survey responses may have not been recorded during the peaks of the pandemic in the respective geographic regions, when PPE would be most at risk of being scarcely available.
Optimization of reuse and of filtering facepiece respirators, return-to-work policies for infected health care professionals, work-from-home policies, attention to stress management, and providing mental health resources are all additional components of an effective cancer care delivery model during the COVID-19 pandemic.
Changes in Cancer Care Delivery
In addition to the drastic changes to everyday life, the COVID-19 pandemic poses unique challenges to the delivery of oncologic care and is forcing us to redefine cancer care delivery models. Key to clinical practice changes is adapting to the specific scenario in which each community exists over time (Harky et al., 2020). Practice patterns will continue to evolve as the locoregional case volume and phases of reopening ebb and flow.
Telehealth
Telehealth has come to the forefront of clinical care during the COVID-19 pandemic. Although it may not be right for every patient and every situation, such as goals of care discussions, it provides a safe and easy way for patients to access their doctors at a time when human contact and mobility present substantial health risks due to the high transmissibility of the SARS-CoV-2 virus.
On March 30, 2020, the US Centers for Medicare & Medicaid Services (CMS) issued the 1135 waiver, loosening regulations and allowing for expansion of coverage for telehealth services to Medicare beneficiaries. Although some centers had been early adopters of telehealth in the pre-COVID era to fill gaps in care that resulted from provider shortages, after the 1135 waiver was issued, nearly all centers reconfigured care delivery models, focusing on the expansion of telehealth capabilities (Royce et al., 2020). The CMS issued further clarification detailing regulatory changes, including lifting of telehealth restrictions on urban areas, new patient visits, HIPAA regulations, remote supervision of services rendered, and supervision of resident physicians (Royce et al., 2020). Perhaps most importantly, CMS clarified that telehealth services paid under the Physician Fee Schedule are the same as in-person services (CMS, 2020). Although these regulations apply to Medicare beneficiaries, many private payers and state Medicaid programs have announced expanded coverage as well. Despite these relaxed policies, challenges remain. For example, a telehealth appointment where the patient is physically in another state from the clinician is considered to cross state lines, and the clinician must have an active license in the state where the patient resides. Emergency licenses have been granted, but whether these programs will continue is unclear (Hollander and Carr, 2020). At a global level, telehealth may impose billing and reimbursement barriers, in addition to a significantly different organization of work, as well legal context.
The degree to which telehealth may affect quality and outcomes in cancer care is still being defined. One can look back to data from the pre-COVID era, which in a brief prepared for the Agency for Healthcare Research and Quality showed evidence of the effectiveness of remote monitoring of patients, communication and counseling for patients with chronic conditions, and providing behavioral support and psychotherapy (Tuckson et al., 2017). Furthermore, a Cochrane systematic review of 93 trials (N = 22,047 participants) evaluated the effectiveness of telehealth as an additive, alternative, or comparison to usual care across several clinical conditions. This review found no difference in all-cause mortality (Flodgren et al., 2015). In fact, the study found an increase in quality of life for those allocated to telehealth compared with usual care and improved disease control in diabetes, hypercholesterolemia, and hypertension. From the oncologic side, results from a meta-analysis of 20 randomized-controlled trials that assessed the effect of telehealth intervention versus usual care in patients with breast cancer showed that telehealth was associated with a higher quality of life and self-efficacy, and less depression and distress, compared with usual care (Chen et al., 2018). Specific to the COVID-19 pandemic, additional advantages of telehealth include reducing PPE demands, limiting exposure, and reducing travel and wait times for patients.
Appropriate patient selection for telehealth oncologic care, however, is key. Both ESMO (ESMO, 2020) and ASCO (ASCO, 2020a) suggest that patients not requiring an in-person exam, treatment, or diagnostics should have telehealth visits during the COVID-19 pandemic. Specifically, telehealth visits are appropriate if the primary reason for the clinic encounter is to follow up on treatment adherence for oral agents, survivorship, genetic counseling, supportive services, or education. Implementation guides and toolkits are published to assist with the rapid integration of outpatient telehealth programs (Smith et al., 2020b).
It is also important to consider the unintended consequences of widespread adoption of technology. There is a serious potential risk of compounding health disparities between patients of different socioeconomic status if telehealth services are mandated. This has been recently highlighted by a multicenter prospective cohort study showing that, among patients with cancer in the northeastern United States, Black and Hispanic patients were less likely to benefit from telehealth visits, compared with White patients (Doroshow et al., 2020). Care should therefore be taken to assess tech literacy and digital services before scheduling services (Elkaddoum et al., 2020), and health care professionals should attempt to minimize disparities in the use of telehealth by evaluating the barriers to adoption.
Screening and Diagnosis during the COVID-19 Pandemic
Global interruptions to cancer screening began with national and state lockdowns. Many patients, providers, and health care institutions temporarily suspended routine cancer screening and diagnostic procedures until the appropriate provisions and safety measures (institutional protocols, testing capabilities, and PPE inventory) were in place. As a result, routine screening dropped by as much as 85%–90% (London et al., 2020). Now, as some health care centers inch toward a new normal, many are struggling with management of the backlog of cases. In the United Kingdom, for example, it is estimated that 1 million people have not been invited to colorectal cancer screening and approximately 8,500 people who received a positive fecal immunochemical test by mail before the national lockdown are currently awaiting an appointment for a follow-up colonoscopy (The Lancet Gastroenterology & Hepatology, 2020). Based on the importance of early screening and detection, combined with the effects of treatment delays, it is perceived, but not characterized, that delays in diagnosis during the pandemic may negatively affect cancer outcomes.
In a population-based modeling study from the United Kingdom assessing the impact of diagnostic delays on survival in breast, colorectal, esophageal, and lung cancer, substantial increases in the number of avoidable cancer deaths are expected to result. In this model, the increase in deaths 5 years after initial diagnosis may be 4%–17% higher, depending on tumor type, due to diagnostic delays during the COVID-19 pandemic compared with pre-pandemic figures (Maringe et al., 2020). Similarly, in another observational modeling study from the United Kingdom, delays of 3–6 months in surgery for incident cancers would decrease life years gained by said surgery by 19% and 43%, respectively, and 26% and 59% when considering resource-adjusted life years gained (Sud et al., 2020).
ASCO (ASCO, 2020a) and ESMO (ESMO, 2020) guidelines recommend that cancer care screening services, including colonoscopies, pap smears, and mammograms, resume in accordance with state and local health authorities. Expanding existing capacity as rapidly as possible should be at the forefront of cancer care delivery systems' efforts.
Cancer-Directed Therapy during the COVID-19 Pandemic
Surgical Oncology
During peak numbers of COVID-19 cases in a community, nearly all non-urgent or “elective” surgeries have been delayed, as reported in studies from the M.D. Anderson Cancer Center (Chang and Liu, 2020) and the University of Alabama-Birmingham (Morrison et al., 2020). The impact on future cancer progression and death as a result of current delays in “elective” cancer surgeries are not yet known. However, investigators from England used hazard ratios from observational studies from 2013 to 2017 to model cancer progression and loss of life years due to surgical delays. They estimated that a 3- or 6-month delay of surgery across all stage 1–3 cancers would cause 4,755 and 10,760 deaths, respectively, among the 94,912 patients undergoing resections for major cancers per year (Sud et al., 2020). For now, many surgical oncologists and multidisciplinary treatment teams are focused on prioritizing surgeries based on clinical rationale, and potential alternative non-surgical management strategies, with decisions being based on locally available resources in line with the American College of Surgeons “Roadmap for Maintaining Essential Surgery during COVID-19 Pandemic” (ACS, 2020b).
Systemic Therapy
Decisions regarding the use of systemic therapy during COVID-19 should be guided by routine oncology care and characteristics of systemic treatment on the immune system. Conversations with patients and shared decisions should focus on evidence-based incremental benefits with the proposed treatment versus the risk of contracting COVID-19 while coming to the clinic for cancer-directed therapy (Gharzai et al., 2020). As outlined by Schrag and colleagues, oncologic treatment generally falls into four categories, those with: (1) curative potential, (2) moderate clinical importance, (3) marginal impact on quality or quantity of life, and (4) survivorship and surveillance (Schrag et al., 2020). Cancer-directed therapy that has curative potential, or dramatic improvements in outcomes, should not be delayed. Treatments indicated for patients in the survivorship and surveillance stage most likely can be continued, given that oral chemotherapy and hormonal therapy can commonly be monitored remotely and require less frequent blood work and in-person visits. However, when the incremental benefit of systemic treatment may be marginal, clinical decision-making is not as clear cut. Considerable uncertainty relative to risks and benefits with each treatment and prevalence of COVID-19 in the local setting are variables in the conversation.
Radiation Therapy
Radiation therapy, a prominent pillar in cancer care given its often curative and palliative role, has similarly been affected by the COVID-19 pandemic. The American Society for Radiation Oncology (ASTRO) surveyed a wide variety of US radiation oncology community and academic practices to assess the initial impact and operational responses to the COVID-19 pandemic. All practices reported uninterrupted operation; however, on average the typical volume had decreased to 68% (range, 10%–95%). Treatment delays were reported by 92% of practices, with the most delays of treatment for low-risk prostate (88%) and early-stage breast cancer (73%). However, the treatment of more aggressive tumor types (such as gastrointestinal, central nervous system, and small cell lung cancers) was delayed by less than 15% of practices, suggesting the safety and importance of providing this essential and non-elective treatment for many cancer types. Adding to this, reports from China and Italy showed that very few patients required treatment interruptions in radiotherapy services, and few patients undergoing radiotherapy were diagnosed with COVID-19 during their treatment course (0.48%, 1 of 209 patients) (Krengli et al., 2020; Xie et al., 2020). As per ASTRO's “COVID-19 Clinical Guidance Resources,” it is imperative that services are provided with robust prevention and that infection control measures be put in place (ASTRO, 2020).
Effect of the COVID-19 Pandemic on Oncologic Clinical Trials
Cancer clinical trials have also been critically affected by the pandemic, with outright halting of all enrollment at some centers during the peak case volume. A cohort study assessing the effects of the pandemic on national enrollment in cancer clinical trials conducted by the SWOG Cancer Research Network showed a decrease in enrollment from 125 to 150 patient enrollments per week in early January (week 1) to 74 enrollments per week near the end of the study period (week 17) (Unger et al., 2020). In March 2020, ASCO surveyed 64 investigators at academic and community practices on the conduct of clinical trials during the COVID-19 pandemic. Nearly 60% of respondents' programs reported halting screening and/or enrollment for certain trials, 50% of respondents' programs ceased research-only blood and/or tissue collections, and 65.6% of site initiation visits were conducted remotely (Waterhouse et al., 2020). In addition, tier-based approaches to prioritizing trials were utilized at 53.1% of respondents' programs. Challenges noted to continuing clinical trial investigations during the pandemic include patients' inability to return to health care centers for study visits, especially if they are traveling from outside the state and have quarantine measures to abide by; the limited availability of ancillary services; and the challenge of holding timely discussions with sponsors and contract research organizations. However, as noted by Waterhouse and co-workers, some positive clinical trial-related lessons are worth perpetuating forward to the post-COVID era. Trialists should continue to streamline visits and create more flexible study timelines, leverage telehealth to limit in-person visits, and use e-signatures for trial documentation (Waterhouse et al., 2020), while trial sponsors should continue to demonstrate increased flexibility with regard to trial protocols. This opportunity to reform how we conduct clinical trials moving forward represents potential areas of improvement for the future (Nabhan et al., 2020). For instance, incorporating telehealth visits and remote consent routinely into clinical trials, as has been done during the pandemic for some clinical trials (Li et al., 2020), could help render trials more accessible and, consequently, trial patient populations more representative of real-world patient populations. Crucially, a recently reported experience from the Dana-Farber Cancer Institute demonstrates that the incorporation of telemedicine and mailing of oral investigational agents during the pandemic was not associated with increases in serious adverse events or major violations related to drug dosing (Tolaney et al., 2020).
The RECOVERY trial, while evaluating COVID-19-directed therapies and not cancer-directed treatments, is a striking example of how randomized controlled trials can be conducted efficiently, cheaply, and collaboratively when regulatory requirements are relaxed and clinical trial protocols are simplified (Emanuel et al., 2020). This trial has thus far shown that dexamethasone is effective in the treatment of patients with COVID-19 who require respiratory support (RECOVERY, 2020a), and that the lopinavir-ritonavir combination (RECOVERY, 2020b) and hydroxychloroquine (Horby et al., 2020) are not effective in the treatment of COVID-19.
There are ongoing large national and international efforts to characterize and understand the impact of the COVID-19 pandemic within the oncologic community. The ASCO Registry is one such survey designed to collect baseline and follow-up data on how the virus affects cancer care and the outcomes of patients with cancer (ASCO, 2020c). Measuring the effects of lower clinical trial recruitment and results on drug development plans in the pharmaceutical industry space will be important to characterize.
Clinical Factors, Biomarkers, and Prognostic Models: Which Patients with Cancer Are Most at Risk?
Although patients with cancer are now recognized to be at increased risk of adverse outcomes with COVID-19 (Garassino et al., 2020; Giannakoulis et al., 2020; Kuderer et al., 2020; Williamson et al., 2020), this patient population is large and relatively heterogeneous. Identifying patients that are at particularly increased risk is essential for risk stratification, tailoring prevention measures, and cancer and COVID-19 treatment. As such, multiple studies have attempted to determine prognostic factors in patients with COVID-19 in general, or specifically within the subgroup of patients with pre-existing diagnoses of cancer (Table 1 ; Box 3 ).
Table 1.
Selected Studies Evaluating Clinical Factors Associated with Adverse Prognosis in Patients with Cancer Who Develop COVID-19
| CCC19 (Kuderer et al., 2020) | UKCCMP (Lee et al., 2020a, Lee et al., 2020c) | OnCovid (Pinato et al., 2020) | TERAVOLT (Garassino et al., 2020) | Nature Cancer 2020 (Albiges et al., 2020) | Nature Medicine 2020 (Robilotti et al., 2020) | Cancer Discovery 2020 (Dai et al., 2020) | Lancet Oncology 2020 (Tian et al., 2020) | Lancet Hematology 2020 (Passamonti et al., 2020) | |
|---|---|---|---|---|---|---|---|---|---|
| Region | International, multicenter | United Kingdom, multicenter | Europe, multicenter | International, multicenter | France, single center | United States, single center | China; multicenter | China; multicenter | Italy; multicenter |
| Cancers included | All invasive cancers | All cancers | All cancers | Lung cancers | All cancers | All cancers | All cancers | All cancers | Hematological cancers |
| Number of patients with cancer and COVID-19 included | 928 | 800 (Lennard Y.W. Lee et al., 2020a) and 1,044 (Lennard Y W Lee et al., 2020a, 2020c) | 890 | 200 | 178 | 423 | 105 | 232 | 536 |
| Lung cancer | x (versus general population) | x (versus other cancers) | |||||||
| Hematologic cancer | x (versus other cancers) | x (versus other cancers) | x (versus other cancers) | x (versus general population) | |||||
| Tumor status | x (progressing versus remission) | x (active malignancy versus remission/no measurable disease) | x (active or metastatic versus remission or localized) | x (metastatic cancer versus non-metastatic cancer or no cancer) | x (stage IV versus stages I–III) | x (progressing versus non-progressing disease) | |||
| Systemic therapy | x (chemotherapy within 4 weeks in patients with hematologic cancers) | x (chemotherapy within the past 3 months) | x (ICI within 90 days) | x (ICI or targeted therapy versus surgery) | |||||
| Increasing age | x | x | x | x | x | x | x | x | |
| Male sex | x | x | x | ||||||
| Comorbidities | x | x | x | x | |||||
| ECOG PS | x | x | x | ||||||
| Smoking | x | x | x |
CCC19, COVID-19 and Cancer Consortium; ICI, immune checkpoint inhibitor; PS, performance status; TERAVOLT, Thoracic Cancers International Covid 19 Collaboration; UKCCMP, UK Coronavirus Cancer Monitoring Project; x, Factor found to be associated with adverse prognosis.
Box 3. Which Patients with Cancer Are Most at Risk?
-
•
Lung cancer and hematologic cancers and advanced or active cancers are associated with adverse outcomes upon SARS-CoV-2 infection.
-
•
There are currently conflicting data on the relationship between receipt of systemic anti-cancer therapies and COVID-19 outcomes in patients with cancer.
-
•
Certain factors found to be associated with adverse outcomes in the general population were also associated with poor outcomes in patients with cancer, including male sex, increasing age, comorbidities, poor performance status, and smoking.
-
•
In patients with cancer who develop COVID-19, markers of inflammation (IL-6, TNF-α), of organ damage (decreased albumin-globulin ratio and increased NT-proBNP), and of immune dysfunction (decreased lymphocytes and CD4+ T cells) were associated with adverse prognosis.
Clinical Factors
Tumor Type, Active Cancer, and Stage
Tumor-related factors appear to be a significant prognostic factor in patients with cancer. In particular, certain tumor types have been found to be associated with particularly poor outcomes with COVID-19. These include lung cancer (Dai et al., 2020; Garassino et al., 2020), potentially due to reduced respiratory reserve, and hematological cancers (Dai et al., 2020; He et al., 2020; Kuderer et al., 2020; Lee et al., 2020a, 2020c; Passamonti et al., 2020; Williamson et al., 2020), perhaps due to the inherent immune suppression that is associated with the cancer itself and the more immune-suppressive therapeutic regimens that are often used to treat these cancers (Rubinstein and Warner, 2020).
Beyond the type of cancer, it is also the status of the tumor (active, stable or progressing, or in remission) that seems to influence the prognosis of patients with cancer who develop COVID-19. Patients with advanced cancer as well as progressing diseases have been consistently found to have worse outcomes (compared with those with localized disease or who are in remission) (Albiges et al., 2020; Dai et al., 2020; Kuderer et al., 2020; Passamonti et al., 2020; Pinato et al., 2020; Tian et al., 2020). It is thus far unclear whether this association is due to the general poorer health status of patients with more advanced cancer disease, affecting treatment strategies adopted while driven by the cancer prognosis (e.g., earlier referral to comfort-based care), or whether advanced or progressing cancers affect the COVID-19 disease course due to intersecting biological mechanisms with SARS-CoV-2, as discussed above.
Receipt of Systemic Cancer-Targeted Therapy
As discussed above, some general guiding principles have emerged with regard to systemic cancer-targeted therapies (Schrag et al., 2020), whereby curative therapies should not be delayed and particularly low-value systemic therapies can be deferred; data are still needed to assess the risk-benefit ratio of specific systemic therapies in the midst of the pandemic. Data on the relationship between specific systemic therapies and COVID-19 outcomes have been sparse thus far, mostly because of the heterogeneity of systemic therapy regimens, the timing of receipt of systemic therapy relative to COVID-19 diagnosis, the presence of multiple possible confounding factors (such as by indication and due to cancer type), and the relatively small numbers of patients on specific regimens who develop COVID-19.
There are currently conflicting data on the association between receipt of systemic therapy and prognosis. In particular, receipt of ICIs (Robilotti et al., 2020; Tian et al., 2020) and chemotherapy (Albiges et al., 2020; Lee et al., 2020a, 2020c) was associated with poor outcomes in patients with cancer and COVID-19 in some studies, but other studies had reported no association (Kuderer et al., 2020; Lee et al., 2020a, 2020c; Luo et al., 2020a, 2020b; Passamonti et al., 2020). However, it should be noted that the absence of conclusive evidence of the association between receipt of systemic therapy and adverse COVID-19 outcomes should not be taken for evidence of absence of an effect. In particular, each of these studies is likely powered only to detect relatively large effects and groups systemic therapies into groups of therapies with related mechanisms of action. The effects of less frequently used systemic therapies (such as experimental therapies and specific combination regimens) are thus far largely unexplored. Moreover, the potential for interaction between systemic therapy regimens and cancers that predispose to worse COVID-19 outcomes (such as hematologic and lung cancers) has also not been evaluated. Therefore, caution should prevail when treating patients with cancer during periods of COVID-19 resurgence, and the benefits and risks of each therapy should be weighed during consultations with patients (Schrag et al., 2020).
Social Determinants of Health
Although the pandemic has, at the time of this writing, hit most countries in the world, patient populations have been unevenly affected, even within the same country or geographic region. In particular, in the United States and in the United Kingdom, race and ethnicity have appeared to be important correlates of rates of infection and of adverse outcomes in patients with COVID-19 in general (Chowkwanyun and Reed, 2020; Williamson et al., 2020; Yancy, 2020). This result mirrors the relationship previously observed between adverse outcomes in other diseases, including cancer, and race and ethnicity (Badal et al., 2020; Marinac et al., 2020; Marinaro et al., 2020; Yedjou et al., 2019). These health disparities are thought to be largely driven by socioeconomic status, lack of sufficient access to care, and area of residence, but biological factors have been implicated in certain instances (although not with COVID-19).
Whether health disparities are also pronounced in patients with cancer who develop COVID-19 has not been fully established. However, it seems plausible that this relationship, between being part of racial or ethnic minorities and adverse COVID-19 outcomes, would hold or even be more pronounced in patients with cancer. In fact, there are early signals to this effect from certain studies of patients with cancer and COVID-19, with one study suggesting that Black patients with cancer are half as likely to receive remdesivir for COVID-19 compared with White patients (Rivera et al., 2020). Furthermore, others suggest that health disparities with regard to cancer care are more likely to become accentuated during the COVID-19 crisis due to socioeconomic factors and decreased access to telehealth services (Balogun et al., 2020).
Comorbidities and General Factors
Beyond cancer-specific, cancer treatment-related, and demographic factors, other general patient factors seem to be important correlates of prognosis for patients with cancer who develop COVID-19. Similar to the general population of patients with COVID-19, the following factors all correlated with poor prognosis in patients with cancer: male sex (Kuderer et al., 2020; Lee et al., 2020a, 2020c; Pinato et al., 2020), older age (Albiges et al., 2020; Garassino et al., 2020; Kuderer et al., 2020; Lee et al., 2020a, 2020c; Passamonti et al., 2020; Pinato et al., 2020; Robilotti et al., 2020; Tian et al., 2020; Westblade et al., 2020), comorbidities (including cardiovascular and respiratory) (Garassino et al., 2020; Kuderer et al., 2020; Lee et al., 2020a, 2020c; Pinato et al., 2020), smoking (Albiges et al., 2020; Garassino et al., 2020; Kuderer et al., 2020; Luo et al., 2020a, 2020b), and poor general performance status (Albiges et al., 2020; Kuderer et al., 2020; Tian et al., 2020). Many of these factors are associated with cancer itself and this may account for the generally poorer prognosis of patients with cancer who develop COVID-19.
Biomarkers of Adverse Prognosis
In addition to clinical factors, prediction of outcomes of patients who develop COVID-19 could be refined by including biomarkers or laboratory results. For instance, a recent study has found that increased IL-6 and TNF-α, which are both thought to be involved in the COVID-19 cytokine storm, can predict adverse outcomes in patients with COVID-19, independent of other clinical factors (Del Valle et al., 2020). Other potential biomarkers have included neutrophil count, lymphocyte count, C-reactive protein level, platelet count, and creatinine, among others (Wynants et al., 2020).
These findings have been corroborated in patients with cancer, whereby inflammation markers (increased in IL-6, TNF-α, C-reactive protein, procalcitonin, ferritin, and D-dimer) (Albiges et al., 2020; Tian et al., 2020), markers of organ damage (decreased albumin, increased cardiac enzymes and lactate dehydrogenase) (Albiges et al., 2020; Tian et al., 2020), and markers of immune dysfunction (decreased lymphocytes, monocytes, and CD4+ T cells) (Albiges et al., 2020; Tian et al., 2020) have all been associated with adverse prognosis. In addition to patient-specific biomarkers, increased SARS-CoV-2 viral load has been recently found to be a predictor of adverse prognosis in patients with or without cancer (Westblade et al., 2020).
Prognostic Models and Collaboration
Whereas these correlates of prognosis are important to inform clinical practice, developing prediction models that accurately predict patient outcomes and that are usable in clinical practice could help triage patients, focus resource utilization, and serve as stratification factors in clinical trials. Unfortunately, a recent review of predictive models for patients with COVID-19 in general concluded that despite the fact that a multitude of models have so far been published, none are currently usable in clinical practice (Wynants et al., 2020), largely due to insufficient reporting and high risk of bias.
In particular, patients with cancer form a relatively small proportion of the overall number of patients infected with COVID-19, and multiple studies have not specifically reported on whether the patients included had a cancer diagnosis. This raises concerns with regard to the generalizability of existing models to patients with cancer. Developing predictive models that are specific to patients with cancer or validating existing models in patients with cancer will require multi-institutional collaborations to ensure adequate statistical power and generalizability. Fortunately, multiple multi-institutional initiatives are currently working on aggregating data on patients with cancer, including the COVID-19 and Cancer Consortium (CCC19) (Rubinstein et al., 2020), ESMO CoCARE, Thoracic Cancers International Covid 19 Collaboration (TERAVOLT) (Whisenant et al., 2020), the UK Coronavirus Cancer-Monitoring Project (Anil et al., 2020), and others, as previously described (Desai et al., 2020; Palmieri et al., 2020).
Impact of COVID-19 on Cancer Research
Funding
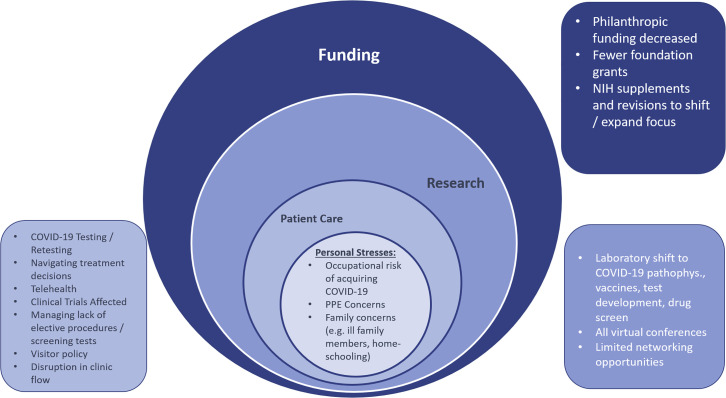
The effects of the COVID-19 pandemic are wide ranging. In addition to its impact on patients and providers, cancer researchers have also had their routine research lives upended. As noted in a letter from the American Association for Cancer Research directors to the US Congress (AACR, 2020), many researchers admirably contributed to the global and national interest by repurposing their cancer research labs to help address the needs of combating the pandemic (Figure 2 ). Their labs and facilities helped develop novel tests, aided in drug screens, conducted vaccine research, and analyzed population cohort data. This shift in research focus has been made possible by increased flexibility in federal grant mechanisms. Specifically, the National Institutes of Health offered Urgent and Emergency competing revisions and administrative supplements to existing grant awards on a rolling basis. When COVID-19 research is proposed, it is considered as “in (or out) of the scope” of the parent grant, with a broad interpretation of “in the scope,” because virtually no NCI grantees have COVID-19 grants (Singer, 2020). Although the federal government is able to support ongoing research due to appropriations, not all funding sources are as flush. COVID-19 has drastically decreased donations to cancer-focused, grant-giving organizations. As such, prominent organizations such as the American Cancer Society (ACS) have not been able to accept award applications for the fall grant cycle due to the financial impact of the pandemic on philanthropy. The ACS expects to experience a $200 million decrease in philanthropic donations during 2020. This represents an approximately 25% decrease in donations relative to the pre-COVID era. Typically, 12%–14% of donations are ear-marked for supporting extramural research (i.e., research that is separate from intramural research activities). ACS funded $37 million worth of extramural research during the pandemic, down from the typical $45–55 million over the same time span in the pre-COVID era. The funding that is currently available is being directed toward Cancer Research Scholar grants that target early-stage investigators and Mission Boost grants that target translation to patients, according to the chief medical and scientific officer, Dr. William Cance (personal communication, September 2020). The ACS is also not alone in having to decrease funding for cancer research, with the Canadian Institutes of Health Research and Cancer Research UK undertaking similar measures (Webster, 2020). The impact on cancer research funding is therefore likely to be felt globally, with governments and philanthropic organizations around the world having to take similar measures due to the unforeseen challenges posed by the pandemic.
Figure 2.
Areas of Disruption to Cancer Care Providers and Researchers
pathophys, pathophysiology.
Science, Home, and Human Interactions
In addition to the shift in focus of laboratory-based research and the effects on funding, cancer researchers have had their scientific investigation, home life, and professional interactions uprooted. In many cases, the ability to perform experiments was altogether halted as institutions and labs were shut down entirely (Colbert et al., 2020). Reopening is largely dependent on local benchmarks and institutional preparedness. After state and local mandates to shelter in place were invoked, many researchers were additionally faced with duties such as caring for family members and home-schooling children. Although this change in work-from-home conditions was universal, using academic metrics to determine whether certain populations have been disproportionately affected (i.e., parents of school-aged children, women, members of underrepresented minority groups, urban versus rural colleagues, earlycareer versus mid-career versus established investigators) will be informative. Levine and Rathmell write in a call-to-action editorial (to support early-career investigators) that “reduced productivity during this time (pandemic closures) can produce an undesirable ‘flattening of the curve’ that is potentially unrecoverable” (Levine and Rathmell, 2020). The in-person connections and networking opportunities that so often define academic conferences have gone by the wayside, with virtual events taking their place in the COVID era. Whether people prefer to consume data and science from their living room or backyard is not as crucial a question as what the long-term losses to the scientific community might be from not interacting, congregating around posters, riffing on pathways, and forging new collaborations. Although many of the virtual events have been highly effective and seamless in delivering material, whether the same enthusiasm and facilitation of future collaborations are born from virtual events is not immediately clear. We must be intentional about creating opportunities to interact spontaneously with one another, especially meeting new people. A useful exercise might be to compare the number of multi-institutional grant applications and publications over the next 1 to 2 years with years past, and to delineate how many of them are new collaborations or include and feature an early-career investigator. Within CCC19, we are intentional about putting early-career investigators and members from underrepresented minority groups out front and featured on prominent publications. It is critical that everyone in the scientific research community recognize that, while the COVID era is difficult for us all, the effects are not shared equally. Now is the time to lift one another up.
And finally, it is worth highlighting that efforts to prevent and treat other non-communicable diseases (NCDs), including cancer, have been upended by the COVID-19 pandemic. Pre-COVID, the World Health Organization prioritized focus and resources on reducing NCD mortality by strengthening health systems delivering NCD services. In a rapid assessment survey of service delivery for NCDs during the COVID-19 pandemic among 163 ministries, 122 countries reported that NCD services are disrupted; thus, now is the time for all countries to strive to build back better systems for the treatment of all patients with NCDs, including cancer (WHO, 2020).
Conclusions
The COVID-19 pandemic has resulted in formidable challenges to patients with cancer as well as clinicians and researchers. As the pandemic rages on across the world, it will be of paramount importance to further study the interactions of cancer and COVID-19 in order to better tailor the management of patients with cancer and minimize disruption to cancer care. The biological mechanisms that underlie the poor outcomes of patients with cancer remain poorly understood and require further research to help target therapeutic interventions in patients with cancer who develop COVID-19. Further research is also needed to determine whether the biological insights derived from the cancer research community can be translated into clinically effective treatments for all patients with COVID-19. Finally, most of the clinical data in this review originate from high-resource countries, reflecting the published literature, but more work is needed to guide the care of patients with cancer during the pandemic in low-resource settings.
Acknowledgments
This work was partly funded by a VUMC CCSG grant, P30 CA068485. The authors would like to thank Dr. William G. Cance, chief medical and scientific officer of the American Cancer Society, for his insights and feedback, and all the COVID-19 and Cancer Consortium investigators for their support (CCC19; https://ccc19.org/collaborators).
Declaration of Interests
Z.B. reports research support from Genentech/imCORE and Bristol-Myers Squibb.
J.E.H. reports no competing interests.
T.K.C. reports research support from AstraZeneca, Alexion, Bayer, Bristol-Myers Squibb/E.R. Squibb and Sons, Cerulean, Eisai, Foundation Medicine, Exelixis, Ipsen, Tracon, Genentech, Roche, Roche Products, F. Hoffmann-La Roche, GlaxoSmithKline, Eli Lilly, Merck, Novartis, Peloton, Pfizer, Prometheus Labs, Corvus, Calithera, Analysis Group, Sanofi/Aventis, and Takeda outside of the submitted work; honoraria from AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol-Myers Squibb/E.R. Squibb and Sons, Cerulean, Eisai, Foundation Medicine, Exelixis, Genentech, Roche, Roche Products, F. Hoffmann-La Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, National Comprehensive Cancer Network (NCCN), Analysis Group, MJH Life Sciences (a health care communications company with several brands such as OncLive, PeerView, and Physicians' Education Resource), Research to Practice, L-path, Kidney Cancer Journal, Clinical Care Options, Platform Q, Navinata Health, Harborside Press, American Society of Medical Oncology, New England Journal of Medicine, The Lancet Oncology, and Heron Therapeutics; and consulting or advisory roles for AstraZeneca, Alexion, Sanofi/Aventis, Bayer, Bristol-Myers Squibb/E.R. Squibb and Sons, Cerulean, Eisai, Foundation Medicine, Exelixis, Genentech, Heron Therapeutics, Eli Lilly, Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, EMD Serono, Prometheus Labs, Corvus, Ipsen, Up-to-Date, NCCN, Analysis Group, Pionyr, Tempest, and Lilly Ventures; owns stocks in Pionyr and Tempest; has leadership roles as director of GU Oncology Division at Dana-Farber, was past president of medical staff at Dana-Farber Cancer Institute, is a member of the NCCN Kidney panel and the GU Steering Committee, was past chairman of the Kidney Cancer Association Medical and Scientific Steering Committee and KidneyCan advisory board, and is Kidney Cancer Research Summit co-chair (2019–present); and has patents, royalties, and other intellectual properties in International Patent Application PCT/US2018/12,209, entitled “PBRM1 Biomarkers Predictive of Anti-immune Checkpoint Response,” filed January 3, 2018 (claiming priority to US Provisional Patent Application 62/445,094, filed January 11, 2017), and International Patent Application PCT/US2018/058430, entitled “Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy,” filed October 31, 2018 (claiming priority to US Provisional Patent Application 62/581,175, filed November 3, 2017). T.K.C.'s institution (Dana-Farber Cancer Institute) might have received additional independent funding of drug companies or royalties potentially involved in research around the subject matter of this article. T.K.C. has mentored several non-US citizens on research projects with potential funding (in part) from non-US sources or foreign components.
S.P. reports that she has received education grants, provided consultation, attended advisory boards, and/or provided lectures for Abbvie, Amgen, AstraZeneca, Bayer, Biocartis, Bioinvent, Blueprint Medicines, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis, Daiichi-Sankyo, Debiopharm, Eli Lilly, F. Hoffmann-La Roche, Foundation Medicine, Illumina, Janssen, Merck Sharp and Dohme, Merck Serono, Merrimack, Novartis, PharmaMar, Pfizer, Regeneron, Sanofi, Seattle Genetics, Takeda, and Vaccibody, from whom she has received honoraria (all fees to institution).
B.I.R. reports that he has received research funding to his institution from Pfizer, Merck, GNE/Roche, Aveo, AstraZeneca, BMS, Exelixis; has consulted for BMS, Pfizer, GNE/Roche, Aveo, Synthorx, Compugen, Merck, Corvus, Surface Oncology, 3DMedicines, Arravive, Alkermes, Arrowhead, GSK, Shionogi; and holds stock in PTC Therapeutics.
J.L.W. reports personal fees from Westat and IBM Watson Health and stock ownership in HemOnc.org LLC, outside the submitted work.
C.A.P., or an immediate family member, currently or during the past 2 years, has owned stock or held an ownership interest in Pfizer, Epizyme, Inovio, OPKO Health, and Roche.
References
- AACR AACR COVID-19 response letter. 2020. https://www.aacr.org/wp-content/uploads/2020/03/AACR-COVID-19-Response-Letter.pdf
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C.M.D., Stark H., Tzankov A., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACS Clinical issues and guidance. 2020. https://www.facs.org/covid-19/clinical-guidance
- ACS Joint statement: roadmap for maintaining essential surgery during COVID-19 pandemic. 2020. https://www.facs.org/covid-19/clinical-guidance/roadmap-maintain-essential-surgery
- Agarwal V., Venkatakrishnan A.J., Puranik A., Lopez-Marquez A., Challener D.W., O Horo J.C., Badley A.D., Halamka J.D., Morice W.G., Soundararajan V. Quantifying the prevalence of SARS-CoV-2 long-term shedding among non-hospitalized COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.06.02.20120774. [DOI] [Google Scholar]
- Al-Shamsi H.O., Coomes E.A., Alrawi S. Screening for COVID-19 in asymptomatic patients with cancer in a hospital in the United Arab Emirates. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albiges L., Foulon S., Bayle A., Gachot B., Pommeret F., Willekens C., Stoclin A., Merad M., Griscelli F., Lacroix L., et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort. Nat. Cancer. 2020:1–11. doi: 10.1038/s43018-020-00120-5. [DOI] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anil I., Arnold R., Benkwitz-Beford S., Branford S., Campton N., Cazier J.B., Cheng V., Curley H., D’Costa J., Edmondson A., et al. The UK Coronavirus Cancer Monitoring Project: protecting patients with cancer in the era of COVID-19. Lancet Oncol. 2020;21:622–624. doi: 10.1016/S1470-2045(20)30230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- Argenzian M.G., Bruc S.L., Slate C.L., Tia J.R., Baldwi M.R., Barr R.G., Chan B.P., Cha K.H., Cho J.J., Gavin N., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASCO . ASCO; 2020. COVID-19 Provider & Practice Information.https://www.asco.org/asco-coronavirus-information/provider-practice-preparedness-covid-19 [Google Scholar]
- ASCO ASCO special report: a guide to cancer care delivery during the COVID-19 pandemic. 2020. https://www.asco.org/sites/new-www.asco.org/files/content-files/2020-ASCO-Guide-Cancer-COVID19.pdf
- ASCO ASCO survey on COVID-19 in oncology (ASCO) registry. 2020. https://www.asco.org/sites/new-www.asco.org/files/content-files/blog-release/documents/COVID19-Registry-Study-Schema-04-16-20.pdf
- ASTRO COVID-19 recommendations and information - American society for radiation oncology (ASTRO) - American society for radiation oncology (ASTRO) 2020. https://www.astro.org/Daily-Practice/COVID-19-Recommendations-and-Information/Clinical-Guidance
- Badal S., Aiken W., Morrison B., Valentine H., Bryan S., Gachii A., Ragin C. Disparities in prostate cancer incidence and mortality rates: solvable or not? Prostate. 2020;80:3–16. doi: 10.1002/pros.23923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogun O.D., Bea V.J., Phillips E. Disparities in cancer outcomes due to COVID-19—a tale of 2 cities. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.3327. [DOI] [PubMed] [Google Scholar]
- Baratchian M., McManus J., Berk M., Nakamura F., Erzurum S., Mukhopadhyay S., Drazba J., Peterson J., Gaston B., Sharifi N. No evidence that androgen regulation of pulmonary TMPRSS2 explains sex-discordant COVID-19 outcomes. bioRxiv. 2020 doi: 10.1101/2020.04.21.051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berraondo P., Sanmamed M.F., Ochoa M.C., Etxeberria I., Aznar M.A., Pérez-Gracia J.L., Rodríguez-Ruiz M.E., Ponz-Sarvise M., Castañón E., Melero I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booeshaghi A.S., Pachter L. Decrease in ACE2 mRNA expression in aged mouse lung. bioRxiv. 2020 doi: 10.1101/2020.04.02.021451. [DOI] [Google Scholar]
- Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., Boodman C., Bello A., Hedley A., Schiffman Z., et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., Huang L., Meng F., Huang L., Wang N., et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020;146:137–146.e3. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . CDC; 2020. Certain Medical Conditions and Risk for Severe COVID-19 Illness.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html [Google Scholar]
- Chang E.I., Liu J.J. Flattening the curve in oncologic surgery: impact of Covid-19 on surgery at tertiary care cancer center. J. Surg. Oncol. 2020 doi: 10.1002/jso.26056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Chen Y.-Y., Guan B.-S., Li Z.-K., Li X.-Y. Effect of telehealth intervention on breast cancer patients’ quality of life and psychological outcomes: a meta-analysis. J. Telemed. Telecare. 2018;24:157–167. doi: 10.1177/1357633X16686777. [DOI] [PubMed] [Google Scholar]
- Chen Y., Shan K., Qian W. Asians do not exhibit elevated expression or unique genetic polymorphisms for ACE2, the cell-entry receptor of SARS-CoV-2. 2020. Preprints 1–13. [DOI]
- Chowkwanyun M., Reed A.L. Racial health disparities and Covid-19 — caution and context. N. Engl. J. Med. 2020;383:201–203. doi: 10.1056/NEJMp2012910. [DOI] [PubMed] [Google Scholar]
- Chung M.K., Karnik S., Saef J., Bergmann C., Barnard J., Lederman M.M., Tilton J., Cheng F., Harding C.V., Young J.B., et al. SARS-CoV-2 and ACE2: the biology and clinical data settling the ARB and ACEI controversy. EBioMedicine. 2020;58:102907. doi: 10.1016/j.ebiom.2020.102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CMS . CMS; 2020. Trump Administration Proposes to Expand Telehealth Benefits Permanently for Medicare Beneficiaries beyond the COVID-19 Public Health Emergency and Advances Access to Care in Rural Areas.https://www.cms.gov/newsroom/press-releases/trump-administration-proposes-expand-telehealth-benefits-permanently-medicare-beneficiaries-beyond [Google Scholar]
- Colbert L.E., Kouzy R., Abi Jaoude J., Ludmir E.B., Taniguchi C.M. Cancer research after COVID-19: where do we go from here? Cancer Cell. 2020;37:637–638. doi: 10.1016/j.ccell.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.R.E., Hastie J., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., Zhang Z., You H., Wu M., Zheng Q., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020:1–8. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Warner J., Kuderer N., Thompson M., Painter C., Lyman G., Lopes G. Crowdsourcing a crisis response for COVID-19 in oncology. Nat. Cancer. 2020;1:473–476. doi: 10.1038/s43018-020-0065-z. [DOI] [PubMed] [Google Scholar]
- Doroshow D., Schmidt A.L., Bakouny Z., Bhalla S., Steinharter J.A., Tremblay D., Awad M.M., Kessler A.J., Bouchard G., Evans M., et al. Disparities in cancer during the COVID-19 pandemic: COVID-19 and cancer outcomes study (CCOS) Ann. Oncol. 2020;31:S1204. [Google Scholar]
- Elkaddoum R., Haddad F.G., Eid R., Kourie H.R. Telemedicine for cancer patients during COVID-19 pandemic: between threats and opportunities. Future Oncol. 2020;16:1225–1227. doi: 10.2217/fon-2020-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel E.J., Zhang C., Diana A. Opinion|where Is America’s Groundbreaking Covid-19 Research? - the New York Times. 2020. https://www.nytimes.com/2020/09/01/opinion/coronavirus-clinical-research.html
- Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- ESMO . ESMO; 2020. Cancer Patient Management during the COVID-19 Pandemic.https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic [Google Scholar]
- Falanga A., Marchetti M., Vignoli A. Coagulation and cancer: biological and clinical aspects. J. Thromb. Haemost. 2013;11:223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Flodgren G., Rachas A., Farmer A.J., Inzitari M., Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst. Rev. 2015;2015:CD002098. doi: 10.1002/14651858.CD002098.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M., Babik J.M. COVID-19 in immunocompromised hosts: what we know so far. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Garassino M.C., Whisenant J.G., Huang L.C., Trama A., Torri V., Agustoni F., Baena J., Banna G., Berardi R., Bettini A.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin M.R., Alvarez C., Miller J.I., Prates E.T., Walker A.M., Amos B.K., Mast A.E., Justice A., Aronow B., Jacobson D. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;9:e59177. doi: 10.7554/eLife.59177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genentech Press Release Genentech: press releases. 2020. https://www.gene.com/media/press-releases/14867/2020-07-28/genentech-provides-an-update-on-the-phas
- Gharzai L.A., Resnicow K., An L.C., Jagsi R. Perspectives on oncology-specific language during the coronavirus disease 2019 pandemic. JAMA Oncol. 2020;6:1424–1428. doi: 10.1001/jamaoncol.2020.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakoulis V.G., Papoutsi E., Siempos I.I. Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Glob. Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. J. Am. Med. Assoc. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harky A., Chiu C.M., Yau T.H.L., Lai S.H.D. Cancer patient care during COVID-19. Cancer Cell. 2020;37:749–750. doi: 10.1016/j.ccell.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Chen L., Chen L., Yuan G., Fang Y., Chen W., Wu D., Liang B., Lu X., Ma Y., et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for covid-19. N. Engl. J. Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., Prudon B., et al. Hydroxychloroquine for COVID-19-preliminary report effect of hydroxychloroquine in hospitalized patients. medRxiv. 2020 doi: 10.1101/2020.07.15.20151852. [DOI] [Google Scholar]
- Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C., Lei H.Y. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDSA COVID-19 resource center. 2020. https://www.idsociety.org/public-health/COVID-19-Resource-Center/ [PubMed]
- Ip A. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-an observational study. medRxiv. 2020 doi: 10.1101/2020.05.21.20109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S.C., Zakowski P., Tran H.P., Smith E.A., Gaultier C., Marks G., Zabner R., Lowenstein H., Oft J., Bluen B., et al. Compassionate use of tocilizumab for treatment of SARS-CoV-2 pneumonia. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidar S., Kaplan M., Gamliel-Lazarovich A. ACE2 of the heart: from angiotensin I to angiotensin (1-7) Cardiovasc. Res. 2007;73:463–469. doi: 10.1016/j.cardiores.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krengli M., Ferrara E., Mastroleo F., Brambilla M., Ricardi U. Running a radiation oncology department at the time of coronavirus: an Italian experience. Adv. Radiat. Oncol. 2020;5:527–530. doi: 10.1016/j.adro.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.Y., Desai A., de Lima Lopes G., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A., Przepiorka D., Farrell A.T., Pazdur R. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.Y.Y. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107:17I–21. doi: 10.1161/01.CIR.0000078466.72504.AC. [DOI] [PubMed] [Google Scholar]
- Lee L.Y.W., Cazier J.B., Angelis V., Arnold R., Bisht V., Campton N.A., Chackathayil J., Cheng V.W.T., Curley H.M., Fittall M.W., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.Y.W., Hill T., Topping O., Tilby M., Baker M., Greig J., Isherwood L., Miller R., Petrenko Y., Desai R., et al. Utility of COVID-19 screening in cancer patients. Cancer Cell. 2020 doi: 10.1016/j.ccell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.Y.W., Cazier J.-B., Starkey T., Briggs S.E.W., Arnold R., Bisht V., Booth S., Campton N.A., Cheng V.W.T., Collins G., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Allen Chan K.C., Hui D.S., Ng E.K.O., Wu A., Chiu R.W.K., Wong V.W.S., Chan P.K.S., Wong K.T., Wong E., et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J. Clin. Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R.L., Rathmell W.K. COVID-19 impact on early career investigators: a call for action. Nat. Rev. Cancer. 2020;20:357–358. doi: 10.1038/s41568-020-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Yin C., Zhou Y., Wang T., Chen J., Liu Y., Chen T., Wang H., Zhang L., Chen X. Digitalized adaptation of oncology trials during and after COVID-19. Cancer Cell. 2020;38:148–149. doi: 10.1016/j.ccell.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Guan W., Chen R., Wang W., Li J., Xu K., Li C., Ai Q., Lu W., Liang H., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Luo S., Libby P., Shi G.P. Cathepsin L-selective inhibitors: a potentially promising treatment for COVID-19 patients. Pharmacol. Ther. 2020;213:107587. doi: 10.1016/j.pharmthera.2020.107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J.W., Fazio-Eynullayeva E., Palchuk M.B., Sankey P., McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin. Cancer Inform. 2020:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J.M., Heinlein C., Kim T., Hernandez S.A., Malik M.S., True L.D., Morrissey C., Corey E., Montgomery B., Mostaghel E., et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Rizvi H., Egger J.V., Preeshagul I.R., Wolchok J.D., Hellmann M.D. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Rizvi H., Preeshagul I.R., Egger J.V., Hoyos D., Bandlamudi C., McCarthy C.G., Falcon C.J., Schoenfeld A.J., Arbour K.C., et al. COVID-19 in patients with lung cancer. Ann. Oncol. 2020;31:1386. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020 doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinac C.R., Ghobrial I.M., Birmann B.M., Soiffer J., Rebbeck T.R. Dissecting racial disparities in multiple myeloma. Blood Cancer J. 2020 doi: 10.1038/s41408-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinaro J., Zeymo A., Egan J., Carvalho F., Krasnow R., Stamatakis L., Lynch J., Hwang J., Williams S., Kowalczyk K. Sex and racial disparities in the treatment and outcomes of muscle-invasive bladder cancer. Urology. 2020 doi: 10.1016/j.urology.2020.06.087. [DOI] [PubMed] [Google Scholar]
- Maringe C., Spicer J., Morris M., Purushotham A., Nolte E., Sullivan R., Rachet B., Aggarwal A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A., Pradhan K., Thota R., Reissman S., Sparano J.A., et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkonen L., Pihlajamaa P., Sahu B., Zhang F.P., Jänne O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol. Cell. Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Min C.K., Cheon S., Ha N.Y., Sohn K.M., Kim Y., Aigerim A., Shin H.M., Choi J.Y., Inn K.S., Kim J.H., et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H., Xiong Y., Cheng Z., Gao S., Liang K., et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll M., Zon R.L., Sylvester PharmD K.W., Chen E.C., Cheng V., Connell MPH N.T., Fredenburgh L.E., Baron R.M., Cho MPH M.H., Woolley A.E., Connors J.M. VTE in ICU patients with COVID-19. CHEST. 2020 doi: 10.1016/j.chest.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V., Carbone G.M., Cavalli A., Pagano F., Ragazzi E., et al. Androgen-deprivation Therapies for Prostate Cancer and Risk of Infection by SARS-CoV-2: A Population-Based Study (N [ 4532) 2020. [DOI] [PMC free article] [PubMed]
- Morrison D.R., Gentile C., McCammon S., Buczek E. Head and neck oncologic surgery in the <scp>COVID</scp> -19 pandemic: our experience in a deep south tertiary care center. Head Neck. 2020;42:1471–1476. doi: 10.1002/hed.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muus C., Luecken M., Eraslan G., Waghray A., Heimberg G., Sikkema L., Kobayashi Y., Vaishnav E.D., Subramanian A., Smilie C., et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv. 2020 doi: 10.1101/2020.04.19.049254. [DOI] [Google Scholar]
- Nabhan C., Choueiri T.K., Mato A.R. Rethinking clinical trials reform during the COVID-19 pandemic. JAMA Oncol. 2020;6:1327–1329. doi: 10.1001/jamaoncol.2020.3142. [DOI] [PubMed] [Google Scholar]
- New York Times Coronavirus Map: Tracking the Global Outbreak - the New York Times. 2020. https://www.nytimes.com/interactive/2020/world/coronavirus-maps.html
- Oberfeld B., Achanta A., Carpenter K., Chen P., Gilette N.M., Langat P., Said J.T., Schiff A.E., Zhou A.S., Barczak A.K., Pillai S. SnapShot: covid-19. Cell. 2020;181:954–954.e1. doi: 10.1016/j.cell.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri C., Palmer D., Openshaw P.J.M., Baille J.K., Semple M.G., Turtle L. Cancer datasets and the SARS-CoV-2 pandemic: establishing principles for collaboration. ESMO Open. 2020;5:e000825. doi: 10.1136/esmoopen-2020-000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti F., Cattaneo C., Arcaini L., Bruna R., Cavo M., Merli F., Angelucci E., Krampera M., Cairoli R., Della Porta M.G., et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V.G., Zhong X., Liaw B., Tremblay D., Tsao C.-K., Galsky M.D., Oh W.K. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann. Oncol. 2020;31:1419–1420. doi: 10.1016/j.annonc.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patell R., Bogue T., Bindal P., Koshy A., Merrill M., Aird W.C., Bauer K.A., Zwicker J.I. Incidence of thrombosis and hemorrhage in hospitalized cancer patients with COVID-19. J. Thromb. Haemost. 2020;18:2349–2357. doi: 10.1111/jth.15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinato D.J., Zambelli A., Aguilar-Company J., Bower M., Sng C., Salazar R., Bertuzzi A., Brunet J., Mesia R., Segui E., et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020 doi: 10.1158/2159-8290.cd-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto B.G.G., Oliveira A.E.R., Singh Y., Jimenez L., Gonçalves A.N.A., Ogava R.L.T., Creighton R., Schatzmann Peron J.P., Nakaya H.I. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J. Infect. Dis. 2020;222:556–563. doi: 10.1093/infdis/jiaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Jeanpierre E., Rauch A., Labreuche J., Susen S. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- Price C.C., Altice F.L., Shyr Y., Koff A., Pischel L., Goshua G., Azar M.M., Mcmanus D., Chen S.-C., Gleeson S.E., et al. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID-19 patients. Chest. 2020 doi: 10.1016/j.chest.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Dexamethasone in hospitalized patients with covid-19 — preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Statement from the Chief investigators of the randomised evaluation of COVid-19 thERapY (RECOVERY) trial on lopinavir-ritonavir. 2020. https://www.recoverytrial.net/files/lopinavir-ritonavir-recovery-statement-29062020_final.pdf
- Regeneron Press Release . Regeneron Pharmaceuticals Inc; 2020. Regeneron and Sanofi Provide Update on Kevzara® (Sarilumab) Phase 3 U.S. Trial in COVID-19 Patients.https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-kevzarar-sarilumab-phase-3 [Google Scholar]
- Reuss J.E., Suresh K., Naidoo J. Checkpoint inhibitor pneumonitis: mechanisms, characteristics, management strategies, and beyond. Curr. Oncol. Rep. 2020 doi: 10.1007/s11912-020-00920-z. [DOI] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera D.R., Peters S., Panagiotou O.A., Shah D.P., Kuderer N.M., Hsu C.-Y., Rubinstein S.M., Lee B.J., Choueiri T.K., de Lima Lopes G., et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: a COVID-19 and Cancer Consortium (CCC19) cohort study. Cancer Discov. 2020;1 doi: 10.1158/2159-8290.cd-20-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnston R., Bernardes M., Bogler Y., Caldararo M., Figueroa C.J., Glickman M.S., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogado J., Obispo B., Pangua C., Serrano-Montero G., Martín Marino A., Pérez-Pérez M., López-Alfonso A., Gullón P., Lara M. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin. Transl. Oncol. 2020 doi: 10.1007/s12094-020-02381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston K.V.I. Infections in cancer patients with solid tumors: a review. Infect. Dis. Ther. 2017 doi: 10.1007/s40121-017-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A., Roshon M., Wrzesinski S.H., Desai J.V., Zarakas M.A., et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce T.J., Sanoff H.K., Rewari A. Telemedicine for cancer care in the time of COVID-19. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein S.M., Steinharter J.A., Warner J., Rini B.I., Peters S., Choueiri T.K. The COVID-19 and cancer Consortium: a collaborative effort to understand the effects of COVID-19 on patients with cancer. Cancer Cell. 2020;37:738–741. doi: 10.1016/j.ccell.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein S.M., Warner J.L. COVID-19 and haematological malignancy: navigating a narrow strait. Lancet Haematol. 2020 doi: 10.1016/s2352-3026(20)30252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A., Armstrong D. Infections in patients with hematologic neoplasms and hematopoietic stem cell transplantation: neutropenia, humoral, and splenic defects. Clin. Infect. Dis. 2011;53:798–806. doi: 10.1093/cid/cir492. [DOI] [PubMed] [Google Scholar]
- Saini K.S., Tagliamento M., Lambertini M., McNally R., Romano M., Leone M., Curigliano G., de Azambuja E. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur. J. Cancer. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag D., Hershman D.L., Basch E. Oncology practice during the COVID-19 pandemic. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6236. [DOI] [PubMed] [Google Scholar]
- Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015 doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., Kochanek M., Böll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. Immunother. Cancer. 2018 doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA. Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- Singer D.S. NCI’s work to advance cancer research while responding to the COVID-19 pandemic. Cancer Cell. 2020;37:746–748. doi: 10.1016/j.ccell.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.C., Sausville E.L., Girish V., Yuan M., Lou, Vasudevan A., John K.M., Sheltzer J.M. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev. Cell. 2020;53:514–529.e3. doi: 10.1016/j.devcel.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W.R., Atala A.J., Terlecki R.P., Kelly E.E., Matthews C.A. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic. J. Am. Coll. Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L., Zhou N., Petty L.A., Baang J.H., Dillman N.O., et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopsack K.H., Mucci L.A., Antonarakis E.S., Nelson P.S., Kantoff P.W. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov. 2020;10:779–782. doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud A., Jones M.E., Broggio J., Loveday C., Torr B., Garrett A., Nicol D.L., Jhanji S., Boyce S.A., Gronthoud F., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020 doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann J.B., Smyth M.J. Immune surveillance of tumors. J. Clin. Invest. 2007 doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.W., Schmitz J.E., Persing D.H., Stratton C.W. Laboratory diagnosis of COVID-19: current issues and challenges. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachey D.T., Rheingold S.R., Maude S.L., Zugmaier G., Barrett D.M., Seif A.E., Nichols K.E., Suppa E.K., Kalos M., Berg R.A., et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet Gastroenterology & Hepatology Resuming bowel cancer screening post-COVID-19. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Yuan X., Xiao J., Zhong Q., Yang C., Liu B., Cai Y., Lu Z., Wang J., Wang Y., et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolaney S.M., Lydon C.A., Li T., Dai J., Standring A., Legor K.A., Caparrotta C.M., Schenker M.P., Glazer D.I., Tayob N., et al. The impact of COVID-19 on clinical trial execution at the dana-farber cancer Institute. J. Natl. Cancer Inst. 2020 doi: 10.1093/jnci/djaa144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckson R.V., Edmunds M., Hodgkins M.L. Telehealth. N. Engl. J. Med. 2017;377:1585–1592. doi: 10.1056/NEJMsr1503323. [DOI] [PubMed] [Google Scholar]
- Unger J.M., Blanke C.D., LeBlanc M., Hershman D.L. Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw. Open. 2020;3:e2010651. doi: 10.1001/jamanetworkopen.2020.10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ürün Y., Hussain S.A., Bakouny Z., Castellano D., Kılıçkap S., Morgan G., Mckay R.R., Pels K., Schmidt A., Doroshow D.B., et al. Survey of the impact of COVID-19 on oncologists’ decision making in cancer. JCO Glob. Oncol. 2020:1248–1257. doi: 10.1200/go.20.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Dhindsa R., Povysil G., Zoghbi A., Motelow J., Hostyk J., Nickols N., Rettig M., Goldstein D.B. TMPRSS2 Transcriptional Inhibition as a Therapeutic Strategy for COVID-19. 2020. [DOI]
- Waterhouse D.M., Harvey R.D., Hurley P., Levit L.A., Kim E.S., Klepin H.D., Mileham K.F., Nowakowski G., Schenkel C., Davis C., et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American society of clinical oncology survey. JCO Oncol. Pract. 2020 doi: 10.1200/op.20.00275. [DOI] [PubMed] [Google Scholar]
- Webster P. How is biomedical research funding faring during the COVID-19 lockdown? Nat. Med. 2020 doi: 10.1038/d41591-020-00010-4. [DOI] [PubMed] [Google Scholar]
- Westblade L.F., Brar G., Pinheiro L.C., Paidoussis D., Rajan M., Martin P., Goyal P., Sepulveda J.L., Zhang L., George G., et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell. 2020 doi: 10.1016/j.ccell.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisenant J.G., Trama A., Torri V., De Toma A., Viscardi G., Cortellini A., Michielin O., Barlesi F., Dingemans A.M.C., Van Meerbeeck J., et al. TERAVOLT: thoracic cancers international COVID-19 collaboration. Cancer Cell. 2020;37:742–745. doi: 10.1016/j.ccell.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO COVID-19 and NCDs. 2020. https://www.who.int/docs/default-source/ncds/ncd-covid-19/for-web---rapid-assessment---30-june-2020-(cleared).pdf?sfvrsn=6296324c_20
- Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters F., van de Bovenkamp J., van den Bosch B., van den Brink S., Broeders M., Chung N.H., Favié B., Goderski G., Kuijpers J., Overdevest I., et al. Multi-center evaluation of cepheid xpert® xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic. J. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern. Med. 2020;180:934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynants L., Van Calster B., Collins G.S., Riley R.D., Heinze G., Schuit E., Bonten M.M.J., Damen J.A.A., Debray T.P.A., De Vos M., et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. 2020;369:18. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Wang X., Liu H., Bao Z., Yu J., Zhong Y., Chua M.L.K. Infection control of 2019 novel corona virus disease (COVID-19) in cancer patients undergoing radiotherapy in wuhan. medRxiv. 2020 doi: 10.1101/2020.03.21.20037051. [DOI] [Google Scholar]
- Yancy C.W. COVID-19 and African Americans. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- Yedjou C.G., Sims J.N., Miele L., Noubissi F., Lowe L., Fonseca D.D., Alo R.A., Payton M., Tchounwou P.B. Advances in Experimental Medicine and Biology. Springer New York LLC; 2019. Health and racial disparity in breast cancer; pp. 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]