To the Editor:
Favipiravir may be an effective option for treating infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), in maintenance dialysis.1 There have been few reports of favipiravir administration in hemodialysis patients. We administered favipiravir to a hemodialysis patient with COVID-19 and found that blood concentrations of favipiravir were similar to those seen in patients not receiving dialysis.
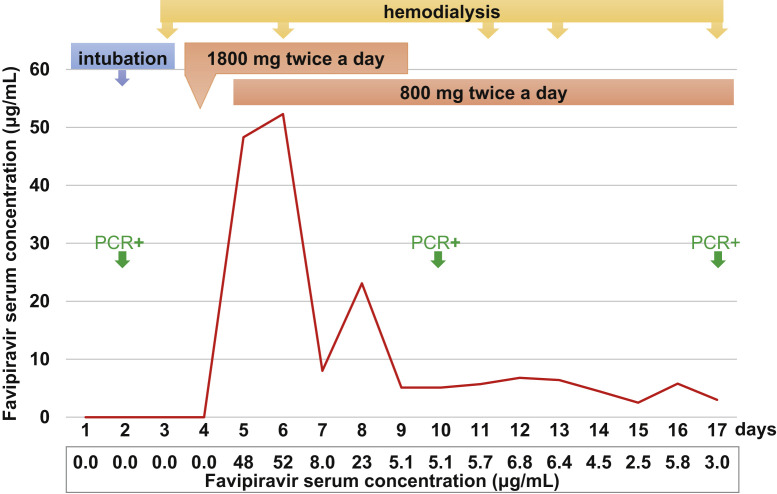
A 72-year-old man presented with fever and cough at an outside hospital. After computed tomography of the chest showed left lower lobe consolidation, he was transferred to our hospital. Medical history included diabetes mellitus and kidney failure treated with hemodialysis since early 2016. A polymerase chain reaction test performed 1 week before hospitalization was negative but a test day 2 of hospitalization proved positive. Oxygenation worsened and he was intubated and ventilated. Favipiravir administration started on day 4. He continued receiving hemodialysis 2 or 3 days a week. Blood concentrations of favipiravir (determined as described in Item S1) are shown in Figure 1 . On day 38, he experienced sudden clinical deterioration and died the next day.
Figure 1.
Blood concentrations of favipiravir. Hemodialysis was performed on days 3, 6, 11, 13, and 17. Abbreviation: PCR, polymerase chain reaction.
The course of the decline in favipiravir blood concentrations that we observed was similar to that reported in patients not receiving hemodialysis.2 , 3 This was unexpected because the molecular weight of favipiravir is 157 Da, 53% to 54% is protein bound, and the volume of distribution is ~20 L, suggesting that dialysis would eliminate favipiravir.
The half-maximal effective concentration of favipiravir against SARS-CoV-2 infection is 9.7 μg/mL, but blood concentrations after day 9 were all below this level.4 Previous reports also indicated favipiravir that blood concentrations were lower than predicted and therapeutic drug monitoring may be necessary.2
Article Information
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received September 10, 2020. Direct editorial input from a Deputy Editor. Accepted in revised form September 22, 2020.
Footnotes
Item S1: Methods for administration of favipiravir and measurement of blood concentration.
Supplementary Material
Item S1: Methods for administration of favipiravir and measurement of blood concentration.
References
- 1.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: a new and emerging antiviral option in COVID-19. Med J Armed Forces India. 2020;76(4):370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irie K., Nakagawa A., Fujita H. Pharmacokinetics of favipiravir in critically ill patients with COVID-19. Clin Transl Sci. 2020;13(5):880–885. doi: 10.1111/cts.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen T.H., Guedj J., Anglaret X. Favipiravir pharmacokinetics in Ebola-infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis. 2017;11(2) doi: 10.1371/journal.pntd.0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1: Methods for administration of favipiravir and measurement of blood concentration.