Abstract
Introduction
The novel coronavirus disease of 2019 (COVID-19) is associated with significant morbidity and mortality. The impact of thrombotic complications has been increasingly recognized as an important component of this disease.
Objective
This narrative review summarizes the thrombotic complications associated with COVID-19 with an emphasis on information for Emergency Medicine clinicians.
Discussion
Thrombotic complications from COVID-19 are believed to be due to a hyperinflammatory response caused by the virus. Several complications have been described in the literature. These include acute limb ischemia, abdominal and thoracic aortic thrombosis, mesenteric ischemia, myocardial infarction, venous thromboembolism, acute cerebrovascular accident, and disseminated intravascular coagulation.
Conclusion
It is important for Emergency Medicine clinicians to be aware of the thrombotic complications of COVID-19. Knowledge of these components are essential to rapidly recognize and treat to reduce morbidity and mortality in these patients.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Thrombotic, Thrombosis, Emergency medicine
1. Introduction
In December of 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) began to infect humans in the city of Wuhan in the Hubei province of China [1]. The virus initially infected individuals linked to a seafood market with a possible animal vector. The disease caused by SARS-CoV-2, which has subsequently become known as the novel coronavirus disease of 2019 (COVID-19), rapidly spread to the rest of the world and was declared a pandemic by the World Health Organization in March of 2020 [2,3]. At the time of this writing, there are 27.3 million cases with nearly 900,000 deaths worldwide and 6.4 million cases with nearly 200,000 deaths in the United States alone [2,3].
Multiple reports have circulated demonstrating increased rates of thromboembolic events in patients with COVID-19 [[4], [5], [6], [7], [8], [9], [10], [11]]. As many of these patients will first present to Emergency Departments (ED), being aware of the thrombotic complications associated with COVID-19 is essential for providers working in EDs. This paper will review the pathophysiology and current understanding of thrombotic complications among COVID-19 patients with an emphasis on information for the Emergency Medicine provider.
2. Methods
This narrative review outlines the underlying pathophysiology and thrombotic complications of COVID-19 in the adult patient with an emphasis on the ED clinician. A literature review of PubMed was performed from inception to June 2020 for articles using the keywords COVID-19, SARS-CoV-2, coronavirus, thrombosis, thrombotic event, limb ischemia, aorta, mesenteric ischemia, myocardial infarction, acute coronary syndrome, acute ischemic stroke, cerebrovascular accident, pulmonary embolism, and deep vein thrombosis for production of this narrative review. Authors included case reports and series, retrospective and prospective studies, systematic reviews and meta-analyses, clinical guidelines, and other narrative reviews. Authors reviewed all relevant articles and decided which studies to include for the review by consensus, with a focus on emergency medicine-relevant articles, including guidelines. A total of 95 resources were selected for inclusion in this review.
3. Discussion
3.1. Pathophysiology
SARS-CoV-2 is a single-stranded RNA virus that belongs to the Coronaviridae family, which it shares with severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and the Middle East respiratory syndrome coronavirus (MERS-CoV) [1,12]. SARS-CoV-1, MERS, and SARS-CoV-2 all bind to angiotensin-converting enzyme 2 (ACE-2), which is a crucial counterregulatory enzyme that converts angiotensin I to angiotensin II [12,13]. ACE-2 is present in nearly all human tissues, including but not limited to endothelial cells from small and large arteries and veins, type I and type II alveolar epithelial cells in lungs, and in the nasal and oral mucosa and the nasopharynx [14]. SARS-CoV-2 has a higher binding affinity for human ACE-2 compared to SARS-CoV-1, which likely contributes to its increased rate of virulence and transmission [15,16]. Angiotensin I, when not broken down by ACE-2, promotes an inflammatory state in the body, as well as causing vasoconstriction, sodium retention, and fibrosis throughout the body. [[17], [18], [19]]. Besides inhibiting ACE-2, COVID-19 may also cause downregulation of the enzyme, based on data from SARS-CoV-1 [20]. This culminates in a diffuse inflammatory state as evidenced by higher plasma levels of cytokines such as IL-2, IL-7, IL-10, granulocyte colony-stimulating factor, IgG-induced protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein 1-alpha, and tumor necrosis factor α [21]. Recent studies have evaluated the role of inflammation in creating hypercoagulable states, possibly via activation of endothelial cells, platelets, and leukocytes inducing tissue factor (TF), and subsequently triggering the coagulation system through binding to the clotting factor VIIa [22,23] This milieu creates hypercoagulability as evidenced by decreased reaction (R) and K values, and increased values of K angle and maximum amplitude (MA) when using thromboelastography (TEG) and the apparent increased incidence of thrombotic events [24]. TEG and rotational thromboelastometry (ROTEM) are viscoelastic assays performed on whole blood that assess time to clot formation, clot strength, and time to clot lysis. These assays are advantageous in that they assess platelets, fibrinogen, and coagulation factors in a single assay. The R value is the time to initial clot formation, the K value and angle reflect the speed of clot formation, and the MA is a measure of clot strength on TEG (Fig. 1 ). Interestingly, an autopsy study of the lungs in 10 patients with COVID-19 found microvascular platelet-rich depositions in the small vessels of the lungs reminiscent of thrombotic microangiopathy [25].
Fig. 1.
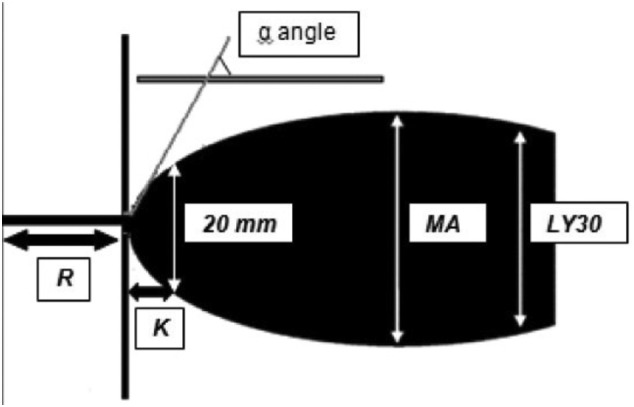
Normal TEG waveform. Obtained from https://en.wikipedia.org/wiki/Thromboelastography#/media/File:Thromboelastography_Waveform.jpg. Open access under Creative Commons 2.0 license.
3.2. Acute limb ischemia
Acute limb ischemia (ALI) is an important consideration in patients with COVID-19 [26]. There have been over a dozen case reports and case series of ALI described in the literature [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. These patients often have multiple thromboses involving different vessels throughout their bodies [27,31,[33], [34], [35], [36], [37], [38],40]. Many of these patients do not have existing peripheral arterial disease [[27], [28], [29], [30], [31],34,36,37,40,41]. Acute limb ischemia can even occur among patients already receiving thromboprophylaxis [34,37,39,41].
Symptoms can include acute limb pain [30,32,40], focal hypothermia [27,30,34,40], skin mottling [26,27,30,35,40], absent pulse [30,34,40], or necrosis of the toes [39]. Patients generally have an elevated D-Dimer [31,33,37,38,40] and may also have an elevated C-reactive protein (CRP) [30]. While a computed tomographic angiogram (CTA) of the extremity is often performed, clinicians should consider adding a CTA of the aorta to evaluate for a concomitant aortic thrombosis [27,37,40]. Treatment involves vascular surgery and interventional radiology consultation, as well as empiric systemic anticoagulation [41,42]. One study of 20 patients found that operative treatment was performed in 17 patients and was able to successfully salvage the limb in 12 (70.6%) [33].
3.3. Abdominal and thoracic aortic thrombosis
Acute abdominal and thoracic aortic thrombosis has also been described in patients with COVID-19 [27,37,40,[43], [44], [45], [46]]. Similar to acute limb ischemia, this has been described in patients who are already receiving thromboprophylaxis [45,47]. This has also been described in a patient with an aortic graft [47].
Symptoms include unilateral distal limb ischemia [27,40], bilateral distal limb ischemia [45,47], bilateral lower extremity weakness [46], bilateral lower extremity loss of sensation [45], and acute periumbilical abdominal pain [43]. Labs are notable for a markedly elevated D-Dimer level, with a greater than 16-fold increase in one study [29,37,43,45,46]. An elevated CRP has also been reported [29]. Treatment involves systemic anticoagulation and consultation with vascular surgery or interventional radiology [42].
3.4. Mesenteric ischemia
Mesenteric ischemia is a less common occurrence with significant morbidity and mortality. This has been described in three case reports of patients with COVID-19 [46,48,49]. Symptoms can include abdominal pain [48,49], vomiting [48], or diarrhea [49]. Labs may demonstrate an elevated D-Dimer [46] or an elevated CRP [48,49]. Imaging should include a CTA of the mesenteric vessels, as a regular CT of the abdomen and pelvis with contrast may not identify this, particularly early in the course of symptoms [48]. Treatment should include systemic anticoagulation and consultation with general surgery, as well as either interventional gastroenterology or interventional radiology [42,48].
3.5. Myocardial infarction
An increased risk of acute coronary syndrome (ACS), including myocardial infarction (MI), is found in viral illnesses, with the greatest risk within the first week of illness due to systemic inflammation resulting in atherosclerotic plaque disruption [[50], [51], [52]]. Literature suggests acute MI may occur in 7–17% of hospitalized patients and over 20% of ICU patients with COVID-19, but the true prevalence is difficult to determine due to underreporting [[51], [52], [53]]. Myocardial injury without direct plaque rupture may also occur due to cytokine storm, hypoxic injury, coronary spasm, and endothelial or vascular injury [54,55]. The presence of preexisting cardiovascular disease increases the morbidity and mortality of acute COVID-19 infection [56]. Patients with ACS in the setting of COVID-19 can present with symptoms other than chest pain, such as respiratory distress or shortness of breath alone [53]. A case series of 18 patients with ST elevation MI on ECG and COVID-19 found 83% were male, with most over the age 60; however, only six patients (33%) had chest pain at the time of ST elevation on ECG [53]. In patients with STEMI, the American College of Cardiology states percutaneous coronary intervention (PCI) is the preferred therapy within 90 min from first medical contact, though fibrinolysis may be considered in patients who are “relatively stable” [57,58]. Patients with equivocal symptoms, atypical electrocardiogram, or delayed presentation and possible but unconfirmed STEMI should undergo further evaluation including echocardiogram and serial ECGs. If the patient has non-STEMI with suspected COVID-19, further testing is recommended prior to catheterization, and in properly selected patients conservative therapy can be sufficient. Patients who are hemodynamically unstable with NSTEMI should be treated similarly as those with STEMI [57,58]. Other medications used in the setting of ACS (e.g., aspirin) are unchanged in COVID-19.
3.6. Venous thromboembolism
As the clinical picture of COVID-19 infection continues to emerge, venous thromboembolism (VTE) is a serious risk, particularly in severe disease. Research has already established that hospitalized patients are prone to deep venous thrombosis (DVT) development. Multiple studies have demonstrated increased rates of DVT in COVID-19 patients. A systematic review and meta-analysis of 20 studies comprising 1988 patients with COVID-19 found a weighted mean prevalence (WMP) of 31.3% for VTE with a WMP of 19.8% for DVT and WMP of 18.9% for pulmonary embolism (PE) [59].
In hospitalized patients not receiving prophylaxis, rates are approximately 0.9% for general admission and 15% to 32% among ICU patients [60,61]. A German study performed consecutive autopsies of 12 deceased patients with COVID-19, finding bilateral DVTs in 7 (58%) cases, none of which were suspected before death [62]. Another study prospectively analyzed venous ultrasound exams on 34 consecutive patients admitted to the ICU with COVID-19 finding DVTs in 22 patients (65%), with 18 patients having bilateral DVTs [63]. On systematic evaluation 48 h after admission, the authors also found that an additional five developed DVTs despite adequate prophylactic anticoagulation [63]. Another retrospective review of 26 consecutive ICU patients in France found DVTs in 18 patients (69%) receiving anticoagulation [64].
Patients hospitalized on the general medical floors also demonstrate an increased risk of DVT. A retrospective review of 71 non-ICU patients in France who received systematic lower extremity doppler exams prior to discharge found 16 patients (22%) developed DVT despite thromboprophylaxis with weight-based enoxaparin [65].
Similar to DVT, studies have also demonstrated a high rate of PE occurrence in patients with COVID-19. Post-mortem examination of 21 consecutive patients in Switzerland found PEs in four (19%) of the patients [66]. A similar autopsy study in Germany found PE was present in 42% of deceased patients, with PE being the cause of death in one-third of patients [62]. Multiple studies have also demonstrated a high prevalence of PE in ICU patients hospitalized with COVID-19. A study of 184 consecutive ICU patients with COVID-19 demonstrated confirmed VTE in 27% of patients by CTPA or compression duplex ultrasonography [67]. The majority (81%) of VTE were PE despite standard pharmacological thromboprophylaxis [67]. Another review of 150 ICU patients found the most significant thromboembolic complication among patients was PE (16.7%) [8]. This same study compared a subgroup of patients with COVID-19 acute respiratory distress syndrome (ARDS) to non-COVID-19 ARDS and found PE rates were significantly higher in the COVID-19 ARDS group, 12% and 2%, respectively [8]. A case series of 107 consecutive patients admitted to the ICU in France demonstrated a PE rate of 20.6% [9]. The authors retrospectively reviewed ICU patients during the same period from the previous year and found a PE rate of 6.1% suggesting patients with severe COVID-19 infections are at higher risk than other non-COVID-19 critically ill patients [9].
Outside of the ICU, studies have demonstrated PE rates of 10% to 22% [11,65]. A retrospective chart review of 327 general floor patients noted 44 patients were tested for VTE with an overall positive rate of 6.4% [4]. A retrospective review of 71 non-ICU COVID-19 patients in France revealed a PE rate of 10% despite receiving adequate thromboprophylaxis. The authors noted a d-dimer threshold of 10,000 μg/L was only moderately predictive of VTE (negative predictive value 90%, positive predictive value 44%) [65]. Another retrospective chart review found d-dimer levels of greater than 2660 μg/L had a 100% sensitivity and 67% specificity for PE [10]. A retrospective chart review of 100 patients hospitalized for COVID-19 who received CTPA found a PE rate of 23%, with a higher prevalence in ICU patients (74% vs 29%) [11]. It is unclear if these patients were receiving anticoagulation. Pre-existing cardiovascular disease was associated with higher incidence of PE [11]. More studies are needed to determine the utility of d-dimer levels for risk stratification of VTE in COVID-19 patients. There is limited data regarding PE prevalence among COVID-19 patients treated in the outpatient setting. However, one recent study in the ED found that among patients receiving a CTPA to evaluate for PE, the positivity rate was similar between COVID-19 patients and those without COVID-19 [68].
When testing for PE in COVID-19 patients, CTPA is the test of choice. If CTPA is contraindicated (e.g., renal failure, severe contrast dye reaction), only the perfusion scintigraphy of the ventilation-perfusion scan should be performed to minimize aerosolization of secretions [69,70].
The American Society of Hematology and the American College of Chest Physicians recommend routine pharmacologic prophylaxis for VTE in patients hospitalized with COVID-19 unless there are pre-existing contraindications [71,72]. Low molecular weight heparin is preferred over unfractionated heparin to reduce healthcare worker exposure to infected patients [71]. If heparin-induced thrombocytopenia develops, fondaparinux should be used. Although some authors have advocated for intermediate or therapeutic dosing, both societies endorse standard prophylaxis dosing until more data is available [71,72]. A review of 150 ICU COVID-19 patients demonstrated a low rate (2.7%) of bleeding complications among patients receiving prophylactic or treatment-based pharmacologic antithrombotic therapy [8]. Although limited, this suggests anticoagulation is relatively safe in COVID-19 patients who do not meet exclusion criteria. A retrospective study of 449 patients with severe COVID-19 infection found an improved 28-day mortality in patients receiving enoxaparin (40–60 mg daily) than those not receiving enoxaparin [73]. Some authors also advocate for the anti-inflammatory role of heparin in severe COVID-19 infection [74]. Heparin is known to decrease inflammation by inhibiting neutrophil activity, expression of inflammatory mediators, and the proliferation of vascular smooth muscle cells [75]. Admitted patients boarding in the emergency department should at minimum receive pharmacologic antithrombotic therapy with a low threshold for additional VTE testing if new symptoms develop.
3.7. Acute cerebrovascular accident
Acute cerebrovascular disease, including ischemic stroke, is a severe neurologic complication of COVID-19 and can be a presenting symptom of this disease [[76], [77], [78], [79]]. Studies suggest a rate of ischemic stroke approaching up to 5% in patients with COVID-19, likely associated with the inflammatory and hypercoagulable state [[77], [78], [79], [80]]. This mirrors other respiratory infections in which the risk of stroke increases by 3.2–7.8 fold within the first several days of infection [81,82]. The risk of mortality in patients with COVID-19 with acute ischemic stroke approaches 38% [78]. However, despite this increased risk of stroke, during the current COVID-19 pandemic, a decrease in the number of acute stroke investigations has been observed, likely due to patient fear of exposure in medical centers [76,[83], [84], [85], [86]].
Patients with COVID-19 who develop an ischemic stroke are usually older, typically over 70 years of age, with significant comorbidities such as liver and renal disease [76,78]. Other factors associated with increased risk of stroke in the setting of COVID-19 include hypertension, diabetes, cancer, lung disease, and prior cerebrovascular disease [76,77,79,80]. However, there are cases of ischemic stroke, even large vessel occlusion, affecting young patients with COVID-19 [87]. Literature suggests the median duration from onset of COVID-19 symptoms to stroke is approximately 10 days [76,78]. Significant coagulation abnormalities in patients with COVID-19 include increased d-dimer, prolonged prothrombin time, and abnormal platelet levels [78,88]. D-dimer levels in patients with stroke and COVID-19 are significantly higher compared to other patients with COVID-19 infection, with one study demonstrating a 12-fold increase in those experiencing stroke [78].
In patients with suspected stroke, protection of healthcare providers is essential even if patients are asymptomatic, which may occur in 18% to 31% of patients ultimately diagnosed with COVID-19 [89,90]. The American Heart Association recommends using appropriate screening guidelines, personal protective equipment, and crisis resource management for evaluating and managing patients with suspected cerebrovascular accidents [86,91]. All patients with suspected stroke should be evaluated as suspected COVID-19 in the prehospital and ED settings [91]. Personal protection equipment including contact and droplet precautions are recommended when caring for non-ventilated patients and those not undergoing aerosol-generating procedures, known as a “protected code stroke” [86]. A face mask or surgical mask should also be placed on the patient. Evaluation includes noncontrast head computed tomography (CT) within 20 min of arrival in the ED. For those with suspected large vessel occlusion, further imaging including CT angiography, CT perfusion, or magnetic resonance imaging (MRI) is recommended. If further imaging will be needed during hospitalization or may impact management (i.e., CT perfusion), then this should be obtained at the time of head CT if able. Similarly, providers should consider using a single test modality (e.g., noncontrast head CT followed by CT angiography of the head and neck), rather than separate imaging modalities (e.g., CT of the head, followed by MRI and carotid ultrasound imaging) to reduce machine and technician utilization [91].
Management of ischemic stroke is unchanged whether COVID-19 is present, with IV alteplase recommended for eligible patients presenting within 3 h (and for highly selected patients within 4.5 h of therapy) [86,91]. As discussed, patients with COVID-19 often demonstrate coagulation system abnormalities and also have risk of hepatic and renal dysfunction. However, the risk of hemorrhage in patients with COVID-19 receiving thrombolysis is unclear. Patients with COVID-19 are eligible for mechanical thrombectomy in the setting of internal carotid artery or middle cerebral artery occlusion if they can be treated within 6 h of symptoms, do not demonstrate extensive ischemic tissue on imaging, and have a NIHSS score ≥ 6 [86,91].
3.8. Disseminated intravascular coagulation
Disseminated intravascular coagulation (DIC) is suggested when there is dysregulation of the coagulation pathways causing both systemic coagulation and hemorrhage associated with thrombocytopenia, elevated fibrin-degradation products (FDP), prolonged PT, and an elevated fibrinogen level [[92], [93], [94]]. DIC commonly presents as thrombosis and hemorrhage in different locations [[92], [93], [94]]. DIC has multiple inciting causes and possesses a mortality rate ranging from 46 to 76% [93]. DIC in COVID-19 patients is also correlated with mortality. Tang et al. published a study of 183 COVID-19 patients admitted to a hospital in Wuhan, China, in whom the presence of DIC was calculated using the International Society on Thrombosis and Haemostasis (ISTH) diagnostic criteria for DIC [92] and compared with mortality [88]. In this population, 71.4% of non-survivors and 0.6% survivors met the criteria of DIC during their hospital stay [88]. A study from Italy that included 388 COVID-19 patients reported DIC according to the ISTH diagnostic criteria in 2.1% of their patients, of whom 88% (7/8) died [4]. Another study of 225 COVID-19 patients found that DIC was present in 6.4% of non-survivors and 0% of survivors [95].
4. Conclusions
COVID-19 is associated with a significant inflammatory response, increasing the risk of arterial and venous thrombosis. These complications may increase the risk of morbidity and mortality and include acute limb ischemia, abdominal and thoracic aortic thrombosis, mesenteric ischemia, myocardial infarction and acute coronary syndrome, venous thromboembolism, acute cerebrovascular accident, and disseminated intravascular coagulation. Knowledge of these conditions in COVID-19 may improve Emergency Medicine clinician recognition and management of these thrombotic complications.
Meetings
None.
Grants
None.
Author contributions
None except listed.
Declaration of Competing Interest
None.
Acknowledgements
This review does not reflect the views or opinions of the U.S. government, Department of Defense, U.S. Army, U.S. Air Force, Brooke Army Medical Center, or SAUSHEC EM Residency Program.
References
- 1.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Coronavirus disease (COVID-19) situation report– 209. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ Available at: Accessed: September 7, 2020.
- 3.COVID-19 Coronavirus Pandemic World o meter. https://www.worldometers.info/coronavirus/?utm_campaign=homeAdUOA?Si Available at. Accessed September 7, 2020.
- 4.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavazzi G., Civardi L., Caneva L., Mongodi S., Mojoli F. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;46(6):1121–1123. doi: 10.1007/s00134-020-06040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poissy J., Goutay J., Caplan M. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 10.Leonard-Lorant I., Delabranche X., Severac F. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020 Apr;23:201561. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020:201561. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoso A., Pranata R., Wibowo A., et al. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2020;S0735–6757(20) doi: 10.1016/j.ajem.2020.04.052. 30280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehr A.R., Channappanavar R., Perlman S. Middle east respiratory syndrome: emergence of a pathogenic human coronavirus. Annu Rev Med. 2017;68:387–399. doi: 10.1146/annurev-med-051215-031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamming I., Timens W., Bulthuis M.L., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai W., He L., Zhang X., et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W., Moore M.J., Vasllieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge X.Y., Li J.L., Yang X.L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H., Penninger J.M., Li Y., et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108(5):1447–1452. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branchford B.R., Carpenter S.L. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6:142. doi: 10.3389/fped.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panigada M., Bottino N., Tagliabue P., et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox S.E., Akmatbekov A., Harbert J.L., et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottlieb M., Long B. Dermatologic manifestations and complications of COVID-19. Am J Emerg Med. 2020 Jun 6 doi: 10.1016/j.ajem.2020.06.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrea V., Gianluca F., Rodolfo P., Paolo T., Alessandro P., Mauro G. Unheralded lower limb threatening ischemia in a COVID-19 patient [published online ahead of print, 2020 May 21] Int J Infect Dis. 2020;S1201–9712(20) doi: 10.1016/j.ijid.2020.05.0607. 30367-2. [DOI] [Google Scholar]
- 28.Rey J.R., Caro-Codón J., Poveda Pineda D., et al. Arterial thrombotic complications in hospitalized patients with COVID-19 [published online ahead of print, 2020 May 23] Rev Esp Cardiol (Engl Ed) 2020;S1885–5857(20) doi: 10.1016/j.rec.2020.05.008. 30205-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyes Valdivia A., Gómez Olmos C., Ocaña Guaita J., Gandarias Zúñiga C. Cardiovascular examination should also include peripheral arterial evaluation for COVID-19 patients [published online ahead of print, 2020 Apr 30] J Vasc Surg. 2020;S0741–5214(20) doi: 10.1016/j.jvs.2020.04.494. 31100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur P., Posimreddy S., Singh B., et al. COVID-19 presenting as acute limb ischaemia. Eur J Case Rep Intern Med. 2020;7(6):001724. doi: 10.12890/2020_001724. 10.12890/2020_001724 Published 2020 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Xiao M., Zhang S., et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur P., Qaqa F., Ramahi A., et al. Acute upper limb ischemia in a patient with COVID-19 [published online ahead of print, 2020 May 13] Hematol Oncol Stem Cell Ther. 2020;S1658–3876(20) doi: 10.1016/j.hemonc.2020.05.001. 30096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellosta R., Luzzani L., Natalini G., et al. Acute limb ischemia in patients with COVID-19 pneumonia [published online ahead of print, 2020 Apr 29] J Vasc Surg. 2020;S0741–5214(20) doi: 10.1016/j.jvs.2020.04.483. 31080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin D.O., Jensen A., Khan M., et al. Arterial thromboembolic complications in COVID-19 in low-risk patients despite prophylaxis [published online ahead of print, 2020 May 6] Br J Haematol. 2020 doi: 10.1111/bjh.16792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou B., She J., Wang Y., Ma X. Venous thrombosis and arteriosclerosis obliterans of lower extremities in a very severe patient with 2019 novel coronavirus disease: a case report. J Thromb Thrombolysis. 2020;50(1):229–232. doi: 10.1007/s11239-020-02084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mestres G., Puigmacià R., Blanco C., Yugueros X., Esturrica M., Riambau V. Risk of peripheral arterial thrombosis in COVID-19 [published online ahead of print, 2020 May 7] J Vasc Surg. 2020;S0741–5214(20) doi: 10.1016/j.jvs.2020.04.477. 31074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kashi M., Jacquin A., Dakhil B., et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Betoule A., Martinet C., Gasperini G., et al. Diagnosis of venous and arterial thromboembolic events in COVID-19 virus-infected patients [published online ahead of print, 2020 Jun 5] J Thromb Thrombolysis. 2020:1–3. doi: 10.1007/s11239-020-02163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantador E., Núñez A., Sobrino P., et al. Incidence and consequences of systemic arterial thrombotic events in COVID-19 patients [published online ahead of print, 2020 Jun 9] J Thromb Thrombolysis. 2020:1–5. doi: 10.1007/s11239-020-02176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh G., Attique H.B., Gadela N.V., Mapara K., Manickaratnam S. COVID-19 related arterial coagulopathy. Cureus. 2020;12(7) doi: 10.7759/cureus.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ilonzo N., Rao A., Safir S., et al. Acute thrombotic manifestations of COVID-19 infection: experience at a large New York City Health System [published online ahead of print, 2020 Aug 31] J Vasc Surg. 2020;S0741–5214(20) doi: 10.1016/j.jvs.2020.08.038. 31922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson R.A., Johnson D.M., Dharia R.N., Merli G.J., Doherty J.U. Anti-coagulant and anti-platelet therapy in the COVID-19 patient: a best practices quality initiative across a large health system [published online ahead of print, 2020 Jun 9] Hosp Pract. 1995;2020:1–11. doi: 10.1080/21548331.2020.1772639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahan K., Kabrhel C., Goldsmith A.J. Abdominal pain in a patient with COVID-19 infection: a case of multiple thromboemboli [published online ahead of print, 2020 May 26] Am J Emerg Med. 2020;S0735–6757(20) doi: 10.1016/j.ajem.2020.05.054. 30391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Berre A., Marteau V., Emmerich J., Zins M. Concomitant acute aortic thrombosis and pulmonary embolism complicating COVID-19 pneumonia. Diagn Interv Imag. 2020;101(5):321–322. doi: 10.1016/j.diii.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Arbelaez D., Ibarra-Sanchez G., Garcia-Gutierrez A., Comanges-Yeboles A., Ansuategui-Vicente M., Gonzalez-Fajardo J.A. Covid-19-related aortic thrombosis: a report of four cases [published online ahead of print, 2020 May] Ann Vasc Surg. 2020;S0890–5096(20) doi: 10.1016/j.avsg.2020.05.031. 30438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vulliamy P., Jacob S., Davenport R.A. Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. Br J Haematol. 2020;189(6):1053–1054. doi: 10.1111/bjh.16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giacomelli E., Dorigo W., Fargion A., Calugi G., Cianchi G., Pratesi C. Acute thrombosis of an aortic prosthetic graft in a patient with severe COVID-19-related pneumonia [published online ahead of print, 2020 Apr 29] Ann Vasc Surg. 2020;S0890–5096(20) doi: 10.1016/j.avsg.2020.04.040. 30366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beccara L.A., Pacioni C., Ponton S., Francavilla S., Cuzzoli A. Arterial mesenteric thrombosis as a complication of SARS-CoV-2 infection. Eur J Case Rep Intern Med. 2020;7(5):001690. doi: 10.12890/2020_001690. 10.12890/2020_001690 Published 2020 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Barry O., Mekki A., Diffre C., Seror M., Hajjam M.E., Carlier R.Y. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia [published online ahead of print, 2020 Apr 29] Radiol Case Rep. 2020;15(7):1054–1057. doi: 10.1016/j.radcr.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwong J.C., Schwartz K.L., Campitelli M.A., et al. Acute myocardial infarction after laboratory- confirmed influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 51.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020 May;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guzik T.J., Mohiddin S.A., Dimarco A., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020 Aug 1;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Welt F.G.P., Shah P.B., Aronow H.D., et al. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from the ACC’s interventional council and SCAI. J Am Coll Cardiol. 2020;75(18):2372–2375. doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahmud E., Dauerman H.L., Welt F.G., et al. Management of acute myocardial infarction during the COVID-19 pandemic [published online ahead of print, 2020 Apr 21] J Am Coll Cardiol. 2020;S0735–1097(20) 35026-9. [Google Scholar]
- 59.Di Minno A., Ambrosino P., Calcaterra I., Di Minno M.N.D. COVID-19 and venous thromboembolism: a meta-analysis of literature studies [published online ahead of print, 2020 Sep 3] Semin Thromb Hemost. 2020 doi: 10.1055/s-0040-1715456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bo H., Li Y., Liu G., Ma Y., Li Z., Cao J., et al. Assessing the risk for development of deep vein thrombosis among Chinese patients using the 2010 Caprini risk assessment model: a prospective multicenter study. J Atheroscler Thromb. 2019 Dec 17 doi: 10.5551/jat.51359. .[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cook D.J., Crowther M.A., Meade M.O., Douketis J. VTE in the ICU workshop participants. Prevalence, incidence, and risk factors for venous thromboembolism in medical-surgical intensive care unit patients. J Crit Care. 2005;20:309–313. doi: 10.1016/j.jcrc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Wichmann D., Sperhake J.P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19 [published online ahead of print, 2020 May 6] Ann Intern Med. 2020:M20–2003. doi: 10.7326/M20-2003. [DOI] [PubMed] [Google Scholar]
- 63.Nahum J., Morichau-Beauchant T., Daviaud F., et al. venous thrombosis among critically Ill patients with Coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3(5):e2010478. doi: 10.1001/jamanetworkopen.2020.10478. Published 2020 May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Llitjos J.F., Leclerc M., Chochois C., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients [published online ahead of print, 2020 Apr 22] J Thromb Haemost. 2020 doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Artifoni M., Danic G., Gautier G., et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50(1):211–216. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menter T., Haslbauer J.D., Nienhold R., et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction [published online ahead of print, 2020 May 4] Histopathology. 2020 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freund Y., Drogrey M., Miró Ò., et al. Association between pulmonary embolism and COVID-19 in emergency department patients undergoing computed tomography pulmonary angiogram: the PEPCOV international retrospective study [published online ahead of print, 2020 Jul 30] Acad Emerg Med. 2020 doi: 10.1111/acem.14096. [DOI] [PubMed] [Google Scholar]
- 69.Williams D.A., Carlson C., McEnerney K., Hope E., Hoh C.K. Technetium-99m DTPA aerosol contamination in lung ventilation studies. J Nucl Med Technol. 1998;26(1):43–44. [PubMed] [Google Scholar]
- 70.Rotzinger D.C., Beigelman-Aubry C., von Garnier C., Qanadli S.D. Pulmonary embolism in patients with COVID-19: time to change the paradigm of computed tomography. Thromb Res. 2020;190:58–59. doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moores L.K., Tritschler T., Brosnahan S., et al. Prevention, diagnosis, and treatment of VTE in patients with Coronavirus disease 2019: CHEST guideline and expert panel report [published online ahead of print, 2020 Jun 2] Chest. 2020;S0012–3692(20) doi: 10.1016/j.chest.2020.05.559. 31625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.American Society of Hematology COVID-19 and VTE/anticoagulation: frequently asked questions. https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation Available at. Accessed September 7, 2020.
- 73.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18:1020–1022. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poterucha T.J., Libby P., Goldhaber S.Z. More than an anticoagulant: do heparins have direct anti-inflammatory effects? Thromb Haemost. 2017;117(3):437–444. doi: 10.1160/TH16-08-0620. [DOI] [PubMed] [Google Scholar]
- 76.Qureshi A.I., Abd-Allah F., Alsenani F., et al. Management of acute ischemic stroke in patients with COVID-19 infection: report of an international panel [published online ahead of print, 2020 May 3] Int J Stroke. 2020 doi: 10.1177/1747493020923234. [DOI] [PubMed] [Google Scholar]
- 77.Bridwell R., Long B., Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. 2020;38(7):1549. doi: 10.1016/j.ajem.2020.05.024. E3-1549.E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y., Li M., Wang M., et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study [published online ahead of print, 2020 Jul 2] Stroke Vasc Neurol. 2020 doi: 10.1136/svn-2020-000431. svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China [published online ahead of print, 2020 Apr 10] JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smeeth L., Thomas S.L., Hall A.J., et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 82.Warren-Gash C., Blackburn R., Whitaker H., et al. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51(3):1701794. doi: 10.1183/13993003.01794-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pasarikovski C.R., da Costa L. The impact of the Covid-19 pandemic on stroke volume. Canad J Neurol Sci/J Canadien des Sci Neurologiques. 2020:1–6. doi: 10.1017/cjn.2020.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kansagra A.P., Goyal M.S., Hamilton S., Albers G.W. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400–401. doi: 10.1056/NEJMc2014816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morelli N., Rota E., Terracciano C., et al. The baffling case of ischemic stroke disappearance from the casualty Department in the COVID-19 era. Eur Neurol. 2020;83(2):213–215. doi: 10.1159/000507666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khosravani H., Rajendram P., Notario L., Chapman M.G., Menon B.K. Protected code stroke: Hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke. 2020;51(6):1891–1895. doi: 10.1161/STROKEAHA.120.029838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mizumoto K., Kagaya K., Zarebski A., et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:1–5. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishiura H., Kobayashi T., Suzuki A., et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dafer R.M., Osteraas N.D., Biller J. Acute stroke Care in the Coronavirus disease 2019 pandemic [published online ahead of print, 2020 Apr 17] J Stroke Cerebrovasc Dis. 2020;29(7):104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor F.B., Jr., Toh C.H., Hoots W.K., Wada H., Levi M. Scientific subcommittee on disseminated intravascular coagulation (DIC) of the international society on thrombosis and haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 93.Kalpatthi R., Kiss J.E. Thrombotic thrombocytopenic purpura, heparin-induced thrombocytopenia, and disseminated intravascular coagulation. Crit Care Clin. 2020;36(2):357–377. doi: 10.1016/j.ccc.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 94.Thachil J. Disseminated intravascular coagulation: a practical approach. Anesthesiology. 2016;125(1):230–236. doi: 10.1097/ALN.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 95.Deng Y., Liu W., Liu K., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J. 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]


