In the August 5, 2020 issue of the Journal, Langer-Gould et al. reported an interesting analysis (Langer-Gould et al., 2020) in which different anti-inflammatory strategies were compared in patients with coronavirus disease 2019 (COVID-19) at different stages of severity, including pre-intensive care unit (ICU) and ICU patients. Based on their analysis, the authors suggest that the timing of initiation of anti-cytokine therapy, with or without corticosteroid therapy, might be much more important than the type of medication used (anakinra or tocilizumab). Specifically, they convincingly demonstrated that aggressive anti-inflammatory treatment should be initiated at the beginning of what the authors call the COVID cytokine storm (COVID-CS) and also suggested simple clinical and laboratory criteria to guide the decision.
These findings are physiologically reasonable, considering the complexity of the immune response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) aggression, as we have summarized previously (de Simone and Mancusi, 2020).
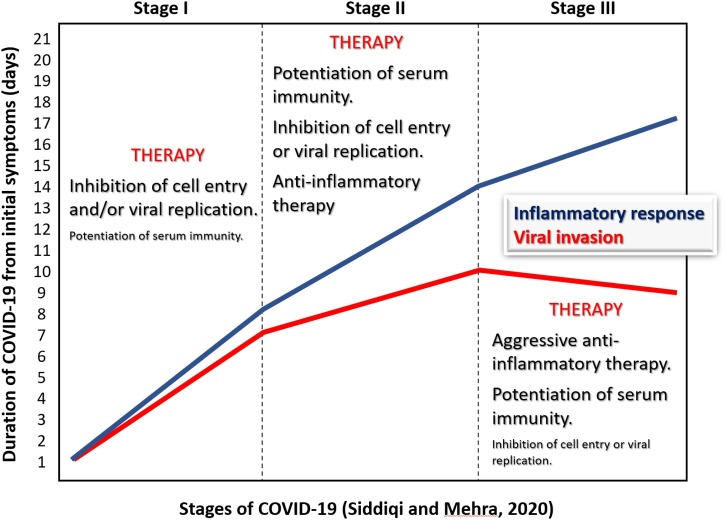
Our emphasis on timing was elicited by important considerations from Siddiqi and Mehra (Siddiqi and Mehra, 2020), who proposed a three-level sequence of SARS-CoV-2 infection stages, based on the evidence that during the clinical course, COVID-19 progresses from a viral infection to a self-maintaining super-inflammatory response. This clinical classification has largely been sustained by a number of observational studies and might be the basis of disappointing findings in some randomized clinical trials (IDSE, 2020, Cavalcanti et al., 2020), as well as near-unexpected results obtained with corticosteroid therapy (The RECOVERY Collaborative Group, 2020).
Therapy for COVID-19 is based on three cornerstones: antiviral therapy, immune potentiation, and aggressive anti-inflammation therapy. Each one should be used according to the clinical conditions and predictive stage of the disease (Figure 1 ), because each one, if provided at the wrong time, might potentially be deleterious. Aggressive anti-inflammatory therapy might be deleterious if administered too early, due to the potential depression of a calibrated immune response (Cicchese et al., 2018), and would certainly be less useful once tissue damage is consolidated and irreversible.
Figure 1.
The three stages of COVID-19, as proposed by Siddiqi and Mehra (Siddiqi and Mehra, 2020), with suggested pathophysiologically appropriate stage-related treatment. The larger the size of the characters within the figure, the stronger the recommendation. The duration of the stages is indicative and can vary substantially. The criteria suggested by Langer-Gould et al. (2020) might substantially help to determine the shift from stage I to stage II.
Conflict of interest
None.
References
- The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDSE Disappointing results from first phase 3 trial of tocilizumab for COVID-19. IDSE. 2020 https://www.idse.net/Covid-19/Article/07-20/Disappointing-Results-From-First-Phase-3-Trial-of-Tocilizumab-for-COVID-19/59141 Online ahead of print. [Google Scholar]
- Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2019014. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchese J.M., Evans S., Hult C., Joslyn L.R., Wessler T., Millar J.A. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol Rev. 2018;285(1):147–167. doi: 10.1111/imr.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Simone G., Mancusi C. COVID-19: timing is important. Eur J Intern Med. 2020;77:134–135. doi: 10.1016/j.ejim.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Gould A., Smith J.B., Gonzales E.G., Castillo R.D., Figueroa J.G., Ramanathan A. Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.07.081. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]