Abstract
Background
Dengue is a major cause of acute febrile illness in Sri Lanka. Dengue has historically been considered an urban disease. In 2012–2013, we documented that acute dengue was surprisingly associated with self-reported rural residence in the Southern Province of Sri Lanka.
Methods
Patients admitted with an acute febrile illness were enrolled from June 2012–May 2013 in a cross-sectional surveillance study at the largest tertiary care hospital in the Southern Province. Acute dengue was diagnosed by serology and virology testing. Site visits were performed to collect residential geographical coordinates. Spatial variation in odds of acute dengue was modeled using a spatial generalized additive model predicted onto a grid of coordinate pairs covering the Southern Province.
Results
Of 800 patients, 333 (41.6%) had laboratory-confirmed acute dengue. Dengue was spatially heterogeneous (local probability of acute dengue 0.26 to 0.42). There were higher than average odds of acute dengue in the rural northeast of the Southern Province and lower than average odds in the urbanized southwest of the Southern Province, including the city Galle.
Conclusions
Our study further affirms the emergence of dengue in rural southern Sri Lanka and highlights both the need for real-time geospatial analyses to optimize public health activities as well as the importance of strengthening dengue surveillance in non-urban areas.
Keywords: dengue, fever, probability, Sri Lanka, urbanization
Introduction
With approximately 390 million dengue infections estimated annually, dengue virus (DENV) is an important cause of undifferentiated fever causing morbidity and mortality in over 100 countries worldwide.1 DENV is a flavivirus that is transmitted by the mosquito vectors Aedes aegypti and Aedes albopictus and has four distinct serotypes (DENV-1-4).2 An individual infected with one serotype of dengue acquires long-term immunity towards that serotype but can be reinfected with another serotype.3 Symptomatic dengue infection can progress to severe disease and ultimately result in mortality.4
Dengue has traditionally been considered an urban disease, with a higher risk of dengue infection in highly connected urban areas and rapidly urbanized areas with inadequate water, waste and sewage systems.1,2 The primary vector for dengue, A. aegypti, is anthropophilic and prefers living among humans in indoor spaces. In addition, increasing numbers of mosquito larval habitats, such as biodegradable plastics and used tires, have contributed to the rise in dengue in urban areas.2 Surveillance and control measures have thus generally focused on urbanized areas; however, recent studies indicate that dengue is becoming increasingly common in rural areas.5,6 Understanding the changing epidemiology of dengue is important in targeting control efforts.
In Sri Lanka, where dengue was first confirmed in 1962, most cases have traditionally been reported from the western, urbanized region. Periodic epidemics with increasing magnitude and severity have been reported since 1989, and all four strains have been found to co-circulate. To document the epidemiology of dengue in the more rural Southern Province of Sri Lanka, we performed surveillance of patients admitted with fever in 2007. We confirmed acute DENV infection in only 6.3% of febrile patients; 54% of patients had serological evidence of acute or past dengue infection and seropositivity was more likely in urban compared with rural inhabitants.7 Following an island-wide increase in dengue cases from 2009 onwards that was associated with the introduction of a new strain of DENV-1, we performed repeat surveillance for dengue in 2012–2013. Interestingly, dengue accounted for 40% of febrile cases and self-reported rural residence was associated with acute dengue.8 Given this surprising temporal evolution in dengue serotype and change in epidemiology, we investigated the association between residential location and dengue infection as well as the association between residential location and duration of fever using geographical information systems analysis and spatial statistical modeling. Our overall aim was to identify locations where dengue transmission comprised a high proportion of fever patients, as well as to identify any associated environmental variables.
Materials and methods
Study design and participants
A cross-sectional surveillance study was conducted among patients presenting with acute febrile illness from June 2012 through May 2013 in adult and pediatric wards at Teaching Hospital Karapitiya, the largest tertiary care hospital (1500 beds) in the Southern Province of Sri Lanka (Figure 1A and B).8 Consecutive patients aged ≥1 y with fever documented at hospital presentation or within 48 h of hospital admission were eligible for enrolment in the study (n=976). Fever was defined as a temperature of ≥100.4°F when measured by study physicians at enrolment or ≥102°F as documented in the medical record (within 48 h of admission). Acute dengue was either confirmed or excluded in 937 of these 976 patients (96.0%). Of these 937 patients, 800 patients had a reported address within 50 km of Teaching Hospital Karapitiya and were included in the geospatial analysis.
Figure 1.
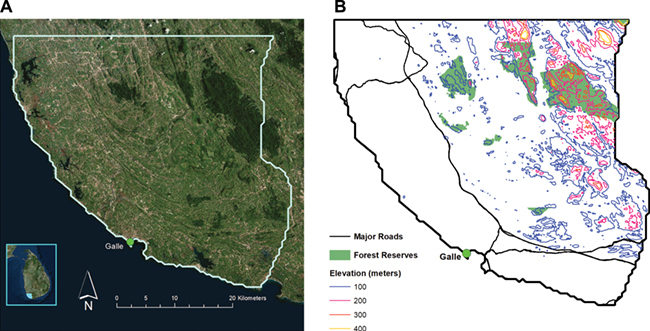
Study area in the Southern Province of Sri Lanka. (A) The referral hospital where patients were evaluated was within the city of Galle (ARCGIS 10.3.1, ESRI, Redlands, CA, USA). (B) Forest reserves, including the Sinharaja forest reserve, are located in the northeastern region of the Southern Province (ARCGIS 10.3.1, ESRI, Redlands, CA, USA).
Study physicians recorded clinical and epidemiological data for patients and phlebotomists collected an acute blood sample from each patient at the time of enrolment in the study. A convalescent blood sample was collected 2–4 wk after enrolment either when a patient returned to the hospital, or the patient was visited at home if his/her address was known. Serum samples were stored promptly at −80°C and shipped to Duke-NUS Graduate Medical School in Singapore on dry ice for further laboratory testing.8 Study team members traveled to patients’ homes to record their longitude and latitude coordinates using global positioning system devices. For those patients whose convalescent blood sample was collected when they returned to the hospital and not at their home, home visits took place in the months following the conclusion of the hospital-based fever study to complete coordinate collection.
Laboratory definitions
Acute dengue infection was defined as (1) IgG seroconversion alone (serologic evidence), (2) PCR and/or isolation positive with positive convalescent IgG (virologic evidence of acute dengue infection with supportive serologic evidence), (3) virus isolation and dengue-specific PCR positive and/or (4) PCR positive with two targets (dengue-specific and pan-flavivirus).8 Presence of IgG in the acute serum sample among patients with acute dengue infection was considered evidence of secondary infection and absence of primary infection. Patients with positive IgG in both acute and convalescent serum samples without evidence of acute infection were classified as having past dengue infection.
Environmental and demographic data
In addition to patient-specific data, we also acquired environmental and demographic data, including classified land use, a digital elevation model and population density (summarized at the level of Grama Niladhari, the smallest government administrative unit in Sri Lanka).9 The land use data source consisted of numerous classifications of both land use and land cover, including agricultural types (e.g. rubber, tea, paddy) and natural features (e.g. stream, marshy), and ‘built up’ in urban environments.
Statistical and spatial analysis
Our unit of statistical analysis was the individual patient. To add environmental variables to each patient's record we sampled them from within a 1 km diameter circle around each patient. This circle was computed for each patient using the Buffer tool in ArcGIS. Vector data (land use and population density) were rasterized to 100 x 100 m cells using the Vector to Raster conversion tool in ArcGIS. We then used zonal statistics, using the Zonal Statistics tool in ArcGIS, to compute the median elevation, median population density and predominant land cover class within the 1 km diameter buffer zone around each patient. We designated the following reference categories for categorical variables: female for gender and ‘garden’ for land use (the most common land use type). Continuous variables were centered on zero by subtracting their mean and standardized by dividing by two standard deviations.10 These procedures improve model stability and inference by transforming all continuous variables to the same numerical scale.
A common geostatistical approach to evaluate data collected at coordinate points are non-parametric smoothing functions to evaluate local variability of a variable in two-dimensional coordinate space. These techniques include generalized additive models (GAM), which can use either local regression estimators or splines, and the closely-related kriging, which uses Gaussian process regression. We used spline-based generalized additive models using the statistical programming language R (www.r-project.org). We chose to use Bayesian estimation methods for their ease in presenting both effect size and uncertainty in the model output. To this end we used the R package brms.11 The brms package facilitates the construction of Bayesian regression models, including generalized additive models, which are then transferred to the program Stan (mc-stan.org) for sampling of the posterior distribution.12
We first evaluated the spatial odds of dengue, using a binomial model in which the outcome variable was a dichotomous dengue test result (either positive or negative) and the primary independent predictor variable was a two-dimensional smooth spline of each patient's longitude-latitude coordinate pair.13,14 To incorporate time into our model we included an additional smooth term for the date (as expressed by integer day counting from the first day of subject recruitment). Using this method we were able to model a locally varying relationship between odds of dengue infection, geographical location and time. For patients with confirmed dengue we constructed a second set of models to evaluate spatial variability in the odds of secondary (vs primary) dengue. Finally, we used similar generalized additive models (GAM) models but with a Poisson link function to evaluate spatial variability in the duration of fever prior to clinical presentation. For all linear predictors and for the model intercepts we specified a normal prior distribution with mean 0 and standard deviation 1.
After fitting each generalized additive model, we predicted the model output onto a dense longitude-latitude grid which covered the extent of the study area of interest: predicted probability (transformed from odds) in the case of the binomial models and predicted days in the case of the Poisson model. We circumscribed regions with contours wherever there was a 95% or greater probability that the local prediction differed from the average.
Finally, we used the R package malariaAtlas to estimate travel times to the referral hospital in Galle.15 This package makes use of a 1 km2 global grid, representing the ease or difficulty of crossing each grid pixel, allowing travel times to be estimated between points. This global ‘friction surface’ takes into account factors such as roads, slope and land cover.
Results
Of the study cohort (n=800), acute dengue was confirmed in 333 (41.6%) using a combination of serological testing, viral isolation and PCR.8 The mean age was 28.8 y (range 1 to 83, IQR 14 to 42) and 510 (63.7%) of the subjects were male.8 Mean duration of fever was 4.9 d (range 0 to 69, IQR 3 to 6): 4.3 d among patients with dengue and 5.1 d among patients without dengue.8 The probability of dengue was highly sensitive to time of year, peaking in September 2012 when 53 of 65 subjects had dengue (81.5%) (Figure 2). By contrast, only eight of 77 subjects (10.4%) had dengue in February 2013.
Figure 2.
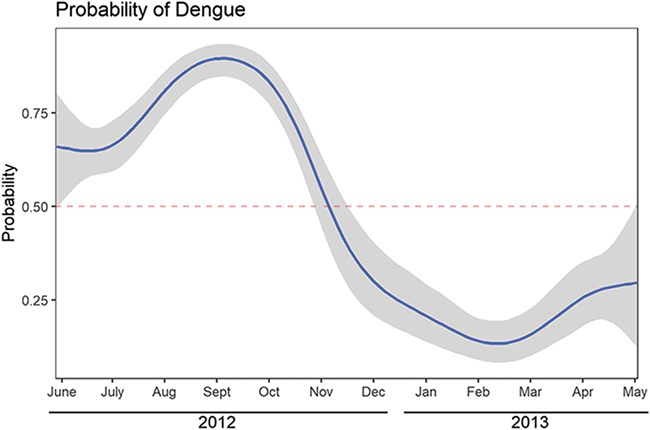
Temporal variability in the probability of dengue among febrile patients. Dengue accounted for as much as 80% of febrile illnesses in August through October 2012, corresponding to the rainy season. During the dry winter months dengue accounted for as little as 10% of febrile illnesses.
Among febrile patients, the probability of finding dengue was highest in the north/northeast and lowest in the south/southwest, along a smooth continuous gradient (Figure 3A). The probability ranged from 0.26 to 0.42 over the extent of the study area. The probability of dengue was lower than average along the coast, in particular in the more urbanized southwest region of the Southern Province, including the city of Galle (Figure 3A). Higher elevation was the only environmental variable associated with a higher probability of dengue (Figure 3B). Land use class and population density, on the other hand, were not associated with dengue. Among individual variables, age and gender were not associated with dengue. However, patients with dengue had a shorter duration of fever (4.3 vs 5.1 d). A 2-fold standard deviation increase in fever duration (approximately 10 d) was associated with odds of dengue of 0.65 (95% credible interval 0.43 to 0.96).
Figure 3.
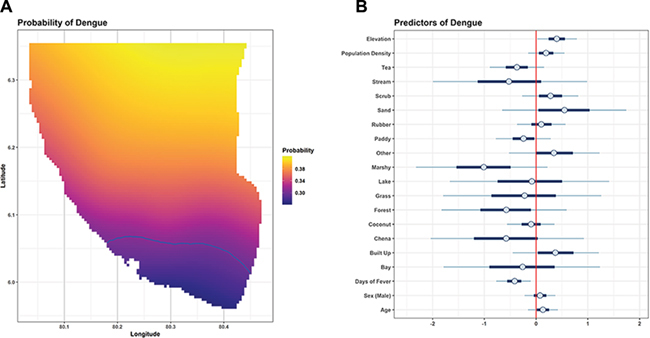
Spatial distribution of dengue among febrile patients and spatial and individual predictors of dengue. (A) The probability of dengue was greatest in the northern/northeastern region of the study area. It was lower along the low-lying coastal areas and lowest in the southern area, including the city of Galle. The blue contour circumscribes an area in which the probability of dengue is significantly lower than the average (ARCGIS 10.3.1, ESRI, Redlands, CA, USA). (B) Symbols represent the mean (circle), 50% highest density interval (thick bars) and 95% highest density interval (thin bars) of the posterior distribution. The x-axis represents the influence of a unit change in each parameter on the log-odds of dengue. In this Bayesian model, a significant predictor is one that does not overlie 0, thus revealing either a positive or negative effect on the probability of dengue. Higher elevation and fewer days of fever were associated with higher probability of dengue. Land cover type, population density, gender and age were not significant.
Among 305 subjects with dengue, 93 had primary infection and 212 had secondary infection. We did not find significant spatial heterogeneity of secondary dengue. Secondary dengue, however, was more frequent among older patients and was associated with higher population density, consistent with our prior finding that pre-existing seropositivity was more common in urban areas.7
Duration of fever was spatially heterogeneous in our study area. Patients with a shorter duration of fever tended to live closer to the city of Galle, whereas those with longer fever duration lived further away (Figure 4A). There were two spatial clusters with significantly longer fever duration, as defined by a 95% or greater probability of local fever duration differing from the mean. These areas were found in the northeast and southeast sectors of our study area. These spatial trends corresponded closely to travel time, with the longest duration fevers occurring in northeastern areas with the longest travel time to reach the referral hospital.
Figure 4.

Spatial distribution of fever duration among febrile patients. (A) The duration of fever was heterogeneous, with shorter duration predominating in the areas surrounding Galle and longer duration in the northern and eastern areas. Contours circumscribe areas in which the duration of fever was significantly longer (red) or shorter (blue) than the overall average (ARCGIS 10.3.1, ESRI, Redlands, CA, USA). (B) Travel time to reach the referral hospital in Galle (white asterisk) ranged from less than 1 to more than 3 h. This distribution appeared to correspond to the duration of fever, represented by contours on this map (ARCGIS 10.3.1, ESRI, Redlands, CA, USA).
Discussion
We found that rural, higher elevation areas of the Southern Province have higher odds of dengue infection compared with low-lying urban areas such as Galle. This finding is somewhat surprising given that dengue has traditionally been considered a more urban disease, associated with peridomestic mosquito exposure and higher population density. However, the results of our study are in accordance with emerging (though limited) data from other tropical developing countries that have reported increasing incidence of dengue cases in rural areas.16–18 In addition, the results of this geospatial analysis are in agreement with our epidemiological analysis from this same cohort showing an association of self-reported rural residence with increased odds of dengue.8 The other environmental variables we investigated, such as land cover and population density, were not associated with odds of dengue. Seasonality, on the other hand, was associated with dengue, with higher odds occurring in the late summer months when monsoon rains may have increased mosquito habitat. Dengue was more likely among patients with shorter fever duration, possibly due to the severity of dengue-related symptoms, leading patients to seek medical care earlier. While we did not find a spatial pattern to secondary dengue cases, we did find that secondary cases were more likely among patients living in areas of higher population density.
One possible explanation for our findings of increased odds of dengue infection in rural areas could be entomological differences between dengue transmission in rural and urban areas. Aedes aegypti, considered as the primary mosquito vector of dengue virus overall, is the primary dengue vector in urban areas, whereas A. albopictus is the vector primarily in rural areas.19 Some studies show evidence of DENV isolates in A. aegypti as well as in A. albopictus spreading to scattered rural areas of India and Sri Lanka.6,20 In addition, entomological studies show the presence of A. albopictus in many of the areas of dengue transmission in Sri Lanka, some at higher frequency than that of A. aegypti, which highlights the importance of considering A. albopictus breeding in transmission-risk areas of dengue.21 Researchers in Sri Lanka have confirmed detection of dengue virus in A. albopictus.21
In addition, studies elsewhere around the world have documented increases in reported dengue infection in rural areas. Sarfraz et al. studied the distribution of dengue infection in the Phitsanulok province of Thailand and found that settlements around gas stations and workshops, swamps and marshes, rice paddies and deciduous forest were among the most favorable areas for dengue vector propagation.22 In particular, A. albopictus can be found in natural containers such as tree holes, cut bamboo stumps and open coconuts, which can retain rainfall water and facilitate breeding.23 Agriculture is the largest driver for land-use change around the world, which could impact the prevalence and incidence of mosquito-borne diseases by influencing mosquitoes' habitat.22 Vegetation such as rubber, coconut, coffee and tea plantations can all serve as breeding sites for A. albopictus. The hotspot of increased odds of dengue infection identified in our study is in close proximity to the Sinharaja Forest reserve, a large rainforest, as well as rubber and tea plantations (Figure 1B). Further entomological studies need to be performed if increased odds of dengue infection in rural areas correlate to increased prevalence of dengue mosquito vectors, particularly A. albopictus.
An additional provocative finding in our study was the direct relationship between fever duration and a longer traveling distance to the referral hospital. This may have several explanations. First, there may be a referral or ascertainment bias of patients from more distant areas. Patients may be more likely to seek care or be referred from a local healthcare facility if they have more protracted or severe illnesses, as Teaching Hospital Karapitiya is the only tertiary care hospital for the entire Southern Province. A travel distance of ≥3 h may dissuade patients from seeking medical care for milder illnesses; consequently, sicker patients may be over-represented among those who live farther away, whereas the closer residents may represent a broader sampling of disease severity. Economics may also have impeded patients from the expense and lost work to seek medical care far from home. Alternatively, the epidemiology of different febrile illnesses (not limited to dengue) may vary geographically, and the longer fever duration in patients from rural areas may represent their greater risk of dengue and/or other febrile illnesses.
One limitation of our study is that patients who lived >50 km from the study center were excluded from the geospatial analysis due to logistical difficulties in visiting those patients' homes. However, we captured data from the majority of patients (800 out of 937 eligible). The association of rural residence with the odds of dengue infection found in this study may have been strengthened if more patients from rural areas were included.
An additional limitation to our study is that many rural inhabitants may be traveling to urban areas for work. Individuals from rural areas are probably more susceptible to primary infection compared with urban dwellers, as they were not exposed to previous urban outbreaks. These mobile individuals traveling back to rural areas could then help catalyze outbreaks of dengue infection in rural areas. Future studies should include work addresses in geospatial analyses to better understand the role of mobility from rural to urban areas in dengue outbreaks.
Conclusions
Our study demonstrates that the odds of dengue infection are significantly higher in rural areas, particularly in the northeast region of the Southern Province of Sri Lanka, suggesting that dengue is no longer a classically urban disease in this area. The implications of our study include emphasizing the importance of public health measures targeted at rural areas to identify current cases as well as to help prevent future dengue outbreaks, as well as performing entomological studies to further elucidate the increase in dengue infection in rural areas of Sri Lanka.
Supplementary Material
Acknowledgments
We would like to thank the patients, laboratory staff and research personnel who made this study possible.
Authors' contributions
CPM, CKB, AN, VD, RK, TA, CWW, LGT and PML designed the study protocol and implemented the study; CPM, ADDS, LGT and PML carried out statistical analyses and PML and MMJ carried out the spatial statistical analyses. CPM, CKB, AN, VD, RK, TA, ADD, MMJ, TØ, DJG, CWW, MER, LGT and PML drafted the manuscript; CPM, CKB, AN, VD, RK, TA, ADD, MMJ, TØ, DJG, CWW, MER, LGT and PML critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. CPM and PML are guarantors of the paper.
Funding
This work was supported by a grant from the Office of Naval Research to the Emerging Infectious Diseases Programme, Duke-NUS Graduate Medical School, Singapore; a National Institutes of Health Research Training Grant funded by the Fogarty International Center and the National Institute of Mental Health [R25 TW009337 to LGT]; by National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIAID) grants [K23AIO83931 to MER and K23AI125677 to LGT]; a Johns Hopkins Center for Global Health Junior Faculty Grant to MER; and by the Hubert-Yeargan Center for Global Health.
Competing interests
None declared.
Ethical approval
Written informed consent was obtained from patients (aged ≥18 y) or their guardians (for patients aged <18 y). Written assent was additionally obtained from patients aged 12–17 y. The institutional review boards of Ruhuna University (Sri Lanka), Duke University Health System (USA) and Johns Hopkins University (USA) all approved the study.
References
- 1. Bhatt S, Gething PW, Brady OJ et al. The global distribution and burden of dengue. Nature 2013;496(7446):504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 1998;11(3):480–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science 1988;239(4839):476–481. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization Dengue Guidelines for Diagnosis, Treatment, Prevention and Control, new edn. Geneva, Switzerland: World Health Organization, 2009. [PubMed] [Google Scholar]
- 5. Mahadev PV, Fulmali PV, Mishra AC. A preliminary study of multilevel geographic distribution and prevalence of Aedes aegypti (Diptera: Culicidae) in the state of Goa, India. Indian J Med Res 2004;120(3):173–182. [PubMed] [Google Scholar]
- 6. Tewari SC, Thenmozhi V, Katholi CR et al. Dengue vector prevalence and virus infection in a rural area in South India. Trop Med Int Health 2004;9(4):499–507. [DOI] [PubMed] [Google Scholar]
- 7. Reller ME, Bodinayake C, Nagahawatte A et al. Unsuspected dengue and acute febrile illness in rural and semi-urban southern Sri Lanka. Emerg Infect Dis 2012;18(2):256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bodinayake CK, Tillekeratne LG, Nagahawatte A et al. Emergence of epidemic dengue-1 virus in the Southern Province of Sri Lanka. PLoS Negl Trop Dis 2016;10(10):e0004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sri Lanka Disaster Risk Information System http://www.riskinfo.lk (accessed 27 August 2018).
- 10. Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat Med 2008;27(15):2865–2873. [DOI] [PubMed] [Google Scholar]
- 11. Bürkner P-C. Brms: an R package for Bayesian multilevel models using Stan. J Stat Softw 2017;80(1):28. [Google Scholar]
- 12. Carpenter B, Gelman A, Hoffman MD et al. Stan: a probabilistic programming language. J Stat Softw 2017;76(1):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wood S. Package 'mgcv' : Mixed GAM Computation Vehicle with GCV/AIC/REML Smoothness Estimation. https://cran.r-project.org/web/packages/mgcv/mgcv.pdf (accessed 29 May 2017).
- 14. Wood SN. Generalized additive models: An Introduction with R, second edition Boca Raton: CRC press, Taylor & Francis, 2017. [Google Scholar]
- 15. Malaria Atlas R Package for Accessing Data. https://map.ox.ac.uk/malariaatlas-r-package-for-accessing-data (accessed 28 October 2018).
- 16. Vong S, Khieu V, Glass O et al. Dengue incidence in urban and rural Cambodia: results from population-based active fever surveillance, 2006–2008. PLoS Negl Trop Dis 2010;4(11):e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mayxay M, Sengvilaipaseuth O, Chanthongthip A et al. Causes of fever in rural southern Laos. Am J Trop Med Hyg 2015;93(3):517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubot-Peres A, Vongphrachanh P, Denny J et al. An epidemic of dengue-1 in a remote village in rural Laos. PLoS Negl Trop Dis 2013;7(8):e2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun W, Xue L, Xie X. Spatial-temporal distribution of dengue and climate characteristics for two clusters in Sri Lanka from 2012 to 2016. Sci Rep 2017;7(1):12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kusumawathie PHD, Fernando WP. Breeding habitats of Aedes aegypti Linnaeus and Albopictus Skuse in a dengue transmission area in Kandy, Sri Lanka. Ceylon J Med Sci 2003;46:51–60. [Google Scholar]
- 21. Hapugoda MD, Gunasekera MB, Silva NR et al. Detection of dengue virus in Aedes albopictus mosquitoes by reverse transcription polymerase-chain reaction-liquid hybridization (RT-PCR-LH) based assay. The Bulletin of the Sri Lanka College of Microbiologists 2003;1(1):30–31. [Google Scholar]
- 22. Sarfraz MS, Tripathi NK, Tipdecho T et al. Analyzing the spatio-temporal relationship between dengue vector larval density and land-use using factor analysis and spatial ring mapping. BMC Public Health 2012;12:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anosike JC, Nwoke BE, Okere AN et al. Epidemiology of tree-hole breeding mosquitoes in the tropical rainforest of Imo state, south-east Nigeria. Ann Agric Environ Med 2007;14(1):31–38. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


