To the Editor:
Chronic obstructive pulmonary disease (COPD) due to cigarette smoking is a major cause of morbidity and mortality worldwide. Given the same amount of exposure to tobacco smoke, women are more likely to develop more severe airflow limitation at an earlier age than men (1). Importantly, at all stages of COPD severity, men have more severe computed tomography–defined emphysema than women (2). Also, the prevalence odds ratio for screen-detectable lung cancer (conditional on age and smoking history) indicates that women have an increased susceptibility to tobacco carcinogens but have a lower rate of fatal outcome of lung cancer compared with men (3).
So far, the reasons why women differ from men in cigarette smoking susceptibility and the pathological and clinical expressions of COPD remain largely unknown. Higher levels of cigarette smoke metabolites, such as polycyclic aromatic hydrocarbons adducts, have been previously found in the lungs of women smokers compared with men (4). Thus, we hypothesize that the differences in the way women and men inhale smoke from cigarettes may, at least in part, contribute to the different clinical and pathological COPD phenotypes between sexes. Using optoelectronic plethysmography (OEP), a noninvasive motion capture method to measure chest wall movements and estimate lung volumes, we assessed the smoking pattern of a matched woman and man population.
Methods
Twenty-eight active smokers, 14 men and 14 women, matched by anthropometric data and smoking habit, and free of pulmonary and other relevant diseases and not under chronic medical treatment, were recruited (see Table 1). First, the breathing pattern during 10 consecutive tidal breaths was assessed. Then, the subjects were invited to puff a cigarette with filter mimicking the way they usually smoke, including the depth of the puff (puff volume), the duration of the puff, and the interval between puffs. To standardize the procedure of data collection, after ignition of the cigarette, the first five puffs were excluded to allow the subject to stabilize his/her puffing pattern. Then, the volume of the following five puffs was measured by OEP. Moreover, to standardize the breathing frequency and to avoid confounding effects on total and compartmental Vt, we excluded the subjects with a smoking puff time longer than 1.5 seconds with a smoking volume >30% of the VC. A dedicated software computed the enclosed volume and its variations during breathing with high accuracy, providing a measurement of the three-dimensional micromovement of the points belonging to the chest wall to compute volume variations of the whole chest wall and the different compartments (5): pulmonary rib cage (RCp), abdominal rib cage (RCa), and the abdomen (Abd). The measurement of the volumes of each hemithorax was provided by midline sensors (anteriorly, along the sternum and continuing caudally below the xiphoid through the umbilicus, and posteriorly, along the spinous processes of the vertebral column).
Table 1.
Baseline Characteristics of the Study Population
| Variable | Women (n = 14) | Men (n = 14) | P Value |
|---|---|---|---|
| Age, yr | 46 ± 11 | 41 ± 9 | 0.182 |
| Height, m | 167 ± 5 | 167 ± 5 | 0.944 |
| Weight, kg | 67 ± 7 | 70 ± 8 | 0.381 |
| BMI, kg/m2 | 24 ± 3 | 25 ± 3 | 0.420 |
| Pack-years | 23 ± 8 | 25 ± 9 | 0.464 |
| FEV1, % pred. | 101 ± 9 | 104 ± 8 | 0.371 |
| FVC, % pred. | 107 ± 8 | 103 ± 7 | 0.232 |
| FEV1/FVC, % pred. | 107 ± 0.2 | 112 ± 0.2 | 0.533 |
| ITGV, % pred. | 118 ± 11 | 112 ± 19 | 0.244 |
| TLC, % pred. | 106 ± 14 | 99 ± 10 | 0.142 |
| SmoV, % VC | 24 ± 3 | 25 ± 3 | 0.352 |
| SmoIT, s | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.533 |
Definition of abbreviations: BMI = body mass index; ITGV = inspiratory thoracic gas volume; pred. = predicted; SmoIT = smoking inhalation time; SmoV = smoking volume.
Statistical analyses included one-way ANOVA tests for continuous variables, followed by pairwise comparisons using Student’s t tests or Mann-Whitney U tests. Pairwise comparisons showed no significant differences in the percentage of men, ages, pack-years, pulmonary function, and anthropometric data, between men and women. Data are presented as mean ± SD.
The software takes into account three main factors: 1) the lung- and diaphragm-apposed parts of the rib cage (RCp and RCa, respectively) are exposed to substantially different pressures on their inner surface during inspiration; 2) the diaphragm acts directly only on RCa; and 3) nondiaphragmatic inspiratory muscles act largely on RCp. Abd volume change is defined as the volume swept by the abdominal wall (Figure 1).
Figure 1.
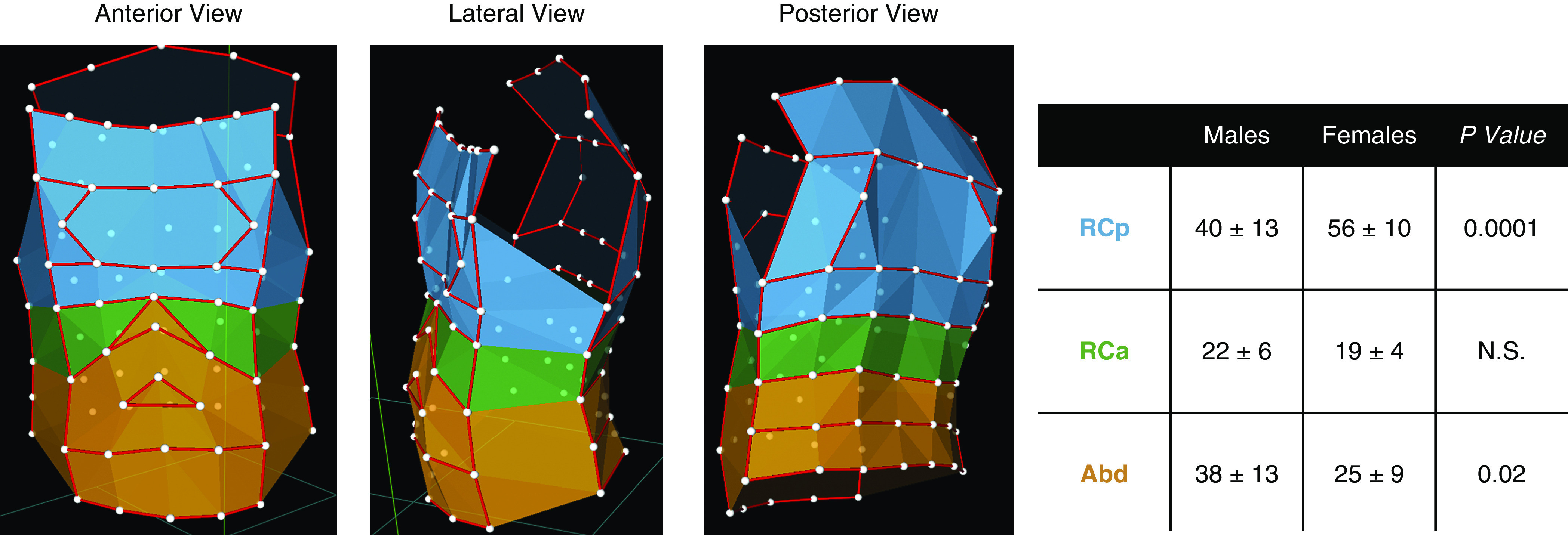
Optoelectronic plethysmography configuration of infrared-reflective markers identifying three main compartments associated with breathing: blue = pulmonary rib cage; green = abdominal rib cage; and orange = abdomen. The left microphotograph shows the anterior view, the middle microphotograph shows the lateral view, and the right microphotograph shows the posterior view. The table shows pairwise comparison (Student’s t test) of the total smoking volume distribution in men and women in the three thoracic compartments. Abd = abdomen; N.S. = not significant; RCa = abdominal rib cage; RCp = pulmonary rib cage.
Results
Men and women had similar anthropometric data and pulmonary function (Table 1). During tidal breathing, both men and women mainly recruited the RCp compartment, to a lesser extent the Abd, and minimally the RCa. The inhaled cigarette smoke volume (25 ± 3% VC in men and 24 ± 3% VC in women; P = 0.352) and the smoke inhalation time (1.1 ± 0.2 s in men and 1.0 ± 0.2 s in women; P = 0.533) were similar between men and women. The compartmental distributions of the smoking volume in the three compartments (pRC, aRC, and Abd) between right versus left hemithoraces within women and men and between women versus men are shown in Tables 2 and 3.
Table 2.
Differences in the Smoking Volume Distributions between Right and Left Hemithoraces in Men and Women
| Compartment | Within Men |
P Value | Within Women |
P Value | ||
|---|---|---|---|---|---|---|
| % SmoV Right Side | % SmoV Left Side | % SmoV Right Side | % SmoV Left Side | |||
| RCp | 21 ± 7 | 20 ± 7 | 0.62 | 30 ± 6 | 26 ± 6 | 0.15 |
| RCa | 10 ± 4 | 11 ± 3 | 0.40 | 9 ± 2 | 10 ± 4 | 0.80 |
| Abd | 19 ± 6 | 19 ± 8 | 0.87 | 12 ± 6 | 13 ± 7 | 0.68 |
| Total | 100 | 100 | ||||
Definition of abbreviations: Abd = abdomen; RCa = abdominal rib cage; RCp = pulmonary rib cage; SmoV = smoking volume.
Differences are expressed as percentage of the total smoking volume puff (% SmoV), in each compartment, between right and left side in women and men.
Table 3.
Differences in Percentage of the Total Smoking Volume Puff Distribution in Each Compartment in the Right and Left Sides between Women and Men
| Compartment | Men vs. Women |
|
|---|---|---|
| % SmoV Right Sides | % SmoV Left Sides | |
| RCp | 0.001 | 0.010 |
| RCa | 0.473 | 0.201 |
| Abd | 0.001 | 0.038 |
For definition of abbreviations, see Table 2.
Data are presented as P values. Bold indicates P values < 0.05.
Instead, the smoke inhalation volume, in each right and left hemithoraces, was mainly concentrating in the RCp and Abd compartments in men. In women, 58% and 54% (right and left side, respectively) of the total smoke inhalation volume was concentrated in the RCp, whereas the remaining volume was distributed between RCa and Abd. This difference in distribution between men and women was emphasized by averaging up both hemithoraces. In men, RCp and Abd shared almost 80% of the smoke inhalation volume (RCp vs. RCa: P < 0.0001; Abd vs. RCa: P < 0.001; RCp vs. Abd: nonsignificant), whereas in women the smoke inhalation volume was mainly collected in RCp (RCp vs. RCa: P < 0.0001; RCp vs. Abd: P < 0.0001; Abd vs. RCa: P < 0.05). The smoking volume collected in RCp and Abd was significantly different between men and women (table within Figure 1).
Discussion
We show for the first time that, while smoking, women preferentially recruit the rib cage with lesser contribution of the abdomen, whereas men preferentially recruit the abdominal compartment. This may be one potential mechanism contributing to the different cigarette smoke susceptibility, and the incidence of lung cancer and COPD, observed between women and men.
Women smokers develop more airflow limitation and more severe COPD, despite lower exposure to tobacco (2), and have a more rapid annual decline in FEV1 than men smokers, even when they smoke fewer cigarettes (6). Furthermore, women with severe COPD have a higher risk of hospitalization and death from respiratory failure and comorbidities (7). However, the underlying reasons are largely unknown.
The different susceptibility to tobacco smoke could reflect a sex difference in the metabolism of cigarette smoke or in the inflammatory response to cigarette smoking owing to hormonal effects (8). We are now adding another mechanism potentially associated with the increased susceptibility to cigarette smoke observed in women: how cigarette smoke is inhaled and distributed throughout the lung.
At birth, the lungs of women are on average smaller than those of men and have fewer respiratory bronchioles. The airways in women are relatively smaller than those in men for the same lung volume, which might induce a higher concentration of tobacco smoke per unit area (9). During growth, the development of new lung parenchyma and airways and the loss of lung elastic recoil occur in women earlier than men (10). However, this maturation process occurs homogeneously throughout the lung and it is unlikely associated with the difference in the smoking pattern that we observe between men and women.
One possible explanation to our findings is that during the biological evolution of the human species, an adaptation of the respiratory system anatomy in women occurred, to allow the gravid uterus to grow the fetus. This possible adaptation might underlie the predominant recruits of the upper areas of the thorax in women while enhanced breathing (smoking). As our data are observational, we cannot provide evidence to support mechanisms underlying the sex differences in the smoking pattern observed in our study. However, it is important to note that in our case study there were not only women who had had at least one pregnancy but also women who had never given birth, and the compartmental smoking distribution between them was similar.
To our knowledge, there are no other described physiological conditions where women use the rib cage compartment more than men. Interestingly, it has been previously demonstrated, by using chest radiographs, that women exhibit a greater inspiratory rib cage muscle contribution and volume expansion during resting breathing than men, presumably reflecting an improved mechanical advantage conferred to these muscles by the greater inclination of ribs (11).
Limitations of the study include the observational nature of the study and the fact that we did not confirm the spatial distribution of the inhaled cigarette smoke by using a tracer. However, OEP is an excellent noninvasive tool to determine compartmental displacement during breathing accurately and has the advantage of allowing accurate observation without complex instrument interference.
To conclude, we show for the first time that, while smoking, women mainly engage the pulmonary ribcage, whereas men mainly engage the abdominal compartment. These findings may help explain the increased susceptibility to cigarette smoke in women versus men, which underlies the increased risk of developing COPD, severe emphysema, and lung cancer in the female population.
Supplementary Material
Footnotes
Supported by funds from the Asthma and Airway Disease Research Center (University of Arizona), Flight Attendants Medical Research Institute grant YFAC141004, and the Parker B. Francis Foundation Fellowship.
Author Contributions: M.P., B.C., and F.P. conceived the project and designed the experiments. M.P., A.C., G.C., F.F., I.M., C.S., E.S., A.A., B.C., and F.P. conducted experiments and/or contributed to data analysis and interpretation. All authors contributed to the writing and editing of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202004-1472LE on June 1, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Jenkins C. More action to address the impact of smoking in women. Am J Respir Crit Care Med. 2017;195:1132–1134. doi: 10.1164/rccm.201701-0030ED. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, et al. National Emphysema Treatment Trial Research Group. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176:243–252. doi: 10.1164/rccm.200606-828OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henschke CI, Yip R, Miettinen OS International Early Lung Cancer Action Program Investigators. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. 2006;296:180–184. doi: 10.1001/jama.296.2.180. [DOI] [PubMed] [Google Scholar]
- 4.Mollerup S, Berge G, Baera R, Skaug V, Hewer A, Phillips DH, et al. Sex differences in risk of lung cancer: expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer. 2006;119:741–744. doi: 10.1002/ijc.21891. [DOI] [PubMed] [Google Scholar]
- 5.Aliverti A, Dellacà R, Pelosi P, Chiumello D, Gatihnoni L, Pedoti A. Compartmental analysis of breathing in the supine and prone positions by optoelectronic plethysmography. Ann Biomed Eng. 2001;29:60–70. doi: 10.1114/1.1332084. [DOI] [PubMed] [Google Scholar]
- 6.Sørheim IC, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, DeMeo DL. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65:480–485. doi: 10.1136/thx.2009.122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prescott E, Bjerg AM, Andersen PK, Lange P, Vestbo J. Gender difference in smoking effects on lung function and risk of hospitalization for COPD: results from a Danish longitudinal population study. Eur Respir J. 1997;10:822–827. [PubMed] [Google Scholar]
- 8.Hardin M, Cho MH, Sharma S, Glass K, Castaldi PJ, McDonald ML, et al. COPDGene and Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-Points Investigators. Sex-based genetic association study identifies CELSR1 as a possible chronic obstructive pulmonary disease risk locus among women. Am J Respir Cell Mol Biol. 2017;56:332–341. doi: 10.1165/rcmb.2016-0172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merkus PJ, ten Have-Opbroek AA, Quanjer PH. Human lung growth: a review. Pediatr Pulmonol. 1996;21:383–397. doi: 10.1002/(SICI)1099-0496(199606)21:6<383::AID-PPUL6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 10.Naeye RL, Freeman RK, Blanc WA. Nutrition, sex, and fetal lung maturation. Pediatr Res. 1974;8:200–204. doi: 10.1203/00006450-197403000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Bellemare F, Jeanneret A, Couture J. Sex differences in thoracic dimensions and configuration. Am J Respir Crit Care Med. 2003;168:305–312. doi: 10.1164/rccm.200208-876OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


