To the Editor:
Right ventricular (RV) adaptation is a major determinant of survival in pulmonary arterial hypertension (PAH) (1). The right ventricle initially compensates for the PAH-induced increase in afterload through hypertrophy and increased contractile function, maintaining RV–pulmonary arterial (PA) coupling. However, as PAH worsens, these compensatory mechanisms fail, leading to RV-PA uncoupling and eventual RV failure (1). In a cardiac magnetic resonance imaging (cMRI) study, female patients with PAH exhibited better RV ejection fraction than their male counterparts (2). RV ejection fraction is load dependent, and pressure–volume (PV) analysis is considered the gold standard to characterize RV-PA coupling (3). To date, no study has directly evaluated whether the superior RV systolic function in female versus male patients with PAH translates into better RV-PA coupling.
We investigated RV-PA coupling using high-fidelity measurements of PV relationships in 57 patients with PAH as part of a prospective study of RV function in PAH and chronic thromboembolic pulmonary hypertension (ClinicalTrials.gov: NCT03403868). The diagnosis of PAH conformed to updated recommendations (4). The study population included a proportion of previously published patients (3, 5, 6). Real-time PV loop assessment was performed as previously described (3), using the Inca PV Loop System (CD Leycom) with a 4F PV catheter (CA-Nr 41063; CD Leycom) inserted in the RV apex. Measurements were repeated during inferior vena cava occlusion for at least 10 seconds at expiratory breath hold for multibeat determinations. Multibeat end-systolic elastance (Ees) was determined by a tangent fitted on the end-systolic portions of the PV loops at decreasing venous return. We calculated arterial elastance (Ea) as end-systolic pressure/stroke volume (SV). RV-PA coupling was defined as the Ees/Ea ratio. Tau, a load-dependent measure of early diastolic relaxation (7), was calculated from the reciprocal of the natural logarithm of the early maximal fall in ventricular pressure during diastole. We calculated end-diastolic elastance (which is load independent) from the relationship dP/dV = αβ × eβ × end-diastolic volume at calculated end-diastolic volumes, where dP/dV is delta pressure/delta volume (1). RV mass index and volume measures were determined by cMRI. Data distributions were tested for normality using Kolmogorov–Smirnov tests and visual assessment of histograms. All studies were approved by the local ethics committee. All patients gave written informed consent.
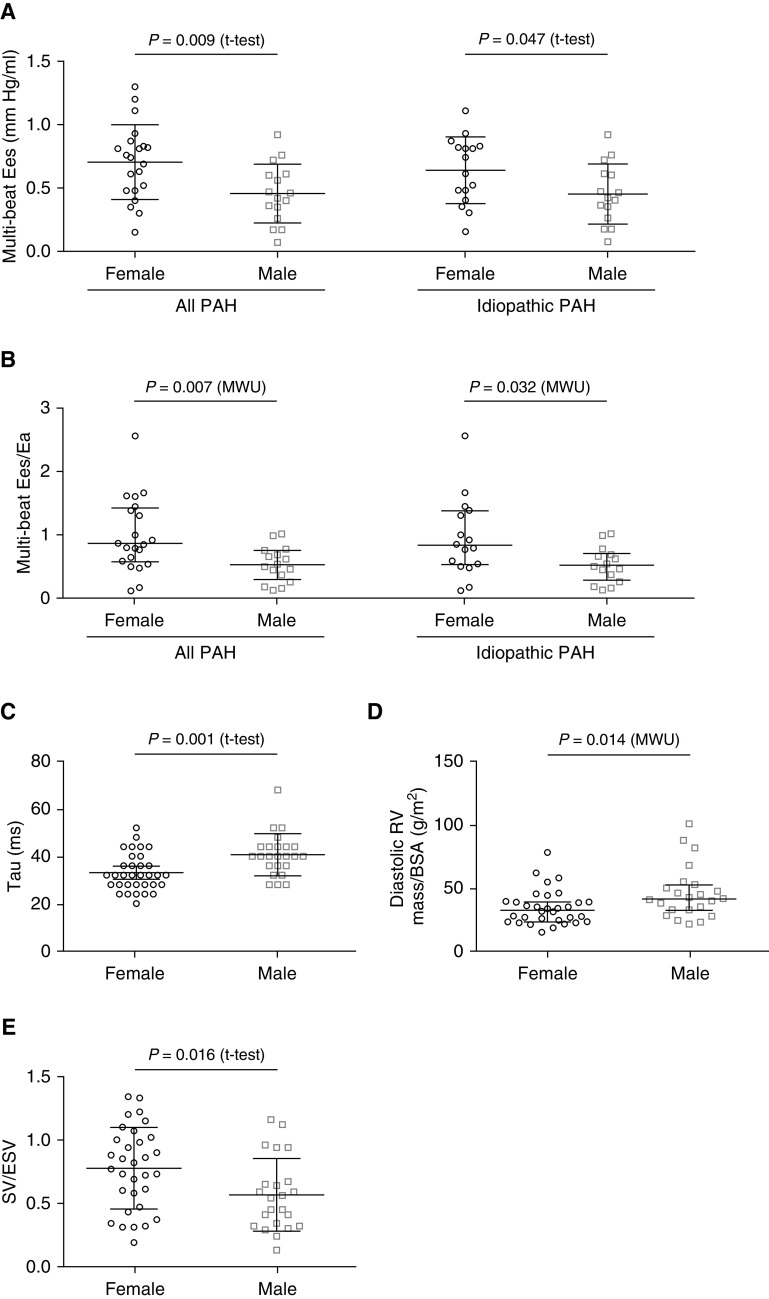
Of the 57 patients with PAH, 37 had multibeat data (Table 1). The study initially included single-beat data only. After establishment of vena cava occlusion, the multibeat method was also used. Therefore, not all patients had multibeat data. Female patients demonstrated significantly higher Ees and Ees/Ea than male patients (Figures 1A and 1B), suggesting higher contractility and better RV-PA coupling despite similar afterload (Ea). Tau and RV mass index were lower in female patients (Figures 1C and 1D), suggesting better diastolic adaptation. However, the mass/volume relationship (which has been associated with clinical worsening in PAH [8]) and end-diastolic elastance showed no significant difference. Although the SV/end-systolic volume ratio was higher in female patients (Figure 1E), we observed no female-to-male differences in other key hemodynamic, echocardiographic, and cMRI parameters (Table 1). Exclusion of patients with associated PAH revealed similar findings (Figure 1). We observed no differences in female patients after stratification for age.
Table 1.
Clinical Characteristics
| Female | Male | P Value | ||
|---|---|---|---|---|
|
n |
33 | 24 | — | |
| Age, yr |
53 ± 14 | 53 ± 12 | 0.98 | |
| PAH subtype, n (%) |
0.40 | |||
| Idiopathic PAH |
24 (72.7) | 22 (91.7) | — | |
| Associated PAH |
9 (27.3) | 2 (8.3) | — | |
| 6-min-walk distance, m (n = 42) |
401 ± 141 | 343 ± 146 | 0.20 | |
| B-type natriuretic peptide, pg/ml (n = 54) |
91 (38–246) | 99 (46–295) | 0.91 | |
| TAPSE/PASP ratio, mm/mm Hg (n = 52) |
0.31 (0.21–0.40) | 0.33 (0.19–0.38) | 0.85 | |
| Prior PAH therapy, n (%) |
||||
| Dual combination therapy |
9 (27.3) | 9 (37.5) | 0.41 | |
| Triple combination therapy |
8 (24.2) | 9 (37.5) | 0.28 | |
| Right heart catheterization |
||||
| Mean pulmonary artery pressure, mm Hg |
42 (36–52) | 44 (34–55) | 0.84 | |
| Pulmonary vascular resistance, Wood units |
7.3 (5.6–9.8) | 6.3 (4.3–11.3) | 0.38 | |
| Cardiac index, L/min/m2 |
2.8 ± 0.7 | 2.9 ± 0.6 | 0.66 | |
| Pulmonary artery wedge pressure, mm Hg |
7 (6–11) | 9 (8–11) | 0.18 | |
| Right atrial pressure, mm Hg |
6 (4–10) | 7 (5–9) | 0.66 | |
| RV pressure–volume loop measurements |
||||
| Single-beat |
||||
| Ea, mm Hg/ml |
0.80 (0.56–0.96) | 0.72 (0.46–1.10) | 0.97 | |
| Ees, mm Hg/ml |
0.68 (0.38–0.91) | 0.48 (0.29–0.63) | 0.03 | |
| Ees/Ea ratio |
0.92 (0.58–1.24) | 0.59 (0.35–0.99) | 0.03 | |
| Eed, mm Hg/ml (n = 51) |
0.14 (0.05–0.23) | 0.16 (0.06–0.24) | 0.84 | |
| Tau, ms |
33 ± 8 | 41 ± 9 | <0.01 | |
| RVSWI, ml · mm Hg/m2 |
1,777 (1,233–2,997) | 1,541 (1,035–2,670) | 0.54 | |
| Multibeat (n = 37, 21 female, 16 male) |
||||
| Ees, mm Hg/ml |
0.70 ± 0.30 | 0.46 ± 0.23 | 0.01 | |
| Ees/Ea ratio |
0.85 (0.56–1.41) | 0.52 (0.29–0.74) | 0.01 | |
| Cardiac magnetic resonance (n = 55) |
||||
| RV mass index, g/m2 |
34 ± 14 | 46 ± 21 | 0.01 | |
| RV end-diastolic volume index, ml/m2 |
96 (80–121) | 105 (89–162) | 0.20 | |
| RV mass/volume ratio |
0.34 (0.27–0.38) | 0.33 (0.28–0.48) | 0.40 | |
| RV ESV index, ml/m2 |
59 (45–92) | 69 (59–107) | 0.17 | |
| RV ejection fraction, % |
40 (35–48) | 35 (27–41) | 0.33 | |
| SV/ESV | 0.78 ± 0.32 | 0.57 ± 0.29 | 0.02 | |
Definition of abbreviations: Ea = arterial elastance; Eed = end-diastolic elastance; Ees = end-systolic elastance; ESV = end-systolic volume; PAH = pulmonary arterial hypertension; PASP = pulmonary artery systolic pressure; RV = right ventricular; RVSWI = right ventricular stroke work index; SV = stroke volume; TAPSE = tricuspid annular plane systolic excursion.
Normally and nonnormally distributed data are presented as means ± SD and medians (interquartile ranges), respectively. Between-group differences were analyzed by Student’s t tests (for normally distributed variables), Pearson chi-square tests (for categorical variables), or Mann-Whitney U tests (for nonnormally distributed variables).
Figure 1.
Sex stratification of pressure–volume loop parameters and cardiac magnetic resonance–derived RV mass index and SV/ESV in patients with PAH. Compared with male patients, female patients had significantly improved (A) RV contractility (multibeat Ees; all PAH, n = 37; idiopathic PAH, n = 31), (B) RV–pulmonary arterial coupling (multibeat Ees/Ea; all PAH, n = 37; idiopathic PAH, n = 31), (C) early diastolic relaxation (Tau; all PAH, n = 57), (D) RV mass index (all PAH, n = 55), and (E) SV/ESV (all PAH, n = 55). Data are presented as (A, C, and E) means and SD for normally distributed variables or (B and D) medians and interquartile ranges for nonnormally distributed variables. Between-group differences were analyzed by Student’s t tests or Mann-Whitney U tests (SPSS, version 25.0; IBM). BSA = body surface area; Ea = arterial elastance; Ees = end-systolic elastance; ESV = end-systolic volume; MWU = Mann-Whitney U test; PAH = pulmonary arterial hypertension; RV = right ventricular; SV = stroke volume.
Previously, only indirect evidence for a sex-based difference in RV-PA coupling was available; in a large retrospective study of veterans with pre- and/or postcapillary pulmonary hypertension, women exhibited lower right atrial pressure despite having higher pulmonary vascular resistance than men, suggesting better RV adaptation to the increased afterload (9). However, most of those patients did not have PAH, and factors such as volume status may have affected right atrial pressure.
The present results show that female patients with PAH have higher contractility and better RV-PA coupling than male patients. In fact, the median Ees/Ea ratio was above the RV-PA uncoupling threshold of 0.8 (3) in female patients but below that threshold in male patients. This might represent a significant contributor to the survival advantage noted in female patients with PAH (2). We observed a consistent pattern using the volume-only method to assess RV-PA coupling (Ees and Ea have a common pressure term, so Ees/Ea can be simplified as a ratio of volumes [SV/end-systolic volume]). However, the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio (previously validated as a noninvasive surrogate for Ees/Ea [5]) was not significantly different in female and male patients. This may be a type II error because of the small sample size and insufficient precision of the ratio. Jacobs and colleagues demonstrated that 40% of the survival advantage in female versus male patients can be explained by a higher RV ejection fraction in female patients after initiation of treatment (2). RV ejection fraction was not significantly higher in women versus men in the present study, but this was also probably a type II error related to the small sample size. However, we assessed several potential confounders, including cMRI data, which did not reveal many sex differences (Table 1), whereas RV-PA coupling did. Especially in light of the relatively small study population, parameters of RV-PA coupling are obviously more sensitive to detect these changes.
Mechanisms underlying the superior RV adaptation in female patients with PAH are not yet known. We hypothesize that the lesser extent of RV hypertrophy in female versus male patients with PAH may make RV cardiomyocytes less prone to substrate depletion and ischemia. We have previously demonstrated in a rodent model that the female sex steroid 17β-estradiol attenuates RV proapoptotic and proinflammatory signaling, mitochondrial abnormalities, and oxidative stress and increases RV abundance of the proangiogenic and procontractile peptide apelin (10). Other sex hormones as well as nonhormonal factors may also contribute. However, estradiol and other sex hormone levels and menopausal status were not defined in the current analysis. Further investigations into mechanisms underlying the superior RV adaptation in female patients are ongoing in our laboratory.
In summary, our data demonstrate superior RV-PA coupling, better diastolic relaxation, and lower RV mass index in female versus male patients with PAH. These differences may underlie the superior survival of female patients. Our observations provide a rationale and basis for further evaluations of sex-based RV structural differences in PAH.
Supplementary Material
Acknowledgments
Acknowledgment
Editorial assistance was provided by Claire Mulligan, Ph.D. (Beacon Medical Communications Ltd.), funded by the University of Giessen.
Footnotes
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) project number 268,555,672; SFB 1213, Project B08; NIH 1R01HL144727–01A1; and VA Merit Review Award BX002042–07.
Author Contributions: K.T., M.J.R., A.Y., H.A.G., R.N., R.V., H.G., R.J.T., W.S., and T.L.: study design. K.T., M.J.R., H.A.G., H.G., and W.S.: patient recruitment. K.T., M.J.R., A.Y., H.A.G., R.N., R.V., H.G., R.J.T., and W.S.: data collection and analysis. K.T., M.J.R., and H.G.: statistical analyses. K.T., M.J.R., A.Y., H.A.G., R.N., R.V., H.G., R.J.T., W.S., and T.L.: drafting of the manuscript. K.T., M.J.R., A.Y., H.A.G., R.N., R.V., H.G., R.J.T, W.S., and T.L.: critical revision of the manuscript for important intellectual content. K.T. was the principal investigator, had access to all the data in the study, and takes full responsibility for the integrity and accuracy of the data analysis.
Originally Published in Press as DOI: 10.1164/rccm.202003-0807LE on June 5, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019;53:1801900. doi: 10.1183/13993003.01900-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, et al. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest. 2014;145:1230–1236. doi: 10.1378/chest.13-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tello K, Dalmer A, Axmann J, Vanderpool R, Ghofrani HA, Naeije R, et al. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail. 2019;12:e005512. doi: 10.1161/CIRCHEARTFAILURE.118.005512. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter MJ, Peters D, Ghofrani HA, Naeije R, Roller F, Sommer N, et al. Evaluation and prognostic relevance of right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med. 2020;201:116–119. doi: 10.1164/rccm.201906-1195LE. [DOI] [PubMed] [Google Scholar]
- 6.Tello K, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, et al. Impaired right ventricular lusitropy is associated with ventilatory inefficiency in pulmonary arterial hypertension. Eur Respir J. 2019;54:1900342. doi: 10.1183/13993003.00342-2019. [DOI] [PubMed] [Google Scholar]
- 7.Sys SU, Brutsaert DL. Diagnostic significance of impaired LV systolic relaxation in heart failure. Circulation. 1995;92:3377–3380. doi: 10.1161/01.cir.92.12.3377. [DOI] [PubMed] [Google Scholar]
- 8.Badagliacca R, Poscia R, Pezzuto B, Nocioni M, Mezzapesa M, Francone M, et al. Right ventricular remodeling in idiopathic pulmonary arterial hypertension: adaptive versus maladaptive morphology. J Heart Lung Transplant. 2015;34:395–403. doi: 10.1016/j.healun.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Ventetuolo CE, Hess E, Austin ED, Barón AE, Klinger JR, Lahm T, et al. Sex-based differences in veterans with pulmonary hypertension: results from the veterans affairs-clinical assessment reporting and tracking database. PLoS One. 2017;12:e0187734. doi: 10.1371/journal.pone.0187734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, et al. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol. 2015;308:L873–L890. doi: 10.1152/ajplung.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.