Acute respiratory distress syndrome (ARDS) is a life-threatening condition characterized by uncontrolled bilateral inflammatory response with infiltrations in the lungs followed by noncardiac hypoxemic respiratory failure (1). Even though major advancements in the management of patients with ARDS have improved survival, this syndrome still accounts for more than 10% of ICU admissions worldwide and has a mortality exceeding 40% in the most severe cases (2). Extracorporeal membrane oxygenation (ECMO) might be considered to stabilize patients with extreme hypoxemia (3) but has not proven superior to conventional therapy for decreasing 60-day mortality (3).
Another growing need is to prevent the long-term neuromuscular, mental, and respiratory complications of the syndrome, which have major socioeconomic consequences (2, 4).
In 2015, we reported the successful use of systemic administration of a single dose of minimally expanded allogeneic bone-marrow (BM) mesenchymal stromal cells (MSCs) from one donor on two patients with severe ARDS on ECMO support (5). In this study, we describe the 5-year follow-up of these two patients regarding health-related quality of life (HRQoL), physical capacity, and pulmonary morphology and function.
Methods
Patients and study design
The regional ethic review board at Uppsala, Sweden (Dnr:2017/255) has approved this 5-year follow-up study of two patients with severe ARDS treated on a compassionate use basis with a single intravenous infusion of thawed, cryopreserved, minimally expanded, HLA-mismatched, allogeneic BM-MSCs (2 × 106 cells/kg body weight) from the same donor (5).
The follow-up evaluation consisted of an in-depth review of the patients’ medical records, a self-assessment test of mental and physical health (the Short Form 36-item health survey [SF-36]), a physical examination, a high-resolution dual-energy computed tomography (DECT) of the lungs, static and dynamic spirometry, and a 6-minute-walk test. All examinations were performed by accredited personnel at Uppsala University Hospital.
Results
Baseline characteristics and initial clinical outcomes
The descriptive data for the two patients at the time of BM-MSC infusion are summarized in Table 1. Despite different etiologies of ARDS, after BM-MSC infusion, there was a subsequent resolution of respiratory and multiorgan failure, leading to weaning off the ECMO system after 24 and 8 days and from mechanical ventilation after 41 and 12 days for patient 1 and 2, respectively.
Table 1.
Presentation of Basal Characteristics as well as Clinical Outcomes Directly and 5 Years after a Single Infusion of BM-MSCs in Two Patients with ARDS
| Characteristics | Patient 1 | Patient 2 | ARDS Survivors at 5 yr |
|---|---|---|---|
| Age at admittance and at 5-yr follow-up, yr | 58 (64) | 40 (45) | — |
| Weight, kg | 116 | 113 | — |
| Height, cm | 179 | 182 | — |
| Body mass index, kg/m2 | 36 | 34 | — |
| Sex | M | M | — |
| Smoker, yes/no | No | No | — |
| Cause of ARDS | H1N1 | Chemotherapy/infection/TRALI | — |
| SOFA score | 18 | 14 | — |
| Comorbidity | |||
| Diabetes, yes/no | No | No | — |
| COPD, yes/no | No | No | — |
| Asthma, yes/no | No | No | — |
| Hypertension, yes/no | Yes | No | — |
| Heart failure, yes/no | No | No | — |
| LVEF, % | >50 | >50 | — |
| Kidney failure, yes/no | Yes | No | — |
| Dialysis, yes/no | Yes | Yes | — |
| Liver failure, yes/no | Yes | No | — |
| Peak Bilirubin concentration, μmol/L | 306 | 199 | — |
| Coagulopathy, yes/no | Yes | Yes | — |
| Platelet count, ×109/L | 47 | 6 | — |
| Initial clinical outcome | |||
| Length of mechanical ventilation, d | 53 | 40 | — |
| Length of ECMO support, d | 30 | 36 | — |
| Length of mechanical ventilation after BM-MSC infusion, d | 41 | 12 | — |
| Length of ECMO support after BM-MSC infusion, d | 24 | 8 | — |
| ICU stay, d | 46 | 42 | — |
| Hospital stay, d | 69 | 79 | — |
| FEV1% predicted | 103 | 95 | — |
| Median | — | — | 83 |
| Interquartile range | — | — | 69–98 |
| Distance walked in 6 min, m | 450 | 565 | |
| Median | 132 | 115 | 436 |
| Interquartile range | — | — | 324–512 |
| Percentage of predicted | — | — | 76 |
| Back to work at 1 yr and 5 yr, % | 100 and 100 | 100 and 100 | 48 and 77 |
| SF-36 score | |||
| Physical functioning | 100 | 85 | 75 |
| Role, physical | 100 | 100 | 88 |
| Bodily pain | 100 | 90 | 74 |
| General health | 80 | 95 | 62 |
| Vitality | 95 | 85 | 55 |
| Social functioning | 100 | 100 | 75 |
| Role, emotional | 100 | 100 | 100 |
| Mental health | 100 | 96 | 76 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; BM-MSC = bone marrow mesenchymal stromal cells; COPD = chronic obstructive pulmonary disease; ECMO = extracorporeal membrane oxygenation; LVEF = left ventricular ejection fraction; SF-36 = Short Form 36-Item Health Survey; SOFA = Sequential Organ Failure Assessment; TRALI = transfusion-related acute lung injury.
Our 5-year clinical data are presented as a comparison to previously reported 5-year follow-up of conventionally treated patients with ARDS by Herridge and colleagues (4). The SF-36 scores range from 0 to 100, with higher scores indicating better health status.
The Sequential Organ Failure Assessment (SOFA) score is an ICU mortality prediction scoring system and is useful in predicting the clinical outcome of critically ill patients (6). The patients in our study had SOFA scores of 18 and 14 (patient 1 and 2, respectively), in which a SOFA score of greater than 12 has a predicted mortality of 95.2% (6).
Physical capacity, HRQoL, and respiratory function
At 5 years, both patients demonstrated normal physical capacity on the 6-minute walk test (Table 1), with the longest recorded distances of 450 m and 565 m, corresponding to 132% and 115% of predicted values based on established norms (7) for patient 1 and 2, respectively. The distance walked in 6 minutes correlated well with the physical component score of the SF-36 and the normalized lung functions on spirometry (Table 1). The two patients also scored highly on the mental health and vitality components of the SF-36 (range 85–100). Both patients were back to 100% work without modification of work schedule within 1 year after the BM-MSC treatment.
Lung parenchymal findings on DECT
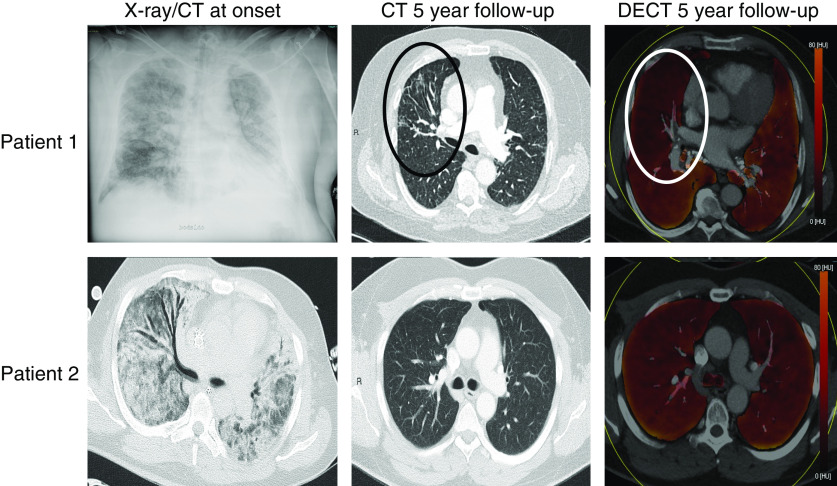
Patient 1 had bilateral infiltrates at admission to the hospital (Figure 1). At the 5-year follow-up, the distribution of deficits in the color-coded perfusion map corresponded with the signs of parenchymal fibrosis, such as segmental traction bronchiectasis and linear stranding of the anterior parts of the right upper lobe. In contrast, although patient 2 had been on ECMO for 28 days before BM-MSC infusion and at that time had massive bilateral pulmonary infiltrates (Figure 1), at the 5-year follow-up, DECT perfusion images were homogeneous without indication of any perfusion deficits or parenchymal fibrosis. No sign of pulmonary embolism was detected in either of the two patients.
Figure 1.
(Top row) Patient 1: 58-year-old man with influenza A H1N1–induced acute respiratory distress syndrome (ARDS). (Left) A supine conventional X-ray on admission with bilateral opacifications. No computed tomography (CT) was performed. (Middle) Five-year follow-up CT, in which the axial morphological image reveals signs of pulmonary injury with traction bronchiectasis and linear strands in the anterior parts of the right upper lobe, marked with black oval. (Right) Axial color-coded perfusion map (DECT) with deficits corresponding with the morphological findings marked with a white circle. (Bottom row) Patient 2: 40-year-old man, in whom a combination of cytokine release after intensive chemotherapy because of acute myeloid leukemia, infection, and massive transfusions most probably caused the ARDS. (Left) Signs of ARDS on CT at admission with bilateral ground glass opacities and consolidations without pleural fluid. (Middle) Axial section demonstrating no pathological findings on the five-year follow-up CT. (Right) Normal-appearing axial color-coded perfusion map (DECT). DECT = dual-energy computed tomography.
Discussion
To our knowledge, this is the first long-term follow-up of patients with the most severe form of ARDS that needed ECMO support in combination with mechanical ventilation in the acute phase and who, at the same time, were treated with a single systemic infusion of allogeneic BM-MSCs (5).
Our main finding is that 5 years after the treatment, both patients have fully recovered their physical and mental capacities, which is unusual for survivors of ARDS (2, 4, 8). Another important finding is that the second patient (patient 2), who had the most severe form of ARDS and was on ECMO support for 28 days before the MSC infusion, had no signs of pulmonary fibrosis 5 years after the MSC treatment, as demonstrated using DECT. Furthermore, because of the immediate response of the MSC infusion in the acute phase, patient 2 could complete treatment for his acute myeloid leukemia and is still in remission 5 years later.
Patient 1, with influenza A H1N1–induced ARDS, exhibited signs of only mild pulmonary injury in the anterior part of the right upper lobe. Such resolutions of severe lung parenchymal injury are rarely seen in long-term follow-up studies of conventionally treated patients with ARDS, in whom computed tomography scans demonstrate residual signs of anterior interstitial fibrosis (4, 9).
The normalization of lung function in the two MSC-treated patients is in line with follow-up reports on patients with conventionally treated ARDS without ECMO support, in whom the lung function usually is described as normal or near normal (2, 4, 8).
MSCs have inborn immunomodulatory and reparative properties that, in preclinical studies, have been demonstrated to affect the different pathological mechanisms involved in ARDS progression (2). In the randomized, multicenter phase IIa START study (10), a single infusion of allogeneic BM-MSCs was demonstrated to be safe in the treatment of patients with moderate to severe ARDS. The efficacy of the treatment was dependent on the viability of MSCs at the time of infusion (10).
In the future, adequately powered randomized trials, with particular attention to quality and viability of MSCs, need to be performed to determine the potential efficacy of BM-MSC treatment of ARDS.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the doctors and nurses at the Department of Cardiothoracic Surgery, Uppsala University Hospital, for their overall support.
Footnotes
Supported by grants from Åke Wiberg’s Foundation, Gullstrand Foundation, Uppsala County Council, RuFu, Lennander’s Foundation, Selander’s Foundation, Uppsala County Association Against Heart and Lung Diseases, and The Swedish Heart and Lung Association.
Author Contributions: O.E.S. contributed to the conception of the work; acquisition, analysis, and interpretation of data; drafting and critical revision of the manuscript; and final approval of the version to be published. E.S. contributed to the conception of the work, analysis and interpretation of data, critical revision of the manuscript, and final approval of the version to be published. T.H. contributed to the acquisition, analysis, and interpretation of data; critical revision of the manuscript; and final approval of the version to be published. J.O.W. contributed to the acquisition, analysis, and interpretation of data and final approval of the version to be published. A.L. contributed to the acquisition, analysis, and interpretation of data; critical revision of the manuscript; and final approval of the version to be published. M.M. contributed to the acquisition, analysis, and interpretation of data; critical revision of the manuscript; and final approval of the version to be published. P.V. contributed to the acquisition, analysis, and interpretation of data; critical revision of the manuscript; and final approval of the version to be published. K.L.B. contributed to the acquisition, analysis, and interpretation of data; critical revision of the manuscript; and final approval of the version to be published. S.R. contributed to the conception of the work; acquisition, analysis, and interpretation of data; drafting and critical revision of the manuscript; and final approval of the version to be published. K.-H.G. contributed to the conception of the work; acquisition, analysis, and interpretation of data; drafting and critical revision of the manuscript; and final approval of the version to be published.
Originally Published in Press as DOI: 10.1164/rccm.202003-0544LE on June 5, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. EOLIA Trial Group, REVA, and ECMONet. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 4.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 5.Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, Dalén M, et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl Med. 2015;4:1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 8.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 9.Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999;210:29–35. doi: 10.1148/radiology.210.1.r99ja2629. [DOI] [PubMed] [Google Scholar]
- 10.Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.