Abstract
Rationale: Tissue Doppler imaging (TDI) is an echocardiographic method that measures the velocity of moving tissue.
Objectives: We applied this technique to the diaphragm to assess the velocity of diaphragmatic muscle motion during contraction and relaxation.
Methods: In 20 healthy volunteers, diaphragmatic TDI was performed to assess the pattern of diaphragmatic motion velocity, measure its normal values, and determine the intra- and interobserver variability of measurements. In 116 consecutive ICU patients, diaphragmatic excursion, thickening, and TDI parameters of peak contraction velocity, peak relaxation velocity, velocity–time integral, and TDI-derived maximal relaxation rate were assessed during weaning. In a subgroup of 18 patients, transdiaphragmatic pressure (Pdi)-derived parameters (peak Pdi, pressure–time product, and diaphragmatic maximal relaxation rate) were recorded simultaneously with TDI.
Measurements and Main Results: In terms of reproducibility, the intercorrelation coefficients were >0.89 for all TDI parameters (P < 0.001). Healthy volunteers and weaning success patients exhibited lower values for all TDI parameters compared with weaning failure patients, except for velocity–time integral, as follows: peak contraction velocity, 1.35 ± 0.34 versus 1.50 ± 0.59 versus 2.66 ± 2.14 cm/s (P < 0.001); peak relaxation velocity, 1.19 ± 0.39 versus 1.53 ± 0.73 versus 3.36 ± 2.40 cm/s (P < 0.001); and TDI-maximal relaxation rate, 3.64 ± 2.02 versus 10.25 ± 5.88 versus 29.47 ± 23.95 cm/s2 (P < 0.001), respectively. Peak contraction velocity was strongly correlated with peak transdiaphragmatic pressure and pressure–time product, whereas Pdi-maximal relaxation rate was significantly correlated with TDI-maximal relaxation rate.
Conclusions: Diaphragmatic tissue Doppler allows real-time assessment of the diaphragmatic tissue motion velocity. Diaphragmatic TDI-derived parameters differentiate patients who fail a weaning trial from those who succeed and correlate well with Pdi-derived parameters.
Keywords: diaphragmatic ultrasonography, velocity of diaphragmatic motion, weaning
At a Glance Commentary
Scientific Knowledge on the Subject
In our article, we present the application of tissue Doppler imaging (TDI) on the diaphragm. This technique allows real-time assessment of the velocity of diaphragmatic muscle motion, a characteristic never studied before in either healthy adults or ICU patients.
What This Study Adds to the Field
Our study demonstrates that weaning success and weaning failure patients have strikingly different diaphragmatic TDI patterns. The former resemble the diaphragmatic TDI characteristics of healthy individuals.
Doppler ultrasound detects the frequency shift of ultrasound signals reflected by moving objects. Tissue Doppler imaging (TDI) is an echocardiographic technique that uses the same Doppler principles to measure the high-amplitude, low-velocity signal of cardiac tissue motion. TDI measurements are robust, easy to obtain, and offer valuable information in various cardiac pathologies.
In recent years, ultrasonography has been used extensively to evaluate diaphragmatic motion characteristics, such as excursion and thickening (1), with derived measurements repeatedly being shown to carry prognostic implications in the outcome of weaning (2–4). However, TDI of diaphragmatic tissue motion has been studied so far very little in adults (5); moreover, no information exists regarding the waveform pattern of diaphragmatic motion velocity and its reference values in healthy subjects and in ICU patients with respiratory failure.
We designed a prospective observational study first to evaluate the waveform pattern of diaphragmatic TDI, measure the velocities of diaphragmatic contraction and relaxation in healthy individuals, report the normal values, and assess the reproducibility of the method. Second, we recorded the diaphragmatic TDI pattern and values in intubated ICU patients during a spontaneous breathing trial, comparing weaning success and weaning failure cases. Finally, in a subgroup of patients, we correlated TDI measurements to parameters derived from the corresponding transdiaphragmatic pressure (Pdi) waveforms, aiming at a better understanding of the clinical significance of the TDI values and their derived parameters. Some of the results of our study have been previously reported in the form of abstracts (6, 7).
Methods
Study Design
The study was conducted from June 2018 to June 2019. TDI was performed on 20 healthy volunteers (10 men and 10 women, aged between 25 and 48 years) to assess the diaphragmatic TDI waveform, acquire the range of normal values of the diaphragmatic motion velocities during inspiration and expiration, and evaluate the reproducibility of the method. For the latter, TDI measurements were performed in two different occasions by a single operator and separately by a second independent operator. A total of over 150 breaths were recorded, accounting for about 8–10 breaths per participant.
After completion of the TDI assessment in healthy volunteers, consecutive ICU patients, mechanically ventilated for more than 48 hours, were screened for participation in the study. Exclusion criteria were age <18 years, neuromuscular diseases, flail chest or multiple rib fractures, chest tubes in place, morbid obesity, spinal cord injuries, and inability to obtain a clear TDI signal of the right hemidiaphragm.
The sonographic examination in the ICU patients was performed at the end of a 30-minute T-piece weaning trial or immediately before reconnection to the ventilator in the case of weaning failure occurring before; however, weaning failure before the end of the weaning trial occurred only in a minority of patients. We assessed our patients during spontaneous breathing because TDI assesses only the absolute tissue velocity and is unable to discriminate passive from active motion. Patients underwent a T-piece trial if they met the following criteria: appropriate mental status and cooperation, adequate oxygenation (oxygen saturation as measured by pulse oximetry > 92%, FiO2 40%) and cough reflex, cardiovascular stability without vasopressor or inotropic support, and normal metabolic status. Reinstitution of mechanical ventilation was initiated if signs of respiratory distress (diaphoresis or tachypnea), hypoxemia and/or hypercapnia, or deterioration of the cardiovascular status (hypertension and tachycardia) were observed. Weaning success was considered if ventilatory support, including noninvasive ventilation, was not required for 48 hours after extubation.
Ultrasound Technique
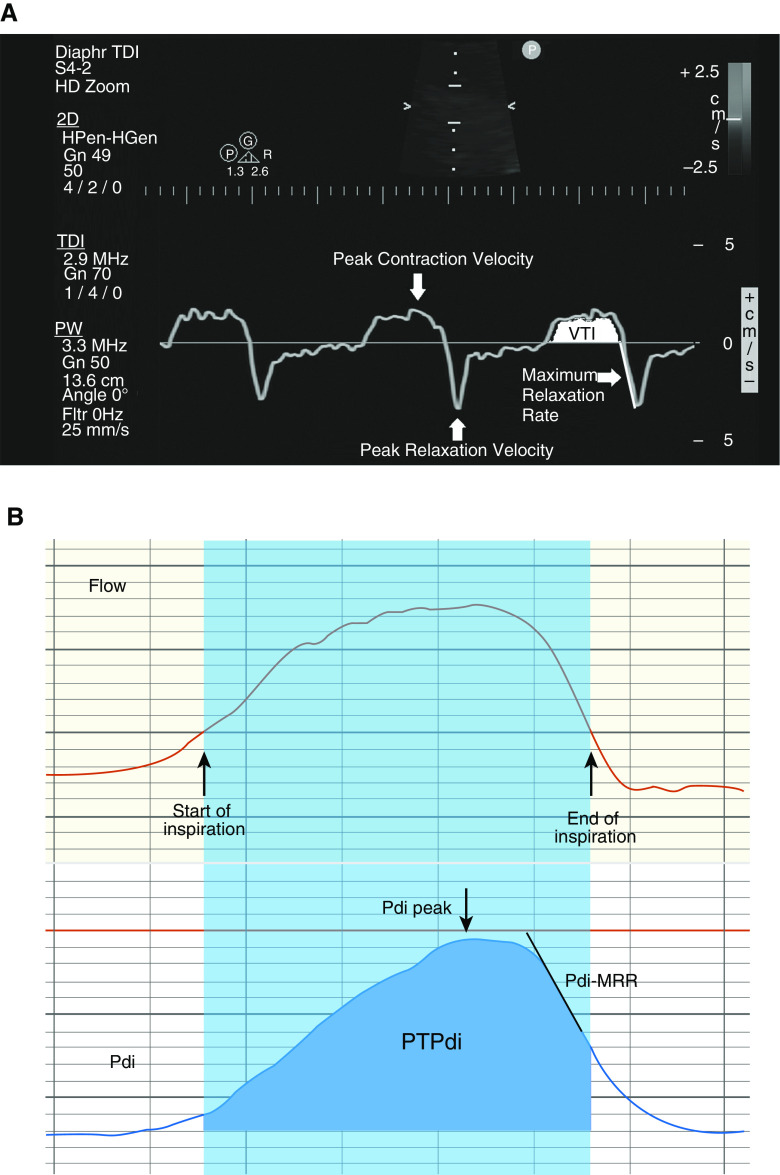
All ultrasound measurements were performed on the right hemidiaphragm because of the limited acoustic window on the left. TDI images were obtained with a phased array 2–4 MHz probe (Philips Sparq ultrasound machine). The probe was placed in the subcostal position between the midclavicular and anterior axillary lines, with the patients being examined while breathing spontaneously laying on a bed with the back elevated at 30°. As TDI measurements are angle dependent, we aimed for the ultrasound beam to reach perpendicularly the middle or posterior third of the hemidiaphragm. The sample volume was initially selected at 18.5 mm and occasionally adjusted to incorporate the whole range of diaphragmatic motion. The velocity scale used was 5 cm/sec to match the lower velocity of the moving diaphragm compared with that of the beating heart (Figure 1). Additional detail on the method for making these measurements is provided in the data supplement (Video E1 in the data supplement and Figures E1A and E1B). Eight to ten breaths per subject were saved for subsequent quantitative analysis.
Figure 1.
Diaphragmatic tissue Doppler imaging (TDI) in a healthy individual breathing quietly. Diaphragmatic TDI exhibits two waves, one during diaphragmatic contraction (above the baseline) and one during diaphragmatic relaxation (below the baseline).
On each TDI waveform, the following parameters were identified and measured (Figure 2A):
-
1.
Peak contraction velocity (PCV), defined as the maximal diaphragmatic velocity during contraction, measured in cm/s;
-
2.
Velocity–time integral (VTI), defined as the area under the TDI curve during inspiration, measured in (cm/s) ⋅ s;
-
3.
Peak relaxation velocity (PRV), defined as the maximal diaphragmatic velocity during relaxation, measured in cm/s;
-
4.
TDI-derived maximal relaxation rate (TDI-MRR), defined as the slope of the initial steepest part of the diaphragmatic motion velocity curve during relaxation, measured in cm/s2.
Figure 2.
(A) Depiction of the tissue Doppler imaging (TDI) parameters measured (peak contraction velocity, peak relaxation velocity, TDI-derived maximal relaxation rate, and the velocity–time integral); to demonstrate the points where measurements were performed, we have used the smooth control option, which softens the appearance of the trace data, allowing the TDI waveform to appear as a single continuous line. Reprinted from Reference 6. (B) Depiction of the transdiaphragmatic pressure (Pdi) parameters measured and correlated (peak Pdi, diaphragmatic pressure–time product, and Pdi-derived maximal relaxation rate) with the TDI parameters. Pdi-MRR = transdiaphragmatic pressure–derived maximal relaxation rate; PTPdi = diaphragmatic pressure–time product; VTI = velocity–time integral.
Pdi Measurements
Pdi measurements were obtained in the same breaths, concomitantly recorded with the TDI measurements (Figure 2B). Additional detail is provided in the online supplement. For the Pdi measurements, double-balloon feeding catheters (Nutrivent) were used, and the position of the catheter was adjusted after performance of a dynamic occlusion test (8). Both balloons were inflated with 2–4 ml of air and connected to a differential pressure transducer (TSD 104A; Biopac Systems Inc.), calibrated before each study with a water manometer.
Parameters measured in each Pdi waveform included (Figure 2B) the following:
-
1.
Peak Pdi, defined as peak value of Pdi measured in cm H2O;
-
2.
Diaphragmatic pressure–time product (PTPdi), measured in cm H2O · s;
-
3.
Pdi-MRR (i.e., the diaphragmatic MRR), defined as the slope of the initial steepest part of the descending part of the Pdi curve measured in cm H2O/s.
Because of the methods’ complexity and high cost, Pdi measurements were performed only in a subgroup of 24 consecutive patients. Inability to obtain a satisfactory Pdi tracing because of the poor quality of the signals, despite good positioning of the double balloon catheter additionally verified with X-ray in six patients, led to exclusion from further assessment.
The study protocol was approved by the scientific and ethics committee of the hospital (research protocol no. 526, June 1, 2018) according to national legislation, and written consent was obtained from the subjects or their surrogates.
Statistical Analysis
Recorded variables, including TDI- and Pdi-derived parameters, are presented as means ± SD or medians with 25th–75th interquartile range (IQR), according to normality of distribution of values, as appropriate. Outcome was assessed as dichotomous categorical variable (weaning success vs. weaning failure). Group comparisons were performed with chi-square, Student’s t test, or Mann-Whitney U test, whereas correlation was examined with Spearman’s rho correlation coefficient, and R2 values were provided.
Our primary hypothesis (that TDI variables differ between weaning success and weaning failure patients) was tested with statistical analysis of comparison of two independent groups. Sample size was calculated in advance with GPower 3.1 statistical program. It was therefore determined for α error probability test of 0.05, statistical power of 0.8, and effect size of 0.5 (medium) at 102 patients (Figure E2).
Finally, reliability analysis included assessment of intra- and interobserver variability with two-way mixed effects for absolute agreement between measurements for each variable separately. Interclass Correlation Coefficient (ICC) and its relative 95% confidence intervals were estimated, and ICC values >0.8 were considered very strong. Reproducibility was also assessed by calculating the mean difference between all pairs of measurements, along with its limits of agreement (means ± 1.96 × SD), which, according to Bland and Altman plotting, reflect the range that has 95% statistical validity. In addition, the coefficient of variance was calculated by the formula (SD/mean) × 100% to assess intrasubject variability.
All statistical analyses and respective graphs were performed using SPSS 25 software (IBM SPSS Statistics version 25.0). P values were considered statistically significant at the 0.05 level.
Results
Healthy Control Subjects
A total 168 different breaths from the control group of 20 healthy volunteers (about 8–10 breaths per participant) were recorded. Mean values for TDI-derived parameters are shown in Table 1.
Table 1.
Reliability Analysis of TDI Measurements (N = 168)
| Interobserver Variability | ||||||
|---|---|---|---|---|---|---|
| Variable | Observer #1 | Observer #2 | Difference | Limits of Agreement | ICC (95% CI) | CV (%) |
| PCV, cm/s | 1.38 ± 0.34 | 1.27 ± 0.34 | 0.105 ± 0.010 | −0.081 to 0.291 | 0.96 (0.95–0.97) | 5.91 |
| VTI, cm | 1.01 ± 0.53 | 1.19 ± 0.55 | 0.095 ± 0.101 | −0.103 to 0.293 | 0.98 (0.98–0.99) | 5.86 |
| PRV, cm/s | 1.21 ± 0.39 | 1.16 ± 0.39 | 0.055 ± 0.077 | −0.096 to 0.206 | 0.98 (0.97–0.99) | 4.09 |
| TDI-MRR, cm/s2 | 3.52 ± 1.97 | 3.84 ± 2.32 | 0.321 ± 0.996 | −1.631 to 2.273 | 0.89 (0.86–0.92) | 8.81 |
| Interobserver Variability | ||||||
|---|---|---|---|---|---|---|
| Variable | Observer #1 First Measure | Observer #1 Second Measure | Difference | Limits of Agreement | ICC (95% CI) | CV (%) |
| PCV, cm/s | 1.38 ± 0.34 | 1.39 ± 0.35 | 0.008 ± 0.076 | −0.141 to 0.157 | 0.98 (0.97–0.98) | 2.70 |
| VTI, cm | 1.01 ± 0.53 | 1.09 ± 0.53 | 0.004 ± 0.073 | −0.139 to 0.147 | 0.99 (0.98–0.99) | 3.18 |
| PRV, cm/s | 1.21 ± 0.39 | 1.21 ± 0.39 | 0.002 ± 0.063 | −0.121 to 0.125 | 0.99 (0.98–0.99) | 2.49 |
| TDI-MRR, cm/s2 | 3.52 ± 1.97 | 3.55 ± 1.92 | 0.025 ± 0.584 | −1.120 to 1.170 | 0.96 (0.94–0.97) | 6.11 |
Definition of abbreviations: CI = confidence interval; CV = coefficient of variance; ICC = interclass correlation coefficient; PCV = peak contraction velocity; PRV = peak relaxation velocity; TDI = tissue Doppler imaging; TDI-MRR = TDI-derived maximal relaxation rate; VTI = velocity–time integral.
Data are presented as means ± SD.
Excellent intra- and interobserver reproducibility was found for all variables, with an ICC above 89% for all measurements. Differences between measurements and limits of agreement (means ± 1.96 × 1.96 SD) are also recorded in Table 1 and were used to create respective Bland and Altman plots (Figures E3A and E3B, E4A and E4B, E5A and E5B, E6A and E5B).
Finally, coefficient of variance values were all <10% (range, 2.49–8.81), implying a narrow range of measurement variability.
Study Population
A total of 116 consecutive ICU patients were enrolled in the study. Sixty-seven patients (57.8%) succeeded the weaning trial, whereas 49 (42.2%) failed. Baseline characteristics are shown in Table 2. Groups did not differ in sex, age, length under mechanical ventilation, or reason for ICU admission.
Table 2.
ICU Patient Baseline Characteristics, Diaphragmatic Evaluations, and Between-Group Comparisons
| Total (n = 116) | Weaning Success (n = 67) | Weaning Failure (n = 49) | P Values | |
|---|---|---|---|---|
| Demographics | ||||
| Sex, M | 87 (75.2) | 47 (70.1) | 40 (81.6) | 0.158 |
| Age, yr | 66 ± 13 | 66 ± 13 | 67 ± 13 | 0.752 |
| ICU admission | ||||
| Length under MV, d | 6 (4–7) | 6 (4–8) | 5 (4–7) | 0.431 |
| Reason for ICU admission |
0.382 | |||
| Respiratory failure | 22 (18.9) | 14 (20.9) | 8 (16.3) | |
| Multiple trauma | 16 (13.8) | 6 (5.2) | 2 (4.1) | |
| Sepsis | 16 (13.8) | 12 (17.9) | 4 (8.2) | |
| Coma | 6 (5.2) | 5 (7.4) | 1 (2.0) | |
| Neurosurgery | 10 (8.6) | 5 (7.4) | 5 (10.2) | |
| Cardiac surgery | 8 (6.9) | 6 (5.2) | 2 (4.1) | |
| Other major surgery | 10 (8.6) | 2 (3.0) | 8 (16.3) | |
| Other | 28 (24.1) | 17 (25.4) | 11 (22.4) | |
| Additional evaluations | ||||
| Respiratory rate | 26.80 ± 0.50 | 25.50 ± 4.00 | 28.50 ± 6.30 | NS |
| Ti | 0.95 ± 0.20 | 0.99 ± 0.22 | 0.86 ± 0.23 | NS |
| Te | 1.32 ± 0.40 | 1.37 ± 0.28 | 1.30 ± 0.45 | NS |
| Ttot | 2.20 ± 0.43 | 2.36 ± 0.40 | 2.16 ± 0.40 | NS |
| Additional diaphragmatic evaluations | ||||
| Displacement, cm | 1.27 ± 0.48 | 1.31 ± 0.44 | 1.22 ± 0.53 | 0.102 |
| Thickening fraction, % | 0.24 ± 0.11 | 0.25 ± 0.11 | 0.23 ± 0.10 | 0.439 |
| TDI measurements | ||||
| PCV, cm/s | 1.55 (1.11–2.35) | 1.36 (1.06–1.79) | 2.02 (1.28–2.89) | 0.001 |
| VTI, cm | 0.78 (0.51–1.01) | 0.73 (0.49–0.96) | 0.89 (0.55–1.11) | 0.178 |
| PRV, cm/s | 1.68 (1.05–2.69) | 1.36 (0.96–1.78) | 2.70 (1.41–4.86) | <0.001 |
| TDI-MRR, cm/s2 | 11.83 (6.70–21.30) | 10.31 (5.66–13.74) | 21.75 (11.83–38) | <0.001 |
| Pdi measurements* | ||||
| Peak Pdi, cm H2O | 22.11 (16.94–29.39) | 16.70 (15.30–19.47) | 29.39 (22.11–48.52) | 0.003 |
| PTPdi, cm H2O · s | 5.30 (3.89–6.95) | 4.42 (3.51–5.22) | 6.44 (4.91–8.91) | 0.043 |
| Pdi-MRR, cm H2O/s | 81.71 (55.30–123.50) | 39.30 (32.30–58.57) | 110.63(85.77–147.52) | <0.001 |
Definition of abbreviations: MV = mechanical ventilation; NS = nonsignificant; PCV = peak contraction velocity; Pdi = transdiaphragmatic pressure; Pdi-MRR = diaphragmatic maximal relaxation rate; PRV = peak relaxation velocity; PTPdi = diaphragmatic pressure–time product; TDI = tissue Doppler imaging; TDI-MRR = tissue Doppler imaging–derived maximal relaxation rate; Te = expiratory time; Ti = inspiratory time; Ttot = total time; VTI = velocity–time integral.
Table entries represent means ± SD, medians (25th–75th interquartile range), or n (%), as appropriate. Comparisons between groups were performed with Student’s t test, Mann-Whitney U test, or chi-square, respectively.
Pdi measurements were performed in 18 patients (8 in the weaning success and 10 in the weaning failure group). Number of repeated measurements was 301 for peak Pdi, 266 for PTPdi, and 283 for Pdi-MRR.
Additional Evaluations and Diaphragmatic Assessments
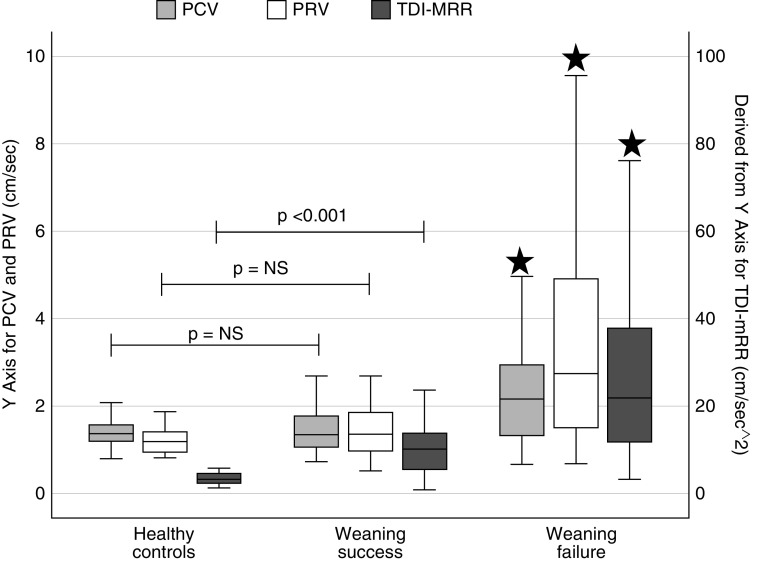
Diaphragmatic assessments in ICU patients included measurements of diaphragmatic displacement and thickening followed by TDI measurements during weaning as well as recordings of respiration timings. Results are shown in Table 2. Statistically significant differences between groups were found in PCVs and PRVs and TDI-MRR (P ≤ 0.001 for all three variables). These differences between the two groups are also clearly demonstrated in their respective TDI pattern (Figures 3A and 3B).
Figure 3.
Diaphragmatic tissue Doppler imaging (TDI) (A) in a weaning success patient and (B) in a weaning failure patient. Weaning failure patients demonstrate higher peak contraction and relaxation velocities and a sharper TDI-derived maximal relaxation rate. A transdiaphragmatic pressure tracing is superimposed to TDI, demonstrating the perfect concordance between the two waveforms concerning the beginning and the end of inspiration and expiration. Pdi = transdiaphragmatic pressure.
Interestingly, when TDI-derived values from ICU patients were compared with those recorded in healthy control subjects, PCVs and PRVs did not differ between weaning success patients and healthy volunteers (median PCV, 1.36; IQR, 1.06–1.79; vs. 1.35 cm/s; IQR, 1.21–1.57; P = 0.984; and median PRV, 1.36; IQR, 0.96–1.78; vs. 1.19 cm/s; IQR, 0.96–1.40, for weaning success and healthy control subjects, respectively). Figure 4 demonstrates the distribution of TDI measurements for all participants.
Figure 4.
Distribution of tissue Doppler imaging measurements in healthy control subjects, weaning success, and weaning failure patients (black stars: comparisons between weaning success and weaning failure patients revealed statistically significant difference). NS = nonsignificant; PCV = peak contraction velocity; PRV = peak relaxation velocity; TDI-MRR = tissue Doppler imaging–derived maximal relaxation rate.
Pdi Measurements
In a subgroup of 18 patients, peak Pdi, PTPdi, and Pdi-MRR were recorded (Table 2). All three Pdi-derived parameters were significantly lower (P ≤ 0.05) in patients who succeeded the weaning trial (n = 8) than in weaning failure patients (n = 10).
Further statistical analysis found significant correlation between peak Pdi and PCV (R2 = 0.727; P < 0.001), PTPdi and PCV (R2 = 0.650; P = 0.007), and Pdi-MRR and TDI-MRR (R2 = 0.634; P < 0.001) (Figures 5A and 5C). A weaker correlation was recorded between VTI and PTPdi (Figure 5D).
Figure 5.

(A) Relationship between peak contraction velocity and peak transdiaphragmatic pressure (Pdi). (B) Relationship between peak contraction velocity and diaphragmatic pressure–time product. (C) Relationship between tissue Doppler imaging–derived maximal relaxation rate and Pdi-derived maximal relaxation rate. (D) Relationship between velocity–time integral and diaphragmatic pressure–time product. PCV = peak contraction velocity; Pdi-MRR = transdiaphragmatic pressure–derived maximal relaxation rate; PTPdi = diaphragmatic pressure–time product; TDI-MRR = tissue Doppler imaging–derived maximal relaxation rate; VTI = velocity–time integral.
Discussion
The contraction and relaxation properties of the diaphragm have not been previously assessed with TDI, and no information exists concerning the diaphragmatic TDI pattern and range of normal values in normal individuals or in ICU patients. Our study provides the TDI pattern of diaphragmatic contraction and relaxation along with their peak corresponding values, the inspiratory VTI, and the TDI-derived relaxation rate in normal individuals and in weaning success and weaning failure patients. We found that weaning failure patients exhibited significantly higher peak contraction and relaxation velocities and higher relaxation rate compared with both healthy participants and weaning success patients (Table 2 and Figure 4).
Studies in weaning from mechanical ventilation have demonstrated that the fundamental abnormality in control of breathing in weaning failure is a shortening of inspiratory time (Ti) (9). The duration of Ti has a direct impact on the work performed by the diaphragm through changes in inspiratory flow and the Ti/Ttotal ratio (10), and incorporation of both diaphragmatic excursion and Ti into a single index has been proposed as a potential weaning predictor (11). Such decreases in inspiratory timing mean that high inspiratory velocities of diaphragmatic motion are expected in weaning failure; interestingly, in our study, no statistical significant difference was found in Ti, Texpiratory, Ttotal, and, therefore, in respiratory rate between weaning success and weaning failure patients (Table 2), suggesting that other mechanisms are possibly involved. However, when we look at Figures 5A and 5C, we observe that the higher the values of PCV and TDI-MRR, the greater the likelihood of weaning failure, and above a certain cutoff point, no patient succeeds. To evaluate the possible use of TDI-MRR as a weaning failure predictor, we estimated the area under the receiver operating characteristic, followed by sensitivity and specificity estimations for various cutoff limits (see online supplement). Area under the receiver operating characteristic was 0.80 (95% confidence interval, 0.71–0.89; P < 0.001) (Figure E7).
Another reason for the high contraction velocities observed in the weaning failure patients of our study is related to the fact that most weaning failure patients also develop an increase in respiratory motor output as they experience progressive ventilatory failure (9). The resulting increase in e can be achieved either through an increase in rate or depth of breathing, but whether an increase in diaphragmatic motion velocity constitutes part of this respiratory response is not known because diaphragmatic velocity has undergone limited investigation to date. This does not seem an unlikely possibility, as the mouth pressure developed 100 ms after occlusion, an index widely used as a measurement of respiratory motor output, is highly dependent on the contractile state of the inspiratory muscles; the latter is also influenced, among other variables, by the velocity of diaphragmatic shortening (12).
Pdi measurement assesses diaphragmatic contractility, but technical limitations and the lack of precise reference values in critically ill patients have restricted widespread clinical application of the method. In cardiac TDI, peak systolic velocity is related to ejection fraction and is used to identify systolic dysfunction; measurement of peak systolic tissue velocities are among the simplest and most robust parameters measured with TDI, with high levels of concordance between observers (13). Therefore, it seemed reasonable to correlate the TDI PCV with peak Pdi and PTPdi, the latter representing indexes of diaphragmatic contractile performance. High correlation coefficients were indeed observed (R2 = 0.727 and 0.650, respectively), suggesting a potential application of this noninvasively acquired parameter to quantify diaphragmatic performance.
Diaphragmatic MRR is traditionally calculated as the slope of a tangent drawn to the steepest portion of the descending part of the Pdi curve. However, the necessity of inserting balloon catheters for this purpose has restricted its clinical use. On the contrary, the TDI-MRR provides real-time rate of change in diaphragmatic velocity. Therefore, with TDI, the diaphragmatic relaxation rate becomes a parameter directly available rather than indirectly derived from the Pdi waveform, obviating the need to assume that changes in the Pdi curve reflect changes in diaphragmatic tension and length (14). Direct measurement of the diaphragmatic relaxation rate can be particularly important in patients in whom alterations in lung mechanics impair the transmission of pleural pressure to the upper airways or when postinspiratory activity of the diaphragm is present (14). Close agreement between Pdi-MRR and TDI-MRR (R2 = 0.634; P < 0.001) may lead to a reappraisal of diaphragmatic relaxation rate in everyday clinical practice and the employment for prognostication.
In our study, higher TDI-MRR and Pdi-MRR values were observed in weaning failure patients compared with weaning success patients (Table 2 and Figure 5C). These high values can be explained by the fact that the diaphragm must return rapidly to its initial length to preserve its pressure-generating capacity. Delayed diaphragmatic relaxation will result in incomplete expiration to functional residual capacity and will place the diaphragm at a mechanical disadvantage for subsequent inspiratory shortening. This suggests that rapid diaphragmatic relaxation may serve as a compensatory mechanism to overcome impending fatigue. This hypothesis is further supported by the fact that diaphragmatic perfusion occurs mainly during relaxation; therefore, rapid return of the diaphragm to its optimal muscle length becomes extremely important for the maintenance of adequate diaphragmatic perfusion, especially under conditions of loaded breathing (15, 16). All these remarks possibly imply that high values of diaphragmatic relaxation are necessary to avoid fatigue.
Another correlation that we looked into was the one between the VTI of the inspiratory part of the diaphragmatic TDI waveform and work of breathing derived from the Pdi tracing. The reason we assumed that this sonographic parameter could possibly represent an index of diaphragmatic work is because it is defined as the area under the diaphragmatic velocity–time curve, similarly to PTPdi being defined as the area under the Pdi-time waveform. However, a wide scatter of VTI and PTPdi values was observed in our study, resulting in a weak correlation between the VTI and PTPdi (Figure 5D); these findings limit the potential of this sonographic index to quantify the diaphragmatic work of breathing. Previous studies that have examined the potential of other ultrasonographic indices, mainly thickening fraction, to quantify diaphragmatic inspiratory effort have demonstrated a significant correlation between thickening fraction and PTPdi (17, 18); however, a considerable overlap between values has also been observed, meaning that a given thickening fraction can be associated with a wide range of PTPdi values. These observations may simply highlight the fact that the degree of diaphragmatic contraction and corresponding work of breathing may vary between subjects (19).
Our study demonstrates that measurement of diaphragmatic motion velocity using TDI is a straightforward method with a fast learning curve. This is an extremely important feature for an ultrasound technique, considering the technical difficulties of other sonographic approaches of diaphragmatic assessment, such as measurement of diaphragmatic thickness and thickening fraction or even the more recently described diaphragmatic assessment by speckle tracking ultrasound. Measurement of diaphragmatic thickness and thickening fraction requires accurate assessment of structures and structural changes of only a few millimeters, inevitably carrying a subsequent low reproducibility ratio (17, 19). Diaphragmatic speckle tracking, which has also recently been used to sonographically assess the diaphragmatic motion and strain, requires dedicated software and considerable time to perform, limiting its potential utility in clinically relevant settings.
There are some limitations in our study. First, we performed TDI measurements on the right hemidiaphragm because it is technically easier. Additionally, in some patients with large diaphragmatic displacement, contamination of the TDI sample volume with liver tissue may be observed. However, it has been demonstrated that adjacent tissues move similarly with the diaphragm during quiet and deep breathing (20). Second, although we found different TDI pattern and indices between weaning success and weaning failure patients, larger studies are needed to prove if TDI can be used as a better screening test during weaning compared with other sonographic indices. Furthermore, the potential of the TDI parameters described, especially contraction and relaxation velocities, remains to be established. Weaning failure represents a multifaceted problem in ICU patients. It would seem highly likely that diaphragmatic TDI patterns will be different in patients who fail because of respiratory muscle fatigue compared with patients who fail weaning because of impaired respiratory drive. Moreover, larger numbers of patients with homogenous lung diseases are needed to assess the TDI pattern and values to better clarify details of respiratory mechanics in different causes of weaning failure.
In conclusion, this study describes a new sonographic approach that allows real-time assessment of the velocity of diaphragmatic motion. This feature of diaphragmatic motion has not been widely evaluated so far, despite some recent studies raising the question of the role of assessment of diaphragmatic velocity in everyday clinical practice (21, 22). Interestingly, a recent multicenter study on diaphragmatic ultrasound failed to demonstrate an association between low values of diaphragmatic displacement and thickening fraction and increased risk of extubation failure (23); such observations underline the need to assess alternative sonographic indices for the evaluation of diaphragmatic function in ICU patients during the weaning process.
Supplementary Material
Footnotes
Author Contributions: D.M. conceived and designed the work. E.S., P.S., D.S., and M.T. made substantial contributions to data acquisition, analysis, and interpretation for the work. S.S. made substantial contributions to data analysis. D.M. made substantial contributions to data analysis and interpretation. E.S. and D.M. drafted the work and revised it critically for important intellectual content. S.S., P.S., D.S., and M.T. revised the manuscript critically for important intellectual content. E.S., S.S., P.S., D.S., M.T., and D.M. gave final approval of the version submitted for publication. E.S., S.S., P.S., D.S., M.T., and D.M. agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201912-2341OC on July 2, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, et al. Sonographic evaluation of the diaphragm in critically ill patients: technique and clinical applications. Intensive Care Med. 2013;39:801–810. doi: 10.1007/s00134-013-2823-1. [DOI] [PubMed] [Google Scholar]
- 2.Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med. 2011;39:2627–2630. doi: 10.1097/CCM.0b013e3182266408. [DOI] [PubMed] [Google Scholar]
- 3.DiNino E, Gartman EJ, Sethi JM, McCool FD. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax. 2014;69:423–427. doi: 10.1136/thoraxjnl-2013-204111. [DOI] [PubMed] [Google Scholar]
- 4.Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006;32:1515–1522. doi: 10.1007/s00134-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 5.Fayssoil A, Nguyen LS, Ogna A, Stojkovic T, Meng P, Mompoint D, et al. Diaphragm sniff ultrasound: normal values, relationship with sniff nasal pressure and accuracy for predicting respiratory involvement in patients with neuromuscular disorders. PLoS One. 2019;14:e0214288. doi: 10.1371/journal.pone.0214288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soilemezi E, Vemvetsou A, Kosmidis K, Dimitroulakis C, Tsagourias M, Matamis D. Evaluation of diaphragmatic function using Tissue Doppler Imaging (TDI) – correlation with transdiaphragmatic (Pdi) derived parameters [abstract] Intensive Care Med Exp. 2018;6(Suppl 2):e0054. [Google Scholar]
- 7.Sotiriou P, Soilemezi E, Kosmidis K, Vemvetsou A, Tsagourias M, Matamis D. Diaphragmatic Tissue Doppler Imaging: a new technique to evaluate diaphragmatic dysfunction? A preliminary study [abstract] Intensive Care Med Exp. 2018;6(Suppl 2):e0515. [Google Scholar]
- 8.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126:788–791. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- 9.Tobin MJ. Principles and practice of mechanical ventilation. 2nd ed. New York: McGraw-Hill Medical Publishing Division; 2006. Weaning from mechanical ventilation; p. 1187. [Google Scholar]
- 10.Vassilakopoulos T, Zakynthinos S, Roussos Ch. Respiratory muscles and weaning failure. Eur Respir J. 1996;9:2383–2400. doi: 10.1183/09031936.96.09112383. [DOI] [PubMed] [Google Scholar]
- 11.Palkar A, Narasimhan M, Greenberg H, Singh K, Koenig S, Mayo P, et al. Diaphragm excursion-time index: a new parameter using ultrasonography to predict extubation outcome. Chest. 2018;153:1213–1220. doi: 10.1016/j.chest.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Roussos C. The Thorax – part B: applied physiology. 2nd ed. Boca Raton. CRC Press; 1995. p. 1111. [Google Scholar]
- 13.Marwick TH. Clinical applications of tissue Doppler imaging: a promise fulfilled. Heart. 2003;89:1377–1378. doi: 10.1136/heart.89.12.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coirault C, Chemla D, Lecarpentier Y. Relaxation of diaphragm muscle. J Appl Physiol (1985) 1999;87:1243–1252. doi: 10.1152/jappl.1999.87.4.1243. [DOI] [PubMed] [Google Scholar]
- 15.Hu F, Comtois A, Grassino AE. Optimal diaphragmatic blood perfusion. J Appl Physiol (1985) 1992;72:149–157. doi: 10.1152/jappl.1992.72.1.149. [DOI] [PubMed] [Google Scholar]
- 16.Hussain SNA, Roussos C, Magder S. Effects of tension, duty cycle, and arterial pressure on diaphragmatic blood flow in dogs. J Appl Physiol (1985) 1989;66:968–976. doi: 10.1152/jappl.1989.66.2.968. [DOI] [PubMed] [Google Scholar]
- 17.Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW, et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med. 2012;38:796–803. doi: 10.1007/s00134-012-2547-7. [DOI] [PubMed] [Google Scholar]
- 18.Umbrello M, Formenti P, Longhi D, Galimberti A, Piva I, Pezzi A, et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care. 2015;19:161. doi: 10.1186/s13054-015-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haaksma M, Tuinman PR, Heunks L. Ultrasound to assess diaphragmatic function in the critically ill-a critical perspective. Ann Transl Med. 2017;5:114. doi: 10.21037/atm.2017.01.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies SC, Hill AL, Holmes RB, Halliwell M, Jackson PC. Ultrasound quantitation of respiratory organ motion in the upper abdomen. Br J Radiol. 1994;67:1096–1102. doi: 10.1259/0007-1285-67-803-1096. [DOI] [PubMed] [Google Scholar]
- 21.Maurizio R, Rinaldi VE, Camerini PG, Salvatori C, Leonardi A, Bini V. Right diaphragmatic peak motion velocities on pulsed wave tissue Doppler imaging in neonates: method, reproducibility, and reference values. J Ultrasound Med. 2019;38:2695–2701. doi: 10.1002/jum.14974. [DOI] [PubMed] [Google Scholar]
- 22.Palkar A, Mayo P, Singh K, Koenig S, Narasimhan M, Singh A, et al. Serial diaphragm ultrasonography to predict successful discontinuation of mechanical ventilation. Lung. 2018;196:363–368. doi: 10.1007/s00408-018-0106-x. [DOI] [PubMed] [Google Scholar]
- 23.Vivier E, Muller M, Putegnat JB, Steyer J, Barrau S, Boissier F, et al. Inability of diaphragm ultrasound to predict extubation failure: a multicenter study. Chest. 2019;155:1131–1139. doi: 10.1016/j.chest.2019.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.