Abstract
Early heart morphogenesis involves a process in which embryonic precursor cells are instructed to form a cyclic contracting muscle tube connected to blood vessels, pumping fluid. Subsequently, the heart becomes structurally complex and its size increases several orders of magnitude to functionally keep up with the demands of the growing organism. Programmed transcriptional regulatory networks control the early steps of cardiac development. However, already during the early stages of its assembly, the heart tube starts to produce electrochemical potentials, contractions, and flow, which are transduced into signals that feed back into the process of morphogenesis itself. Heart morphogenesis, thus, involves the interplay between progressively changing genetic networks, function, and shape. Morphogenesis is evolutionarily conserved, but species-specific differences occur and in mouse, for instance, distinct phases of development become overlapping and compounded in an extremely fast gestation. Here, we review the early morphogenesis of the chambered heart that maintains a circulation supporting development of an organism rapidly growing in size and requirements.
GENERALIZED VIEW OF EVOLUTIONARILY CONSERVED VERTEBRATE CHAMBERED HEARTS
The vertebrate heart is a unidirectional muscular pump controlled by an intrinsic electrical signal. It consists of an outer layer of muscle and inner layer of endothelial endocardium. During development, an expanding cell layer covers the muscle to form the epicardium and invades the muscle wall to form interstitial fibroblasts and other cell types and helps building the coronary vasculature (Cao et al. 2019). In all vertebrates with a chambered heart (from fish to mammals), the heart tube forms a relative constriction between the expanding atrial and ventricular compartment, the atrioventricular (AV) canal (Fig. 1; Kim et al. 2001; Moorman and Christoffels 2003; Jensen et al. 2013). At this position, on the inside of the tube, cushions and unidirectional valves will form that support one-way blood flow and prevent regurgitation to the atrium during ventricular systole. Moreover, at the inflow tract, small and relatively weak valves form, and at the outlet, sturdy valves form that perform similar functions to prevent filling of diastolic compartments by downstream blood (Fig. 1). In all vertebrate species examined, the electrical impulse that sets calcium-mediated contraction in motion is generated at the atrial inflow, travels rapidly over the atria causing them to contract, propagates slowly through the AV canal myocardium, and then travels rapidly through the ventricle causing this strong muscle to contract subsequently (Van Mierop 1967; Silverman et al. 2006; Park and Fishman 2017; van Eif et al. 2018; Tyser and Srinivas 2019). In amphibians, reptiles, birds, and mammals, the atrium is divided by a septum in a right compartment that receives blood from the systemic circulation, and a left compartment that receives blood from the pulmonary circulation. In mammals, birds, and some reptiles (e.g., crocodiles) a muscular ventricular septum is present that divides the ventricular compartment in a high-pressure systemic (left) and low-pressure pulmonary (right) compartment (Crossley et al. 2016). The septum is associated with a specialized AV conduction system, consisting of an AV node, AV bundle, and bundle branches (Silverman et al. 2006; Jensen et al. 2018). Although fish, amphibians, and reptiles have a predominantly trabecular ventricular wall, mammals and birds have thick compact ventricular walls with comparatively few trabeculations at the luminal side, and a Purkinje system that rapidly transmits and distributes the electrical signal to the ventricular working myocardium (Sedmera 2011; Boukens et al. 2019). Besides cardiac muscle cells, many other cell types are present, such as fibroblasts, endocardial cells, endocardium-derived cell types, smooth muscle, resident macrophages, etc., which play roles in structural and functional homeostasis of the organ (Pinto et al. 2016). After almost two decades of claims, raising hope with misleading data, it has now been widely accepted that resident stem cells for cardiomyocytes are not present in numbers that allow for significant regeneration after injury, muscle loss, and scarring (Cai and Molkentin 2017; Eschenhagen et al. 2017). Nevertheless, mature hearts of particular fish and amphibian species show a striking ability to regenerate after extensive damage, by reinitiating cell divisions of surviving cardiomyocytes, as do mice and possibly humans until shortly after birth. In contrast, postnatal mammals, in general, do not regenerate lost muscle, but will respond to injury by activating stress, survival, and scar-forming processes (Vivien et al. 2016; Tzahor and Poss 2017). The muscle cells formed shortly after birth will last for the duration of mammalian postnatal life with very little renewal of cardiomyocytes (Bergmann et al. 2015).
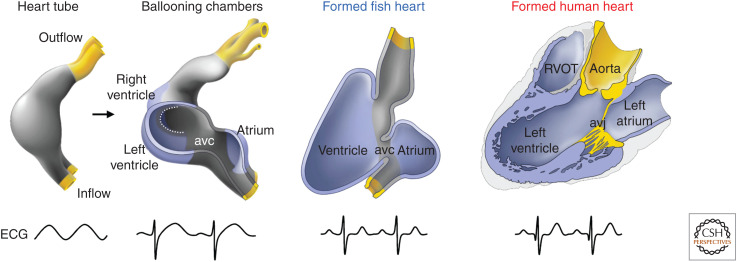
Figure 1.
The building plan of the vertebrate heart. All vertebrate hearts develop from a heart tube with intrinsic pacemaking caudally and in which the electrical propagation is slow giving rise to a sinusoid electrocardiogram (ECG). During “ballooning,” chambers form locally on the outer curvature of the heart tube. Because fast electrical propagation is part of the chamber program, the heart now acquires alternate regions of slow and fast propagation and the resultant ECG is adult-like. Much of this configuration persists in the formed hearts of vertebrates from fish to human. Note that the chamber configuration of the chambered embryonic heart, fish heart, and adult human heart are essentially the same. (avc) Atrioventricular canal (myocardial), (avj) atrioventricular junction (most of the myocardium is disrupted by the insulating plane), (RVOT) right ventricular outflow tract. (Modified from Jensen et al. 2013, with permission, from Elsevier © 2013.)
In this review, we briefly summarize general and evolutionarily conserved aspects of heart morphogenesis from the specification of heart field mesoderm to the formation of the chambered heart with valves and a conduction system. We emphasize (1) formation of the initial heart tube and its elongation by addition at the inflow and outflow of cardiomyocytes from rapidly proliferating progenitor pools; (2) patterning of the heart tube and localized differentiation and growth underlying chamber morphogenesis; (3) the formation of the sinus venosus, AV canal, and outflow tract and associated valves; (4) the development of the pacemaker and conduction system; and (5) the impact of developing heart function on morphogenesis. Cells comprising structures in the embryonic heart are not the precursors of equivalent structures in the mature heart. For example, particular second heart field (SHF) progenitor cells will initially form the embryonic outflow tract, and perform outflow tract functions (sphincter, slow conduction, long-lasting contracted state), whereas they will differentiate into ventricular working cardiomyocytes and will be incorporated into the right ventricle in the formed heart. The implications of this insight will be highlighted as well.
EARLY HEART TUBE FORMATION AND ELONGATION: SWITCHING FROM PROLIFERATION TO DIFFERENTIATION
Cardiac morphogenesis involves a series of iterative steps of progenitor cell expansion, intercellular signaling, transcriptional response, patterning, and differentiation into more specialized cell types. This cyclic process is at the basis of the formation of structures comprised of different cell types with specialized functions. The exact moment heart development starts is debatable. Gastrulation produces ectoderm, primitive endoderm, and, in between the axial and lateral plate, mesoderm from the epiblast. The mesoderm is the main source of the cells of the heart. Localized Wnt, Fgf, and BMP signaling from the neural and dermal ectoderm and from the endoderm localize induction of specification of cardiogenic mesoderm to the anterolateral zones of the intraembryonic mesoderm (Harvey 2002). Cells expressing transcription factor Mesp1 in mesoderm are specified into cardiac precursors, activate Smarcd3 (encoding an SWI/SNF-related chromatin-modifying enzyme), and subsequently cardiogenic transcription factors, including Isl1, Tbx5, Gata family members, and Nkx2-5, causing the further specification of these cells, which are referred to as the heart field(s) (Evans et al. 2010; Devine et al. 2014; Meilhac et al. 2015). Expression of these factors in the heart fields is not homogeneous. For example, Tbx1 and Fgf10 will be activated in cells fated to form the outflow tract and right ventricle, Tbx5 will be activated in the cardiogenic progenitors giving rise to components upstream of the future right ventricle (everything but the right ventricle and outflow tract), whereas Tbx18 will be activated selectively in progenitors fated to form sinus venosus (sinus horns, sinus node, venous valves) and epicardium (Meilhac et al. 2015). Nkx2-5 is switched off in the sinus venosus progenitors before their differentiation (Mommersteeg et al. 2010), and Isl1 expression is switched off in progenitors that differentiate and form the cardiac crescent and heart tube, but maintained at high levels in the progenitors and the formed sinus node (Cai et al. 2003). Furthermore, many mesodermal cells fated to form heart will only activate such cardiogenic transcription factors at later stages, indicating the heart fields expand during development (Bressan et al. 2013). Taken together, heart fields are heterogeneous in both time and in space.
During the process of heart field specification, the embryo folds around the endoderm, which forms a primitive foregut (Fig. 2). Formation of the foregut and heart tube from fusing bilateral fields are intimately related and conserved across species and particularly well-studied in chicken (Abu-Issa and Kirby 2007; Hosseini et al. 2017). The heart fields migrate with the invaginating foregut such that the lateral edges of the heart fields (cardiac crescent) fuse at the midline, from anterior to posterior (Fig. 2). This forms a trough, closed at the ventral side but open and in direct communication with the ventral foregut endoderm. This trough is often already referred to as the primitive heart tube (Fig. 3). Simultaneously, endocardial cells that differentiated and formed small tubes in the crescent fuse and form an endocardial tube that becomes enveloped by the embryonic cardiomyocytes. At the dorsal side, the myocardial trough wall forms a continuum with the pericardial wall epithelium (the SHF cells; see below) (Figs. 2 and 3). Subsequently, the trough also closes dorsally, and the ridge that connects the tube to the dorsal pericardial wall, the dorsal mesocardium, is disrupted, leaving the tube connected only at the inflow and outflow to the pericardial wall (Fig. 2). Heart tube formation from progenitors in zebrafish and Xenopus frogs, two model organisms used frequently in developmental studies, differs in a number of details, but is essentially comparable (Smith et al. 2008; Evans et al. 2010). The underlying regulatory networks are conserved between vertebrate species, and even in model organisms like tunicates (Ciona) and fruit fly (Drosophila) homologous network components were found to control heart tube formation (Zaffran and Frasch 2002; Wang et al. 2019).
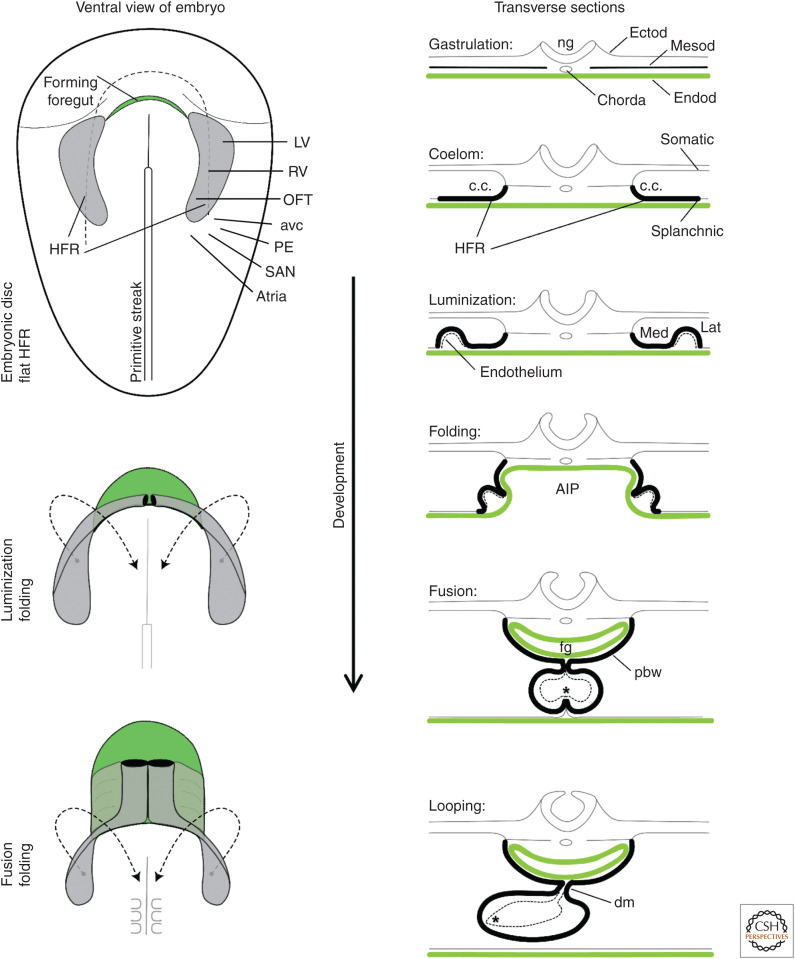
Figure 2.
Early heart development and morphological changes. On the left, it is illustrated that the folding of the embryo associates with foregut formation (green) and that the classically recognized heart-forming region ([HFR], gray, including first heart field [FHF] and second heart field [SHF]) swings toward ventral and medial to progressively fuse at the midline. Within the HFR are cells of the future left ventricle (LV), right ventricle (RV), and the outflow tract (OFT). Contiguous to this HFR are cells that will come to form the atrioventricular canal (avc), proepicardium (PE), the atria, and the sinus venosus/sinus node (SAN). On the right, the illustrations show schematic transverse sections of the changes that occur in association with the folding of the embryo. Note the relation between the lateral HFR rim and the ventral heart tube. The medial HFR becomes the pericardial back wall (pbw), or SHF. (AIP) Anterior intestinal portal, (c.c.) coelomic cavity, (dm) dorsal mesocardium, (Ectod) ectoderm, (Endod) endoderm, (fg) foregut, (Lat) lateral, (Med) medial, (Mesod) mesoderm, (ng) neural groove. The asterisk indicates contact between endocardium and myocardium. (Modified from van den Berg and Moorman 2009, with permission from the authors. Fate-mapping of structures based on data in Bressan et al. 2013 and Dyer and Kirby 2009.)
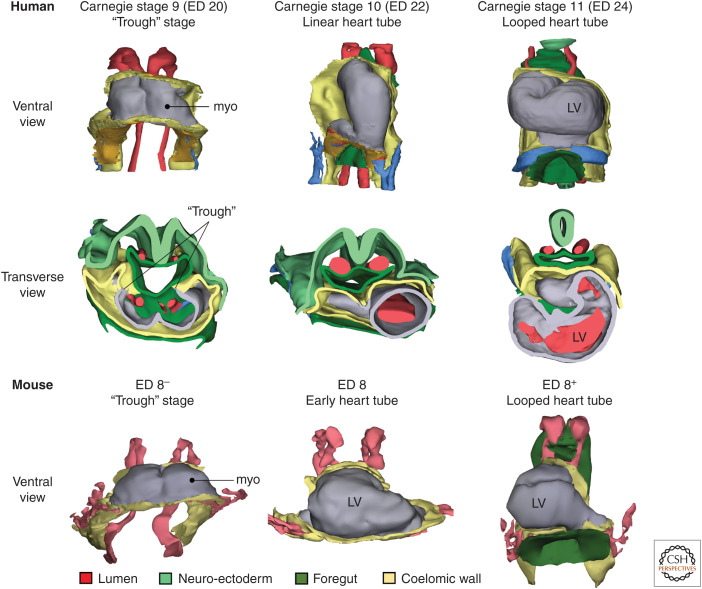
Figure 3.
Early heart morphogenesis in human and mouse. In Carnegie stage 9, or approximately day 20 of gestation, the heart resembles a “trough” because the heart has not yet closed dorsally. By Carnegie stage 10, or approximately day 22 of gestation, the linear heart tube has formed around a single lumen and the heart tube has liberated itself from the coelomic wall, except at the inflow and outflow. By Carnegie stage 11, or approximately day 24 of gestation, the heart tube has looped and early stages of ballooning of the left ventricle (LV) can be seen. In mouse, within few hours around embryonic day 8, the heart transitions from a trough to a looped tube with early chamber formation. (myo) Myocardium. (The figure is based on 3D models in Sizarov et al. 2011 and de Boer et al. 2012.)
The heart tube increases four- to fivefold in length during the next stages of development, but the cardiomyocytes of the tube do not proliferate (van den Berg et al. 2009). Early fate map studies by de la Cruz indicate that the early chicken heart tube gives rise to the left ventricle, and perhaps the AV canal, whereas the remainder of the cardiac components are added to the heart tube thereafter (de la Cruz et al. 1977, 1989; de la Cruz and Markwald 1998). Virágh and Chalice (1973) proposed that the splanchnic mesothelial (mesodermal) cells (dorsal pericardial cavity wall epithelium) continuously differentiate into myocardium to form the outflow tract, reminiscent to the contributions of coelomic epithelium-derived cells to organogenesis in general (Ariza et al. 2016). Studies from several laboratories identified subpopulations of the progenitor pool in the dorsal pericardial wall and pharyngeal region of mouse and chicken from which cardiomyocytes are added to the outflow of the heart tube, to give rise to the right ventricle and outflow tract (Dyer and Kirby 2009; Kelly et al. 2014). Retrospective clonal analysis studies have indicated that two distinct lineages segregating early from a common progenitor contribute to the mouse E8.5 heart tube. The presumptive left ventricle and outflow tract (which will become right ventricular 1–2 days later), respectively, are derived from a single lineage, whereas the remainder of the heart tube compartments are colonized by both lineages (Meilhac et al. 2004a). The population of progenitors giving rise to myocardium (and endocardium) of all heart components expresses Isl1 and Nkx2-5 (Ma et al. 2008). Isl1 expression is strikingly maintained in all progenitors but switched off during myocardial differentiation, allowing the visualization of the progenitors in relation to the forming heart tube (Cai et al. 2003). In sharp contrast to the crescent/heart tube, the progenitors in the pericardial cavity wall dorsally and medially from the heart tube proliferate at high rates, and dye labeling in chicken embryos has visualized their migration and expansive growth and contribution to either the outflow tract or inflow tract (Fig. 4; van den Berg et al. 2009). Thus, two progenitor cell populations can be identified, those that differentiate early, cease proliferation, and form the cardiac crescent/heart tube by E7.5–E8 of mouse development (mainly future left ventricle and AV canal; see below), and those that lie medially to the crescent and have maintained the progenitor state (undifferentiated, rapid proliferation) to be added progressively to the elongating heart tube later and form the other structures. Just before their myocardial differentiation and addition to the cardiac trough/heart tube, these progenitors also cease proliferation (Fig. 4). The sources of these cells have been named first heart field (FHF) and SHF, respectively (Meilhac and Buckingham 2018).
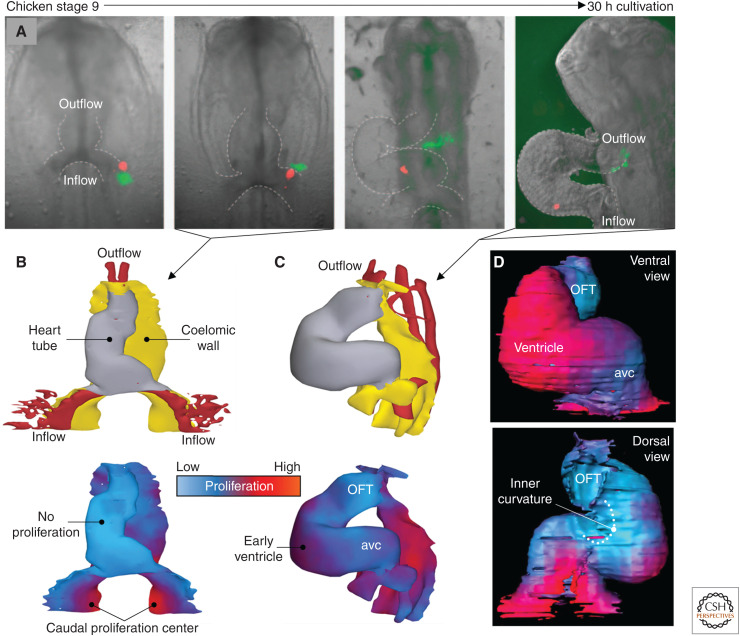
Figure 4.
Additions of cells to the heart tube by cells of the coelomic wall. (A) Fluorescent labeling of cells in a cultured chicken embryo (Hamburger–Hamilton stage 9) to track the substantial movement of cells from the highly proliferative coelomic wall. (B) In Hamburger–Hamilton stage 10, corresponding to the second image from the left in A, the linear heart tube shows virtually no proliferation (1 hour after BrdU exposure), whereas the coelomic wall is highly proliferative. (C) At Hamburger–Hamilton stage 14, corresponding approximately to rightmost image in A, proliferation is starting to reinitiate on the outer curvature of the looped heart tube (1 hour after BrdU exposure). (D) At Hamburger–Hamilton stage 12, long exposure to BrdU (10 hours) reveals pronounced differences in proliferation between the forming ventricle (high, ventral view) and the inner curvature (low, dorsal view). (avc) Atrioventricular canal, (OFT) outflow tract. (A–C based on modified images and 3D models in van den Berg et al. 2009; D based on a 3D model in Soufan et al. 2006.)
Several additional genetic markers identified subpopulations within the FHF and SHF, also highlighting that the SHF cells added to the outflow (anterior SHF) are molecularly distinguishable (e.g., Fgf10+, Tbx1+, Mef2-SHF-enhancer+) from those that contribute to the inflow tract (posterior SHF; e.g., Tbx5+, Osr1+, Tbx18+) (Meilhac and Buckingham 2018). Strict boundaries between the FHF and SHF do not exist, as heart tube assembly is progressive, with SHF cells continuously differentiating and adding to the heart tube. The distinction between the fields was chosen arbitrarily at E7.5, when the FHF has differentiated to form the cardiac crescent. Indeed, the descendants of cells marked by different genetic markers for (sub)heart fields show a unique pattern in the heart with distinct borders (Cai et al. 2003; Verzi et al. 2005; Xu et al. 2005; Christoffels et al. 2006; Tian et al. 2010; Bertrand et al. 2011; Liang et al. 2013; Devine et al. 2014; Zhou et al. 2015, 2017; Meilhac and Buckingham 2018). However, a border is present between the anterior and posterior SHF contributions to the outflow and inflow side, respectively (see below). Moreover, a less-well-defined border has also been identified by several genetic lineage markers at the level of the interventricular septum (Devine et al. 2014). This is also implied by the retrospective clonal analysis, if we assume that the right ventricle of the E8.5 mouse heart tube is largely taken up by the definitive interventricular septum, and the E8.5 outflow tract forms the definitive right ventricle. Equivalent heart fields/progenitor cells have been identified in zebrafish and Ciona tunicates (de Pater et al. 2009; Zhou et al. 2011; Felker et al. 2018; Wang et al. 2019). In fish, the (less-well-defined and lineage-marker-dependent) border between the FHF and SHF contribution is found halfway in the ventricle (Zhou et al. 2011; Felker et al. 2018), suggesting that perhaps the proximal fish ventricle (closer to the AV canal) and distal fish ventricle (closer to the outflow tract) are homologs to the left and right ventricle, respectively, of mammals and birds, and that the interventricular septum evolved at this border. Although somewhat oversimplified, the SHF has been a valuable concept that has helped to understand the mechanisms underlying particular congenital defects of the outflow (e.g., tetralogy of Fallot or transposition of the great arteries) or atria and AV canal.
PATTERNING, GROWTH, AND CHAMBER FORMATION
The heart tube can be considered to undergo four major processes: (1) elongation and directional looping to acquire an asymmetric S shape, or left-handed spiral around an imaginary caudocranial axis facing the inner curvature, which sets the definitive configuration of the chambers; (2) localized differentiation and expansion of ventricular and atrial myocardium at the outer curvatures of the S shape (so-called “ballooning”); (3) formation of valves and septa; and (4) formation and ingression of the epicardium and coronary vasculature. Of note, mouse heart tube morphogenesis looks different from that in chicken, human, or fish; as the distance between the neural fold and anterior intestinal portal is relatively small compared with embryo size in mice, the initial heart tube (at ∼E7.5–8) is much shorter and broader than in the other species. Therefore, it may appear similar to the developmentally earlier crescent in human or chicken, even though fusion is already advanced and chamber differentiation may already have been initiated. The latter species, but also fish and amphibians, go through a proper (and attractive to study) straight heart tube stage before extensive looping and chamber differentiation occurs.
The heart tube is asymmetric along all three spatial axes, and the asymmetry in morphology becomes more and more apparent as chambers develop. Atria develop dorsally and caudally, ventricles ventrally and cranial of the atria (right ventricle cranial of the left ventricle). Moreover, the atria and sinus venosus show pronounced left–right asymmetry as do the great arteries, and the heart tube loops always in the same direction (called “rightward”). This requires patterning along all three axes before or during heart tube formation (Harvey 2002; Moorman and Christoffels 2003). Although the heart tube elongates, the distance between its connections with the body, the inflow tract and outflow tract stay largely fixed. As a consequence, the heart tube has to fold, or loop, within the pericardial cavity as soon as the dorsal mesocardial connection has been disrupted (Männer 2009). The initial ventral direction of looping is probably caused by ventral-specific reinitiation of proliferation (see below) of the middle portion of the tube. The direction of looping is evolutionarily conserved and always the same in uncompromised individuals. In mammals and birds, the inflow will point slightly to the left, the main bulk of the tube (future left ventricle) then crosses from left to right (“rightward looping”), and the most cranial, downstream part (future interventricular septum and right ventricle) then turning from right to left and the last portion to the right again to maintain a perpendicular connection to the outflow orifice (Fig. 3). The left side of the embryo has been subject to leftness signaling (Nodal, Lefty, etc.) during and after gastrulation (Grimes and Burdine 2017). This converges on selective Pitx2c expression in the left heart field, which impacts on asymmetric morphogenesis of the heart (Desgrange et al. 2018). A recent study proposed that asymmetries at the fixed heart poles generating opposite deformations, associated with the progressive release of the heart tube by disruption of the dorsal mesocardium, are sufficient to generate looping of a tube growing between fixed poles. This model explains how initial left–right differences between heart precursors are amplified, resulting in directional looping morphogenesis (Le Garrec et al. 2017).
As soon as heart tube formation is initiated, the ventral region initiates the expression of a chamber myocardium–associated program (mouse E8.5). This program includes Nppa, Nppb and Gja5, Smpx, Bmp10, Atp2a2 (Serca2a), among others (Christoffels et al. 2000). During and after looping, the chamber myocardium, now at the outer curvature, will expand dramatically to form most of the future left ventricle (Fig. 5). The future right ventricle, also at the outer curvature (former ventral side) too initiates this program, albeit to a lesser extent. At mouse E9–9.5, the dorsocaudal portion of the heart tube also initiates a chamber myocardium–associated program (Fig. 5). These regions represent the future atrial myocardium. The initiation of this program is also associated by reinitiation of the cell cycle, which happens only in chamber myocardium, causing it to expand exponentially (Blausen et al. 1990; Soufan et al. 2006; Butcher et al. 2007; Sizarov et al. 2011; de Boer et al. 2012). On their differentiation and reinitiation of proliferation, the ventricular chambers immediately start to form trabecules under control of Notch, Neuregulin, Ephrin, and Bmp10 signaling, etc. (del Monte-Nieto et al. 2018; MacGrogan et al. 2018). The chambers expand exponentially in size and cell number (de Boer et al. 2012). The growth of the cardiomyocytes is clonal (daughter cells do not disperse) and oriented, with specific orientations in the different compartments, which likely contributes to compartment-specific morphogenesis (Meilhac et al. 2003, 2004b). The expansion and growth of the (compact) wall are under control of intrinsic and extrinsic signals from the epicardium that will envelop the heart muscle at the pericardial lumen side. Epicardial cells will invade the wall, form interstitial fibroblast and contribute to the coronary vasculature that will develop from these stages onward (He et al. 2019). The inflow (sinus venosus) and outflow portions of the elongated tube, the inner curvatures of the ventricular and atrial zones, and the now visible AV canal between the atria and ventricles maintain their embryonic phenotype, and do not initiate the rapid proliferative phase. Suppression of differentiation and proliferation along with induction of cushion formation at these sites involves a complex BMP-Gata-Tbx2/3-Notch regulatory network (Stefanovic et al. 2014; MacGrogan et al. 2018; van Eif et al. 2018). The concept of chamber differentiation and expansion at the outer curvatures of the looping primary heart tube has been named the “ballooning model” (Christoffels et al. 2000).
Figure 5.
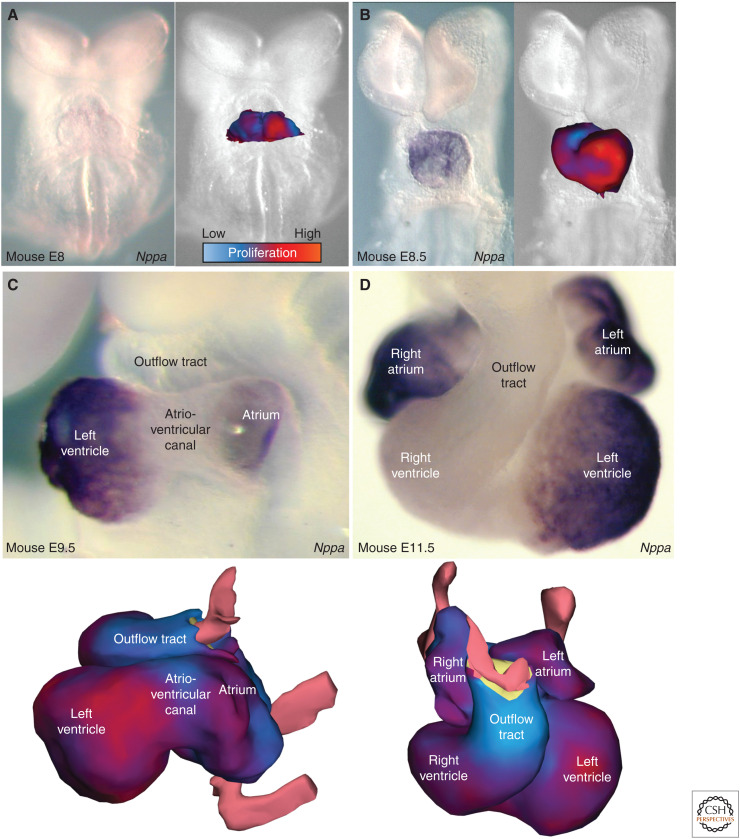
Ballooning chambers by proliferation (1 hour Brdu exposure) and formation of the building plan of the heart. (A) In mouse embryonic day 8, ventral view, the heart is trough-shaped but unlike in human and chicken, local high levels of proliferation are already revealing the future left ventricle. (B) In mouse embryonic day 8.5, ventral view, expression of Nppa and high levels of proliferation identifies the developing left ventricle. (C) By 9.5 days of embryonic mouse development, viewed from the left, the atrium is also expressing Nppa, which again is mirrored in higher levels of proliferation (bottom). In contrast, the atrioventricular canal and the outflow tract maintain a heart-tube-like phenotype. (D) Much of the building plan of the heart is established by embryonic day 11.5 in mouse. (Modified from images in Christoffels et al. 2000 and de Boer et al. 2012, with permission, from Elsevier © 2000.)
The dorsoventral patterning underlying ventral ventricular and dorsal atrial chamber differentiation is not well known. The ventral side of the heart tube is derived from the lateral rims of the heart fields (Fig. 2), which could provide localized signals at that stage. Moreover, it could also be a matter of timing. The ventral side is the earliest mesoderm to differentiate to myocardium, because the caudal side still forms a continuity with the SHF progenitors in the pericardial wall that add myocardium dorsally until the mesocardium is disrupted. The transcription factor Hand1, required for cardiac development (George and Firulli 2019), and expressed in the outer curvature of the chamber-forming heart, shows a beautiful ventral-restricted pattern in the heart tube stage (Christoffels et al. 2000).
The most prominent pattern of the heart tube is that along the anteroposterior, or craniocaudal, axis. Markers like atrial-specific myosin, Myh6, Myh7, Myl2 (Mlc2v), Tbx5, Nr2f2 (Coup-TFII), and Irx4 were among the first to reveal these patterns, as they were expressed in anteroposterior patterns but not differentially expressed along dorsoventral or left–right axes (and as such also not strictly associated with chamber myocardium, but with an anteroposterior segment of the tube within which either ventricular or atrial chamber myocardium develops) (Christoffels et al. 2000). As discussed above, the tube elongates at both cranial and caudal poles. The cranially added cells will form in chronological order the definitive interventricular septum, right ventricle, right and left ventricular outlets, and noncardiomyocyte intrapericardial portions of the great arteries (Dyer and Kirby 2009; Mohan et al. 2018). Of note, all of the cardiomyocytes added to the outlet side briefly obtained the phenotype and function of embryonic outflow tract before they differentiated in accordance with their definitive fate such as right ventricle. The caudally added cardiomyocytes that will form definitive AV canal (AV node and transitional cells), atria, and sinus venosus, first acquired an inflow tract phenotype during caudal expansion, including dominant pacemaker activity (Hcn4 expression), before they moved on to their final destiny such as (Hcn4-negative) atrial working myocardium. Retinoic acid signaling likely plays a key role in providing anteroposterior identity. During and after specification of the heart fields, the caudal portion of the embryo expresses Raldh2, the rate-limiting retinoic acid synthesis enzyme in the embryo (Simões-Costa et al. 2005). The caudal cardiac precursors overlap with this domain, receive retinoic acid signaling, and acquire a caudal identity. Tbx5 is a target of retinoic acid and is expressed in and required for the development of the caudal heart components (De Bono et al. 2018). Hox genes are targets of retinoic acid signaling as well, expressed in caudal domains, and also involved in the development of caudal structures (Bertrand et al. 2011). The nuclear receptor Coup-TFII (NR2F2) was identified as a retinoic acid receptor. It is expressed caudally and required for formation of the caudal portion of the heart tube (Pereira et al. 1999). Interestingly, Coup-TFII is required and sufficient for atrial identity of chamber myocardium (Wu et al. 2013). Whereas Tbx5, Osr1, etc. control deployment of the posterior SHF, Tbx1, Fgf8, and Fgf10, etc. control anterior SHF deployment. The border between anterior and posterior SHF progenitors in the dorsal pericardial wall is maintained by RA signaling, Coup-TFII, Tbx1, Tbx5, Hedgehog/Gli, Osr1, and other factors (Pereira et al. 1999; Niederreither et al. 2001; Ryckebusch et al. 2008; Lin et al. 2012; Xie et al. 2012; Rana et al. 2014; Zhou et al. 2015; De Bono et al. 2018). Disruption of any of these factors causes border disruption, dysfunction of the respective SHF, and defects in outflow- and inflow-derived structures.
THE DEFINITIVE HEART CONFIGURATION, WITH VALVES AND SEPTA
From mouse E9 onward, chamber differentiation is well underway. Looping has completed by E9.5 and between E9.5 and 10.5 the AV canal expands (shifts) to the right to maintain the connection of the right atrium with the right ventricle. By mouse E10-11 (human 4–5 weeks), the basic building plan of the heart and the definitive configuration of the chambers has been established (Fig. 5). The first morphological signs of interventricular septum formation occur at E9.25, when trabecular ventricular myocardium visibly forms and a few trabecular ridges seemingly fuse in between the future left and right ventricles (Mohun and Anderson 2019). The septum grows by proliferation at the same speed as the walls of the ventricles expand (Sedmera et al. 2003). The jelly that initially filled the space between the myocardial mantle and endocardial lining disappears where chambers differentiate, causing the endocardium and myocardium to make contact. The jelly at the level of the AV canal, inner curvatures, and outflow tract, in contrast, are maintained, and even expand. Here, endocardial cells transition to mesenchyme and populate the jelly and contribute to the growth and morphogenesis of the cushions. In addition, mesenchymal cells derived from the cardiac neural crest migrate into and populate the distal outflow tract cushions (Waldo et al. 1998). These cushions play crucial roles in valve formation and septation of the AV junction and the systemic and pulmonary arterial outlets. In addition, starting at E9.5, a “protrusion” is seen to expand in the dorsal wall of the atrium, the dorsal mesenchymal protrusion, or vestibular spine (Mommersteeg et al. 2006; Snarr et al. 2007). This protrusion is associated with the forming pulmonary vein and additionally forms a complex with the superior and inferior AV cushions and together ensures correct septation of the AV junction (Briggs et al. 2012). Errors in dorsal mesenchymal protrusion patterning or proliferation or cause severe AV septal defects. Involved transcriptional networks have been described, including Tbx1, Tbx5, Osr1, Hedgehog, etc. (e.g., Xie et al. 2012; Rana et al. 2014; Zhou et al. 2015). Although the superior and inferior AV cushions form from mouse E9.5 onward, the mural cushions (along the left and right AV canal wall) only become prominent later, from E11.5 onward, and will form the mural leaflets of the mitral and tricuspid valves. The outflow tract cushions, or ridges, will remodel, the neural crest–derived cells will aid in the septation of the single outflow channel into two vessels (aorta and pulmonary trunk) and be removed, the proximal parts will partake in ventricular septation and proper connection of the great arteries to the respective ventricle, whereas the more distal portion will also aid in the septation and remodel into semilunar valves with three leaflets at the entrance to each great artery.
DEVELOPMENT OF HEART FUNCTION AND CONDUCTION SYSTEM DEVELOPMENT
The first spontaneous rhythmic action potentials were observed in the three somite rat (E9.5) and mouse (E8.5) and stage 7 chicken cardiac crescents, immediately followed by the first contractions (Goss 1938; Fujii et al. 1981; Hirota et al. 1985; Navaratnam et al. 1986). High-resolution live imaging of mouse embryos established Ca2+ oscillations in the E7.75 crescent preceding contractions at E8 (Tyser et al. 2016). From that stage onward, spontaneous action potentials become more regular and synchronized. In heart tube stages, dominant pacemaker activity is always observed in the caudal end (but left or right side not fixed), the impulse and induced slow peristaltic contraction waves migrating toward the cranial outflow (Van Mierop 1967). Thus, as soon as the heart tube is established, pacing, synchronization, and unidirectional contraction waves and flow have been established. Taking into account that the initial heart tube is comprised of little more than the left ventricular primordium, and perhaps the AV canal at its inflow (Aanhaanen et al. 2009), this means that during tube elongation the cardiac cells added to the caudal end take over dominant pacemaker activity, and the actual definitive pacemaker (sinus node in the sinus venosus) still has to be formed from the caudal SHF. The latter will occur between mouse E9.5–E11.5, when the sinus venosus and sinus node primordia are being added from Tbx5+ Tbx18+ Hcn4+ posterior SHF progenitors (Mommersteeg et al. 2007; van Eif et al. 2018). During this time, the dominant pacemaker activity will gradually move toward the definitive sinus node region (Vicente-Steijn et al. 2010; Yi et al. 2012).
The differentiating ventricular and atrial chamber myocardium acquire properties of fast intercellular conduction, driven by induced expression of Gja5 (Cx40), Gja1 (Cx43) subunits of high-conductance gap junctions, Scn5a (Nav1.5), and other ion-handling proteins (Moorman and Christoffels 2003; Gros et al. 2004). Furthermore, functional sarcoplasmic reticulum (SR) develops in chamber myocardium, along with the expression of Serc2a, and their contractile apparatus becomes more abundant and organized (Moorman et al. 2000). In contrast, the nonchamber differentiating myocardium of the sinus venosus, AV canal, inner curvatures, and outflow tract maintain the embryonic slow conducting and poorly developed SR and contractility phenotype (de Jong et al. 1992; van Eif et al. 2018). It is also in this period of chamber differentiation that the electrocardiogram (ECG) transforms from sinusoidal (uniform slow impulse propagation in tube) to one that resembles that of the mature heart, with a P wave (fast atrial activation and contraction), PR interval (slow propagation through AV canal myocardium), QRS complex (fast ventricular activation and contraction), and a “T” wave-like signal resulting from slow activation of the proportionally large embryonic outflow tract (this wave is not the equivalent of the T wave in the adult heart) (Fig. 1). Because of this functionality resembling the mature heart, together with the acquisition of the definitive configuration of the chambers by these stages (see above), one could argue that the basic building plan of the vertebrate heart has been established.
The development of the conduction system components in the different vertebrate species has been extensively reviewed (Christoffels and Moorman 2009; Miquerol et al. 2011; Jongbloed et al. 2012; Munshi 2012; Jensen et al. 2013; Park and Fishman 2017; van Eif et al. 2018). Briefly, the sinus node develops in the (right) sinus venosus, connected to the (right) atrium. The AV canal myocardium is maintained, but in mammals and chicken remodeled to a discrete AV node and extensions/ring bundles. The crest of the ventricular septum comprises the AV bundle, the flanks of the septum the bundle branches, and parts of the trabecular myocardium of the ventricles remodel into the Purkinje fiber network. Consistent with the concept that progenitors for future compartments are progressively added to the poles of the elongating heart tube, the early heart tube outflow comprises the AV bundle progenitors, whereas the early heart tube inflow tract comprises the AV canal/AV node progenitors (Mohan et al. 2018). The sinus node progenitors (along with those of the atria and AV canal) are initially found caudal/lateral of the first differentiating (and cardiogenic factor-expressing) heart field progenitors (Fig. 2) and are added relatively late to the caudal pole of the heart tube (Mommersteeg et al. 2010; Bressan et al. 2013; Ren et al. 2019).
FUNCTION INSTRUCTS DIFFERENTIATION AND MORPHOGENESIS
The early embryo is so small, in human and mouse just under 2–3 mm crown to rump length (de Bakker et al. 2016), that diffusion is sufficient for much of the exchange of nutrients and gasses for development to proceed (Pelster and Burggren 1996). The viability of zebrafish embryos remain independent of a circulatory system for a longer developmental period (Pelster and Burggren 1996) enabling investigations of cardiac developmental processes in fish embryos with highly compromised cardiac function or structure. However, mammalian embryonic development becomes dependent on heart function soon after it is initiated (e.g., Papadatos et al. 2002; Huang et al. 2003; Stieber et al. 2003). With proceeding development, cardiac morphogenesis increasingly relies on both its electrical and mechanical function, implicating them as integral network components of the morphogenetic process. Indeed, both functions have been shown to influence particular aspects of morphogenesis, and to cause structural defects when perturbed. The mechanisms underlying the interaction between function and morphogenesis of the vertebrate heart are currently being explored (Andrés-Delgado and Mercader 2016; Happe and Engler 2016; Tyser et al. 2016; Yalcin et al. 2017; Sidhwani and Yelon 2019). Blood flow through the heart causes shear stress, pressure differences, and stretch, all of which are sensed by the different cell types of the heart tube. During development, embryo size, flow, and pressure will change. Moreover, the remodeling of the heart, chamber expansion, and alignment, etc., cause changes in shape and local differences in shear stress, pressure, and stretch. Chamber formation, cell size, endocardium formation, valve formation, and cardiomyocyte proliferation and maturation are influenced by these mechanical cues. Primary cilia, transient receptor potential Ca2+ channels and integrins in the endocardium are among the mechanosensors that in response to forces change intracellular Ca2+ concentrations, activity of transcription factor Klf2 among others, Rho, Tgfb, and Hippo signaling in endocardium or underlying myocardium. This, again, affects cell proliferation, size, shape, differentiation, maturation, extracellular matrix production, etc. (Heallen et al. 2019) For example, a recent study showed that increased expansion of the atrial chamber volume increases junctional forces within endocardial cells, and via biomechanical signaling triggers nuclear localization Yap1 (of the Hippo pathway) and endocardial proliferation (Bornhorst et al. 2019).
As gestation proceeds, the increased demand for blood flow is largely met by increases in heart size and more specifically by increases in ventricular stroke volume (Kenny et al. 1986). This is because heart rate is more or less constant across gestational stages in human, mouse, and chicken once it has accelerated in the early embryo to ∼200 beats per minute (Bogue 1933; Ishiwata et al. 2003; Lindsey et al. 2014). Mean arterial blood pressure in the systemic circulation also undergoes a pronounced gestational increase from ∼1 mmHg in the first days of chicken heart development (Van Mierop and Bertuch 1967) to ∼10 mmHg at the embryonic–fetal transition in mouse (∼E14.5) (Ishiwata et al. 2003) and reaches ∼40 mmHg around hatching in chicken. Much has still to be learned about the relative importance of myocardial mass and myocardial maturation in sustaining the rapid increases in ventricular work (the product of stroke volume and blood pressure), but hypoplastic heart syndromes can be extremely severe conditions for the developing fetus (Feinstein et al. 2012).
Taken together, heart morphogenesis is the product of an interplay between developing genetic networks, shape, size, and biomechanical and electrophysiological input in the context of a constantly changing demand for pump function. The complexity of these interacting adapting networks is daunting if one appreciates how the exponentially growing embryo's changing and increasing demand for flow and pressure need to be delivered by a constantly growing and remodeling heart pump.
Footnotes
Editors: Benoit G. Bruneau and Paul R. Riley
Additional Perspectives on Heart Development and Disease available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Aanhaanen WT, Brons JF, Domínguez JN, Rana MS, Norden J, Airik R, Wakker V, de Gier-de Vries C, Brown NA, Kispert A, et al. 2009. The Tbx2+ primary myocardium of the atrioventricular canal forms the atrioventricular node and the base of the left ventricle. Circ Res 104: 1267–1274 . 10.1161/CIRCRESAHA.108.192450 [DOI] [PubMed] [Google Scholar]
- Abu-Issa R, Kirby ML. 2007. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol 23: 45–68. 10.1146/annurev.cellbio.23.090506.123331 [DOI] [PubMed] [Google Scholar]
- Andrés-Delgado L, Mercader N. 2016. Interplay between cardiac function and heart development. Biochim Biophys Acta 1863: 1707–1716. 10.1016/j.bbamcr.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza L, Carmona R, Cañete A, Cano E, Muñoz-Chápuli R. 2016. Coelomic epithelium-derived cells in visceral morphogenesis. Dev Dyn 245: 307–322. 10.1002/dvdy.24373 [DOI] [PubMed] [Google Scholar]
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, et al. 2015. Dynamics of cell generation and turnover in the human heart. Cell 161: 1566–1575. 10.1016/j.cell.2015.05.026 [DOI] [PubMed] [Google Scholar]
- Bertrand N, Roux M, Ryckebüsch L, Niederreither K, Dollé P, Moon A, Capecchi M, Zaffran S. 2011. Hox genes define distinct progenitor sub-domains within the second heart field. Dev Biol 353: 266–274. 10.1016/j.ydbio.2011.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blausen BE, Johannes RS, Hutchins GM. 1990. Computer-based reconstructions of the cardiac ventricles of human embryos. Am J Cardiovasc Pathol 3: 37–43. [PubMed] [Google Scholar]
- Bogue JY. 1933. The electrocardiogram of the developing chick. J Exp Biol 10: 286–292. [Google Scholar]
- Bornhorst D, Xia P, Nakajima H, Dingare C, Herzog W, Lecaudey V, Mochizuki N, Heisenberg CP, Yelon D, Abdelilah-Seyfried S. 2019. Biomechanical signaling within the developing zebrafish heart attunes endocardial growth to myocardial chamber dimensions. Nat Commun 10: 4113 10.1038/s41467-019-12068-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukens BJD, Kristensen DL, Filogonio R, Carreira LBT, Sartori MR, Abe AS, Currie S, Joyce W, Conner J, Opthof T, et al. 2019. The electrocardiogram of vertebrates: evolutionary changes from ectothermy to endothermy. Prog Biophys Mol Biol 144: 16–29. 10.1016/j.pbiomolbio.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Bressan M, Liu G, Mikawa T. 2013. Early mesodermal cues assign avian cardiac pacemaker fate potential in a tertiary heart field. Science 340: 744–748. 10.1126/science.1232877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs LE, Kakarla J, Wessels A. 2012. The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion. Differentiation 84: 117–130. 10.1016/j.diff.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher JT, Sedmera D, Guldberg RE, Markwald RR. 2007. Quantitative volumetric analysis of cardiac morphogenesis assessed through micro-computed tomography. Dev Dyn 236: 802–809. 10.1002/dvdy.20962 [DOI] [PubMed] [Google Scholar]
- Cai CL, Molkentin JD. 2017. The elusive progenitor cell in cardiac regeneration: slip slidin’ away. Circ Res 120: 400–406. 10.1161/CIRCRESAHA.116.309710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. 2003. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 5: 877–889. 10.1016/S1534-5807(03)00363-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cao Y, Duca S, Cao J. 2019. Epicardium in heart development. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a037192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels VM, Moorman AFM. 2009. Development of the cardiac conduction system. Why are some regions of the heart more arrhythmogenic than others? Circ Arrhythm Electrophysiol 2: 195–207. 10.1161/CIRCEP.108.829341 [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Habets PEMH, Franco D, Campione M, de Jong F, Lamers WH, Bao ZZ, Palmer S, Biben C, Harvey RP, et al. 2000. Chamber formation and morphogenesis in the developing mammalian heart. Dev Biol 223: 266–278. 10.1006/dbio.2000.9753 [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Mommersteeg MTM, Trowe MO, Prall OWJ, de Gier-de Vries C, Soufan AT, Bussen M, Schuster-Gossler K, Harvey RP, Moorman AFM, et al. 2006. Formation of the venous pole of the heart from an Nkx2-5-negative precursor population requires Tbx18. Circ Res 98: 1555–1563. 10.1161/01.RES.0000227571.84189.65 [DOI] [PubMed] [Google Scholar]
- Crossley DA II, Burggren WW, Reiber CL, Altimiras J, Rodnick KJ. 2016. Mass transport: circulatory system with emphasis on nonendothermic species. Compr Physiol 7: 17–66. 10.1002/cphy.c150010 [DOI] [PubMed] [Google Scholar]
- de Bakker BS, de Jong KH, Hagoort J, de Bree K, Besselink CT, de Kanter FE, Veldhuis T, Bais B, Schildmeijer R, Ruijter JM, et al. 2016. An interactive three-dimensional digital atlas and quantitative database of human development. Science 354: aag0053 10.1126/science.aag0053 [DOI] [PubMed] [Google Scholar]
- de Boer BA, van den Berg G, de Boer PA, Moorman AF, Ruijter JM. 2012. Growth of the developing mouse heart: an interactive qualitative and quantitative 3D atlas. Dev Biol 368: 203–213. 10.1016/j.ydbio.2012.05.001 [DOI] [PubMed] [Google Scholar]
- De Bono C, Thellier C, Bertrand N, Sturny R, Jullian E, Cortes C, Stefanovic S, Zaffran S, Théveniau-Ruissy M, Kelly RG. 2018. T-box genes and retinoic acid signaling regulate the segregation of arterial and venous pole progenitor cells in the murine second heart field. Hum Mol Genet 27: 3747–3760. 10.1093/hmg/ddy266 [DOI] [PubMed] [Google Scholar]
- de Jong F, Opthof T, Wilde AA, Janse MJ, Charles R, Lamers WH, Moorman AF. 1992. Persisting zones of slow impulse conduction in developing chicken hearts. Circ Res 71: 240–250. 10.1161/01.RES.71.2.240 [DOI] [PubMed] [Google Scholar]
- de la Cruz MV, Markwald RR. 1998. Living morphogenesis of the heart. Birkhäuser, Boston, MA. [Google Scholar]
- de la Cruz MV, Sánchez Gómez C, Arteaga MM, Argüello C. 1977. Experimental study of the development of the truncus and the conus in the chick embryo. J Anat 123: 661–686. [PMC free article] [PubMed] [Google Scholar]
- de la Cruz MV, Sánchez-Gómez C, Palomino M. 1989. The primitive cardiac regions in the straight tube heart (stage 9) and their anatomical expression in the mature heart: an experimental study in the chick embryo. J Anat 165: 121–131. [PMC free article] [PubMed] [Google Scholar]
- del Monte-Nieto G, Ramialison M, Adam AAS, Wu B, Aharonov A, D'Uva G, Bourke LM, Pitulescu ME, Chen H, de la Pompa JL, et al. 2018. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature 557: 439–445. 10.1038/s41586-018-0110-6 [DOI] [PubMed] [Google Scholar]
- de Pater E, Clijsters L, Marques SR, Lin YF, Garavito-Aguilar ZV, Yelon D, Bakkers J. 2009. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 136: 1633–1641. 10.1242/dev.030924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrange A, Le Garrec JF, Meilhac SM. 2018. Left–right asymmetry in heart development and disease: forming the right loop. Development 145: dev162776 10.1242/dev.162776 [DOI] [PubMed] [Google Scholar]
- Devine WP, Wythe JD, George M, Koshiba-Takeuchi K, Bruneau BG. 2014. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLife 3: 1–23. 10.7554/eLife.03848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA, Kirby ML. 2009. The role of secondary heart field in cardiac development. Dev Biol 336: 137–144. 10.1016/j.ydbio.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisén J, Giacca M, Hare JM, Houser S, Lee RT, et al. 2017. Cardiomyocyte regeneration: a consensus statement. Circulation 136: 680–686. 10.1161/CIRCULATIONAHA.117.029343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Yelon D, Conlon FL, Kirby ML. 2010. Myocardial lineage development. Circ Res 107: 1428–1444. 10.1161/CIRCRESAHA.110.227405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JA, Benson DW, Dubin AM, Cohen MS, Maxey DM, Mahle WT, Pahl E, Villafañe J, Bhatt AB, Peng LF, et al. 2012. Hypoplastic left heart syndrome: current considerations and expectations. J Am Coll Cardiol 59: S1–S42. 10.1016/j.jacc.2011.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker A, Prummel KD, Merks AM, Mickoleit M, Brombacher EC, Huisken J, Panáková D, Mosimann C. 2018. Continuous addition of progenitors forms the cardiac ventricle in zebrafish. Nat Commun 9: 2001 10.1038/s41467-018-04402-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Hirota A, Kamino K. 1981. Optical indications of pacemaker potential and rhythm generation in early embryonic chick heart. J Physiol 312: 253–263. 10.1113/jphysiol.1981.sp013627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George RM, Firulli AB. 2019. Hand factors in cardiac development. Anat Rec (Hoboken) 302: 101–107. 10.1002/ar.23910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss CM. 1938. The first contractions of the heart in rat embryos. Anat Rec 70: 505–524. 10.1002/ar.1090700502 [DOI] [Google Scholar]
- Grimes DT, Burdine RD. 2017. Left-right patterning: breaking symmetry to asymmetric morphogenesis. Trends Genet 33: 616–628. 10.1016/j.tig.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros D, Dupays L, Alcoléa S, Meysen S, Miquerol L, Théveniau-Ruissy M. 2004. Genetically modified mice: tools to decode the functions of connexins in the heart-new models for cardiovascular research. Cardiovasc Res 62: 299–308. 10.1016/j.cardiores.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Happe CL, Engler AJ. 2016. Mechanical forces reshape differentiation cues that guide cardiomyogenesis. Circ Res 118: 296–310. 10.1161/CIRCRESAHA.115.305139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RP. 2002. Patterning the vertebrate heart. Nat Rev Genet 3: 544–556. 10.1038/nrg843 [DOI] [PubMed] [Google Scholar]
- *.He L, Lui KO, Zhou B. 2019. The formation of coronary vessels in cardiac development and disease. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a037168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Heallen TR, Kadow ZA, Wang J, Martin JF. 2019. Determinants of cardiac growth and size. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a037150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota A, Kamino K, Komuro H, Sakai T, Yada T. 1985. Early events in development of electrical activity and contraction in embryonic rat heart assessed by optical recording. J Physiol 369: 209–227. 10.1113/jphysiol.1985.sp015897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini HS, Garcia KE, Taber LA. 2017. A new hypothesis for foregut and heart tube formation based on differential growth and actomyosin contraction. Development 144: 2381–2391. 10.1242/dev.145193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Sheikh F, Hollander M, Cai C, Becker D, Chu PH, Evans S, Chen J. 2003. Embryonic atrial function is essential for mouse embryogenesis, cardiac morphogenesis and angiogenesis. Development 130: 6111–6119. 10.1242/dev.00831 [DOI] [PubMed] [Google Scholar]
- Ishiwata T, Nakazawa M, Pu WT, Tevosian SG, Izumo S. 2003. Developmental changes in ventricular diastolic function correlate with changes in ventricular myoarchitecture in normal mouse embryos. Circ Res 93: 857–865. 10.1161/01.RES.0000100389.57520.1A [DOI] [PubMed] [Google Scholar]
- Jensen B, Wang T, Christoffels VM, Moorman AF. 2013. Evolution and development of the building plan of the vertebrate heart. Biochim Biophys Acta 1833: 783–794. 10.1016/j.bbamcr.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Jensen B, Boukens BJ, Crossley DA II, Conner J, Mohan RA, van Duijvenboden K, Postma AV, Gloschat CR, Elsey RM, Sedmera D, et al. 2018. Specialized impulse conduction pathway in the alligator heart. eLife 7: e32120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloed MR, Vicente Steijn R, Hahurij ND, Kelder TP, Schalij MJ, Gittenberger-de Groot AC, Blom NA. 2012. Normal and abnormal development of the cardiac conduction system; implications for conduction and rhythm disorders in the child and adult. Differentiation 84: 131–148. 10.1016/j.diff.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Kelly RG, Buckingham ME, Moorman AF. 2014. Heart fields and cardiac morphogenesis. Cold Spring Harb Perspect Med 4: a015750 10.1101/cshperspect.a015750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny JF, Plappert T, Doubilet P, Saltzman DH, Cartier M, Zollars L, Leatherman GF, St John Sutton MG. 1986. Changes in intracardiac blood flow velocities and right and left ventricular stroke volumes with gestational age in the normal human fetus: a prospective Doppler echocardiographic study. Circulation 74: 1208–1216. [DOI] [PubMed] [Google Scholar]
- Kim JS, Virágh S, Moorman AFM, Anderson RH, Lamers WH. 2001. Development of the myocardium of the atrioventricular canal and the vestibular spine in the human heart. Circ Res 88: 395–402. 10.1161/01.RES.88.4.395 [DOI] [PubMed] [Google Scholar]
- Le Garrec JF, Domínguez JN, Desgrange A, Ivanovitch KD, Raphaël E, Bangham JA, Torres M, Coen E, Mohun TJ, Meilhac SM. 2017. A predictive model of asymmetric morphogenesis from 3D reconstructions of mouse heart looping dynamics. eLife 6: e28951 10.7554/eLife.28951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Wang G, Lin L, Lowe J, Zhang Q, Bu L, Chen Y, Chen J, Sun Y, Evans SM. 2013. HCN4 dynamically marks the first heart field and conduction system precursors. Circ Res 113: 399–407. 10.1161/CIRCRESAHA.113.301588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FJ, You LR, Yu CT, Hsu WH, Tsai MJ, Tsai SY. 2012. Endocardial cushion morphogenesis and coronary vessel development require chicken ovalbumin upstream promoter-transcription factor II. Arterioscler Thromb Vasc Biol 32: e135–e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SE, Butcher JT, Yalcin HC. 2014. Mechanical regulation of cardiac development. Front Physiol 5: 318 10.3389/fphys.2014.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Zhou B, Pu WT. 2008. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev Biol 323: 98–104. 10.1016/j.ydbio.2008.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGrogan D, Münch J, de la Pompa JL. 2018. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat Rev Cardiol 15: 685–704. 10.1038/s41569-018-0100-2 [DOI] [PubMed] [Google Scholar]
- Männer J. 2009. The anatomy of cardiac looping: a step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin Anat 22: 21–35. 10.1002/ca.20652 [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Buckingham ME. 2018. The deployment of cell lineages that form the mammalian heart. Nat Rev Cardiol 15: 705–724. 10.1038/s41569-018-0086-9 [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Kelly RG, Rocancourt D, Eloy-Trinquet S, Nicolas JF, Buckingham ME. 2003. A retrospective clonal analysis of the myocardium reveals two phases of clonal growth in the developing mouse heart. Development 130: 3877–3889. 10.1242/dev.00580 [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. 2004a. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell 6: 685–698. 10.1016/S1534-5807(04)00133-9 [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kerszberg M, Moss JE, Buckingham ME. 2004b. Oriented clonal cell growth in the developing mouse myocardium underlies cardiac morphogenesis. J Cell Biol 164: 97–109. 10.1083/jcb.200309160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhac SM, Lescroart F, Blanpain C, Buckingham ME. 2015. Cardiac cell lineages that form the heart. Cold Spring Harb Pespect Med 5: a026344 10.1101/cshperspect.a026344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Beyer S, Kelly RG. 2011. Establishment of the mouse ventricular conduction system. Cardiovasc Res 91: 232–242. 10.1093/cvr/cvr069 [DOI] [PubMed] [Google Scholar]
- Mohan RA, Mommersteeg MTM, Domínguez JN, Choquet C, Wakker V, de Gier-de Vries C, Boink GJJ, Boukens BJ, Miquerol L, Verkerk AO, et al. 2018. Embryonic Tbx3+ cardiomyocytes form the mature cardiac conduction system by progressive fate restriction. Development 145: dev167361 10.1242/dev.167361 [DOI] [PubMed] [Google Scholar]
- *.Mohun TJ, Anderson RH. 2019. Understanding ventricular septation. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a037143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommersteeg MTM, Soufan AT, de Lange FJ, van den Hoff MJB, Anderson RH, Christoffels VM, Moorman AFM. 2006. Two distinct pools of mesenchyme contribute to the development of the atrial septum. Circ Res 99: 351–353. 10.1161/01.RES.0000238360.33284.a0 [DOI] [PubMed] [Google Scholar]
- Mommersteeg MTM, Hoogaars WMH, Prall OWJ, de Gier-de Vries C, Wiese C, Clout DEW, Papaioannou VE, Brown NA, Harvey RP, Moorman AFM, et al. 2007. Molecular pathway for the localized formation of the sinoatrial node. Circ Res 100: 354–362. 10.1161/01.RES.0000258019.74591.b3 [DOI] [PubMed] [Google Scholar]
- Mommersteeg MT, Domínguez JN, Wiese C, Norden J, de Gier-de VC, Burch JB, Kispert A, Brown NA, Moorman AF, Christoffels VM. 2010. The sinus venosus progenitors separate and diversify from the first and second heart fields early in development. Cardiovasc Res 87: 92–101. 10.1093/cvr/cvq033 [DOI] [PubMed] [Google Scholar]
- Moorman AFM, Christoffels VM. 2003. Cardiac chamber formation: development, genes and evolution. Physiol Rev 83: 1223–1267. 10.1152/physrev.00006.2003 [DOI] [PubMed] [Google Scholar]
- Moorman AFM, Schumacher CA, de Boer PA, Hagoort J, Bezstarosti K, van den Hoff MJ, Wagenaar GT, Lamers JM, Wuytack F, Christoffels VM, et al. 2000. Presence of functional sarcoplasmic reticulum in the developing heart and its confinement to chamber myocardium. Dev Biol 223: 279–290. 10.1006/dbio.2000.9752 [DOI] [PubMed] [Google Scholar]
- Munshi NV. 2012. Gene regulatory networks in cardiac conduction system development. Circ Res 110: 1525–1537. 10.1161/CIRCRESAHA.111.260026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnam V, Kaufman MH, Shepper JN, Barton S, Guttridge KM. 1986. Differentiation of the myocardial rudiment of mouse embryos: an ultrastructural study including freeze-fracture replication. J Anat 146: 65–85. [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dollé P. 2001. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128: 1019–1031. [DOI] [PubMed] [Google Scholar]
- Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, et al. 2002. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci 99: 6210–6215. 10.1073/pnas.082121299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Fishman GI. 2017. Development and function of the cardiac conduction system in health and disease. J Cardiovasc Dev Dis 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelster B, Burggren WW. 1996. Disruption of hemoglobin oxygen transport does not impact oxygen-dependent physiological processes in developing embryos of zebra fish (Danio rerio). Circ Res 79: 358–362. 10.1161/01.RES.79.2.358 [DOI] [PubMed] [Google Scholar]
- Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. 1999. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev 13: 1037–1049. 10.1101/gad.13.8.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D'Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, et al. 2016. Revisiting cardiac cellular composition. Circ Res 118: 400–409. 10.1161/CIRCRESAHA.115.307778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana MS, Théveniau-Ruissy M, De Bono C, Mesbah K, Francou A, Rammah M, Domínguez JN, Roux M, Laforest B, Anderson RH, et al. 2014. Tbx1 coordinates addition of posterior second heart field progenitor cells to the arterial and venous poles of the heart. Circ Res 115: 790–799. 10.1161/CIRCRESAHA.115.305020 [DOI] [PubMed] [Google Scholar]
- Ren J, Han P, Ma X, Farah EN, Bloomekatz J, Zeng XI, Zhang R, Swim MM, Witty AD, Knight HG, et al. 2019. Canonical Wnt5b signaling directs outlying Nkx2.5+ mesoderm into pacemaker cardiomyocytes. Dev Cell 50: 729–743.e5. 10.1016/j.devcel.2019.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckebusch L, Wang Z, Bertrand N, Lin SC, Chi X, Schwartz R, Zaffran S, Niederreither K. 2008. Retinoic acid deficiency alters second heart field formation. Proc Natl Acad Sci 105: 2913–2918. 10.1073/pnas.0712344105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmera D. 2011. Function and form in the developing cardiovascular system. Cardiovasc Res 91: 252–259. 10.1093/cvr/cvr062 [DOI] [PubMed] [Google Scholar]
- Sedmera D, Reckova M, DeAlmeida A, Coppen SR, Kubalak SW, Gourdie RG, Thompson RP. 2003. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat Rec 274A: 773–777. 10.1002/ar.a.10085 [DOI] [PubMed] [Google Scholar]
- Sidhwani P, Yelon D. 2019. Fluid forces shape the embryonic heart: Insights from zebrafish. Curr Top Dev Biol 132: 395–416. 10.1016/bs.ctdb.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman ME, Grove D, Upshaw CB Jr. 2006. Why does the heart beat? The discovery of the electrical system of the heart. Circulation 113: 2775–2781. 10.1161/CIRCULATIONAHA.106.616771 [DOI] [PubMed] [Google Scholar]
- Simões-Costa MS, Vasconcelos M, Sampaio AC, Cravo RM, Linhares VL, Hochgreb T, Yan CY, Davidson B, Xavier-Neto J. 2005. The evolutionary origin of cardiac chambers. Dev Biol 277: 1–15. 10.1016/j.ydbio.2004.09.026 [DOI] [PubMed] [Google Scholar]
- Sizarov A, Ya J, de Boer BA, Lamers WH, Christoffels VM, Moorman AF. 2011. Formation of the building plan of the human heart: morphogenesis, growth, and differentiation. Circulation 123: 1125–1135. 10.1161/CIRCULATIONAHA.110.980607 [DOI] [PubMed] [Google Scholar]
- Smith KA, Chocron S, von der Hardt S, de Pater E, Soufan A, Bussmann J, Schulte-Merker S, Hammerschmidt M, Bakkers J. 2008. Rotation and asymmetric development of the zebrafish heart requires directed migration of cardiac progenitor cells. Dev Cell 14: 287–297. 10.1016/j.devcel.2007.11.015 [DOI] [PubMed] [Google Scholar]
- Snarr BS, O'Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, Wessels A. 2007. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res 101: 971–974. 10.1161/CIRCRESAHA.107.162206 [DOI] [PubMed] [Google Scholar]
- Soufan AT, van den Berg G, Ruijter JM, de Boer PAJ, van den Hoff MJB, Moorman AFM. 2006. Regionalized sequence of myocardial cell growth and proliferation characterizes early chamber formation. Circ Res 99: 545–552. 10.1161/01.RES.0000239407.45137.97 [DOI] [PubMed] [Google Scholar]
- Stefanovic S, Barnett P, van Duijvenboden K, Weber D, Gessler M, Christoffels VM. 2014. GATA-dependent regulatory switches establish atrioventricular canal specificity during heart development. Nat Commun 5: 3680 10.1038/ncomms4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieber J, Herrmann S, Feil S, Loster J, Feil R, Biel M, Hofmann F, Ludwig A. 2003. The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci 100: 15235–15240. 10.1073/pnas.2434235100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED, et al. 2010. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell 18: 275–287. 10.1016/j.devcel.2010.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Tyser RCV, Srinivas S. 2019. The first heartbeat—origin of cardiac contractile activity. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a037135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyser RC, Miranda AM, Chen CM, Davidson SM, Srinivas S, Riley PR. 2016. Calcium handling precedes cardiac differentiation to initiate the first heartbeat. eLife 5: e17113 10.7554/eLife.17113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Poss KD. 2017. Cardiac regeneration strategies: staying young at heart. Science 356: 1035–1039. 10.1126/science.aam5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg G, Moorman AF. 2009. Concepts of cardiac development in retrospect. Pediatr Cardiol 30: 580–587. 10.1007/s00246-008-9369-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg G, Abu-Issa R, de Boer BA, Hutson MR, de Boer PA, Soufan AT, Ruijter JM, Kirby ML, van den Hoff MJ, Moorman AF. 2009. A caudal proliferating growth center contributes to both poles of the forming heart tube. Circ Res 104: 179–188. 10.1161/CIRCRESAHA.108.185843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eif VWW, Devalla HD, Boink GJJ, Christoffels VM. 2018. Transcriptional regulation of the cardiac conduction system. Nat Rev Cardiol 15: 617–630. 10.1038/s41569-018-0031-y [DOI] [PubMed] [Google Scholar]
- Van Mierop LHS. 1967. Localization of pacemaker in chick embryo heart at the time of initiation of heartbeat. Am J Physiol 212: 407–415. 10.1152/ajplegacy.1967.212.2.407 [DOI] [PubMed] [Google Scholar]
- Van Mierop LH, Bertuch CJ Jr. 1967. Development of arterial blood pressure in the chick embryo. Am J Physiol 212: 43–48. 10.1152/ajplegacy.1967.212.1.43 [DOI] [PubMed] [Google Scholar]
- Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. 2005. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol 287: 134–145. 10.1016/j.ydbio.2005.08.041 [DOI] [PubMed] [Google Scholar]
- Vicente-Steijn R, Kolditz DP, Mahtab EA, Askar SF, Bax NA, Van Der Graaf LM, Wisse LJ, Passier R, Pijnappels DA, Schalij MJ, et al. 2010. Electrical activation of sinus venosus myocardium and expression patterns of RhoA and Isl-1 in the chick embryo. J Cardiovasc Electrophysiol 21: 1284–1292. 10.1111/j.1540-8167.2010.01790.x [DOI] [PubMed] [Google Scholar]
- Virágh S, Challice CE. 1973. Origin and differentiation of cardiac muscle cells in the mouse. J Ultrastruct Res 42: 1–24. 10.1016/S0022-5320(73)80002-4 [DOI] [PubMed] [Google Scholar]
- Vivien CJ, Hudson JE, Porrello ER. 2016. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen Med 1: 16012 10.1038/npjregenmed.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. 1998. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev Biol 196: 129–144. [DOI] [PubMed] [Google Scholar]
- Wang W, Niu X, Stuart T, Jullian E, Mauck WM III, Kelly RG, Satija R, Christiaen L. 2019. A single-cell transcriptional roadmap for cardiopharyngeal fate diversification. Nat Cell Biol 21: 674–686. 10.1038/s41556-019-0336-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SP, Cheng CM, Lanz RB, Wang T, Respress JL, Ather S, Chen W, Tsai SJ, Wehrens XH, Tsai MJ, et al. 2013. Atrial identity is determined by a COUP-TFII regulatory network. Dev Cell 25: 417–426. 10.1016/j.devcel.2013.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Hoffmann AD, Burnicka-Turek O, Friedland-Little JM, Zhang K, Moskowitz IP. 2012. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev Cell 23: 280–291. 10.1016/j.devcel.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Cerrato F, Baldini A. 2005. Timed mutation and cell-fate mapping reveal reiterated roles of Tbx1 during embryogenesis, and a crucial function during segmentation of the pharyngeal system via regulation of endoderm expansion. Development 132: 4387–4395. 10.1242/dev.02018 [DOI] [PubMed] [Google Scholar]
- Yalcin HC, Amindari A, Butcher JT, Althani A, Yacoub M. 2017. Heart function and hemodynamic analysis for zebrafish embryos. Dev Dyn 246: 868–880. 10.1002/dvdy.24497 [DOI] [PubMed] [Google Scholar]
- Yi T, Wong J, Feller E, Sink S, Taghli-Lamallem O, Wen J, Kim C, Fink M, Giles W, Soussou W, et al. 2012. Electrophysiological mapping of embryonic mouse hearts: mechanisms for developmental pacemaker switch and internodal conduction pathway. J Cardiovasc Electrophysiol 23: 309–318. 10.1111/j.1540-8167.2011.02191.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffran S, Frasch M. 2002. Early signals in cardiac development. Circ Res 91: 457–469. 10.1161/01.RES.0000034152.74523.A8 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cashman TJ, Nevis KR, Obregon P, Carney SA, Liu Y, Gu A, Mosimann C, Sondalle S, Peterson RE, et al. 2011. Latent TGF-β binding protein 3 identifies a second heart field in zebrafish. Nature 474: 645–648. 10.1038/nature10094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Liu J, Olson P, Zhang K, Wynne J, Xie L. 2015. Tbx5 and Osr1 interact to regulate posterior second heart field cell cycle progression for cardiac septation. J Mol Cell Cardiol 85: 1–12. 10.1016/j.yjmcc.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wang J, Guo C, Chang W, Zhuang J, Zhu P, Li X. 2017. Temporally distinct Six2-positive second heart field progenitors regulate mammalian heart development and disease. Cell Rep 18: 1019–1032. 10.1016/j.celrep.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]