Abstract
Objective.
While a certain fraction of endometriomas can develop de novo epithelial ovarian cancer (EOC) such as clear cell carcinoma (OCCC), there is currently no useful biomarker available for early detection of OCCC from endometriomas. The aim of this study was to describe the diagnostic utility of a novel biomarker for EOC especially for OCCC to distinguish from endometrioma.
Methods.
More than 100,000 glycan structures of serum glycoproteins obtained from 134 pretreatment all stage EOC patients (including 45 OCCCs) and 159 non-cancer control women (including 36 endometriomas) were explored for a mass spectrum approach. Diagnostic accuracy of identified biomarker was compared to the one of CA-125 by comparing area under curve (AUC) and positive/negative predictive values (PPV and NPV).
Results.
A2160, a fully-sialylated alpha-chain of complement 4-binding protein, was identified as a candidate target marker. A2160 was significantly elevated in all stages of OCCC compared to with endometriomas. Diagnostic accuracy of A2160 (cutoff 1.6 U/mL) to distinguish early stage OCCC from endometrioma is significantly higher than that of CA-125 (cutoff 35 IU/L): AUC for A2160 versus CA-125,0.92 versus 0.67; PPV 95% versus 64%; and NPV 85% versus 58%. In addition, fully-sialylated glycans had a higher accuracy for diagnosing EOC as compared to partially-sialylated glycans of alpha-chain of complement 4-binding protein.
Conclusion.
Our study suggested that A2160 may be a useful biomarker to distinguish early-stage OCCC from endometrioma. This new biomarker can be potentially applied for the monitoring of endometrioma patients, making possible the early diagnosis of OCCC.
Keywords: Complement 4-binding protein, Full sialylation, Ovarian clear cell carcinoma, Endometrioma: early detection
1. Introduction
Epithelial ovarian cancer (EOC) remains the leading cause of death among the gynecological cancers in the United States [1]. Accurate diagnosis and early detection of EOC is always challenging, and only 15% of EOC patients are diagnosed with early-stage disease. The remaining large fraction of EOC patients are at an advanced-stage at the time of diagnosis [2]. Early-stage EOC has a nearly 90% 5-year survival rate as opposed to less than 30% in advanced-stage disease, so developing a useful screening method is of utmost importance in EOC. Among EOC patients those with clear cell carcinoma (OCCC) were significantly more likely to have FIGO Stage I disease than were those with Serous adenocarcinoma (SAC) (48.5% vs. 16.6%). A high recurrence rate was noted in those patients with Stage IC CCC (37%), however, the survival rates for these patients were lower than those for patients with SAC [3].
Various attempts have been made at ovarian cancer screening following the identification of the cancer antigen 125 (CA-125) as a biomarker for ovarian cancer more than 30 years ago. These have included CA-125 combined with transvaginal ultrasonography [4]. and CA-125 combined with other biomarkers [5]. However, the results of CA-125-based screening approaches for ovarian cancer diagnosis have as of yet, not been proven to be of efficacious. In addition, numerous studies including proteomic and nucleic acid–based analyses have been conducted to identify biomarkers for ovarian cancer with little benefit seen over the utility of CA-125 [6]. A fundamental limitation of CA-125 in ovarian cancer diagnosis is that it is often elevated in nonmalignant gynecologic conditions such as in endometriosis.
Ovarian endometriosis is a prevalent gynecologic disease, particularly in young reproductive aged women [7]. Consequences of endometriosis may include infertility, ectopic pregnancy, and chronic pelvic pain. Due to the fact that endometriomas are commonly seen in reproductive aged women, usual management includes either conservative surgery or close observation in order to preserve ovarian function. The most concerning issue surrounding the management of endometriomas is that there is a risk of malignant degeneration to OCCC, one of the more aggressive subtypes of ovarian cancer [8].
Common strategies for the discovery of new cancer biomarkers, include identification of anomalous alternative mRNA splicing products of an expressed gene, as well as genes which are re-expressed following silencing during normal differentiation. Although identification of sugar chain alterations produced by cancer cells represents another strategy for biomarker discovery, the low productivity of antibodies for the specific sugar chains, previously discovered sugar chain markers are the “tip of the iceberg” of numerous cancer-specific markers [9].
Dramatic improvements in mass spectrum (MS) technology in sensitivity, resolution, throughput, and dynamic range have revolutionized the approach to biomarker analysis in this decade. With this novel technology, we investigated not only sugar chain alterations but also the combination of protein and glycan changes. However, direct exhaustive analysis of serum glycoproteins has remained difficult because of the numerous peaks in MS data [10]. To solve this problem, highly efficient and enriched strategies to isolate glycopeptides with new MS data analyzing methods had been required.
The aim of this study was to establish a novel technology to explore new glycoprotein markers for EOC, and among them to find a highly specific marker for the early diagnosis of EOC that could overcome the weaknesses of the current EOC markers.
2. Materials and methods
2.1. Patient samples (Table 1 and Table S1)
Table 1.
Summary of patients.
| Participants | Samples | Age | ||
|---|---|---|---|---|
| Ovarian cancer | Serous | 30 | 56.8 +/−12.5 | |
| Clear cell | 45 | 134 | ||
| Endometrioid | 22 | (14) | ||
| Mucinous | 12 | |||
| Unclassified | 25 | |||
| Controls | Endometrioma | 36 | 37.5 +/−7.6 | |
| Uterine fibroid | 21(2) | 47.2 +/−7.7 | ||
| Ovarian cystoma | 23 | 52.3 +/−15.0 | ||
| Uterus cancer | 16 | 159(16) | 57.6 +/−11.2 | |
| Menstruation | 19 | 36.2 +/−8.9 | ||
| Healthy women | 44(14) | 46.9 +/−10.1 |
() Caucasian.
During the study period (2010–2014) total of 293 consecutive serum samples were obtained at the time of ovarian mass detection and prior to the initiation of any treatment. These included EOC patients (n = 134) and a control group (n = 159). The mean age of EOC patients was 56.8 (SD ± 12.5). The ethnicity of the EOC group consisted of Asian (n = 120, 89.6%) and Caucasian (n = 14, 10.4%). The most common histologic subtypes of EOC was OCCC (n = 45, 33.6%), followed by serous (n = 30, 22.4%), unclassified (n = 25, 18.7%), endometrioid (n = 22, 16.4%), and mucinous adenocarcinoma (n = 12, 9.0%). The non-cancer control group comprised both healthy women (n = 63) and women with gynecologic diseases, including endometrioma which may also demonstrate elevated CA125 levels (n = 96). Among 63 healthy women, there were 19 (30.2%) pre-menopausal women in whom blood sampling was obtained during the menstrual period, and the remaining 44 (69.8%) healthy women had blood sampling outside of their menstrual periods (pre-menopausal n = 24, post-menopausal n = 11, and unknown menstrual status n = 9). Among 96 patients with gynecologic disease other than ovarian cancer, there were 36 (37.5%) cases of endometrioma. The mean age of the control group, stratified by diagnosis, included: endometrioma 37.5 (±7.6), uterine myomas 47.2 (±7.7), benign ovarian cyst 52.3 (±15.0), uterine cancer 57.6 (±11.2), healthy menstruating women 36.2 (±8.9), and healthy women without menstruation 46.9 (±10.1). In addition, fluid samples from the cystic component of EOC (n = 8) and from benign ovarian tumor (n = 5) were also collected. Additional blood samples were obtained 1 year after completion of the initial ovarian cancer treatment for nine randomly selected EOC patients that demonstrated elevated A2160 levels at the initial diagnosis. All blood samples were collected by venous puncture from patients before surgery and from healthy individuals at their first hospital admission. Samples were centrifuged and stored at −80 °C for further examination, with avoidance of repeated freeze-thaw cycles.
2.2. Glycopeptide profiling and marker screening
The outline schema of the process is shown in Fig. 1A. The detailed descriptions of the techniques are described in Supplemental Appendix. Briefly, after extracting serum glycoproteins and treatment with proteases, glycopeptides were enriched by filtration and AAL lectin column chromatography, followed by liquid chromatography mass spectrometry (LC-MS). The errors of peak intensity in the raw MS data were corrected by internal glycopeptide standards, and all peak areas were then normalized to those of one healthy woman (HEA1433), which was set at one unit. The reason why we chose HEA1433 for normalization was that MS profile of HEA1433 showed typical normal healthy pattern among controls, and there were enough sera as a normalizing standard. All processes were monitored by utilization of QC (Quality Control) samples prepared by pooling all patient and control sera. QCs were analyzed every 15 samples and if the values became abnormal, that batch including the QC was reanalyzed.
Fig. 1.
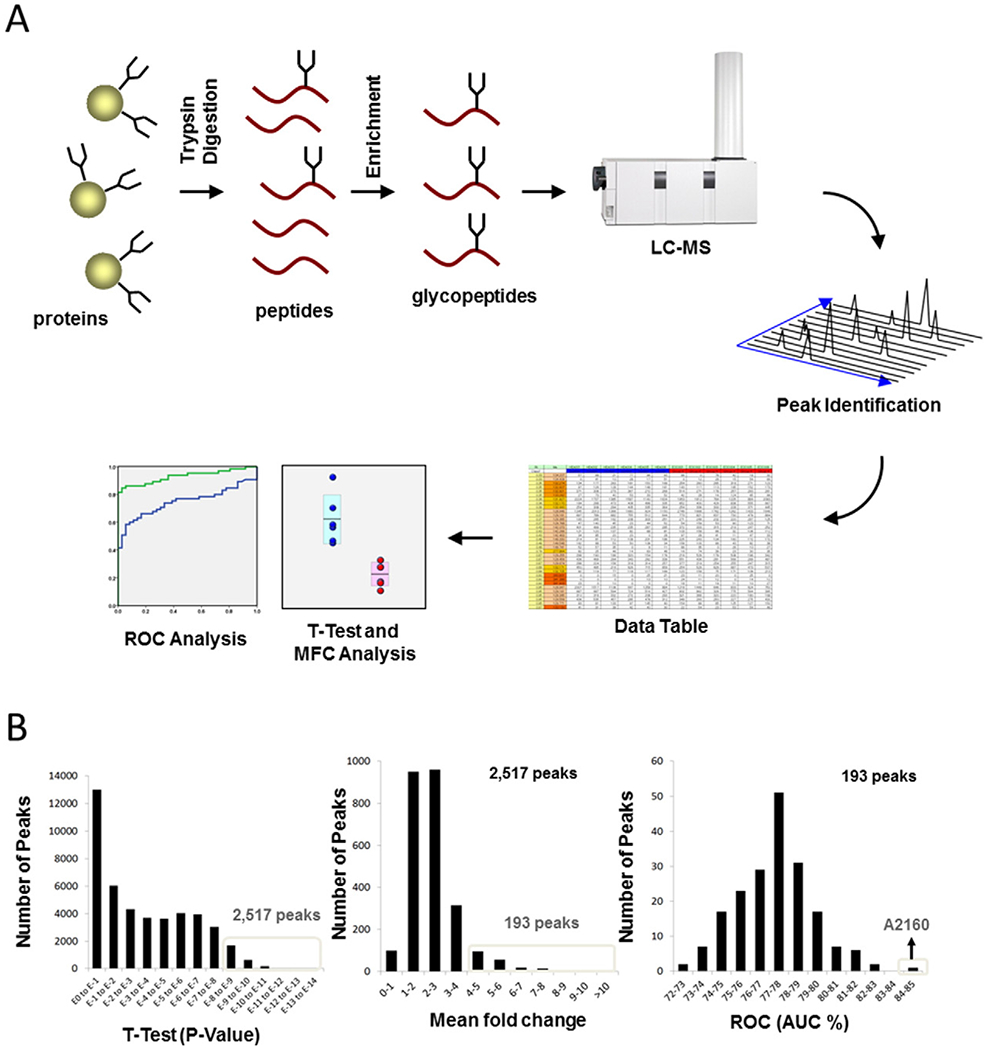
Schema of gyloprotein profiling strategy and data mining (A) Glycopeptide profiling strategy is shown. After serum glycoproteins were treated with proteases, glycopeptides were enriched by filtration and AAL lectin column chromatography, and then were analyzed by LC-MS. The position and area of each peak were calculated by our original software. Then all peak areas were normalized to those of a healthy woman (HEA1433), which was set at one unit. A potential new marker of epithelial ovarian cancer (EOC) was identified through investigation of over 100,000 glycopeptide peaks by using t-test, mean fold change (MFC) analysis, and receiver-operator-characteristic (ROC) characteristics analysis for comparison between the EOC and control groups. (B) Data Mining is shown. Glycopeptide A2160 was identified from among more than 100,000 glycopeptide peaks by analysis with t-test (2517 peaks with P < 10−8 compared with controls), MFC analysis (193/2517 peaks with mean values ≥ 4 times those of controls), and ROC analysis (identifying the peak with the highest AUC among 193 peaks).
2.3. Peptide sequencing and analysis of sugar chains
The outline schema of the process is shown in Figure S7. Glycans were removed from the potential marker glycopeptide, and were subjected to analysis by LC-MS/MS. The peptide fragment pattern was assessed with MASCOT (Matrix Science, Boston, MA). Sugar chain structures were determined by LC-MS analysis of marker glycopeptide fragments and by LC analysis of N-glycans released from the glycopeptide.
2.4. Essential glycan structure of the new marker
The outline schema of the process is shown in Figure S7. Serum glycoproteins obtained from EOC patients (n = 19) and control subjects (healthy women, n = 10; endometrioma, n = 19) were digested with specific enzymes to generate fragments of our new marker glycopeptide with one glycan site on each peptide. These fragments were analyzed by LC-MS, and AUCs were compared between the EOC and control subjects.
2.5. Statistical analysis
Continuous variables were expressed as mean (±standard deviation [SD]) or median (range), and the statistical significance was determined by t-test or mean fold change (MFC) analysis, as appropriate. Categorical variables were expressed with number (%). Diagnostic accuracy of biomarkers for EOC over control counterparts was examined with ROC curve analysis comparing AUC with 95% CI. Based on the results, the cutoff value of biomarker was determined and sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for diagnosing EOC or OCCO over control group were calculated. All statistical analyses were two-tailed, and P < 0.05 was considered as statistical significance. Statistical Package for the Social Sciences (SPSS, version 17.0, Chicago, IL, USA) and our original statistical software were used for the analysis.
2.6. Study approval
Institutional Review Board (IRB) approval was obtained at Tokai University (IRB registration number, 09R-082) and Mitsubishi Chemical Group Science and Technology Research Center, Inc. (IRB registration number, 090,819–1) for the use of patient’s clinical information and serum/tumor samples. Upon obtaining these master IRB approvals, the following participating institutions obtained the IRB for the patient accrual: Tokai University Hospital (Kanagawa, Japan), the Leading Project for Personalized Medicine of the Ministry of Education, Culture, Sports, Science and Technology (Tokyo, Japan), Kanagawa Cancer Research and Information Association (Kanagawa, Japan), Sho Hospital (Tokyo, Japan), SOIKEN (Osaka Japan), and ProteoGenex, Inc.(California, America). Signed informed consents were obtained from all participants in the study.
3. Results
3.1. Glycopeptide profiling and screening for candidate markers
As a potential EOC marker, Glycopeptide A2160 was identified by screening over 100,000 glycopeptide peaks using the t-test (2517 peaks had P < 10−8 compared with controls), MFC analysis (193/2517 peaks had mean values ≥4 times that of controls), and receiver-operator-characteristics (ROC) analysis (identifying one peak [A2160] with the highest area-under-curve [AUC] among 193 peaks, Fig. 1A–B). Prior to this study, we conducted a validation of this method. The differences of inter-day or intra-day reproducibility (CV%) of the results of the assay were within 20%. Similarly, the differences of error for the assay results between operators or labs were also within 20%.
3.2. Diagnostic utility for EOC: A2160 versus CA-125
After A2160 was identified as the most potent biomarker for EOC, the diagnostic accuracy of A2160 was determined by comparing it to CA-125, a traditional biomarker for EOC. First, ROC curve analysis was performed comparing EOC patients (n = 134) to healthy subjects not currently on their menstrual period (n = 44). Based on the results, the cutoff value of A2160 to maximize the sensitivity and specificity for diagnosing EOC was set as 1.6 U/mL. With this cutoff value for A2160, there were 112 (84%) cases of EOC above the value. Diagnostic utility of A2160 for EOC over healthy control was: sensitivity 84%, specificity 82%, positive predictive value (PPV) 93%, negative predictive value (NPV) 62%, and accuracy 83%, respectively. In the same comparison, elevated CA-125 (>35 IU/L) was seen in 105 (78.4%) cases of EOC, and the diagnostic utility of CA-125 was: sensitivity 78%, specificity 100%, PPV 100%, NPV 60%, and accuracy 84%, respectively (Those performances with 50 or 200 IU/L of CA-125 cutoff were shown in Table S2). Next, serum A2160 and CA-125 levels were plotted across the various disease types included EOC, other gynecologic conditions, and healthy women (Fig. 2A–B). The results showed that diagnostic utility of distinguishing EOC from endometrioma (plotted as EM) was superior to that of CA-125.
Fig. 2.
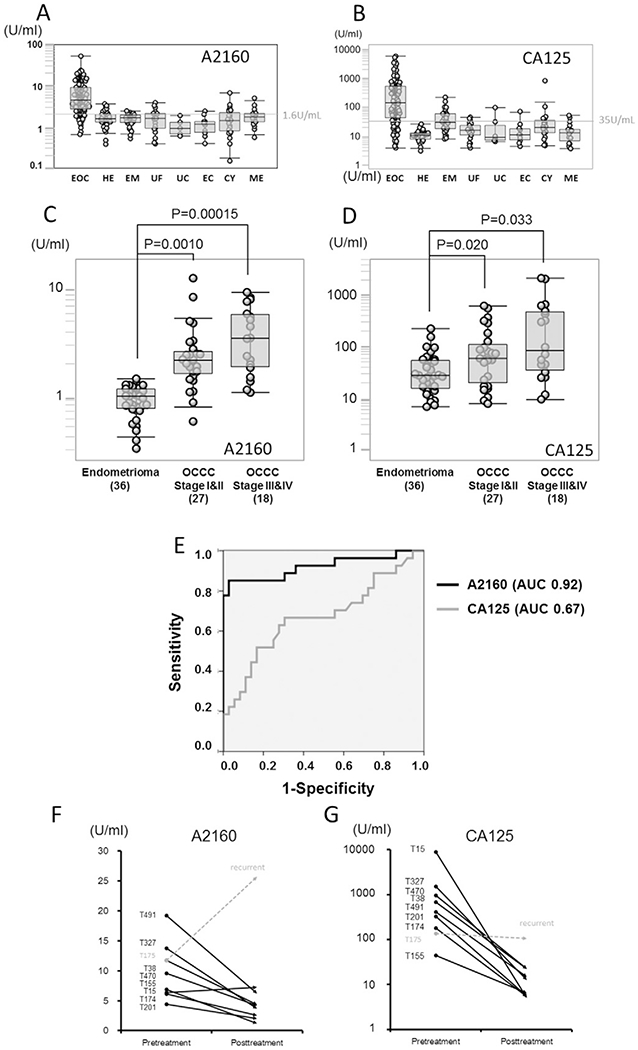
Comparison of A2160 and CA-125 levels in diagnosing ovarian cancer (A–B) Box-whisker plots for A2160 and CA-125 levels in healthy subjects and various gynecological diseases are shown. The cut-off value of A2160 was set to maximize the sensitivity and specificity, and was found to be 1.6 U/mL. Superiority of A2160 over CA-125 was demonstrated by comparison of the EOC group and the control group including patients with falsely elevated CA-125 level, especially in endometrioma. The range of CA-125 values (1–10,000 U/mL) in EOC patients was significantly wider than that of A2160 values (1–50 U/mL). EOC: epithelial ovarian cancer, HE: Healthy women, EM: endometrioma, UF: uterine fibroid, UC: uterine cervical cancer, EC: endometrial cancer, CY: ovarian cyst, ME: menstruation. (C–D) A2160 and CA-125 levels are shown for endometrioma, stages I–II OCCC and stage III-IV OCCC. Magnitude ofstatistical significance of A2160 between stages I–II OCCC and healthy subjects (P = 0.001, t-test) was larger than that of CA-125 (P = 0.02). (E) ROC curve analysis of A2160 and CA125 between stages I–II OCCC (n = 27) and endometrioma (n = 36). AUC for A2160 (0.92, 95%CI 0.84–0.99) was markedly larger than AUC for CA-125 (0.67, 95%CI 0.53–0.81) in distinguishing OCCC from endometrioma. (F–G) Interval changes of A2160 and CA125 levels in EOC patients before and one year after treatment are shown. Eight patients with elevated A2160 levels went into remission after tumor resection, and A2160 levels paralleled with CA-125 level. There was one patient (T175) developed EOC recurrence: only A2160 level was elevated at recurrence, and CA-125 levels did not show elevation.
ROC curve analysis for A2160 and CA-125 was then performed comparing the EOC group and various subtypes of the control group (Table S3). Among the 7 comparisons between A2160 and CA-125, AUC of A2160 was superior or comparable in the majority of comparisons. The most meaningful difference of AUCs between A2160 and CA-125 existed in the comparison of EOC over endometrioma: A2160, AUC 0.92 (95% confidence interval [CI] 0.88–0.96, P < 10−15); and CA-125, AUC 0.77 (95% CI 0.70–0.84, P < 10−6). Of note, among the subtypes of the control group, endometrioma had the highest median CA-125 level with the nearly half of endometrioma cases having abnormally elevated CA-125 values higher than 35 IU/L (Fig. 2B). A2160 levels between EOC and healthy subjects were compared across ethnicity groups (Figure S1). Both Asian and Caucasian groups showed similar trends and EOC patients had significantly elevated A2160 compared to healthy controls. Across the histologic subtypes of EOC, the average level of serous adenocarcinoma patients (7.5 U/mL) was little higher than that of the other EOCs (4.7 U/mL) patients (P < 0.05), however, A2160 levels were similarly elevated when compared to healthy and endometrioma subjects (Figure S2). A2160 levels obtained from the cystic fluid of ovarian tumors were further compared between EOC (n = 8) and benign ovarian tumors (n = 5). The results showed that A2160 levels were higher in EOC compared to benign ovarian tumors (Figure S3).
This study specifically examined the diagnostic accuracy of A2160 and CA-125 for early-stage OCCC (stages I–II, n = 27) compared to endometriomas (n = 36, Fig. 2C–D). Both A2160 and CA-125 levels were statistically significantly higher in women with OCCC than in women with endometriomas; however, the magnitude of statistical significance was larger with A2160 as compared to CA-125 (P = 0.001 versus P = 0.02). When ROC curves were constructed comparing stages I–II OCCC to endometrioma, the diagnostic accuracy for A2160 (AUC 0.92, 95%CI 0.83–0.99) was clinically meaningfully higher than that of CA-125 (AUC 0.67, 95%CI 0.53–0.81, Fig. 2E). With a cutoff value of 1.6 U/mL, the diagnostic utility of A2160 for stage I-IV OCCC over endometrioma was: sensitivity 87%, specificity 94%, PPV 95%, NPV 85%, and accuracy 85%, respectively. With a cutoff value of 35 IU/L, diagnostic utility of CA-125 for stage I-IV OCCC over endometrioma was: sensitivity 71%, specificity 50%, PPV 64%, NPV 58%, and accuracy 62%, respectively. PPV of A2160 to diagnose OCCC versus endometrioma (95%) was markedly higher than that of CA-125 (64%). The PPV and NPV of A2160 were also higher than those of CA-125 when the cutoff value of CA-125 was changed to 50 IU/L or 200 IU/L (Table S2).
The utility of A2160 as a surveillance maker for EOC progression was examined (Fig. 2F–G). At the time of data analysis, nine EOC patients with elevated A2160 levels at the time of the initial diagnosis in whom A2160 levels were available at one year postoperative follow-up were randomly chosen. Of those, there were eight (88.8%) patients who were in remission after one year from treatment, and all except for one patient (T15) had markedly decreased A2160 levels compared to pre-treatment levels. There was one patient (T175) who developed disease recurrence. In this case, serum A2160 level was significantly elevated at the time of recurrence without elevation of CA-125.
3.3. Combination assay of A2160 and CA125
There was a weak correlation observed between A2160 and CA125 (r = 0.60), however, the relationship was complementary, and CA125 negative (less than 35 UI/mL) patients could be judged as positive based on A2160 levels. Furthermore the combination with A2160 and CA125, which is calculated by the following equation, showed much higher AUC (0.93) compared to solo A2160 or CA125. (The factors were optimized by Microsoft Excel Solver). Combination index = Log10(CEA) × 0.3 + Log10(A2160) × 0.7, The sensitivity and specificity of the combination index became 81% and 95% respectively when the cut-off was defined as 0.70 U/mL (Figure S4).
3.4. Peptide and glycan structural analysis of A2160
The MS/MS fragmentation pattern of A2160 peptide coincided with the alpha-chain of complement 4 binding protein (C4BP), and it was determined to match positions 499 to 529 of the amino acid sequence (Fig. 3A). The peptide had 2 N-glycan binding sites (Asn506 and Asn528). The peptide was bound to 2 glycans with 11 hexoses, 9 HexNAcs, 5 N-acetyl neuraminic acids, and 1 fucose, based on the molecular weight difference between before and after removing the N-glycans from A2160 glycopeptide. Judging from the molecular weight of the A2160 fragment glycopeptides separated at the middle of 2 glycan sites, the proposed glycan structures were determined to be A3G3S3F and A2G2S2, which were bound to Asn 506 and 528, respectively (Fig. 3B).
Fig. 3.
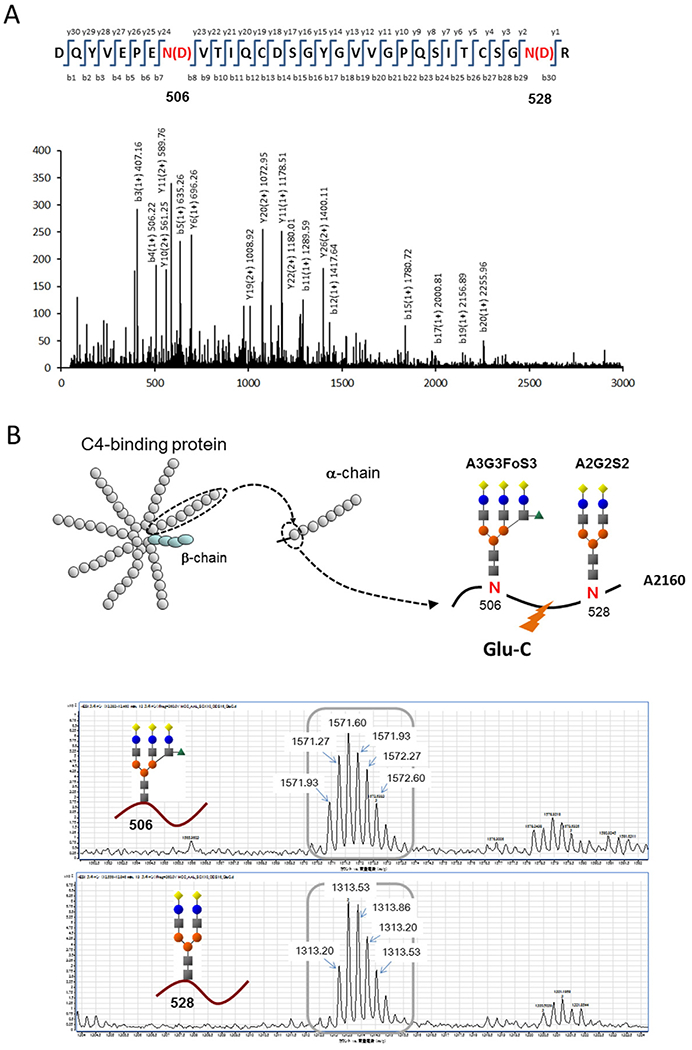
Identification and sequencing of A2160 (A) Peptide sequence analysis and identification of A2160 are shown. The MS/MS fragmentation pattern of A2160 peptide (after removing glycans) coincided with the alpha-chain of complement 4 binding protein (C4BP) from positions 499 to 529 of the amino acid sequence. Asn (N) was changed to Asp (D) by hydrolysis of sugar chains. (B) A2160 glycopeptide analysis and proposed structure are shown. The molecular weights of the glycopeptide fragments (detected as triply charged ions) revealed that the sugar chains binding to Asn 506 and 528 were A3G3S3F and A2G2S2, respectively.
3.5. Key alteration of glycan chains in the C4BP alpha-chain
Comparison of AUC values between the EOC patients and the control group revealed that glycopeptides with fully-sialylated glycans showed significantly higher AUC values compared to glycopeptides with partially-sialylated glycans (Figs. 4A–B and S5). This difference was observed regardless of the binding site (Asn506 or 528) or fucose binding (Fig. 4C). The difference between full and partial sialylation was not significant in other proteins like alpha-1 -acid glycoprotein (Figure S6). Collectively, these results suggest that the fully-sialylated alpha-chain of C4-binding protein is a novel biomarker for EOC, and this is especially potent in distinguish OCCC from endometrioma when compared to CA-125.
Fig. 4.
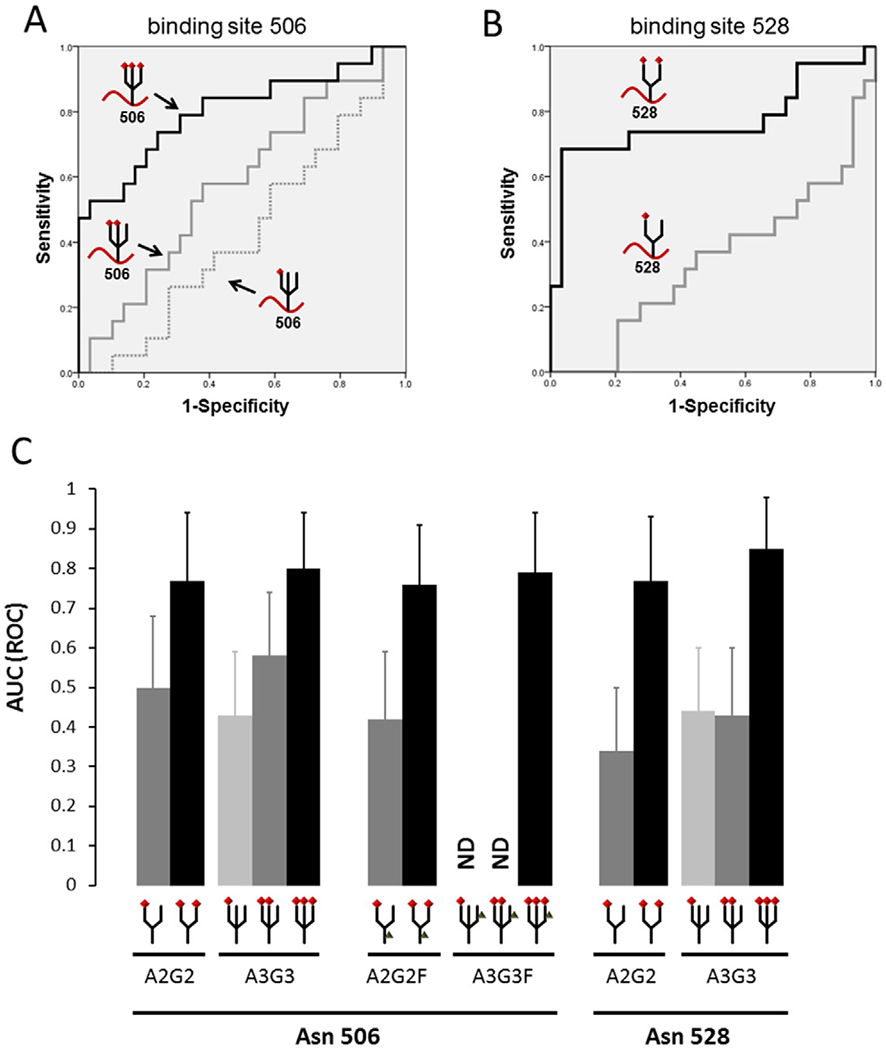
Comparison of fully versus partially sialylated alpha-chain of C4BP Area-under-curves (AUCs) of various glycans at the 506 and 528 sites of the C4BP alpha-chain in the serum of EOC (n = 19) and control subjects (healthy women n = 10, and endometrioma n = 19) were examined. (A) ROC curves of tri-antennary glycans on Asn 506 in the C4BP alpha-chain are shown. Fully-sialylated glycopeptides (binding 3 sialic acids) showed a significantly higher AUC than partially-sialylated glycans. (B) Receiver-operator-characteristic (ROC) curves of bi-antennary glycans on Asn 528 in the C4BP alpha-chain are shown. Fully-sialylated glycopeptides (binding 2 sialic acids) showed a markedly higher AUC than monosialylated glycans. (C) AUC values of each glycopeptide derived from the C4BP alpha-chain are shown. Comparing AUCs between epithelial ovarian cancer patients and the control group revealed that glycopeptides with fully-sialylated glycans showed significantly higher AUCs than those with partially-sialylated glycans. This trend was observed regardless of the binding sites and fucose binding. Error bars indicate 95% confidence interval of AUC. Red diamond-shape: sialic acids, Gray triangle-shape: fucose, A2G2: bi-antennary N-glycan, A3G3: tri-antennary N-glycan, A2G2F: bi-antennary N-glycan with one fucose, A3G3F: tri-antennary N-glycan with one fucose.
4. Discussion
Key findings of our study were that A2160 is a potentially valuable biomarker of EOC and that A2160 can be especially useful in distinguishing early-stage OCCC from endometriomas. However, the true predictive value of A2160 would have to be subjected to prospective analysis. Several of the salient topics merit further discussion.
Endometrioma is a form of endometriosis defined as the presence of ectopic endometrial tissue in the ovary. Overall, endometriosis can be seen in 6–10% of reproductive aged women (7). Recent epidemiologic and genomic studies have shown that endometrioma can be associated with an increased risk of future development of OCCC particularly in women with a long-stang history of endometriosis [11–12]. For this reason, women with endometriomas benefit from close observation for long-term monitoring. Becase CA-125, a traditional biomarker for ovarian cancer diagnosis, can be abnormally elevated in more than half of endometriomas (Fig. 2B and Table S2), the novel biomaker A2160 could satisfy an unmet need in allowing proper diagnosis of ovarian cancer in women with endometriosis and a pelvic mass. Our study is the first to demonstrate a higher diagnostic utility of A2160 than CA-125 to identify stages I–II OCCC over endometrioma, indicating that there is a clinical implication of A2160 for early detection of ovarian cancer (Fig. 2E).
Our data speculate that A2160 is likely produced from the ovarian cancer cells. A subset analysis of our clinical samples showed that the fluid aspirated from cystic counterparts of ovarian cancer had a trend of higher A2160 level compared to the fluid obtained from benign ovarian cysts (Figure S3). This is indeed supported by the results of a preclinical study by others demonstrating an expression of alpha-chain of C4BP in ovarian cancer cell lines whereas the normal ovarian tissue expresses beta-chain of C4BP [13–14]. One direction of interest may be therefore to examine the survival impact of elevated A2160 as a predictive marker of disease progression. In addition, a group of our patients with longterm follow-up showed that A2160 paralleled to the disease status, implying that A2160 can be a possible disease monitoring marker after the treatment (Fig. 2F). There was one case (T175) of recurrence with elevated A2160 level without elevation of CA-125. Further study will be needed to answer if A2160 is superior to CA-125 to detect ovarian cancer recurrence.
The role of C4BP in cancer biology remains incompletely understood. A2160 is a component of C4BP that is a large glycoprotein (500 kDa) mainly produced in the liver [14]. The main structure of the C4BP molecule in the blood circulating system consists of 7 identical alpha-chains and additional 1 unique beta-chain (Fig. 3B). Functionally, C4BP is a member of the complement cascade, in which it acts as an inhibitory player. That is, C4BP suppresses the overactivation of the complement system as part of the process of innate immunity. This is of a particular interest in a view of cancer immunity as it is speculated that ovarian cancer cells may produce A2160 to allow the cell to escape the host innate immune response via C4BP’s inhibitory function of the membrane attack complex (MAC) in the complement pathway.
A possible mechanism by which the ovarian cancer cell escapes from host immunity is via the A2160-driven protection, which merits further speculation. C4BP is known to bind strongly to ovarian cancer cells as shown in an in-vitro study, and this binding capacity is based on the electric balance regulation between C4BP and the cancer cell [15]. Our results showed that the fully-sialylated alpha-chain of C4BP was more potent in diagnosing ovarian cancer as compared to the partially-sialylated counterpart (Fig. 4A–C). Because sialic acid is a negatively charged molecule, it is reasonably theorized that the fully-charged sialylated form has larger affinity for ovarian cancer cells than the partially-sialylated form ofalpha-chain ofC4BP. As a consequence, ovarian cancer cells covered by A2160, the fully-sialylated form, may be more protected from the complement-induced tumor immune system.
Detection of early ovarian cancer has been challenging given that very few qualitative and quantitative circulating biomarkers have been available. In this setting, full-sialylation of the C4BP alpha-chain at sites 506 and 528, is a better candidate for the early detection of ovarian cancer, as compared to partial-sialylation, as full-sialylation is associated with delayed liver metabolism via the hepatocyte asialoglycoprotein receptor, and results in a prolonged half-life in the blood circulation [15–17]. Sustained blood levels of fully-sialylated A2160, therefore, enables us to detect OCCC in early-stage more readily than does CA-125 (Fig. 2C–D).
A2160 was identified by the extensive glycopeptide analysis in this study that required us to develop a novel methodological approach in mass spectrum technology. During this exploration of ovarian cancer biomarkers, we also examined several other candidates, in addition to A2160, for glycan sialylation that demonstrated substantial AUC values for EOC markers (Figure S6). Notably, we found that there was no other marker whose specificity was affected by the level of sialylation other than A2160. In the past, increased fucosylation has been found in the analysis of glycopeptides of haptogloblin in pancreatic cancer [18]. Another study developed a comprehensive method for serum glycoprotein analysis by combining lectin arrays with mass spectrometry [19]. However, their technologies only targeted certain glycoproteins and did not screen all the peptides with bound sugar chains. The main difficulty of performing comprehensive glycopeptide profiling by using MS is the complexity of analyzing a huge number of peaks [9]. The estimated number of glycopeptide patterns can be more than 100,000 when serum protein (more than 1000) and sugar chains (more than 100) are combined together, and it has been extremely difficult to analyze such mega data. We developed highly efficient enrichment strategies for glycopeptides by using a special filtration and a lectin column (manuscript in preparation), as well as novel software to analyze MS mathematically and macroscopically by using specific internal standards without labeling (manuscript in preparation).
In summary, the fully-sialylated alpha-chain of C4-binding protein, A2160, is a newly identified biomarker for ovarian cancer. The potential role of A2160 for both diagnostic and therapeutic implications could significantly impact the care of women with pelvic masses at risk of ovarian cancer. That is, A2160 can be valuable for early diagnosis and monitoring of ovarian cancer; and understanding the function of A2160 has therapeutic implications in terms of overcoming cancer immune resistance. Validation and further studies are warranted.
Supplementary Material
HIGHLIGHT.
More than 100,000 glycan structure of serum glycoprotein in ovarian cancer patients were screened.
A2160, fully-sialylated alpha-chain of C4BP, was isolated as a novel biomarker for ovarian cancer.
A2160 is particularly useful to distinguish early-stage clear cell carcinoma from endometrioma.
Acknowledgments
This work was supported in part by a Grant-in-aid for scientific research from Ministry of Education, Culture, Sports, Science and Technology (No. 26462538), Ministry of Education, Culture, Sports, Science and Technology-Supported Program (S1201001) for the Strategic Research Foundation at Private Universities, 2012–2014, grants from Mr. Minoru Sano Memorial Fund and Tokai University Research Aid and Ensign Endowment for Ovarian Cancer Research. We thank Dr. Brendan Grubbs for the technical support for the study.
Footnotes
Disclosure
The authors have declared that no conflict of interest exists.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2015.10.012.
References
- [1].Siegel R, Naishadham D Jemal A Cancer statistics, 2013. CA Cancer J. Clin 2013;63: 11–30. [DOI] [PubMed] [Google Scholar]
- [2].American Cancer Society, www.cancer.org 2015. (accessed March 23, 2015).
- [3].Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, et al. , Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy, Cancer 88 (2000) 2584–2589. [PubMed] [Google Scholar]
- [4].Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, et al. , Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial, JAMA 305 (2011) 2295–2303. [DOI] [PubMed] [Google Scholar]
- [5].Kobayashi E, Ueda Y, Matsuzaki S, Yokoyama T, Kimura T, et al. , Biomarkers for screening, diagnosis, and monitoring of ovarian cancer, Cancer Epidemiol. Biomark. Prev 21 (2012) 1902–1912. [DOI] [PubMed] [Google Scholar]
- [6].L Clarke-Pearson D, Clinical practice. Screening for ovarian cancer, N. Engl. J. Med 61 (2009) 170–177. [DOI] [PubMed] [Google Scholar]
- [7].Bulun SE, Endometriosis N Engl. J. Med 360 (2009) 268–279. [DOI] [PubMed] [Google Scholar]
- [8].Kobayashi H, Sumimoto K, Moniwa N, Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan, Int. J. Gynecol. Cancer 17 (2007) 37–43. [DOI] [PubMed] [Google Scholar]
- [9].Narimatsu H, Sawaki H, Kuno A, Kaji H, Ito H, Ikehara Y, et al. , A strategy for discovery of cancer glyco-biomarkers in serum using newly developed technologies for glycoproteomics, FEBS J. 277 (2010) 95–105. [DOI] [PubMed] [Google Scholar]
- [10].Zhang Y, Yin H, Lu H, Recent progress in quantitative glycoproteomics, Glycoconj. J 29 (2012) 249–258. [DOI] [PubMed] [Google Scholar]
- [11].Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, et al. , Association between endometrioma and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies, Lancet Oncol. 13 (2012) 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A, Cancer risk after a hospital discharge diagnosis of endometriosis, Am. J. Obstet. Gynecol 176 (3) (1997) 572–579. [DOI] [PubMed] [Google Scholar]
- [13].Criado-Garcia O, Fernaud-Espinosa I, Bovolenta P, Sainz dela Cuesta R, Rodriguez de Cordoba SS, Expression of the beta-chain of the complement regulator C4b-binding protein in human ovary, Eur. J. Cell Biol 78 (1999) 657–664. [DOI] [PubMed] [Google Scholar]
- [14].Nozaki H, in: Technology NloAI. S.a (Ed.) Differential marker of epithelial ovarian cancer, vol. JP 2012-145500 2012, pp. 1–58 (Japan). [Google Scholar]
- [15].Holmberg MT, Blom AM, Meri S, Regulation of complement classical pathway by association of C4b-binding protein to the surfaces of SK-OV-3 and Caov-3 ovarian adenocarcinoma cells, J. Immunol 167 (2001) 935–939. [DOI] [PubMed] [Google Scholar]
- [16].Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G, The role of sialic acid in determining the survival of glycoproteins in the circulation, J. Biol. Chem 246 (1971) 1461–1467. [PubMed] [Google Scholar]
- [17].Sorensen AL, Rumjantseva V, Nayeb-Hashemi S, Clausen H, Hartwig JH, et al. , Role of sialic acid for platelet life span: exposure ofβ-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes, Blood 114 (2009) 1645–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Miyoshi E, Shinzaki S, Moriwak K, Matsumoto H, Identification of fucosylated haptoglobin as a novel tumor marker for pancreatic cancer and its possible application for a clinical diagnostic test, Methods Enzymol. 478 (2010) 153–164. [DOI] [PubMed] [Google Scholar]
- [19].Sogabe M, Nozaki H, Tanaka N, Kubota T, Kaji H, et al. , Novel glycobiomarker for ovarian cancer that detects clear cell carcinoma, J. Proteome Res 13 (2014) 1624–1635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


