Abstract
Currently, the world is suffering with one of the biggest pandemics of recent history. Caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus disease 2019 (COVID-19) is provoking devastating consequences on economic and social fields throughout all continents. Therefore, pathophysiological knowledge about COVID-19 is imperative for better planning of preventive measures, diagnosis, and therapeutics of the disease. Based on previous studies, this work proposes new hypothesis related to the role of the renin-angiotensin system on the pathophysiology of COVID-19, and its purpose is to enrich the discussion and to offer alternative ways for experimental and clinical studies aiming at the formulation of new diagnosis and/or treatment methods.
Keywords: SARS-CoV-2, COVID-19, Renin-angiotensin system, ACE2
Background
The renin-angiotensin system (RAS) is currently one of the focuses of worldwide research due to coronavirus 2019 (COVID-19), a pandemic that has destabilized the world and created devastating consequences for economic and social areas [1], [2], [3]. Amongst the components of rennin-angiotensin system (RAS), the angiotensin-converting enzyme 2 (ACE2) has gained great prominence for being directly associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the coronavirus related to COVID-19 [4], [5]. Thus, the protagonism of ACE2 is being debated amongst researchers with the aim of establishing a role of RAS on the pathophysiologic context of COVID-19 [6], [7], [8].
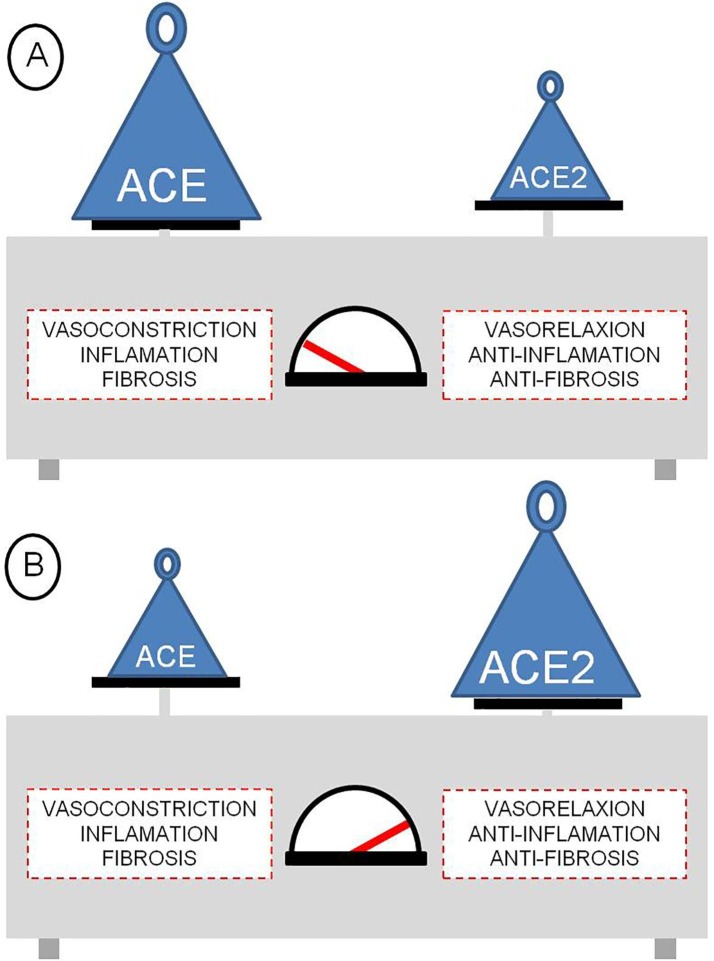
RAS was initially described as a regulating component of blood pressure and hydric balance, being called “classic pathway”. Subsequently, with the discovery of new components and finding of a “local tissue RAS”, the existence of a more complex system was observed with endocrine, paracrine and intracrine characteristics [9], [10], [11]. RAS elucidation continues to expand, new components are being discovered and also new ways of interaction between the local and systemic pathways, as well as interactions with the external environment [12]. Nowadays, RAS is known as a complex system, with systemic and local (tissue) activities which communicate between themselves and involve different sign pathways, leading to different results depending on the chosen pathway [9], [12]. This system’s balance is imperative for homeostasis, in which the angiotensin-converting enzyme (ACE) and ACE2 are the main weights in this scale (Fig. 1 ). When the scale plate tends to the ACE side, the production of angiotensin II (Ang II) and the activation of its respective receptors (AT1) increases. The activation of the detrimental pathway (ACE – Ang II – AT1R) stimulates actions such as cell proliferation, inflammation, fibrosis, and thrombosis. On the other hand, when the scale plate tends to the ACE2 side, the production of angiotensin 1–7 (Ang 1–7) increases, as well as the activation of its respective receptors (Mas) [13]. The activation of the protective pathway (ACE2 – Ang 1–7 – Mas) neutralizes the detrimental pathway, stimulating actions such as anti-inflammatory, anti-fibrotic, and anti-thrombotic effects [9], [14].
Fig. 1.
Renin-angiotensin system regulation. (A) – Prevalence of angiotensin-converting enzyme (ACE), accentuating the harmful effects from the pathway ACE/Ang II/AT1. (B) – Prevalence of angiotensin-converting enzyme 2 (ACE2), accentuating the beneficial effects from pathway ACE2/Ang 1–7/Mas.
Hypothesis
Renin-angiotensin system and the coronavirus
Coronaviruses are classified into four different genus, in which 3 species (α HCoV-NL63, SARS-CoV, and SARS-CoV-2) have ACE2 as a receptor [15]. The process of cell invasion occurs similarly amongst the different species. Briefly, the virus-cell interaction occurs through the ACE2 receptor coupling, and the internalization of the virus then occurs through fusion or endocytosis, with the participation of other components (clathrin, TMPRSS2, and others) [16], [17], [18]. Although they belong to the same subfamily Coronavirinae and share the same receptor, these viruses have significant differences, as presented in Table 1 . Amongst these differences, it is important to highlight in SARS-CoV-2 (the virus related to COVID-19) the longer incubation period and the faster viral peak, observed a few days after the beginning of symptoms.
Table 1.
Comparison amongst different Coronaviruses which use ACE2 as receptor.
| Species | Genus | Receptor | Affinity (Receptor) | Incubation period | Viral peak (after onset of symptoms) |
|---|---|---|---|---|---|
| SARS-CoV 2 | Betacoronavirus | ACE 2 | Very high | 2–14 days | 3 days |
| SARS-CoV 1 | Betacoronavirus | ACE 2 | High | 2–7 days | 10 days |
| HCoV-NL63 | Alpha-Coronavirus | ACE 2 | low | – | 2–6 days |
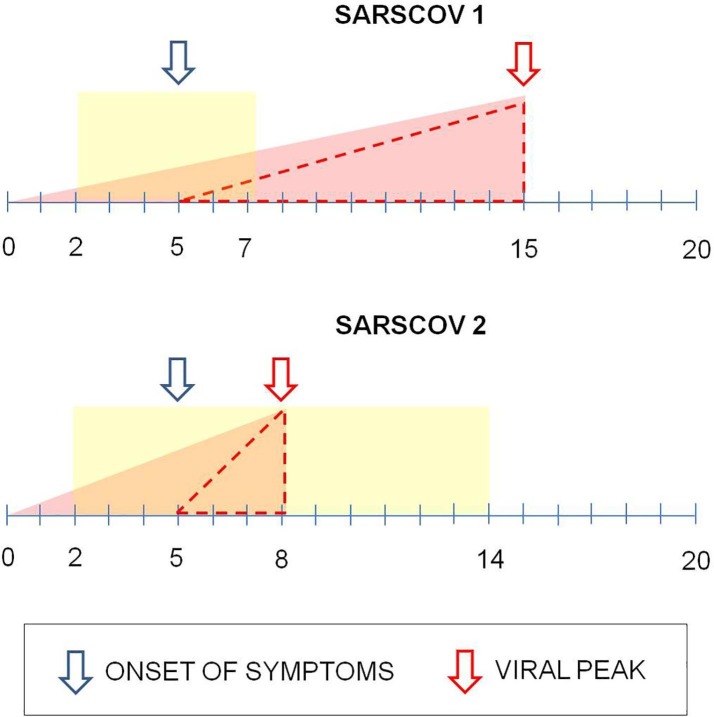
Such characteristics might be related to the fact that SARS-CoV-2 presents the most extreme deficiency of CpG amongst the known betacoronavirus genomes. The reduction of the CpG index may have allowed the virus to escape from immune response mediated by human ZAP (zinc finger antiviral protein), thus becoming a severe human pathogen [24]. Furthermore, SARS-CoV-2 has a much higher infection and replication capacity than SARS-CoV, however without inducing in a significant way the increase in interferons type I, II or III, and inflammatory mediators (pro-inflammatory cytokines/chemokines) [25]. Such characteristics of SARS-CoV-2 are imperative for the understanding of COVID-19 pathophysiology. The high replication rate associated with low inflammation leads to the viral peak even at the onset of symptoms, which makes early disease identification difficult, and as a consequence, makes treatment with antivirals ineffective because the effectiveness of their action occurs during the rise of the replication curve, the most recommended phase for such medicine [25]. Although SARS-CoV and SARS-CoV-2 use ACE2 as cell coupling receptor, the pathogenic characteristics of SARS-CoV-2 provide a great difference on disease progression, as shown in Fig. 2 .
Fig. 2.
Comparison between infection with SARS-CoV and SARS-CoV-2 and disease progression. For comparison purposes, the same day was considered for viral infection and beginning of symptoms for both viruses. The rectangle represents the incubation period. The small window (dotted triangle) between beginning of symptoms (left arrow) and viral peak (right arrow) hinders the action of antiviral drugs on COVID-19.
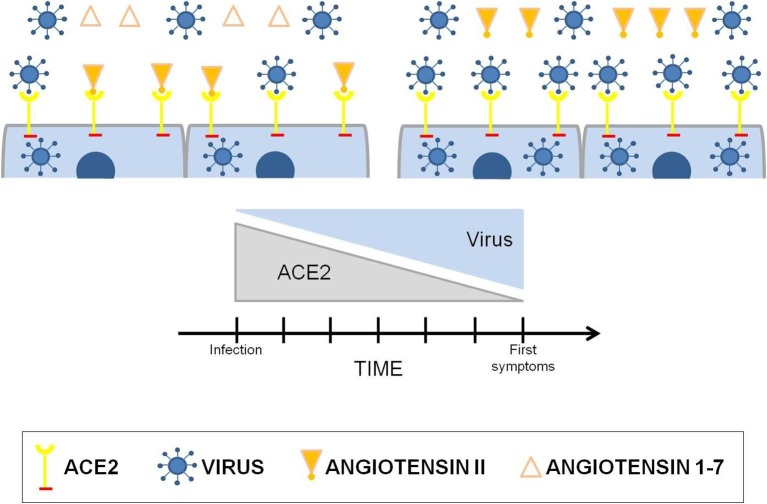
ACE2 is a fundamental piece in the pathophysiology of COVID-19, since the high replication capacity of SAR-CoV-2 is directly related to the coupling to ACE2 and cell infection. Studies show that the virus causes a reduction of ACE2 in the infected organs [26]. Therefore, the higher the amount of virus, the higher will be the use of ACE2 by it, creating an inverse correlation between the amount of SARS-CoV-2 and ACE2. At a certain time, the constant and gradual reduction of ACE2 will cause a RAS imbalance, increasing the action of ACE in detriment of the decrease in the action of ACE2, thus prevailing the harmful factors (Fig. 3 ).
Fig. 3.
Correlation between SARS-CoV-2 infection and the renin-angiotensin system. Because of ACE2 decreasing due to the gradual increase in viral load, imbalance of the renin-angiotensin system occurs, thus prevailing the harmful effects of the pathway ACE/Ang II/AT1.
The ACE2 level reduction caused by SARS-CoV-2 infection may be directly related to the pathogenesis of COVID-19 [26]. The Ang 1–7 decrease and the lower activation of Mas receptors and/or the Ang II production increase, with subsequent activation of AT1 receptor, is widely known for the triggering of inflammation and fibrosis [27]. For example, a study with mice has shown that acute lung lesion results in a marked decrease of ACE2, and the use of recombinant ACE2 has a protective effect against the lung lesion. The same study noted that ACE, Ang II, and the Ang II receptor 1 (AT1) provoke the disease pathogenesis. Thus, the important role of RAS on acute lung lesion pathogenesis is demonstrated [28]. Histologically, patients with COVID-19 present a pattern of diffuse alveolar damage and perivascular lymphocyte infiltration, similar to what is observed in influenza cases. A surprising histopathological finding on COVID-19 was the lung angiogenesis, which is 2.7 times greater than on the lungs of patients with influenza [29]. Angiogenesis, a complex process by which new blood vessels are formed from existing, is induced by hypoxia. Hypoxia is a condition in which tissues are not properly oxygenated, resulting in considerable cell stress and adaptive responses. The transcriptional responses to hypoxia are mostly mediated by the hypoxia-inducible factor (HIF), a transcription factor that acts as oxygen sensors and are related to the activation of VEGF-A expression [30], [31]. Under hypoxia, HIF influences ACE and ACE2 in different ways, positively regulating the expression of ACE and negatively of ACE2 [32], [33]. In COVID-19, such circumstances may worsen the clinical condition, for stimulating even more the inflammatory, fibrotic, and thrombogenic pathways of RAS. Although COVID-19 is clinically classified as an acute respiratory syndrome, the increased angiogenesis suggests a chronic cell adaptation towards progressive hypoxia. The chronic adaptation to hypoxia could explain the higher resistance of some patients towards low O2 saturation, in which such patients do not present an involuntary increase of ventilation, even when great lung damage is present (“ground-glass opacity”).
The replicative cycle of SARS-CoV-2 causes a local immune response as a result of cell death and tissue damage. In some cases, the immune response occurs in an unregulated manner, triggering a cytokine storm, and consequently, generalized pulmonary inflammation [34]. As with COVID-19, other acute respiratory diseases (SARS, MERS, Influenza) can also present the clinical picture of cytokine storm [35]. High virus titers and dysregulation of the cytokine/chemokine response cause an inflammatory cytokine storm, mainly related to the influx of inflammatory mononuclear macrophages. The activation of these macrophages by interferon-α/β produces more chemotactic factors for monocytes (CCL2, CCL7 and CCL12), resulting in the additional accumulation of mononuclear macrophages, and subsequently, an increase in the levels of pro-inflammatory cytokines (TNF, IL-6, IL1-β and inducible nitric oxide synthase), thus increasing the severity of the disease [35].
In severe cases of COVID-19, patients demonstrate elevated plasma levels of IL-2, IL-7, IL-10, granulocyte colony stimulating factor (G-CSF), IP-10, MCP1, macrophage inflammatory protein 1α (MIP1α) and tumor necrosis factor (TNF) [34]. Viral infection and replication in airway epithelial cells can cause high levels of pyroptosis. Pyroptosis is a highly inflammatory form of programmed cell death seen in cytopathic viruses such as SARS-CoV-2. IL-1β, an important cytokine released during pyroptosis, is elevated in infection by SARS-CoV-2, thus being a possible trigger for the cytokine storm [34].
The excessive or uncontrolled release of pro-inflammatory cytokines, characteristic of the cytokine storm, is also associated with non-infectious diseases, such as autoimmune diseases [36]. Yiguo Qiu et al. analyzed RAS in mice with autoimmune uveitis. The study demonstrated that the administration of Ang 1–7 antagonist reversed the protective effects of ACE2 on inflammatory signals and on the production of inflammatory cytokines, as well as on the regulation of local immune responses. The inhibition of Ang 1–7 increased the production of the pro-inflammatory cytokines IL-6, IL-1β, TNF-α, and MCP-1 [37]. This study demonstrates the importance of Ang 1–7 in mediating the inflammatory process, as its inhibition can cause an increase in the production of pro-inflammatory cytokines, similar to that observed in the cytokine storm in COVID-19. ACE2 plays a crucial role in RAS because it neutralizes ACE activity by degrading Ang I in Ang (1–9), as well as hydrolyzing Ang II, producing Ang (1–7) [38]. Thus, ACE2 is essential to stimulate the beneficial effects of the protective axis of RAS, ACE2/Ang (1–7)/Mas, and to mitigate the deleterious effects of the harmful axis of RAS, ACE/Ang II/AT1. Maintaining normal levels of ACE2 in the lung is beneficial for combating inflammatory lung disease. The reduction in ACE2 expression may be related to pulmonary inflammation and subsequent cytokine storm seen in patients with severe COVID-19. ACE2 maintains the proper function of the heart and kidneys, and the negative regulation of ACE2 by SARS-CoV-2 can compromise this protective characteristic and contribute to the damage caused by the infection of these organs [38].
Renin-angiotensin system and the self-limitative replication of SARS-CoV-2
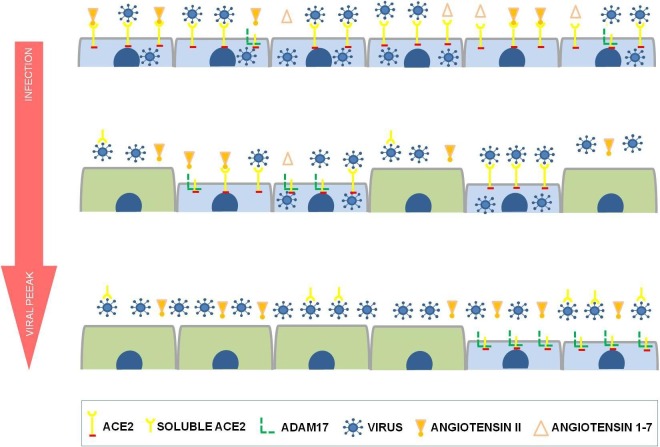
RAS is classified as systemic (classic) and local, and the interaction between them is fundamental to homeostasis [9], [10], [39]. This interaction between local and systemic RAS probably occurs through the circulation of soluble ACE and ACE2. Although they are transmembrane proteins, ACE and ACE2 may undergo a cleavage process by ACE secretase and ADAM17, respectively, releasing an active extracellular domain. This soluble component is capable of converting angiotensin I into Ang II (ACE) and Ang II into Ang 1–7 (ACE2) [39], [40]. The balance between the amount of transmembrane ACE2 and soluble ACE2 is imperative, because the excessive release of the soluble form to the extracellular environment could lead to local tissue injury, since it will cause misbalance on the production of local Ang II and Ang 1–7. The local RAS misbalance makes the harmful pathway effects prevail (ACE – Ang II – AT1R) on the referred organ or tissue [41]. In an ACE2 receptor-dependent viral infection (Coronavirus NL63), the reduction of ACE2 cellular level occurs without changes in ACE levels [42]. This finding, plus the fact that SARS-CoV-2 elicits a low immune and inflammatory response, even when compared to SARS-CoV [25], is more evidence that COVID-19 pathogenesis is more related to RAS misbalance than to the viral action itself. Mossel et al. (2005) noted that ACE2 expression resulted in SARS-CoV replication in all cell lineages refractory to viral replication, thus demonstrating the direct dependence of the viral replication on the receptor. However, it was noted that a small number of cells with high ACE2 expression levels did not endure viral replication. The justification for such a fact is the high expression level of cell ACE2, resulting in the formation of enough soluble ACE2 to block the infectiousness of the inoculum and/or the first generation progenies [43]. Studies show that the use of human soluble ACE2 or human recombinant ACE2 have the capability to block SARS-CoV-2 infection [44], [45]. Hong Peng Jia et al. (2009) demonstrated that in airways infections by SARS-CoV, ACE2 present on the surface liquid of the airways was active and even capable of connecting to the virus. The increase in ACE2 release, due to endotoxins and pro-inflammatory cytokines, is a sign that the production of soluble ACE2 may have defensive functions in the host [12]. Based on these findings the hypothesis of the self-limitative viral replication is suggested, and in consequence, of cellular infection. This would happen for two reasons: (1) decrease in the availability of ACE2 on a cell surface caused by the viral replication itself (viral peak); (2) virus neutralization due to soluble ACE2 release to the extracellular environment as a defense of the infected cell itself (Fig. 4 ). Young cells express a lower quantity of ACE2, related mainly to the need of epithelial polarization. Thus, undifferentiated cells are less susceptible to infection by SARS-CoV [46]. Such a fact probably prevents the infection of new cells arising from regenerative tissue response, thus decreasing the possibility of viral replication in COVID-19. Furthermore, the virus in the extracellular environment becomes more vulnerable to the neutralizing action of soluble ACE2, stimulated by the viral infection itself, as a cellular defense mechanism likely mediated by the increase in the activity of ADAM17 [12], [23], [47].
Fig. 4.
Renin-angiotensin system and self-limitative replication of SARS-CoV-2. The short cells represent differentiated cells and the tall represent undifferentiated cells, which do not yet express ACE2. The ACE2 expression on a cell surface is imperative for cell infection, and in consequence, viral replication of SARS-CoV-2.
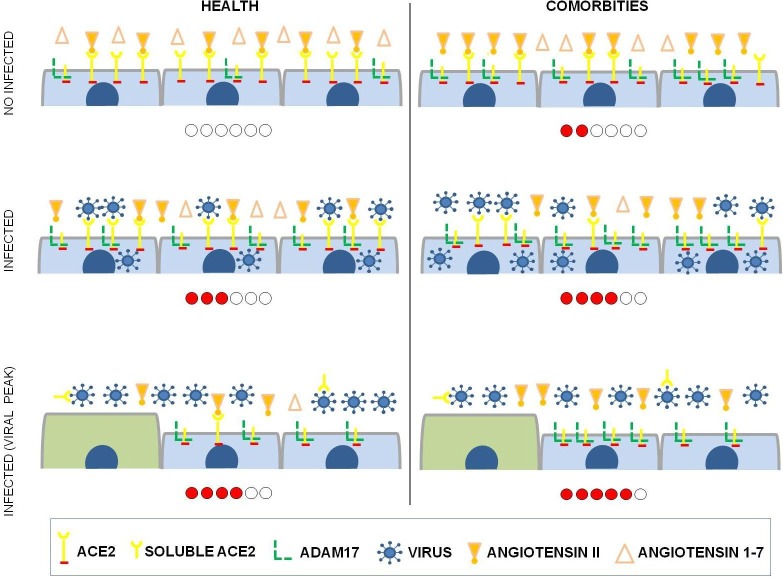
Renin-angiotensin system and SARS-CoV-2 infection in subjects with previous comorbidities
Studies show that diabetes, cardiovascular diseases, obesity, and other comorbidities are risk factors for COVID-19 [48], [49]. When what these comorbidities have in common is analyzed, it is observed that individuals affected by these comorbidities have a higher expression of ADAM17 [50], [51], [52], [53]. ADAM17 is a protease with a single transmembrane domain and a cytoplasmic portion. ADAM17 releases extracellular domains of transmembrane proteins to produce soluble bioactive signaling proteins that participate in autocrine and paracrine signaling. ADAM17 substrates include adhesion proteins, transmembrane mucins, membrane-bound cytokines (TNF-α), growth factors (TGF-α), and cytokine receptors (IL-6R, TNF-R) [53], [54]. ADAM17 has a pro-inflammatory and pro-fibrotic role in chronic kidney disease, and is also involved in other pathological processes such as inflammatory diseases, neurological diseases, cardiovascular diseases, and diabetes [51]. In addition to the previously mentioned substrates, ADAM17 is also responsible for the cleavage of the ACE2 ectodomain, forming soluble ACE2. When ADAM17 catalyzes the release of ectodomain from ACE2, the protective axis of RAS mediated by ACE2 is compromised [51]. The increase in soluble ACE2 (systemic) may cause an imbalance of the tissue RAS (local), decreasing the action of the axis ACE2/Ang 1–7 and accumulating Ang II, and thus prevailing the deleterious effects of RAS. Therefore, individuals with previous comorbidities can naturally present an inflammatory state due to the pro-inflammatory role of ADAM17, as well as due to its action in RAS, interfering in the tissue balance of ACE2/ACE. SARS-CoV-2 infection negatively regulates ACE2 receptors, with loss of the catalytic effect of these receptors on the external site of the membrane [26]. In individuals with previous comorbidities, SARS-CoV-2 infection may potentiate the existing RAS imbalance, further suppressing the protective axis of ACE2 and accentuating the harmful axis of ACE, thus leading to the worsening of the clinical picture in a patient with comorbidities (Fig. 5 ).
Fig. 5.
Renin-angiotensin system in healthy subjects or in subjects with previous comorbidity infected with SARS-CoV-2. The short cells represent differentiated cells and the tall represent undifferentiated cells which do not yet express ACE2. The misbalance of the renin-angiotensin system, with prevalence of ACE – Ang II – AT1 pathway, is represented by the amount of dots on the level bar below each scheme.
Although the protease activity of ADAM17 over ACE2 is observed, the same is not observed in ACE [55], [56], [57]. Interestingly, ACE can regulate the activity of ADAM17, since it can be triggered from the activation of the AT1 receptor by Ang II [58]. Thus, ACE directly regulates the formation of Ang II from Ang I, and interferes via the action of ADAM17 in ACE2, in the tissue formation of Ang 1–7 from Ang II. Therefore individuals with greater expression of tissue ACE, and consequently, greater production of Ang II, will present a decrease in tissue ACE2 and an increase in soluble ACE2 (systemic), due to greater activation of ADAM17 and ACE2 cleavage. This interaction between ACE, ADAM17 and ACE2 can explain the difference in COVID-19 involvement between men and women. Androgens increase renin levels and ACE activity, while estrogen decreases renin levels, ACE activity, and AT1 receptor density, and is also related to increased levels of Ang 1–7 [59], an important actor in the protective axis of RAS. This explains the fact that men have higher circulating (soluble) ACE2 levels than women [60], due to the greater action of ADAM17 via androgens/ECA/Ang II/AT1. Thus, due to higher levels of circulating ACE2, men may present a lower level of tissue ACE2, becoming more vulnerable to local RAS imbalance after SARS-Cov-2 infection. This argument gains more strength when we observe that children [61] and the elderly [62] do not show a difference in the involvement of COVID-19 between genders, probably due to the lack of hormonal interference before puberty and also due to the loss of protection from estrogen in women after menopause.
Renin-angiotensin system and SARS-CoV-2 infection in adults, children, and dogs
A particularity of SARS-CoV-2 infection in humans is infection development without symptoms, or with light symptoms in children [63], [64]. Such a fact demonstrates that physiological differences between adults and children provoke different responses to SARS-CoV-2 infection. It is important to highlight that SARS-CoV-2 infection is not exclusive to humans, being observed also in other mammals [65]. A better understanding of the infection in other species is imperative to the understanding of the infection in humans. Sit et al. (2020) reported the infection of two dogs by SARS-CoV-2 with suggestive evidence of human-animal transmission [66]. The animals infected with SARS-CoV-2 were asymptomatic during the whole course of the disease, similarly to what is observed in children. When RAS in adults, children, and dogs are analyzed, interesting correlations and differences can be noted. In all three cases, there is an alteration in the RAS balance during comorbidities such as heart disease and/or chronic kidney disease. Heart disease and/or chronic kidney disease in adults and children cause an increase of plasmatic Ang 1–7, which is probably a defense mechanism of the body towards injury [67], [68], [69]. Currently, the plasma measurement of ACE2, the enzyme responsible for the formation of Ang 1–7, is being studied as a potential biomarker for heart and kidney alterations [40], [70]. Larouche-Lebel et al. (2019) studied the heart alterations in dogs and observed the same Ang 1–7 behavior, which presented high levels when compared to healthy dogs [71]. Although it presents the same behavior, the intensity of the level increase of Ang 1–7 is different. Dogs and children show similar responses to the disease, presenting a great increase in the production of Ang 1–7 when compared to the control group [67], [71]. In sick adults, the Ang 1–7 level is also high when compared to healthy subjects [68], [69], but the response intensity is much lower when compared to dogs and children. The distinct adequacy of RAS towards SARS-CoV-2 infection could explain the different forms of manifestations of COVID-19 in adults, children, and dogs. Children and dogs infected with SARS-CoV-2 succeeded to preserve adequate levels of ACE2 and Ang 1–7 during viral infection, thus maintaining RAS balance. Due to low efficiency of the response towards injury, adults might present a great decrease of ACE2, thus prevailing RAS harmful pathway effects (ACE – Ang II – AT1R). Although they are susceptible to infection by SARS-CoV-2, children and dogs do not develop the symptomatology of COVID-19 observed in adults due to the greater capability of ACE2 expression and Ang 1–7 production towards injury.
Testing the hypothesis
Experimental and clinical approaches can be used to test the hypothesis. The assessment merit of the hypothesis could be performed by plasma ACE2 and Ang 1–7 measurements in patients positive for SARS-CoV-2. In hospitalized patients, blood collection is part of the clinical routine, so plasma evaluation of the components of RAS would not cause any harm to the patient, or difficulties for the clinical team. Comparative analysis could be performed in a dependent manner, measuring the daily plasma levels of ACE2 and Ang 1–7 and correlating with the evolution of the patient's clinical condition, as well as could be performed independently, comparing patients infected with SARS-CoV-2 and the control group (non-infected). Another approach could be through the relation between ACE2 and ACE, thus comparing the anti-inflammatory and pro-inflammatory actions of RAS. Additionally, if the measurement is feasible, the analysis of RAS in respiratory secretions can be tested. The feasibility of this test may allow the early diagnosis of COVID-19, even before the beginning of symptoms. The use of soluble ACE2 as well as drugs that stimulate ACE2 production should also be considered as possible therapeutic strategies. Comparative studies between adults and children, as well as between humans and other mammals, could be conducted to better understand the correlation between RAS and COVID-19.
Conclusion
Although this is only a hypothesis, there is strong evidence that the inflammatory, fibrotic, and thrombotic processes observed in COVID-19 might be more related to the tissue ACE2 decrease, and consequent RAS misbalance, than to the inflammatory process caused by the virus itself. Thus, this hypothesis seeks to reaffirm the important role of ACE2 on the pathophysiology of COVID-19. A better understanding of COVID-19 pathophysiology is imperative to elaborate strategies during the pandemic. Thus, this work seeks to enlarge the discussion related to COVID-19, offering alternative ways for experimental and clinical studies aiming at the formulation of new diagnosis and/or treatment methods.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thanks to Isabela Tanuri Bessa for her helpful comments. Thanks also to the researchers from different knowledge areas for all their work undertaken as the basis for the construction of this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110330.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Fagoonee I., Pellicano R. Covid-19 brings the world economy to its knees. Minerva Med. 2020 doi: 10.23736/S0026-4806.20.06603-3. [DOI] [PubMed] [Google Scholar]
- 2.McKee M., Stuckler D. If the world fails to protect the economy, COVID-19 will damage health not just now but also in the future. Nat Med. 2020;26(5):640–642. doi: 10.1038/s41591-020-0863-y. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M., Mckee M., Mossialos E. Developing a sustainable exit strategy for COVID-19: health, economic and public policy implications. J R Soc Med. 2020;113(5):176–178. doi: 10.1177/0141076820925229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin–Angiotensin–Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–Angiotensin–Aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao L, Sakagami H, Miwa N. ACE2: The key Molecule for Understanding the Pathophysiology of Severe and Critical Conditions of COVID-19: Demon or Angel? Viruses. 04 2020;12(5)doi:10.3390/v12050491. [DOI] [PMC free article] [PubMed]
- 7.Liabeuf S, Moragny J, Bennis Y, et al. Association between renin-angiotensin system inhibitors and COVID-19 complications. Eur Heart J Cardiovasc Pharmacother. Jun 2020;doi:10.1093/ehjcvp/pvaa062. [DOI] [PMC free article] [PubMed]
- 8.K. Lanza L.G. Perez L.B. Costa T.M. Cordeiro V.A. Palmeira V.T. Ribeiro A.C. Simões e Silva Covid-19: the renin–angiotensin system imbalance hypothesis 134 11 2020 2020 1259 1264 https://portlandpress.com/clinsci/article/134/11/1259/225184/Covid19-the-reninangiotensin-system-imbalance. [DOI] [PMC free article] [PubMed]
- 9.Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. Sep 2008;264(3):224-36. doi:10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed]
- 10.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A Novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87(5) doi: 10.1161/01.RES.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 11.Turner A.J., Tipnis S.R., Guy J.L., Rice G.I., Hooper N.M. ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can J Physiol Pharmacol. 2002;80(4):346–353. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- 12.Jia H.P., Look D.C., Tan P., Shi L., Hickey M., Gakhar L., Chappell M.C., Wohlford-Lenane C., McCray P.B., Jr Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos R.A.S., e Silva A.C.S., Maric C., Silva D.M.R., Machado R.P., de Buhr I., Heringer-Walther S., Pinheiro S.V.B., Lopes M.T., Bader M., Mendes E.P., Lemos V.S., Campagnole-Santos M.J., Schultheiss H.-P., Speth R., Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. PNAS. 2003;100(14):8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaduganathan M, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Covid-19. Reply. N Engl J Med. 06 2020;382(24):e92. doi:10.1056/NEJMc2013707. [DOI] [PubMed]
- 15.Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 06 2012;4(6):1011-33. doi:10.3390/v4061011. [DOI] [PMC free article] [PubMed]
- 16.Milewska A, Nowak P, Owczarek K, et al. Entry of Human Coronavirus NL63 into the Cell. J Virol. 02 2018;92(3)doi:10.1128/JVI.01933-17. [DOI] [PMC free article] [PubMed]
- 17.Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. JVI. 2010;84(24):12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Res. 2020;178:104792. doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L.M., Law K.I., Tang B.S.F., Hon T.Y.W., Chan C.S., Chan K.H., Ng J.S.C., Zheng B.J., Ng W.L., Lai R.W.M., Guan Y., Yuen K.Y. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/s0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Hoek L, Sure K, Ihorst G, et al. Croup is associated with the novel coronavirus NL63. PLoS Med. Aug 2005;2(8):e240. doi:10.1371/journal.pmed.0020240. [DOI] [PMC free article] [PubMed]
- 21.Xie M., Chen Q. Insight into 2019 novel coronavirus — An updated interim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., Simmons G., Hofmann H., Kuri T., Weber F., Eichler J., Drosten C., Pöhlmann S. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. JVI. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia X. Extreme genomic CpG deficiency in SARS-CoV-2 and evasion of host antiviral defense. Mol Biol Evol. Apr 2020;doi:10.1093/molbev/msaa094. [DOI] [PMC free article] [PubMed]
- 25.Chu H, Chan JF, Wang Y, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. Apr 2020;doi:10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed]
- 26.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;06(76):14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.AlQudah M., Hale T.M., Czubryt M.P. Targeting the renin-angiotensin-aldosterone system in fibrosis. Matrix Biol. 2020;91-92:92–108. doi: 10.1016/j.matbio.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai Y., Kuba K., Rao S., Huan Y.i., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.-C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S.H., Manandhar S., Lee Y.M. Roles of RUNX in hypoxia-induced responses and angiogenesis. Adv Exp Med Biol. 2017;962:449–469. doi: 10.1007/978-981-10-3233-2_27. [DOI] [PubMed] [Google Scholar]
- 31.Kumar H., Choi D.-K. Hypoxia inducible factor pathway and physiological adaptation: a cell survival pathway? Mediators Inflamm. 2015;2015:1–11. doi: 10.1155/2015/584758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R., Wu Y., Zhao M. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297(4):L631–L640. doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R., Su H., Ma X., Xu X., Liang L.i., Ma G., Shi L. MiRNA let-7b promotes the development of hypoxic pulmonary hypertension by targeting ACE2. Am J Physiol Lung Cell Mol Physiol. 2019;316(3):L547–L557. doi: 10.1152/ajplung.00387.2018. [DOI] [PubMed] [Google Scholar]
- 34.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm' in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu Y., Tao L., Zheng S., Lin R.u., Fu X., Chen Z., Lei C., Wang J., Li H., Li Q., Lei B.o. AAV8-mediated angiotensin-converting enzyme 2 gene delivery prevents experimental autoimmune uveitis by regulating MAPK, NF-κB and STAT3 Pathways. Sci Rep. 2016;6(1) doi: 10.1038/srep31912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Datta P.K., Liu F., Fischer T., Rappaport J., Qin X. SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10(16):7448–7464. doi: 10.7150/thno.48076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleming I. Signaling by the angiotensin-converting enzyme. Circ Res. 2006;98(7):887–896. doi: 10.1161/01.RES.0000217340.40936.53. [DOI] [PubMed] [Google Scholar]
- 40.Anguiano L., Riera M., Pascual J., Soler M.J. Circulating ACE2 in cardiovascular and kidney diseases. Curr Med Chem. 2017;24(30):3231–3241. doi: 10.2174/0929867324666170414162841. [DOI] [PubMed] [Google Scholar]
- 41.Ortiz-Pérez JT, Riera M, Bosch X, et al. Role of circulating angiotensin converting enzyme 2 in left ventricular remodeling following myocardial infarction: a prospective controlled study. PLoS One. 2013;8(4):e61695. doi:10.1371/journal.pone.0061695. [DOI] [PMC free article] [PubMed]
- 42.Dijkman R, Jebbink MF, Deijs M, et al. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J Gen Virol. Sep 2012;93(Pt 9):1924-1929. doi:10.1099/vir.0.043919-0. [DOI] [PubMed]
- 43.Mossel E.C., Huang C., Narayanan K., Makino S., Tesh R.B., Peters C.J. Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. J Virol. 2005;79(6):3846–3850. doi: 10.1128/JVI.79.6.3846-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alhenc-Gelas F., Drueke T.B. Blockade of SARS-CoV-2 infection by recombinant soluble ACE2. Kidney Int. 2020;97(6):1091–1093. doi: 10.1016/j.kint.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B., Jr ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. JVI. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hintz K.A., Rassias A.J., Wardwell K. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol. 2002;72(4):711–717. [PubMed] [Google Scholar]
- 48.Fang X., Li S., Yu H., Wang P., Zhang Y., Chen Z., Li Y., Cheng L., Li W., Jia H., Ma X. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12(13):12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poblador-Plou B, Carmona-Pírez J, Ioakeim-Skoufa I, et al. Baseline Chronic Comorbidity and Mortality in Laboratory-Confirmed COVID-19 Cases: Results from the PRECOVID Study in Spain. Int J Environ Res Public Health. 07 2020;17(14)doi:10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed]
- 50.Junyent M., Parnell L.D., Lai C.Q. ADAM17_i33708A>G polymorphism interacts with dietary n-6 polyunsaturated fatty acids to modulate obesity risk in the Genetics of Lipid Lowering Drugs and Diet Network study. Nutr Metab Cardiovasc Dis. 2010;20(10):698–705. doi: 10.1016/j.numecd.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palau V., Pascual J., Soler M.J., Riera M. Role of ADAM17 in kidney disease. Am J Physiol Renal Physiol. 2019;317(2):F333–F342. doi: 10.1152/ajprenal.00625.2018. [DOI] [PubMed] [Google Scholar]
- 52.Rizza S., Copetti M., Cardellini M., Menghini R., Pecchioli C., Luzi A., Di Cola G., Porzio O., Ippoliti A., Romeo F., Pellegrini F., Federici M. A score including ADAM17 substrates correlates to recurring cardiovascular event in subjects with atherosclerosis. Atherosclerosis. 2015;239(2):459–464. doi: 10.1016/j.atherosclerosis.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 53.Stolarczyk M., Scholte B.J. The EGFR-ADAM17 axis in chronic obstructive pulmonary disease and cystic fibrosis lung pathology. Mediators Inflamm. 2018;2018:1–22. doi: 10.1155/2018/1067134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zunke F., Rose-John S. The shedding protease ADAM17: Physiology and pathophysiology. Biochim Biophysi Acta (BBA) – Molecular Cell Research. 2017;1864(11):2059–2070. doi: 10.1016/j.bbamcr.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Lambert D.W., Yarski M., Warner F.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280(34):30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allinson T.M.J., Parkin E.T., Condon T.P., Schwager S.L.U., Sturrock E.D., Turner A.J., Hooper N.M. The role of ADAM10 and ADAM17 in the ectodomain shedding of angiotensin converting enzyme and the amyloid precursor protein. Eur J Biochem. 2004;271(12):2539–2547. doi: 10.1111/j.1432-1033.2004.04184.x. [DOI] [PubMed] [Google Scholar]
- 57.Parvathy S., Karran E.H., Turner A.J., Hooper N.M. The secretases that cleave angiotensin converting enzyme and the amyloid precursor protein are distinct from tumour necrosis factor-alpha convertase. FEBS Lett. 1998;431(1):63–65. doi: 10.1016/s0014-5793(98)00726-1. [DOI] [PubMed] [Google Scholar]
- 58.Kawai T., Forrester S.J., O'Brien S., Baggett A., Rizzo V., Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol Res. 2017;125(Pt A):4–13. doi: 10.1016/j.phrs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Komukai K., Mochizuki S., Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol. 2010;24(6):687–698. doi: 10.1111/j.1472-8206.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- 60.Anguiano L., Riera M., Pascual J., Valdivielso J.M., Barrios C., Betriu A., Mojal S., Fernández E., Soler M.J. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol Dial Transplant. 2015;30(7):1176–1185. doi: 10.1093/ndt/gfv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong Y., Mo X.i., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 62.Posso M., Comas M., Román M., Domingo L., Louro J., González C., Sala M., Anglès A., Cirera I., Cots F., Frías V.-M., Gea J., Güerri-Fernández R., Masclans J.R., Noguès X., Vázquez O., Villar-García J., Horcajada J.P., Pascual J., Castells X. Comorbidities and mortality in patients with COVID-19 aged 60 years and older in a university hospital in spain. Arch Bronconeumol. 2020 doi: 10.1016/j.arbres.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhopal S.S., Bagaria J., Bhopal R. Risks to children during the covid-19 pandemic: some essential epidemiology. BMJ. 2020;369 doi: 10.1136/bmj.m2290. [DOI] [PubMed] [Google Scholar]
- 64.Khan E. COVID-19 in children: epidemiology, presentation, diagnosis and management. J Pak Med Assoc. 2020;70(Suppl 3):1. doi: 10.5455/JPMA.10.5455/JPMA.25. [DOI] [PubMed] [Google Scholar]
- 65.Luan J., Lu Y., Jin X., Zhang L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem Biophys Res Commun. 2020;526(1):165–169. doi: 10.1016/j.bbrc.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.T.H.C. Sit C.J. Brackman S.M. Ip K.W.S. Tam P.Y.T. Law E.M.W. To V.Y.T. Yu L.D. Sims D.N.C. Tsang D.K.W. Chu R.A.P.M. Perera L.L.M. Poon M. Peiris Infection of dogs with SARS-CoV-2 10.1038/s41586-020-2334-5 http://www.nature.com/articles/s41586-020-2334-5. [DOI] [PMC free article] [PubMed]
- 67.Suessenbach F.K., Burckhardt B.B. Levels of angiotensin peptides in healthy and cardiovascular/renal-diseased paediatric population—an investigative review. Heart Fail Rev. 2019;24(5):709–723. doi: 10.1007/s10741-019-09797-y. [DOI] [PubMed] [Google Scholar]
- 68.Zhou X., Zhang P., Liang T., Chen Y., Liu D., Yu H. Relationship between circulating levels of angiotensin-converting enzyme 2-angiotensin-(1–7)-MAS axis and coronary heart disease. Heart Vessels. 2020;35(2):153–161. doi: 10.1007/s00380-019-01478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basu R., Poglitsch M., Yogasundaram H., Thomas J., Rowe B.H., Oudit G.Y. Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol. 2017;69(7):805–819. doi: 10.1016/j.jacc.2016.11.064. [DOI] [PubMed] [Google Scholar]
- 70.Anguiano L., Riera M., Pascual J., Valdivielso J.M., Barrios C., Betriu A., Clotet S., Mojal S., Fernández E., Soler M.J. Circulating angiotensin converting enzyme 2 activity as a biomarker of silent atherosclerosis in patients with chronic kidney disease. Atherosclerosis. 2016;253:135–143. doi: 10.1016/j.atherosclerosis.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 71.Larouche‐Lebel É., Loughran K.A., Oyama M.A., Solter P.F., Laughlin D.S., Sánchez M.D., Assenmacher C.-A., Fox P.R., Fries R.C. Plasma and tissue angiotensin‐converting enzyme 2 activity and plasma equilibrium concentrations of angiotensin peptides in dogs with heart disease. J Vet Intern Med. 2019;33(4):1571–1584. doi: 10.1111/jvim.15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.