Abstract
The presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in water and wastewater has recently been reported. According to the updated literature, the stools and masks of the patients diagnosed with coronavirus disease (COVID-19) were considered as the primary route of coronavirus transmission into water and wastewater. Most coronavirus types which attack human (possible for SARS-CoV-2) are often inactivated rapidly in water (i.e., the survival of human coronavirus 229E in water being 7 day at 23 °C). However, the survival period of coronavirus in water environments strongly depends on temperature, property of water, concentration of suspended solids and organic matter, solution pH, and dose of disinfectant used. The World Health Organization has stated that the current disinfection process of drinking water could effectively inactivate most of the bacterial and viral communities present in water, especially SARS-CoV-2 (more sensitive to disinfectant like free chlorine). A recent study confirmed that SARS-CoV-2 RNA was detected in inflow wastewater (but not detected in outflow one). Although the existence of SARS-CoV-2 in water influents has been confirmed, an important question is whether it can survive or infect after the disinfection process of drinking water. To date, only one study confirmed that the infectivity of SARS-CoV-2 in water for people was null based on the absence of cytopathic effect (CPE) in infectivity tests. Therefore, further studies should focus on the survival of SARS-CoV-2 in water and wastewater under different operational conditions (i.e., temperature and water matrix) and whether the transmission from COVID-19-contaminated water to human is an emerging concern. Although paper-based devices have been suggested for detecting the traces of SARS-CoV-2 in water, the protocols and appropriate devices should be developed soon. Wastewater and sewage workers should follow the procedures for safety precaution against SARS-CoV-2 exposure.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Enveloped virus, Sewage, Wastewater
Highlights
-
•
SARS-CoV-2 coronavirus detected in water and wastewater.
-
•
Transmission route of SARS-CoV-2 into water through stool and mask of infected patient.
-
•
Coronavirus often inactivated rapidly in water.
-
•
Paper-based devices suggested for detecting traces of SARS-CoV-2 in water.
-
•
Existing disinfection processes possibly sufficient to kill SARS-CoV-2 in water.
1. Introduction
An abrupt epidemic outbreak of coronavirus disease 2019 (COVID-19) (WHO, 2019), which was resulted from severe acute respiratory syndrome coronavirus (abbreviated as SARS-CoV), has currently caused enormous global concerns within the scientific and healthcare community and general population alike due to the unavailability of human coronavirus vaccines. The causative agent of this pandemic was permanently named as SARS-CoV-2 (tentatively named as 2019-nCOV) to distinguish it from the SARS-CoV-1 virus that was first recognized in 2002 (Chaudhry and Sachdeva, 2020; WHO, 2019; Zhu et al., 2020). The first case of infection from SARS-CoV-2 was initially identified in Wuhan city, Hubei Province, China, in December 2019 (Zhu et al., 2020). Recently, Lam et al. (2020) identified that the SARS-CoV-2-related coronaviruses in Malayan pangolins (Manis javanica) in China. As a result, the pangolins were blamed as a possible intermediate host in the emergence of COVID-19 outbreak in human population. A similar conclusion was also drawn by Zhang et al. (2020b) who reported that the pangolin species is a natural reservoir of SARS-CoV-2-like coronavirus. The infection of COVID-19 strain has since spread from China to approximately 216 countries and territories around the world. This outbreak is estimated to cause more than 1,115,000 deaths and 40,000,000 coronavirus-infected cases (data were updated until October 19th, 2020) (WHO, 2020a; Worldometer, 2020).
Similar to the Middle East respiratory syndrome coronavirus (MERS-CoV; identified in 2012), the SARS-CoV-1 and SARS-CoV-2 viruses mainly transmit through the small respiratory droplets of disease carriers generated from sneezing and coughing by humans. Such a route is recognized as human-to-human transmission (Chan et al., 2020) or respiratory transmission (Wu et al., 2020b). This means that super spreaders (SARS-CoV-2) can rapidly transmit the infection to many others, especially through routine international travel or mass gatherings in public places. Although the faecal-oral transmission of SARS-CoV-2 is possible (Arslan et al., 2020; Heller et al., 2020; Wu et al., 2020b), there are not any experimental data or robust evidences to confirm the faecal-oral hypothesis. In essence, three kinds of coronavirus (i.e., MERS-CoV, SARS-CoV-1, and SARS-CoV-2) share a similarity in their biochemical and physical properties (Chaudhry and Sachdeva, 2020; Race et al., 2020; Zhu et al., 2020). In addition, they are classified as an enveloped virus that contains the bits of protein and genetic material enclosed by a lipid host cell membrane (Chan et al., 2020; WHO, 2020c). Therefore, to some extent, previous studies on SARS-CoV-1 and MERS-CoV coronavirus and other enveloped viruses can give a close reference for SARS-CoV-2 (van Doremalen et al., 2020).
Traces of SARS-CoV-2 coronavirus (its nucleic acid fragments) have been recently detected in wastewater treatment plant-derived sludges (Alpaslan Kocamemi et al., 2020), municipal sewage (Ahmed et al., 2020a; Medema et al., 2020) or wastewater (Haramoto et al., 2020; Pineda, 2020), medical wastewater (Zhang et al., 2020), wastewater from commercial cruise ship and commercial passenger aircraft (Ahmed et al., 2020b), non-potable water (Monde, 2020), secondary-treated wastewater (Haramoto et al., 2020), and river water (Guerrero-Latorre et al., 2020; Haramoto et al., 2020; Rimoldi et al., 2020); thus, further investigations are necessary and must be given more priority (Chaudhry and Sachdeva, 2020; WHO, 2020b). In order to assess the effectiveness of wastewater treatment technological solutions for the current pandemic scenario, this review focused on the following key questions: (1) what the transmission routes of the SARS-CoV-2 coronavirus into sewage or wastewater are, (2) what methods are suggested for the detection of SARS-CoV-2 in sewage or wastewater, (3) where SARS-CoV-2 in raw sewage or wastewater is found (case report) in sewage or wastewater, (4) how long it can survive in sewage or wastewater, (5) what the main factors affecting the survival of SARS-CoV-2 in water are, (6) whether coronavirus can survive after drinking water disinfection process, and (7) how to protect people working around wastewater from COVID-19 infection.
2. Transmission route of SARS-CoV-2 into water and wastewater
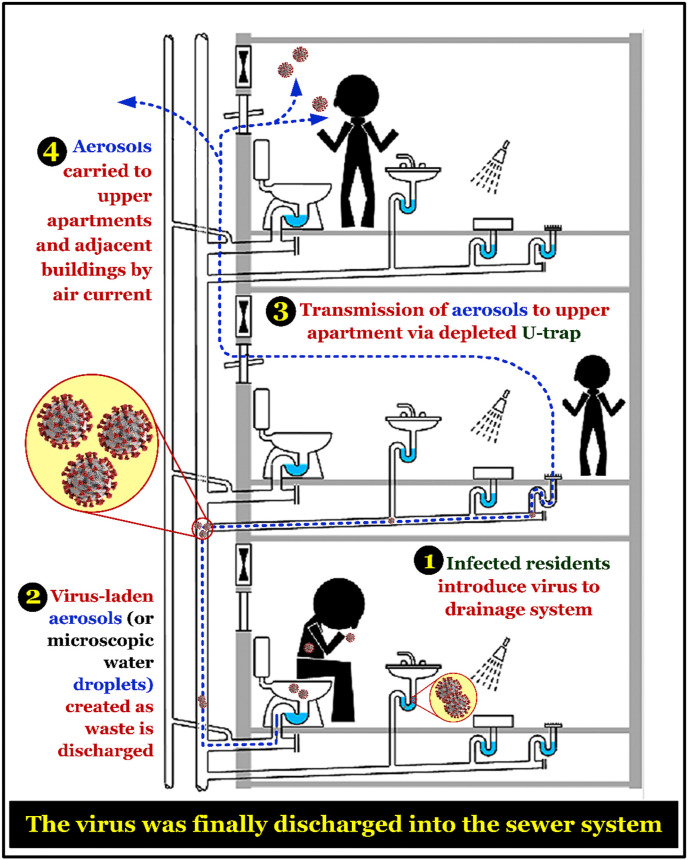
For the coronavirus-infected communities living in apartment buildings, wastewater plumbing systems have been considered as a potential pathway for transmitting the SARS-CoV-1 coronavirus into the sewer system since 2013 (Gormley et al., 2017; McKinney et al., 2006). Similar to SARS-CoV-1, the SARS-CoV-2 virus can be spread via aerosols or microscopic water droplets (Leung et al., 2020; WHO, 2020c). In fact, van Doremalen et al. (2020) reported that the SARS-CoV-2 and SARS-CoV-1 viruses share a similarity in their stability in aerosols and on surfaces. Depending on the inoculum shed, the viruses can remain viable and infectious on surfaces (up to a few days) and in aerosols (for hours). Similarly, Ong et al. (2020) investigated the survival of SARS-CoV-2 in air, surface, and personal protective equipment of disease carriers and healthcare workers. They found that the samples collected from air outlet fans, door handles, sinks, and toilet bowls were positive, which confirms that SARS-CoV-2 can be transmitted through the stools of infected people. Furthermore, Hu et al. (2020) collected the high-touch surface samples of a quarantine room (23 sites) and found that the percentage of collected samples was positive for SARS-CoV-2 as follows: 70% (in the bedroom) > 50% (bathroom) > 33% (corridor). They also concluded that the most contaminated sites with the highest viral loads were identified at the inner walls of the toilet bowl and the sewer inlet of the room (Hu et al., 2020). Such a transmission pathway, through the sanitary (or wastewater) plumbing system, might be likely responsible for environmental contamination and spread of COVID-19 in the communities (Fig. 1 ). Therefore, Gormley et al. (2020) recently provided several recommendations to ensure that transmission through the wastewater plumbing system is minimized. Fig. 2 summarizes some valuable suggestions to avoid the risk of spreading the pathogen through wastewater plumbing system in the buildings.
Fig. 1.
A transmission route of SARS-CoV-1 virus (possible for SARS-CoV-2 virus) at the buildings through the sanitary (or wastewater) plumbing system. Figure was adapted from Gormley et al. (2017) with some modifications.
Fig. 2.
Suggestions to mitigate the pathogen spread through the wastewater plumbing system in the building proposed by Gormley et al. (2020).
Some recent studies have showed that the stool specimens collected from infected patients (including asymptomatic children) had the SARS-CoV-2 virus (Holshue et al., 2020; Tang et al., 2020; Wang et al., 2020; Wu et al., 2020b; Xiao et al., 2020; Yeo et al., 2020). This is attributed to the existence of COVID-19 infection in the gastrointestinal tract of patients and can be excreted from the gastrointestinal tract via their faeces (Xiao et al., 2020). This suggested that the SARS-CoV-2 virus is usually excreted in the stools of an infected person. A recent investigation demonstrated that the median lifespan of SARS-CoV-2 in the stool the specimens of the patients was up to 22 days (interquartile range of 17 and 31 days) (Zheng et al., 2020), which was remarkable longer than that of SARS-CoV-1 (only 4 days) (Lai et al., 2005). The study also indicated that SARS-CoV-2 can survive longer in the stool specimens (22 days, 17–31 days) than that in respiratory (18 days, 13–29 days) and serum (16 days, 11–21 days) ones (Zheng et al., 2020). In general, the faeces and urine from some COVID-19-infected patients are discharged into sewer systems and subsequently enter wastewater and sewage treatment systems/plants (Ahmed et al., 2020a; Collivignarelli et al., 2020; Qu et al., 2020). This can be considered as the primary route of SARS-CoV-2 transmission to water and wastewater (Barcelo, 2020; Collivignarelli et al., 2020; Chaudhry and Sachdeva, 2020) because SARS-CoV can remain its infectious in the tool specimens for >7 days at 20 °C (Lai et al., 2005). Schematic route of transmission for the SARS-CoV-2 virus is presented in Fig. 3 (Wigginton et al., 2015).
Fig. 3.
A route of transmission for coronavirus (SARS-CoV-2). Figure was adapted from Wigginton et al. (2015) with some modifications.
Because the novel COVID-19 pandemic causes severe respiratory illness and related causalities, people are always encouraged or enforced to follow a compulsory policy to wear face masks (i.e., cloth, surgical, or even self-made masks) in public places as a precaution against the spread and infection of SARS-CoV-2 coronavirus (Alizargar, 2020; Chaib, 2020; Feng et al., 2020; Liu et al., 2020). Interestingly, Ho et al. (2020) reported that there was not significant difference between the commercial surgical mask and self-designed triple-layer cotton mask for preventing droplets during coughing. Therefore, cotton masks can serve as a promising substitute for medical surgical in inhibiting the transmission of respiratory droplets in micro-environments (Ho et al., 2020). A recent study demonstrated that surgical face masks can inhibit the direct transmission of influenza viruses and human coronaviruses from the virus-borne airborne particles, droplets, and body fluids of infected people (Leung et al., 2020). In contrast, Bae et al. (2020a) concluded that “surgical and cotton masks seem to be ineffective in preventing the dissemination of SARS–CoV-2 from the coughs of patients with COVID-19 to the environment and external mask surface”. However, this study has been retracted as requested by the editor (Bae et al., 2020b) because their interpretation of experimental results was misleading. Notably, Chin et al. (2020) reported that the SARS-CoV-2 virus can still exist at a detectable level of infection on the outer layer of a surgical face mask for up to a week. If approximately 20% of the total world population wears face (cloth or surgical) masks daily, it can be estimated that approximately 1.6 billion of masks (especially surgical masks) are used per day (Ho et al., 2020). In the United States, if each person wears one surgical mask per day, it will be necessary to supply more than 100 billion of masks (Liu et al., 2020). A similar estimation that reported the urgent global shortage of face mask during the coronavirus pandemic outbreak was published by other scholars (Wu et al., 2020a). To meet the global rising demand of using surgical masks during the COVID-19 outbreak, the World Health Organization (WHO) has encouraged the relevant industries to increase manufacturing by approximately 40% (Chaib, 2020), especially in China, in which accounts for approximately 50% of the global production of face masks (Wu et al., 2020a). Therefore, the management of discarded face masks that might offer a highly potential transmission route of SARS-CoV-2 into water must be given priority for investigation. This is especially important because none of the masks used are collected and treated as hazardous wastes, especially in developing countries or countries overloaded with infected patients. It has been reported that some of them might have been thrown away or disposed carelessly into the surface water (Kalina and Tilley, 2020). The presence of coronavirus-carrying masks discarded into water can create a new transmission pathway (Fig. 3). However, the further studies should recheck such high assumption.
As aforementioned, one of the transmission routes of COVID-19 into water and wastewater is through the large amount of face masks used worldwide by general public, patients and health workers. Following their use, those face masks have been disposed without treatment or disinfection, thus raising concerns about the potential health risks and threatening to the environment. Some methods have been recommended to disinfect the used masks for reusing or before disposing to minimise the hazardous wastes (Doan, 2020; Li et al., 2020a; Liu et al., 2020; Mechler, 2020; Xiang et al., 2020). For example, Doan (Doan, 2020) suggested to use the microwave technique for sanitising the disposable medical and used cloth masks. The method involves an antiseptic solution (i.e., 0.9% physiological saline) being sprayed on the mask to maintain the moisture. The moist masks were then transferred into a microwave oven with a default capacity of 800 W and subsequently heated for around 1 min. This disinfection protocol has been found to effectively kill 99.9% of viruses (Doan, 2020).
Another physical method (dry heat pasteurization) for disinfecting the surgical face masks and N95 respirators used has been recently reported by Xiang et al. (2020). Six species of respiratory bacteria (Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumonia, Acinetobacter baumannii, and Corynebacterium pseudodiphtheria), one fungi species (Candida albicans), and one H1N1 indicator virus (an RNA-enveloped virus similar to SARS-CoV-2) were selected as target pathogens. The authors concluded that the dry heat of used surgical face masks and N95 respirators at 70 °C for 1 h in an electric oven can warrant the effective disinfection of them. The sterile masks and respirators can be consecutively used at least three rounds of the heating without significantly changing their filtering efficiencies and physical features (i.e., shape) (Xiang et al., 2020).
A shorter steam treatment has been also reported as effective method for the rapid decontamination of methicillin-resistant Staphylococcus aureus and bacteriophage MS2 on the surface of the N95 respirators and medical face masks (Li et al., 2020a). Briefly, the inoculated N95 respirators and medical masks were placed into a steamer (100 °C) for different steam times of 2, 10, or 30 s. The results indicated that the steam times of 10 and 30 s were sufficient for decontaminating Staphylococcus aureus and bacteriophage MS2 on both respirators and medical masks, whereas the opposite was true for the 2-s steam treatment. However, the method of steam treatment did not effectively decontaminate to Geobacillu stearothermophilus spores in the surface of respirators and medical face masks.
3. Suggested methods/devices for detecting SARS-CoV-2 in water and wastewater
The most common methods for the detection of SARS-CoV-2 in different kinds of samples (including river water, sewage, and wastewater) are the reverse transcription-polymerase chain reaction (RT-PCR) and the reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) (Ahmed et al., 2020a; Guerrero-Latorre et al., 2020; Haramoto et al., 2020; Medema et al., 2020; Sherchan et al., 2020). For example, Wang et al. (2020) applied RT-PCR to detect SARS-CoV-2 in a variety of specimens from multiple sites (i.e., faeces, urine, sputum, blood, and nasal discharge) of 205 hospitalized patients diagnosed with COVID-19. Similarly, viral nucleic acid detection using RT-PCR has been widely used as the standard for detecting genetic traces of SARS-CoV-2 virus from patient specimens (Fang et al., 2020). A similar finding was reported by other scholars for the detection of coronavirus—SARS-CoV-1 (Wang et al., 2005a; Wang et al., 2005b) and SARS-CoV-2 (Young et al., 2020)—in water and wastewater samples. In addition, those methods have been successfully applied for detecting adenovirus and enterovirus in river water and sewage (Girones et al., 1995). Although the RT-PCR and RT-qPCR techniques have been applied as gold standard for the detection of pathogens including SARS-CoV-2, they are not able to distinguish between infectious and inactive fractions (WEF, 2020a). This is also a current challenge that needs to be addressed in further studies.
Recently, an overview of the approach utilizing paper-based devices (PADs) for the detection of infectious diseases and pathogens in water and wastewater was suggested by Mao et al. (2020). According to the authors, such devices can quickly and accurately detect various pathogens and infectious diseases such as malaria, Escherichia coli, HIV, Zika virus, and bovine infectious reproductive diseases at each point of the collected wastewater. Therefore, the authors assumed that PADs can be also applied to accurately detect the presence of SARS-CoV-2 in water environments. Similarly, Yang et al. (2020) recommended to use the paper-based diagnostic device for detecting the presence of SARS-CoV-2. However, the sensitivity and selectivity of PADs for various sensing applications are a big challenge that should be continuously improved in the future (Liu et al., 2019).
Currently, researchers at Cranfield University are developing an innovative PADs for detecting SARS-CoV-2 in wastewater from communities infected with the virus (Yang, 2020). Such a device should be inexpensive (less than US$1.3), portable, disposable, and easily operated even by non-expert or laymen. In essence, the method involves filtration of the nucleic acids of pathogens from the samples collected from wastewater/water by such a paper-based device. After that, a common biochemical reaction can be carried out with certain reagents to detect the presence of SARS-CoV-2 viral nucleic acid. A result of such a method can be observed macroscopically, for example, as a green circle (confirming positive) and a blue circle (demonstrating negative) (Yang, 2020). Furthermore, a recent review on nanoscale analytical tools and biosensors for identifying some important prognostic features of pathogens (i.e., SARS-CoV-2) was published by Bhalla et al. (2020). Although the existing tools (i.e., biosensors) have been widely used for characterizing and detecting SARS-CoV-2 virus in different environmental samples (i.e., blood, water, food, etc.), PADs has strongly recommended for in-situ quantitatively analysing SARS-CoV2 in water environments because of its quickness, low cost, accuracy, simplicity, and sensitivity.
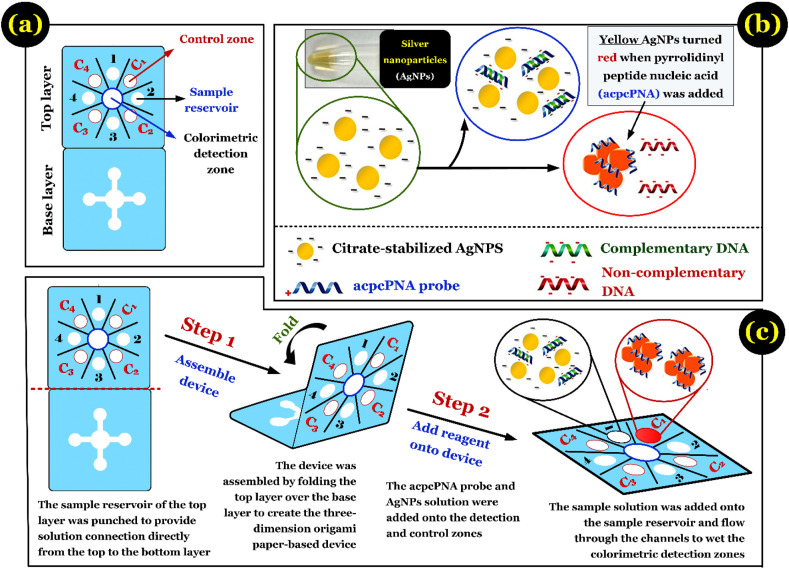
Earlier, Teengam et al. (2017) demonstrated that a PADs (Fig. 4 a) can serve as a simple, fast, sensitive, and selective method for detecting the DNA of MERS-CoV, Mycobacterium tuberculosis, and human papillomavirus. The authors developed a multiplex colorimetric PADs using silver nanoparticles (AgNPs) as a target colorimetric reagent for detecting DNA based on the pyrrolidinyl peptide nucleic acid (acpcPNA)-induced nanoparticle aggregation. The process of acpcPNA-induced AgNP aggregation in the presence of complementary and non-complementary DNA is briefly summarised in Fig. 4b. Such a multiplex paper-based colorimetric DNA sensor was successfully applied for screening and simultaneous detection of the oligonucleotides of MERS-CoV virus, human papillomavirus, and Mycobacterium tuberculosis (Fig. 4c). However, whether PADs accurately detects the SARS-CoV-2 virus in water and wastewater (concentrated and trace sources) is challenge and should be confirmed by continuous experiments.
Fig. 4.
(a) Design of a typical paper-based analytical device; (b) the process of pyrrolidinyl peptide nucleic acid (acpcPNA)-induced AgNP aggregation in the presence of complementary DNA and non-complementary DNA; and (c) brief operation of such device. Figure was adapted from Teengam et al. (2017) with some modifications.
To summarize, some methods/devices have been applied for detecting the presence of SARS-CoV-2 in different water environments (i.e., river and wastewater), but the detailed and standardized protocols and validations for such methods/devices are not yet available in the public domain.
4. Case report for detecting SARS-CoV-2 in raw sewage, river water, and wastewater
The most popular route of COVID-19 transmission into sewage and wastewater is through the excreta from disease carriers as discussed earlier (Section 2). In the past, Wang et al. (2005b) concentrated and detected the SARS-CoV-1 coronavirus in sewage samples collected from two hospitals (the 309th Hospital of the Chinese People's Liberation Army and Xiao Tang Shan Hospital) in China, where the patients with SARS were identified. They found that the SARS-CoV-1 coronavirus was detected in the raw sewage (before disinfection) of the two hospitals. However, the low concentration of disinfectants used might not be enough to completely destroy the coronavirus; therefore, the presence of SARS-CoV-1 was still detected in some samples from the 309th hospital after the disinfection process. They concluded that the virus could survive for 2 days in sewage at 20 °C and for 14 days at 4 °C. In particular, although the virus was inactivated, RNA could still be detected in the sewage after 8 days (Wang et al., 2005b).
The first study on the presence of SARS-CoV-2 in sewage in the Netherlands was conducted by Medema et al. (2020). Sewage water samples were collected from the wastewater treatment plants (WWTPs) (i.e., Amsterdam, Den Haag, Utrecht, Apeldoorn, Amersfoort, Schiphol, and Tilburg) of six cities and Schiphol airport within the Netherlands. The results indicated that the SARS-CoV-2 virus was detected in untreated wastewater in the Amsterdam Schiphol Airport in Tilburg WWTP and the wastewater treatment plant in Kaatsheuvel. The wastewater samples from the municipal wastewater treatment plants were collected and monitored because the wastewater was treated in the town where first case (person with COVID-19) in the Netherlands lived. The results indicated that the SARS-CoV-2 virus was detected in the water samples collected from two places. Notably, the authors also reported that the first water sample containing the virus was monitored for four days after the first person in Amsterdam Schiphol Airport had been tested positively for SARS-CoV-2 coronavirus. This was an important and interesting finding in the context of the whole pandemic that is spreading across the globe (Medema et al., 2020). An analogous observation was reported by other researchers (Xiao et al., 2020). Those authors collected stool specimens from 73 SARS-CoV-2-infected hospitalized patients (aged between 10 months and 78 years). Their result showed that the stool sample tested positive for SARS-CoV-2 were detected during the initial 12 days. Importantly, approximately 23.3% of stool specimens remained COVID-19 positive although their respiratory samples were found to be negative (Xiao et al., 2020).
Similarly, Sherchan et al. (2020) collected six grab and nine composite samples from two WWTPs at a four-month period (from January to April 2020). Those collected samples included the untreated wastewater (influent), secondary-treated effluent wastewater (before chlorination), and final effluent (after chlorine disinfection). The results demonstrated that the SARS-CoV-2 RNA was detected in approximately 13% (2/15) of the untreated wastewater samples (positive) using with two reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assays. However, the secondary-treated effluent wastewater and final effluent samples were negatively detected for SARS-CoV-2 RNA. The results suggested that the SARS-CoV-2 coronavirus was removed through the wastewater treatment processes to undetectable levels.
In addition, Haramoto et al. (2020) investigated the existence of SARS-CoV-2 virus in different water matrixes from March 17th to May 7th, 2020. The water samples were collected from WWTP (influent and secondary-treated wastewater) and a local river (surface water) in Yamanashi Prefecture, Japan. The result of RT-qPCR analysis demonstrated that the concentration of SARS-CoV-2 RNA in five secondary-treated wastewater samples (before chlorination) was 2.4 × 103 copies/L. In contrast, the fragments of SARS-CoV-2 RNA was not detected in the influent wastewater (n = 5) and reviewer (n = 3) samples. On the basis of the limit of detection (LOD), the authors explained reasons why SARS-CoV-2 was quantitatively detected in the secondary-treated wastewater samples but not in the influent ones (Haramoto et al., 2020). The LOD value for the influent (4.0 × 103–8.2 × 104 copies/L) was remarkably higher than that for the secondary-treated wastewater (1.4 × 102–2.5 × 103 copies/L). This is because the former (200 mL) had a lower filtration volume than the latter (5000 mL).
A recently daily news/sources in France informed that minuscule traces of virus was found in the Paris' non-potable water supply (Lesté-Lasserre, 2020; Monde, 2020). The non-potable water network that was drawn from the Ourcq canal and Seine river without intensive treatments was only used for a myriad of other activities, such as cleaning streets, watering the city's parks, gardens, and wood, and supplying to ornamental public fountains. The staffs from the Paris water authority's laboratory collected the 27 multiple water samples around the Paris' capital. The result demonstrated that the small amounts of SARS-CoV-2 virus (infinitesimal traces) were detected in the four samples (~15%). The authors, however, also noted that the transmission route of SARS-CoV-2 into non-potable water was unclear. However, they assured that drinking water is currently consumed without any potential health risk because the supply source of non-potable and potable waters to the city was from a completely different source (Monde, 2020). Two independent water networks which supply for drinking water (2000 km from Paris) and non-drinking water (1800 km) in Paris were a unique legacy inherited from Baron Haussmann in the 19th century.
Similarly, the fragments of viral RNA from SARS-CoV-2 coronavirus have been detected in untreated wastewaters (sewage) from WWTPs at two cities (Milan and Rome), Italy (La Rosa et al., 2020). The authors reported that the six per twelve samples collected from the influent sewage had a positive result with SARS-CoV-2 (its genetic material being detected). Although the viral RNA in wastewater was detected in wastewater, it did not imply that SARS-CoV-2 coronavirus was current active and caused an infection transmission. Notably, the SARS-CoV-2 fragments in one sewage sample (the Milan city) were detected a few days after Higher Institute of Health had affirmed the first case of SARS-CoV-2 infection. This also suggested that the daily epidemiological monitoring of wastewater can become an indicator of the circulation the virus (i.e., SARS-CoV-2), its recurrence, and its epidemic outbreaks to communities (La Rosa et al., 2020). Another confirmation of the SARS-CoV-2 genome detected in the raw wastewater samples from three WWTPs in Italy was investigated by Rimoldi et al. (2020). However, according to the infectivity test, the authors confirmed that the pathogenicity of SARS-CoV-2 coronavirus in wastewater was worthless due to the absence of cytopathic effect (CPE). In fact, viruses are often killed or inactivated during water treatment or purification processes.
Furthermore, Ahmed et al. (2020a) initially reported the detection of SARS-CoV-2 in untreated wastewater (sewage) samples (accounting for 22.2% of the total investigated samples). The samples were collected at one suburban pumping station and two wastewater treatment plants (representing urban catchments) in South east Queensland, Australia. In addition, the authors applied the Monte Carlo simulation to estimate the number of infections. The simulation will be conducted if the water samples are scored to be a positive result with SARS-CoV-2. The result of simulation indicated that the number of SARS-CoV-2-infected individuals in the catchment basin with the population of 600,000 ranged from 171 to 1090 infected persons. Therefore, the existence of SARS-CoV-2 in untreated wastewater can serve as an early warning signal for COVID-19 infections in communities (Ahmed et al., 2020a).
A first detection of SARS-CoV-2 RNA virus in untreated wastewater from six WWTPs (located in Murcia, Cartagena, Molina de Segura, Lorca, Cieza, and Totana) in Spain has recently reported by Randazzo et al. (2020). The water samples, which included 42 influents, 18 secondary-treated effluents, and 12 tertiary-treated effluents), were collected early in the morning from March 12th to April 14th, 2020 for monitoring the existence of this coronavirus. The results (detected SARS-CoV-2 titers from the RT-qPCR) indicated the presence of the fragments of SARS-CoV-2 (positively-tested results) in the influent water samples (83%) and the secondary-treated water samples (11%), whereas the opposite was true for the tertiary effluent water samples (0%). They also found that the water samples had been detected positively 12–16 days before the COVID-19 infectious cases were announced in the Cieza, Totana, and Lorca municipalities (Randazzo et al., 2020).
Finally, the first occurrence of SARS-CoV-2 in river water from the Quito city in Ecuador (a low-sanitation country) was confirmed by Guerrero-Latorre et al. (2020). The authors explained the existence of SARS-CoV-2 in the urban river derived from directly discharging wastewaters of the city into the natural streams. A similar result was reported by Rimoldi et al. (2020) for detecting the SARS-CoV-2 virus in water samples in the Lambro River, Italy. In contrast, the SARS-CoV-2 RNA was not detected in the water samples of one river (Yamanashi Prefecture, Japan) (Haramoto et al., 2020) and two rivers (Vettabbia and Lambro Meridionale, Italy) (Rimoldi et al., 2020). The findings suggested the important role of sanitation in preventing the pandemic dissemination of COVID-19. Notably, although the viral RNA of SARS-CoV-2 was found in rivers, the infectivity experiment on culture cells demonstrated that the infectivity of the coronavirus was null (Rimoldi et al., 2020). This confirmed a low potential health risk from the infection of SARS-CoV-2 coronavirus in river waters.
In general, several case studies (Ahmed et al., 2020a; Medema et al., 2020; Xiao et al., 2020) did not confirm that the SARS-CoV-2 virus could survive after water and wastewater disinfection using common disinfectants. However, to some extent, the surveillance of SARS-CoV-2 RNA in sewage and wastewater (before disinfection), which is also known as wastewater-based epidemiology (WBE), may be an early important indicator of the appearance of SARS-CoV-2 virus and its spreading in the population (Ahmed et al., 2020a; Ahmed et al., 2020b; Barcelo, 2020; Chaudhry and Sachdeva, 2020; Medema et al., 2020; Orive et al., 2020; Randazzo et al., 2020; Wang et al., 2005b). In other words, routine wastewater monitoring can help identify a non-invasive early warning sign to alert communities to new SARS-CoV-2 infections (Ahmed et al., 2020a; Collivignarelli et al., 2020; Mallapaty, 2020; Orive et al., 2020; WHO, 2020b). This is because of a large number of SARS-CoV-2 carriers without specific symptomatic symptoms (i.e., fever, cough, dyspnoea, fatigue, sore throat, and myalgias) (Al-Tawfiq, 2020) or undocumented infection (Li et al., 2020b).
4.1. Survival period of coronaviruses in water and wastewater
To date, no study on the persistence and survivability of SARS-CoV-2 in water or wastewater is available in the public domain (Race et al., 2020; WHO, 2020b). However, the survival period of the SARS-CoV-1 and MERS-CoV coronaviruses previously investigated in the literature can be considered for reference and comparison purpose.
For example, Wang et al. (2005a) investigated the resistance of SARS-CoV-1 in different water matrices at 4 °C and 20 °C. The results demonstrated that SARS-CoV-1 only survived for two days in dechlorinated tap water, hospital wastewater, and domestic sewage at 20 °C (Table 1 ). This clearly highlights the fact that SARS-CoV-1 was rapidly inactivated in water at ambient temperatures. A similar conclusion was made by other scholars (Gundy et al., 2008). The authors (Gundy et al., 2008) reported that coronaviruses (human coronavirus 229E and animal feline infectious peritonitis coronavirus) died off very rapidly in wastewater samples (primary effluent filtered, primary effluent unfiltered, and secondary effluent), with a 99% reduction in approximately two days and 99.9% reduction in two to 4 day at 23 °C (Table 2 ). In addition, Ye et al. (2016) estimated the survivability of the two human enveloped viruses (murine hepatitis coronavirus and Pseudomonas phage cystovirus) in untreated municipal wastewater. They also found that the estimated time for reaching 90% inactivation of two model enveloped occurred very rapidly at 25 °C, with more than 0.5 days for murine hepatitis coronavirus and two days for Pseudomonas phage cystovirus (Table 3 ).
Table 1.
Persistence of the SARS-CoV-1 virus in different water matrixes at 4 °C and 20 °C (data were adapted from Wang et al., 2005a,b).
| Water samples | Detection time (day) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 8 | 14 | |
| 1. Water temperature at 4 °C | |||||||||
| 309th hospital wastewater | Post. | Post. | Post. | Post. | Post. | Post. | Post. | Post. | Post. |
| Domestic sewage | Post. | Post. | Post. | Post. | Post. | Post. | Post. | Post. | Post. |
| Dechlorinated tap water | Post. | Post. | Post. | Post. | Post. | Post. | Post. | Post. | Post. |
| PBS | Post. | Post. | Post. | Post. | Post. | Post. | Post. | Post. | Post. |
| 2. Water temperature at 20 °C | |||||||||
| 309th hospital wastewater | Post. | Post. | Post. | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. |
| Domestic sewage | Post. | Post. | Post. | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. |
| Dechlorinated tap water | Post. | Post. | Post. | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. |
| PBS | Post. | Post. | Post. | Post. | Post. | Post. | Post. | Post. | Post. |
Note: Post. (positive for SARS-CoV) and Neg. (negative for SARS-CoV); phosphate-buffered saline (abbreviated as PBS).
Table 2.
The time (day) required for infectivity reduction of viruses in different water samples at 23 °C and 4 °C (data were adapted from Gundy et al., 2008).
| Reduction (99%) |
|||
|---|---|---|---|
| HCoV | FIPV | PV-1 | |
| 1. Temperature (23 °C) | |||
| Tap water (filtered) | 6.76 (10.1) | 6.76 (10.1) | 43.3 (64.9) |
| Tap water (unfiltered) | 8.09 (12.1) | 8.32 (12.5) | 47.5 (71.3) |
| Primary effluent (filtered) | 1.57 (2.35) | 1.60 (2.40) | 23.6 (35.5) |
| Primary effluent (unfiltered) | 2.36 (3.54) | 1.71 (2.56) | 7.27 (10.9) |
| Secondary effluent (unfiltered) | 1.85 (2.77) | 1.62 (2.42) | 3.83 (5.74) |
| 2. Temperature (4 °C) | |||
| Tap water (filtered) | 392 (588) | 87.0 (130) | 135 (203) |
Note: Human coronavirus 229E (HCoV), (animal) feline infectious peritonitis virus (FIPV), and poliovirus 1 (PV-1); Data in parenthesis (day) represents the reduction rate of 99.9%. The Primary effluent was collected after settling; meanwhile the secondary effluent was collected before chlorination.
Table 3.
The estimated time (hour) for reaching 90% inactivation of two model enveloped viruses (MHV and φ6) in unpasteurized and pasteurized wastewater at 25 °C and 10 °C (data were adapted from Ye et al., 2016).
| Estimated inactivation (90%) |
||
|---|---|---|
| MHV | φ6 | |
| 1. Temperature (25 °C) | ||
| Untreated wastewater | 13 | 7 |
| Pasteurized wastewater | 19 | 53 |
| 2. Temperature (10 °C) | ||
| Untreated wastewater | 36 | 28 |
| Pasteurized wastewater | 149 | 146 |
Note: Two human enveloped virus: murine hepatitis coronavirus (MHV) and Pseudomonas phage (φ6) cystovirus.
Furthermore, a previous study on the survival and persistence of two surrogate human coronaviruses—transmissible gastroenteritis (TGEV) and mouse hepatitis (MHV)—in reagent-grade water, lack water, and pasteurized settled sewage (or settled human sewage) at 25 °C and 4 °C has been conducted by Casanova et al. (2009). They concluded that approximately 99% of the two kinds of coronaviruses from the water died at 25 °C after 22 days for TGEV and 17 days for MHV; meanwhile, their die-off percentage in the sewage was approximately 99% after 9 days of TGEV and 7 days of MHV (Table 4 ).
Table 4.
The time (day) required for infectivity reduction of viruses in reagent-grade water (pH 6.0, turbidity 0.1 NTU), pasteurized settled sewage (obtained from drinking water treatment plant), and lake water (pH 7.5, turbidity 1.73 NTU) at 25 °C and 4 °C (data were adapted from Casanova et al., 2009).
| Experimental result |
Estimated result |
|||||
|---|---|---|---|---|---|---|
| Reduction (99%) |
Reduction (99.9%) |
Reduction (99.99%) |
||||
| TGEV | MHV | TGEV | MHV | TGEV | MHV | |
| 1. Temperature (25 °C) | ||||||
| Reagent-grade water | 22 | 17 | 33 | 26 | 44 | 35 |
| Pasteurized settled sewage | 9 | 7 | 14 | 10 | 19 | 14 |
| Lake water | 13 | 10 | – | – | – | – |
| 2. Temperature (4 °C) | ||||||
| Reagent-grade water | 220 | >365 | 330 | >365 | 330 | >365 |
| Pasteurized settled sewage | 79 | 70 | 73 | 105 | 98 | 139 |
Note: transmissible gastroenteritis (TGEV) and mouse hepatitis (MHV) coronaviruses settled human sewage.
4.2. Main factors affecting the survival of coronavirus in water and wastewater
The existence of coronavirus in water is possible. Thus, several factors affecting its survival in the aqueous environment should be considered. Those include aqueous temperature, the concentration of suspended solid and organic matter, solution pH, and the dose of disinfectant used.
The details of effect of water temperature on the survival of coronavirus in water are provided in Table 1, Table 2, Table 3, Table 4. Clearly, temperature is an extremely important factor influencing the survival of coronaviruses. The coronavirus often tends to be rapidly inactivated at high temperatures (i.e., 20 °C) rather than at low temperatures (i.e., 4 °C). For example, Table 1 shows that in the same experiment, SARS-CoV-1 can survive in different types of water samples (hospital wastewater, domestic sewage, and dechlorinated tap water) at 4 °C for 14 days, while it only persists in water samples for 2 days at 20 °C (Wang et al., 2005a). Furthermore, Gundy et al. (2008) investigated the survival of three types of coronaviruses (HCoV, FIPV, and PV-1) in filtered tap water at 4 °C and 23 °C. The authors proposed that human 229E coronavirus can survive for approximately 7 day at 23 °C, but up to more than 1 year at 4 °C (Table 2). A similar observation was reported by other investigators (Casanova et al., 2009), although the TGEV and MHV coronaviruses survived and remained infectious at 4 °C and 25 °C. The titer of the infectious coronaviruses decreased more rapidly at 25 °C than at 4 °C (Table 4). Therefore, it can be concluded that the coronaviruses (possibly including SARS-CoV-2) in water are more sensitive to temperature.
The effect of suspended solids and organic matters on the survival of coronaviruses has also been studied (Gundy et al., 2008). The authors reported that coronaviruses can survive longer in primary wastewater (i.e., ~11 days for PV-1) than in secondary (activated sludge) wastewater (~six days for PV-1). This is because the latter contained a higher level of suspended solids (110–220 mg/L) in water than did the former (5.5–22 mg/L). This suggested that the suspended solids can protect coronaviruses (also known as solids-associated coronaviruses) from inactivation (Table 2). This finding is consistent with the literature data (Table 3, Table 4). Similarly, Zhang et al. (2020a) reported that the organic matters in the patient's stools can protect the SARS-CoV-2 virus from the disinfection process of medical wastewaters. In other words, the survival of coronaviruses in water is strongly dependent on the properties of the respective water matrices.
Notably, Chin et al. (2020) investigated the stability of SARS-CoV-2 under different environmental conditions and concluded that SARS-CoV-2 was extremely stable in a wide pH range from 3.0 to 10 at room temperature. In contrast, Lai et al. (2005) found that SARS-CoV-1 in stool specimens can survive for 1 day at pH 8.0, 5 days at pH 9.0, but only for 3 h at pH 6. Moreover, the dose of disinfectant used also plays an extremely important role in inactivating coronaviruses as demonstrated by Wang et al. (2005a). The authors found that an increase in the dose of chlorine dioxide (5, 10, 20, and 40 mg/L) resulted in an increase in the inactivation rate of SARS-CoV-1 in wastewater (0%, 94.38%, 82.22%, and 99.9999%, respectively).
To sum up, the survival period of coronavirus (also for SARS-CoV-2) in water is strongly dependent on water temperature, water property, solution pH, and the presence of disinfectant.
5. Consideration whether SARS-CoV-2 exists after the disinfection process of drinking water
According to WHO (WHO, 2020c), there are no available evidences which confirm the survival of SARS-CoV-2 virus after the disinfection process for both wastewater and drinking water. This assumption is also consistent with the reports of the Water Environment Federation (WEF, 2020a,b) and several recent studies (Sherchan et al., 2020). Similarly, the United States Environmental Protection Agency (USEPA, 2020) reported that SARS-CoV-2 virus has not been detected in drinking-water supplies after disinfection process. Recently, researchers from the University of Arizona collected wastewater samples from the County Wastewater Treatment Plant and explore whether SARS-CoV-2 virus is tracked in the samples (Pineda, 2020). The results indicated that the RNA gene fragments of the SARS-CoV-2 virus were not detected in the treated wastewater.
In general, the virus is divided into two groups: enveloped virus and non-enveloped virus (large and small non-enveloped viruses). SARS-CoV-2 is a typical enveloped virus (surrounded by a fragile outer lipid membrane) that has been acknowledged as the easiest virus to be killed when comparing with large or small non-enveloped virus (WHO, 2020c). Because the MERS-CoV and SARS-CoV-1 viruses are derived from the same family of the SARS-CoV-2 coronaviruses, they exbibit similar biochemical and physical properties. Therefore, to some extent, they can be considered as a typical example, and this has been discussed below within this section.
Among the existing methods (i.e., adsorption, ozonation, chlorination, membrane, ultraviolet light, and advanced oxidation processes) which are applied for inactivation of coronaviruses (Naddeo and Liu, 2020) or enveloped viruses (Lénès et al., 2010), the UV (ultraviolet) radiation and chlorination are the most common methods used for disinfecting water supplies, especially in developing countries (HPSC, 2020). Wang and co-workers (Wang et al., 2005a) applied chlorine dioxide (ClO2) and sodium hypochlorite (NaClO) as the target disinfectants to explore the inactivation of SARS-CoV-1 in wastewater. They found that SARS-CoV-1 was extremely sensitive to selective disinfectants. A similar conclusion was highlighted by Lénès et al. (2010) who assessed the removal and inactivation of two enveloped viruses (H5N1 and H1N1) by different disinfectants (i.e., chlorine, chlorine dioxide, and ozone). Although both disinfectants can inactivate SARS-CoV-1 virus in water, NaClO was better than ClO2 in terms of inactivation (Wang et al., 2005a). For example, in the same low-concentration disinfectants (10 mg/L), the rates of SARS-CoV-1 inactivation using ClO2 and NaClO were found to be 99.999% and 68.38%, respectively after 10 min of contact time. However, under the same experimental conditions, both disinfectants were less effective in killing bacteria (i.e., Escherichia coli) with the low inactivation rates of 17.4% for ClO2 and 14.3% for NaClO. This confirmed that SARS-CoV-1 coronavirus was more sensitive to disinfectants than E. coli (Wang et al., 2005a). Therefore, the treated water was suggested to be safe for the consumers.
Recently, Zhang et al. (2020a) investigated the effective deactivation of SARS-CoV-2 in medical wastewater from the (influent and effluent) septic tanks of the Fangcang hospital by the disinfectants (i.e., sodium hypochlorite). Although the study and wastewater samples were conducted and collected at the hospital, the results can be considered as a close reference to evaluate the effect of disinfectants on the survival of SARS-CoV-2. The results indicated that using free chlorine >0.5 mg/L (contact time of 1.5 h) cannot secure a complete disinfection of the SARS-CoV-2 virus in medical wastewaters, whereas the opposite was true for using 6700 g/m3 dosage of sodium hypochlorite. Although the SARS-CoV-2 viral RNA was not detected within the over-dosage of sodium hypochlorite used, a high level of disinfection by-product residuals can cause some risks to ecological system and threats to human health (Zhang et al., 2020a).
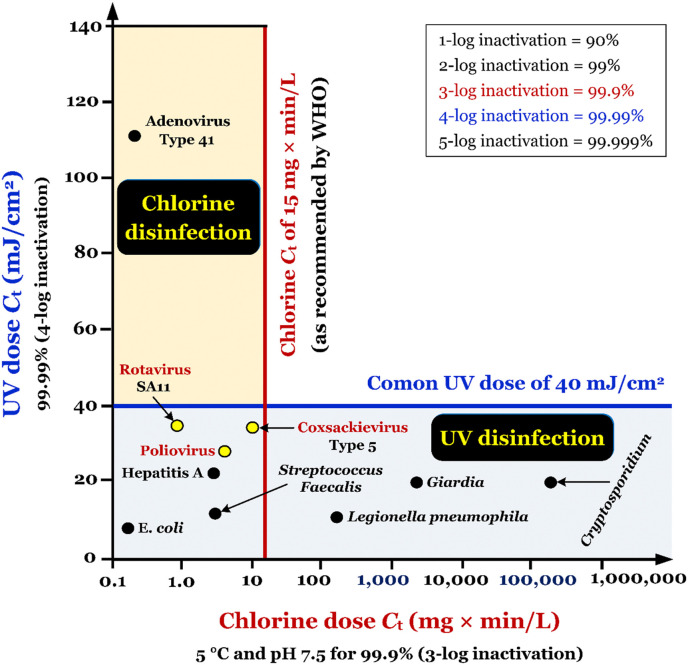
Fig. 5 represents the disinfection efficiency of various pathogenic microorganisms in water by chemical disinfectant (chlorination) and non-chemical disinfectant (UV light). Clearly, non-enveloped viruses (i.e., poliovirus, Coxsackievirus, and Rotavirus) can be inactivated by two methods. In particular, non-enveloped viruses can be inactivated at a chlorine dose (C t) of less than 15 mg×min/L. Therefore, it is expected that the enveloped SARS-CoV-2 virus will be effectively inactivated by chlorination even at a lower chlorine C t dose of 15 mg×min/L (EPA, 2011; HPSC, 2020). Similarly, a previous study demonstrated that enveloped viruses (i.e., Pseudomonas virus φ6) were more susceptible than non-enveloped viruses (i.e., bacteriophage MS2) under free chlorine disinfection (prepared from NaClO) and UV254 radiation (Ye et al., 2018). Similarly, Lénès et al. (2010) found that H5N1 (an enveloped virus) was very sensitive to UV radiation (>5.5-log inactivation obtained within a low UV fluence of 25 mJ/cm2), whereas the opposite was true for bacteriophage MS2 (1.87-log inactivation). Although each disinfection method is efficient for inactivating the enveloped virus (also for SARS-CoV-2), the combination of such methods is always recommended (EPA, 2011). This is because chlorination-based disinfection is not efficient in inactivating protozoan parasites (i.e., Cryptosporidium), whereas the opposite is true for UV light-based disinfection ( Fig. 5 ).
Fig. 5.
Synergistic utilization of the common disinfection systems: UV (ultra-violet) light and chlorination. Figure was adapted from EPA, 2011 with some modifications. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Notably, some researchers recently reported that the RNA of SARS-CoV-2 virus was detected in untreated wastewaters in WWTP such as influents (Randazzo et al., 2020; Rimoldi et al., 2020) and secondary-treated water samples (Randazzo et al., 2020). However, its RNA was not detected in tertiary effluent samples of WWTPs after the current disinfection process with alone NaClO (Randazzo et al., 2020), the combination of NaClO and UV (Randazzo et al., 2020), peracetic acid (Randazzo et al., 2020), or high intensity UV lamps (Rimoldi et al., 2020). Although the current disinfection process from WWTPs in Spain Randazzo et al. (2020) and Italy (Rimoldi et al., 2020) killed totally SARS-CoV-2 virus, the researchers did not report the detail conditions of the disinfection process (i.e., the used disinfectant dosage and contact time) and the effects of those conditions on the survival of such coronavirus (Randazzo et al., 2020; Rimoldi et al., 2020).
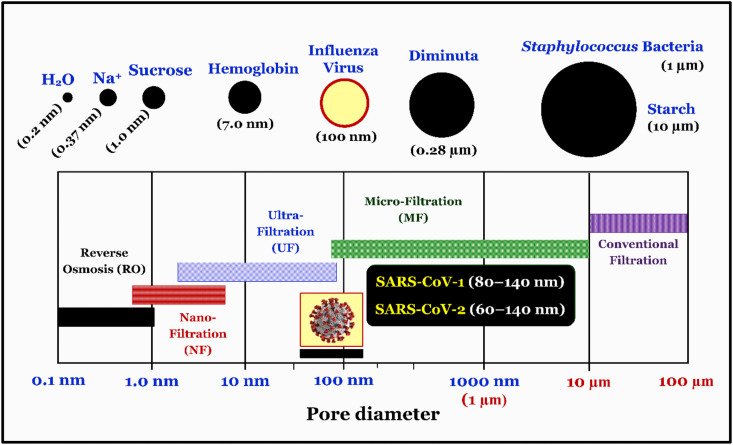
Membrane technology has been widely employed as a conventional disinfection method for drinking water (Bodzek et al., 2019; Lénès et al., 2010). In this method, the size of the viruses plays an important role in selecting the appropriate kinds of membranes. Each virion (particle) of coronaviruses (i.e., SARS-CoV-1 and MERS-CoV related to the Coronaviridae family in the Nidovirales order) varied from 80 nm to 220 nm in diameter (Burrell et al., 2017; Lénès et al., 2010). Recently, Zhu et al. (2020) reported that the diameter of SARS-CoV-2 virion ranged from 60 nm to 140 nm (Race et al., 2020), which is similar to that of SARS-CoV-1 from 80 to 140 nm (Ksiazek et al., 2003). A comparison of microorganisms' sizes with the pore sizes of the membranes is illustrated in Fig. 6 . According to the diameter of each SARS-CoV-2 virion and membranes, it is highly recommended that the ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO) membranes are appropriate for inactivating (or rejecting) the coronaviruses in SARS-CoV-2-contaminated water (Hai et al., 2018; Lénès et al., 2010; Zhu et al., 2020).
Fig. 6.
Comparison of micro-organisms sizes including SARS-CoV-1 (Ksiazek et al., 2003) and SARS-CoV-2 (Zhu et al., 2020) coronaviruses with the pore size diameters of membranes. Figure adapted from Hai et al. (2018).
For adsorption method, Ciejka et al. (2017) developed the biopolymeric material (i.e., the cations-modified chitosan-based nano/microspheres) and applied it for the selective and reversible adsorption of different pathogenic coronaviruses from aqueous suspensions. The target coronaviruses included two human coronaviruses (HCoV-NL63 and HCoV-OC43) and mouse hepatitis coronavirus (MHV). Their results demonstrated that the biopolymeric material can adsorb the HCoV-NL63 (strongly) and MHV (moderately) coronavirus from water, but cannot adsorb HCoV-OC43 coronavirus. The desorption study using 2.0 M NaCl indicated that the desorbed HCoV-NL63 coronavirus can be desorbed from the laden biopolymeric material. The number of viral RNA copies that was desorbed from the laden biopolymeric material was 2.4 ± 0.9 × 106 (copies/mL). Notably, the HCoV-NL63 particles desorbed were still infectious (i.e., the retention of virus virulence) (Ciejka et al., 2017). However, whether the biopolymeric material can effectively adsorb SARS-CoV-2 coronavirus in water is a current challenge that should be confirmed by further studies.
To sump, although until now, there is no evidence on the survival of SARS-CoV-2 virus in treated water, future studies should be conducted to thoroughly approve this supposition. As aforementioned discussion, the treatment processes of existing disinfection might be sufficient to kill SARS-CoV-2 in water. However, the protocols for disinfecting the SARS-CoV-2 virus in drinking water treatment are missing.
6. Suggestion for protecting the health of wastewater plant operators
The protection of the health of the wastewater plant operators is imperative given the potential health risk that could be posed by COVID-19 if the wastewater contains the virus. As recommended by Casanova et al. (2009), the coronaviruses (i.e., TGEV and MHV) can remain in its infectious state a for long period in pasteurized settled sewage, lake water and reagent-grade water (Table 4). Therefore, the coronaviruses-contaminated water can be considered as a potential vehicle for human exposure if aerosols or microscopic water droplets are produced (Barcelo, 2020; Casanova et al., 2009). Although epidemiological study or evidence to confirm that wastewater is a route of transmission is missing (Arslan et al., 2020; Collivignarelli et al., 2020; WEF, 2020a), the employees involved in wastewater management operations should be specifically protected from such virus (Ahmed et al., 2020a). Fig. 7 provides a summary regarding some suggestions for protecting the health of relevant workers during the COVID-19 outbreak as recommended by World Health Organization (WHO, 2020c), Water Research Australia (WRU, 2020), and Water Environment Federation (WEF, 2020a).
Fig. 7.
Recommendations for protecting wastewater treatment plant operators and sewer workers from the potential exposure of SARS-CoV-2 (WEF, 2020; WHO, 2020c; WRU, 2020).
Furthermore, studies on which disinfectants against enveloped SARS-CoV-2 virus are appropriate have been lacking and hitherto neglected. However, the effective disinfectants for other coronaviruses (e.g. SARS and MERS) can be still used for the reference. For example, a recent review on the disinfection of coronavirus in histopathology was provided by Henwood (2020). The authors collected data from various coronaviruses (i.e., SARS-CoV-1 and MERS-CoV) similar to SARS-CoV-2 and they concluded that coronaviruses can be effectively deactivated by 70% ethanol or 0.1% sodium hypochlorite. A similar finding was reported by Kampf et al. (2020) who reviewed the inactivation of coronaviruses (i.e., SARS-CoV-1 and MERS-CoV) by different types of biocidal agents. It was found that using 62%–71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite (NaOCl) as surface disinfection rapidly reduced the infectivity of such coronaviruses (also possible for SARS-CoV-2) on different surfaces within 1 min exposure time (Kampf et al., 2020). However, recently, WHO (WHO, 2020c) recommended to use a higher concentration of NaOCl (i.e., 0.5%; equivalent to 5000 ppm or 1-part household bleach with 5% NaOCl to 9 parts water) or 70% ethyl alcohol for disinfecting surfaces. Therefore, the ethanol and sodium hypochlorite are highly recommended for killing SARS-CoV-2 and protecting water workers from COVID-19 infection (Fig. 7).
7. Conclusions
The review revealed that to-date, although the existence of SARS-CoV-2 coronavirus in river water and untreated wastewater is confirmed, a strong evidence of its survival time in water environments is missing. Only one study confirmed that the infectivity of SARS-CoV-2 coronavirus in water was worthless based on the infectivity test (absence of cytopathic effect). Future studies should be conducted to strongly confirm the survival time of SARS-CoV-2 coronavirus in different water conditions (temperature, pH, organic matter, etc.) as well as its infectivity. The current disinfection processes might be enough to efficiently inactivate SARS-CoV-2 in water. The protocols for disinfection of SARS-CoV-2 virus should be established by the relevant scientific communities. The appearance of novel SARS-CoV-2 coronavirus in water and wastewater is highly likely as reported in several parts of the world. Almost all coronaviruses are sensitive to temperature and are rapidly inactivated in the water environment. The most common transmission route of SARS-CoV-2 into water, sewage, and wastewater is through stools of symptomatic people. The paper-based devices might be a promising solution for fast and accurate detection of traces of SARS-CoV-2 in water, sewage, and wastewater samples. Current disinfection methods used in the drinking water treatment process highly inactivate and efficiently destroy SARS-CoV-2 in water. Frequent monitoring of sewage and wastewater can provide an early warning medium for the emergence of the SARS-CoV-2 coronavirus a population (i.e., neither city nor smaller municipality), which results in mitigating the pathogen transmission and threat to public health. If the SARS-CoV-2 coronavirus is detected in wastewater, the water workers should be specifically protected from such virus (especially following the safety procedures). In addition, some training courses for those workers should be routine opened to prevent their exposure to wastewater. Appropriate disinfectants against enveloped SARS-CoV-2 virus should be confirmed by future studies.
An important point to consider here is that studies have shown that SARS-CoV-2 coronavirus has been detected in river water, sewage, and wastewater samples, but the risk posed to the human health and the environment is minimal. The persistence of the virus is also negligible and mostly gets destroyed in the ambient temperature and climatic conditions. However, the occurrence and distribution of the virus in wastewater plants across the cities around the world would enable us to identify the source and location that people are being infected. Therefore, early warning signs in terms of detection of the virus would go a long way to manage the epidemic better and help alert the respective government authorities to take precautionary measures before the issue becomes serious.
CRediT author statement
Hai Nguyen Tran: Conceptualization, Methodology, Software, Validation, Visualization, Writing-Original Draft. Giang Truong Le: Funding acquisition, Conceptualization. Dong Thanh Nguyen: Funding acquisition, Methodology. Ruey-Shin Juang: Formal analysis, Writing-Review & Editing. Jörg Rinklebe: Formal analysis, Validation, Writing-Review & Editing. Amit Bhatnagar: Formal analysis, Validation, Writing-Review & Editing. Eder C. Lima: Formal analysis, Writing-Review & Editing. Hafiz M. N. Iqbal: Formal analysis, Writing-Review & Editing. Ajit K Sarmah: Formal analysis, Validation, Writing-Review & Editing. Huan-Ping Chao: Funding acquisition, Formal analysis, Writing-Review & Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., Edson J., Ehret J., Gyawali P., Hamilton K., Hosegood I., Hugenholtz P., Jiang G., Kitajima M., Sichani H.T., Shi J., Shimko K.M., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas Dsc K.V., Verhagen R., Zaugg J., Mueller J.F. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travelers. J. Trav. Med. 2020 doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq J.A. Asymptomatic coronavirus infection: MERS-CoV and SARS-CoV-2 (COVID-19) Trav. Med. Infect. Dis. 2020;35:101608. doi: 10.1016/j.tmaid.2020.101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizargar J. Wearing masks and the fight against the novel coronavirus (COVID-19) Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpaslan Kocamemi B., Kurt H., Sait A., Sarac F., Saatci A.M., Pakdemirli B. SARS-CoV-2 detection in Istanbul wastewater treatment plant sludges. medRxiv. 2020;2005 2020. 2012.20099358. [Google Scholar]
- Arslan M., Xu B., Gamal El-Din M. Transmission of SARS-CoV-2 via fecal-oral and aerosols–borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S., Kim M.C., Kim J.Y., Cha H.H., Lim J.S., Jung J., Kim M.J., Oh D.K., Lee M.K., Choi S.H., Sung M., Hong S.B., Chung J.W., Kim S.H. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: a controlled comparison in 4 patients. Ann. Intern. Med. 2020;173(1):M20–M1342. doi: 10.7326/M20-1342. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bae S., Kim M.C., Kim J.Y., Cha H.H., Lim J.S., Jung J., Kim M.J., Oh D.K., Lee M.K., Choi S.H., Sung M., Hong S.B., Chung J.W., Kim S.H. Notice of retraction: effectiveness of surgical and cotton masks in blocking SARS-CoV-2. Ann. Intern. Med. 2020;173(1):79. doi: 10.7326/L20-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo D. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. J. Environ. Chem. Eng. 2020;8(4) doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla N., Pan Y., Yang Z., Payam A.F. Opportunities and challenges for biosensors and nanoscale analytical tools for pandemics: COVID-19. ACS Nano. 2020;14(7):7783–7807. doi: 10.1021/acsnano.0c04421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodzek M., Konieczny K., Rajca M. Membranes in water and wastewater disinfection–review. Arch. Environ. Protect. 2019;45(1):3–18. [Google Scholar]
- Burrell C.J., Howard C.R., Murphy F.A. fifth ed. Academic Press; London: 2017. Fenner and White's Medical Virology; pp. 437–446. [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejka J., Wolski K., Nowakowska M., Pyrc K., Szczubiałka K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater. Sci. Eng. C. 2017;76:735–742. doi: 10.1016/j.msec.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collivignarelli M.C., Collivignarelli C., Carnevale Miino M., Abbà A., Pedrazzani R., Bertanza G. SARS-CoV-2 in sewer systems and connected facilities. Process Saf. Environ. Protect. 2020;143:196–203. doi: 10.1016/j.psep.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaib F. Shortage of personal protective equipment endangering health workers worldwide. 2020. https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S., Tsoi H.-W., Lo S.K.-F., Chan K.-H., Poon V.K.-M., Chan W.-M., Ip J.D., Cai J.-P., Cheng V.C.-C., Chen H., Hui C.K.-M., Yuen K.-Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A.K., Sachdeva P. Coronavirus disease 2019 (COVID-19): a new challenge in untreated wastewater. Can. J. Civ. Eng. 2020;47:1005–1009. [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1(1):E10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan H.N. Medical face masks can be reused with microwave method: expert. 2020. https://vietnamnews.vn/society/654072/medical-face-masks-can-be-reused-with-microwave-method-expert.html
- Epa I. Environmental Protection Agency Wexford; Ireland: 2011. Water Treatment Manual: Disinfection. [Google Scholar]
- Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P., Ji W. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296:E115–E117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Shen C., Xia N., Song W., Fan M., Cowling B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir. Med. 2020;8(5):434–436. doi: 10.1016/S2213-2600(20)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A. COVID-19: mitigating transmission via wastewater plumbing systems. The Lancet Global Health. 2020;8(5):E63. doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A., Rodriguez-Gil C. Pathogen cross-transmission via building sanitary plumbing systems in a full scale pilot test-rig. PloS One. 2017;12(2) doi: 10.1371/journal.pone.0171556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2008;1(1):10. [Google Scholar]
- Girones R., Puig M., Allard A., Lucena F., Wadell G., Jofre J. Detection of adenovirus and enterovirus by PCR amplification in polluted waters. Water Sci. Technol. 1995;31(5–6):351–357. [Google Scholar]
- Hai F.I., Yamamoto K., Lee C.-H. IWA Publishing; 2018. Membrane Biological Reactors: Theory, Modeling, Design, Management and Applications to Wastewater Reuse. [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral transmission: are we asking the right questions? Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henwood A.F. Coronavirus disinfection in histopathology. J. Histotechnol. 2020:1–3. doi: 10.1080/01478885.2020.1734718. [DOI] [PubMed] [Google Scholar]
- Ho K.-F., Lin L.-Y., Weng S.-P., Chuang K.-J. Medical mask versus cotton mask for preventing respiratory droplet transmission in micro environments. Sci. Total Environ. 2020;735 doi: 10.1016/j.scitotenv.2020.139510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HPSC Advice note to EHS on COVID-19 in chlorinated drinking water supplies and chlorinated swimming pools. 2020. https://www.lenus.ie/handle/10147/627346 Health Service Executive.
- Hu X., Xing Y., Ni W., Zhang F., Lu S., Wang Z., Gao R., Jiang F. Environmental contamination by SARS-CoV-2 of an imported case during incubation period. Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalina M., Tilley E. “This is our next problem”: cleaning up from the COVID-19 response. Waste Manag. 2020;108:202–205. doi: 10.1016/j.wasman.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.-E., Humphrey C.D., Shieh W.-J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.-Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.Y.Y., Cheng P.K.C., Lim W.W.L. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. 2005;41(7):e67–e71. doi: 10.1086/433186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.T.-Y., Shum M.H.-H., Zhu H.-C., Tong Y.-G., Ni X.-B., Liao Y.-S., Wei W., Cheung W.Y.-M., Li W.-J., Li L.-F., Leung G.M., Holmes E.C., Hu Y.-L., Guan Y. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- Lénès D., Deboosere N., Ménard-Szczebara F., Jossent J., Alexandre V., Machinal C., Vialette M. Assessment of the removal and inactivation of influenza viruses H5N1 and H1N1 by drinking water treatment. Water Res. 2010;44(8):2473–2486. doi: 10.1016/j.watres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Lesté-Lasserre C. Coronavirus found in Paris sewage points to early warning system. Science. 2020;368:6489. 6410.1126/science.abc3799. [Google Scholar]
- Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K.-H., McDevitt J.J., Hau B.J.P., Yen H.-L., Li Y., Ip D.K.M., Peiris J.S.M., Seto W.-H., Leung G.M., Milton D.K., Cowling B.J. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.F., Cadnum J.L., Redmond S.N., Jones L.D., Pearlmutter B., Haq M.F., Donskey C.J. Steam treatment for rapid decontamination of N95 respirators and medical face masks. Am. J. Infect. 2020;48(7):855–857. doi: 10.1016/j.ajic.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Yang D., Liu G. Signal amplification strategies for paper-based analytical devices. Biosens. Bioelectron. 2019;136:60–75. doi: 10.1016/j.bios.2019.04.043. [DOI] [PubMed] [Google Scholar]
- Liu Y., Leachman S.A., Bar A. Proposed approach for reusing surgical masks in COVID-19 pandemic. J. Am. Acad. Dermatol. 2020;83(1):e53–e54. doi: 10.1016/j.jaad.2020.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580:176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- Mao K., Zhang H., Yang Z. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environ. Sci. Technol. 2020;54(7):3733–3735. doi: 10.1021/acs.est.0c01174. [DOI] [PubMed] [Google Scholar]
- McKinney K.R., Gong Y.Y., Lewis T.G. Environmental transmission of SARS at amoy gardens. J. Environ. Health. 2006;68(9):26–30. [PubMed] [Google Scholar]
- Mechler S. Covid-19 pandemic: Face mask disinfection and sterilization for viruses. 2020. https://consteril.com/covid-19-pandemic-disinfection-and-sterilization-of-face-masks-for-viruses/
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Monde L. Tiny traces" of SARS-CoV-2 in non-potable water in the city of Paris. 2020. https://www.lemonde.fr/sante/article/2020/04/19/des-traces-infimes-du-sars-cov-2-dans-l-eau-non-potable-de-la-ville-de-paris_6037099_1651302.html
- Naddeo V., Liu H. Editorial perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ. Sci. Water Res. Technol. 2020;6(5):1213–1216. [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020;732:139298. doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda P. ASU, UA researchers look for traces of COVID-19 in Tempe and Tucson wastewater. 2020. https://www.azcentral.com/story/news/local/tempe/2020/04/02/asu-researchers-look-traces-covid-19-tempe-wastewater-could-be-early-warning-system/5109746002/
- Qu G., Li X., Hu L., Jiang G. An imperative need for research on the role of environmental factors in transmission of novel Coronavirus (COVID-19) Environ. Sci. Technol. 2020;54(7):3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- Race M., Ferraro A., Galdiero E., Guida M., Núñez-Delgado A., Pirozzi F., Siciliano A., Fabbricino M. Current emerging SARS-CoV-2 pandemic: potential direct/indirect negative impacts of virus persistence and related therapeutic drugs on the aquatic compartments. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A., Tong Z., Wang H., Dai Y., Li K., Liu J., Wu W., Yuan C., Yu M., Li P. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Inf. Disp. 2020;26(6):1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teengam P., Siangproh W., Tuantranont A., Vilaivan T., Chailapakul O., Henry C.S. Multiplex paper-based colorimetric DNA sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal. Chem. 2017;89(10):5428–5435. doi: 10.1021/acs.analchem.7b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA . 2020. Coronavirus and Drinking Water and Wastewater.https://www.epa.gov/coronavirus/coronavirus-and-drinking-water-and-wastewater [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N., Xiao W.-J., Yin J., Wei W., Wang G.-J., Si B.-y., Guo B.-Z., Liu C., Ou G.-R., Wang M.-N., Fang T.-Y., Chao F.-H., Li J.-W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J., Guo T., Zhen B., Kong Q., Yi B., Li Z., Song N., Jin M., Xiao W., zhu X., Gu C., Yin J., Wei W., Yao W., Liu C., Li J., Ou G., Wang M., Fang T., Wang G., Qiu Y., Wu H., Chao F., Li J. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan hospital and the 309th hospital of the Chinese people's liberation Army. Water Sci. Technol. 2005;52(8):213–221. [PubMed] [Google Scholar]
- WEF Current priority: coronavirus. 2020. https://www.wef.org/coronavirus
- WEF The water professional's guide to COVID-19. 2020. https://www.wef.org/news-hub/wef-news/the-water-professionals-guide-to-the-2019-novel-coronavirus/
- WHO Country & technical guidance-coronavirus disease. 2019. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it COVID-19.
- WHO . 2020. Coronavirus Disease 2019 (COVID-19) Situation report–184. [Google Scholar]
- WHO Status of environmental surveillance for SARS-CoV-2 virus. 2020. https://www.who.int/news-room/commentaries/detail/status-of-environmental-surveillance-for-sars-cov-2-virus
- WHO . 2020. Water, Sanitation, Hygiene, and Waste Management for COVID-19; pp. 227–406. Technical Brief. [Google Scholar]
- Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ. Sci. Water Res. Technol. 2015;1(6):735–746. [Google Scholar]
- Worldometer Worldometer COVID-19 data. 2020. https://www.worldometers.info/coronavirus/
- WRU SARS-CoV-2 water and sanitation. 2020. https://www.waterra.com.au/publications/latest-news/2020/sars-cov-2-water-and-sanitation-factsheet-update/
- Wu H.-l., Huang J., Zhang C.J.P., He Z., Ming W.-K. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: reflections on public health measures. EClinicalMedicine. 2020;21:100329. doi: 10.1016/j.eclinm.2020.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. The lancet. Gastroenterol. Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Song Q., Gu W. Decontamination of surgical face masks and N95 respirators by dry heat pasteurization for one hour at 70°C. Am. J. Infect. 2020;48(8):880–882. doi: 10.1016/j.ajic.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Wang Y.-C., Shen C.-F., Cheng C.-M. Point-of-care RNA-based diagnostic device for COVID-19. Diagnostics. 2020;10(3):165–167. doi: 10.3390/diagnostics10030165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. News from Cranfield University; 2020. Wastewater test could provide early warning of COVID-19.https://www.cranfield.ac.uk/press/news-2020/wastewater-test-could-provide-early-warning-of-covid-19 [Google Scholar]
- Ye Y., Chang P.H., Hartert J., Wigginton K.R. Reactivity of enveloped virus genome, proteins, and lipids with free chlorine and UV254. Environ. Sci. Technol. 2018;52(14):7698–7708. doi: 10.1021/acs.est.8b00824. [DOI] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? The Lancet Gastroenterol. Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.-T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K., Anderson D.E., Chan K.S., Tan T.Y., Ng T.Y., Cui L., Said Z., Kurupatham L., Chen M.I.-C., Chan M., Vasoo S., Wang L.-F., Tan B.H., Lin R.T.P., Lee V.J.M., Leo Y.-S., Lye D.C., Team, f.t.S.N.C.O.R. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. J. Am. Med. Assoc. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X., Wang X., Liu Y., Li G., Qu J. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30(7):1346–1351. doi: 10.1016/j.cub.2020.03.022. e1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., Chen W., Wang Q., Zhang D., Liu Y., Gong R., Ma Z., Lu S., Xiao Y., Gu Y., Zhang J., Yao H., Xu K., Lu X., Wei G., Zhou J., Fang Q., Cai H., Qiu Y., Sheng J., Chen Y., Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]