Highlights
-
•
Canine chaphamaparvovirus (CaChPV) is a novel parvovirus recently discovered in dogs;
-
•
Herein, stool samples from dogs with or without enteric signs were screened for CaChPV;
-
•
CaChPV DNA was found either in diarrhoeic (1.9 %) or asymptomatic (1.6 %) dogs;
-
•
The nearly complete genome sequences were determined for two strains;
-
•
The Italian CaChPV strains tightly clustered with the American reference viruses.
Keywords: Canine chaphamaparvovirus 1, Parvoviridae, Dogs, Enteric samples
Abstract
Canine chaphamaparvovirus (CaChPV) is a newly recognised parvovirus discovered by metagenomic analysis during an outbreak of diarrhoea in dogs in Colorado, USA, in 2017 and more recently detected in diarrhoeic dogs in China. Whether the virus plays a role as canine pathogen and whether it is distributed elsewhere, in other geographical areas, is not known. We performed a case-control study to investigate the possible association of CaChPV with enteritis in dogs. CaChPV DNA was detected both in the stools of diarrhoeic dogs (1.9 %, 3/155) and of healthy animals (1.6 %, 2/120). All the CaChPV-infected dogs with diarrhea were mixed infected with other enteric viruses such as canine parvovirus (formerly CPV-2), canine bufavirus (CBuV) and canine coronavirus (CCoV), whilst none of the asymptomatic CaChPV positive animals resulted co-infected. The nearly full-length genome and the partial capsid protein (VP) gene of three canine strains, Te/36OVUD/19/ITA, Te/37OVUD/19/ITA and Te/70OVUD/19/ITA, were reconstructed. Upon phylogenetic analyses based on the NS1 and VP aa sequences, the Italian CaChPV strains tightly clustered with the American reference viruses. Distinctive residues could be mapped to the deduced variable regions of the VP of canine and feline chaphamaparvoviruses, considered as important markers of host range and pathogenicity for parvoviruses.
1. Introduction
Parvoviridae are small (∼25 nm diameter), non-enveloped, single-stranded and negative-sense DNA viruses of 3.9–6.3 kb in length, with the coding region bracketed by terminal repeats that can fold into hairpin-like structures (Berns and Parrish, 2013). They have a large host spectrum, spanning from invertebrates to mammals (Pénzes et al., 2019). Parvoviruses have long been known in dogs, since the identification of canine minute virus, or canine parvovirus (CPV) type 1 (CPV-1; genus Bocaparvovirus), in 1967 from the faecal samples of healthy dogs (Binn et al., 1970). CPV-1 infection is responsible for reproductive disorders and occasionally for respiratory and gastrointestinal signs in young dogs (Decaro et al., 2012). A second CPV (CPV-2; genus Protoparvovirus) was reported in the 1970s in Europe and North America in puppies with signs of haemorrhagic gastroenteritis and myocarditis (Appel et al., 1979). CPV-2 is currently regarded as the major causative agent of severe gastroenteritis in puppies (Decaro and Buonavoglia, 2012). Recent advances in molecular technologies have been allowed the discovery of novel parvoviruses in dogs, including two additional bocaparvoviruses species, CBoV-2 and CBoV-3 identified respectively from healthy and sick dogs respiratory samples (Kapoor et al., 2012) and from the liver of a dog with multiorgan failure (Li et al., 2013), and the still unclassified carnivore protoparvoviruses like-bufaviruses (CBuVs) (Martella et al., 2018) detected in stool samples of dogs with or without enteric disease and in the nasal and oropharyngeal swabs of animals with respiratory signs. More recently, assessing faecal samples from an unexplained outbreak of diarrhoea in Colorado (USA), a novel canine virus has been discovered by metagenomic approach (Fahsbender et al., 2019). Upon sequence analysis of the nearly complete genome of the two strains identified, Cachavirus-1A strain IDEXX1 (GenBank accession number MH893826) and Cachavirus-1B strain IDEXX2 (MK448316), the highest genetic relatedness (65.4–74.4 % nt identity) was found to members of the genus Chaphamaparvovirus, previously described under an unofficial umbrella term “Chapparvovirus” (Pénzes et al., 2019). On the basis of the International Committee on Taxonomy of Viruses (ICTV) classification criteria for Parvoviridae species demarcation, both the canine strains were classified within the species Carnivore chaphamaparvovirus 1 (Pénzes et al., 2019).
The genus Chaphamaparvovirus includes viruses infecting vertebrate hosts that are more closely related to invertebrate-infecting parvoviruses than to members of the Parvovirinae. The discovery of ChPVs has compelled a radical rethink on the evolution and genetic relationships among Parvoviridae, leading to a recent taxonomical re-classification characterised by the introduction of the novel subfamily Hamaparvovirinae that encompasses divergent densoviruses and vertebrate-infecting parvoviruses (Pénzes et al., 2019). In the last few years, ChPVs have been identified in several animal species including bats, rodents, birds, pigs and domestic cats (Baker et al., 2013; Reuter et al., 2014; Yang et al., 2016; Palinski et al., 2016; Lima et al., 2019; de Souza et al., 2017; Yinda et al., 2018; Roediger et al., 2018; Williams et al., 2018; Li et al., 2020). However, information on their epidemiology and/or ability to cause disease in their natural hosts are still limited. Preliminary epidemiological data collected by Fahsbender et al. (2019) address the question on the possible role of canine ChPVs (CaChPVs) in the aetiology of dog enteritis. Molecular analysis by quantitative PCR (q-PCR) of a large set of enteric samples revealed the presence of CaChPVs DNA in the faeces of dogs with (4.35 %) or without (1.47 %) diarrhoea, although a clear association with enteric signs was not demonstrated. In a more recent study performed in China (Hu et al., 2020), the detection rate of CaChPVs DNA was 0 % and 1.55 % in healthy or diarrhoeic dogs, respectively. However, association between CaChPVs and gastrointestinal disease was not supported by statistical analysis.
In order to gather additional information on the distribution of this novel parvovirus in dogs and to investigate its possible association with enteritis, during the year 2019 a surveillance study was initiated by implementing with CaChPV-specific assays the diagnostic algorithms of cases of acute gastro-enteritis admitted to the veterinary hospital of the Faculty of Veterinary Medicine, University of Teramo (Italy).
2. Materials and methods
2.1. Sampling
A case-control study was conducted using two subsets of dogs selected on the basis of the presence of acute gastroenteric signs for clinical cases and the absence of enteritis for controls. A total of 155 rectal swab samples (subset A) was collected from household dogs suffering from gastro-enteric disorders. The inclusion criteria were the presence of mild to severe diarrhoea and age comprised between 2 and 6 months old. Rectal specimens (n. 120) randomly recruited among healthy dogs during routine visits in 2019 and matching with cases for living condition and age were included in the study as control group (subset B). Informed consent was obtained from all animal owners.
2.2. Molecular screening
The collected swabs were immersed in 1 mL of viral transport medium consisting of Dulbecco’s modified Eagle’s medium (D-MEM), and subsequently clarified by centrifuging at 2500 g for 10 min. DNA and RNA were extracted from 200 μl of viral suspension by using the QIAamp Cador Pathogen Mini Kit (Qiagen S.p.A., Milan, Italy), following the manufacturer’s instructions. All faecal samples were screened for CPV-2, CBuV, canine coronavirus (CCoV), canine kobuvirus (CaKoV) and norovirus (NoV) by conventional PCR and reverse transcription (RT)-PCR (Pratelli et al., 1999; Buonavoglia et al., 2001; Vennema et al., 2002; Di Martino et al., 2013; Martella et al., 2018). The presence of CaChPV DNA was assessed by nested-PCR using diagnostic primer sets CPV_625F/CPV_948R and CPV18_687FN/CPV_911RN, following chemical and thermal conditions previously described (Fahsbender et al., 2019). A positive control, constructed by cloning the 323-nt synthetised fragment of the non-structural (NS1) protein encoding gene of the prototype strain Cachavirus-1B IDEXX2 (MK448316) (Fahsbender et al., 2019) into TOPO XL PCR vector (Invitrogen, Ltd, Milan, Italy), was included in each PCR assay. Specific primers and probe (CachaRT-For 5′ AACAATCCTTACAATTGGCT 3′, CachaRT-rev 5′ TTCCTGTTCTAGACAAGGAT 3′ and Pb 5′ Fam GACAATACAAGCTCAGTTTG BHQ1 3′) were designed to amplify a 75 bp region on the NS1 for quantitative detection in real-time PCR (qPCR). A standard curve was generated using 10° to 109 copies per reaction of CaChPV plasmid DNA containing the synthetised fragment. Viral DNA quantification was performed using TaqMan Fast Advanced (ThermoFisher Scientific, US) reaction master mix in a 25-μl volume comprising 200 nM of each primer, 250 nM of probe, 5 μl of extracted DNA and 20 μl of master mix. Thermal cycling conditions consisted of Taq DNA polymerase activation at 95 °C for 2 min, followed by 45 cycles of denaturation at 95 °C for 10 s and annealing/extension at 45 °C for 30 s. The specificity of the assay was evaluated with a panel of canine DNA viruses (CPV-1, CPV-2, CBuV, canine adenovirus type 1 and type 2). The qPCR assay was able to detect >101 DNA copies/5 μL of standard DNA and 4.0 × 101 DNA copies/5 μL of DNA template extracted from clinical samples.
2.3. Genome sequencing and phylogenetic analysis
In order to gather sequence information from the CaChPVs detected in our study, attempts were made to generate the complete genome on all the samples containing quantifiable viral DNA by using specific primers designed based on available CaChPV genome sequences (Table 1 ). The PCR assays were performed with TaKaRa La Taq polymerase (Takara Bio Europe S.A.S. Saint-Germain-en-Laye, France). The amplicons were purified and cloned using a TOPO XL Cloning Kit (Life Technologies). Consensus sequences were generated by sequencing at least three clones for each PCR fragment.
Table 1.
List of primers used in this study. Nucleotide position refers to the sequence of Carnivore chaphamaparvovirus 1 prototype strain CaChPV-1B IDEXX2 (GenBank accession no. MK448316).
| Oligonucleotide | Position | Sequence (5′ to 3′) | Sense | Reference |
|---|---|---|---|---|
| CaChPV_1F | 1−20 | CTAGCACACTCGGCGCAATG | + | This study |
| CaChPV_197F | 197−216 | TGCCTCCTGCTAAACAAGCA | + | This study |
| CaChPV_620F | 620−641 | ATGCAAGCAGAAATGGAACGTG | + | This study |
| CaChPV_1302F | 1302−1323 | GCATGAAATGTGCGCCTGATAT | + | This study |
| CaChPV_1566R | 1547−1566 | GGACCCCATAAAACTAATGT | – | This study |
| CPV_625F | 1998−2017 | CAACTAGCCGAATGCAGGGA | + | Fahsbender et al., 2019 |
| CPV18_687FN | 2060−2079 | AGCTCAGTTTGGCCCAGATC | + | Fahsbender et al., 2019 |
| CPV_911RN | 2265−2284 | AGAGGGATCGCTGGATCTGT | – | Fahsbender et al., 2019 |
| CPV_948R | 2302−2321 | CGATAACATCCCCGGACTGG | – | Fahsbender et al., 2019 |
| CaChPV_2611R | 2590−2611 | TCAGCCATATTGCTGTTGTAAA | – | This study |
| CaChPV_2604F | 2604−2625 | ATGGCTGAAGATGTATCTTTTA | + | This study |
| CaChPV_3027F | 3027−3048 | TCTGGAAGCGACGGTACAACTC | + | This study |
| CaChPV_3874R | 3855−3874 | CTACGTTGGTCCATGTTCGTCT | – | This study |
| CaChPV_4226R | 4207−4226 | GGATACACAGGCGCCAGTAC | – | This study |
Sequencing was carried out using BigDye Terminator Cycle chemistry (Applied Biosystems, Foster City, California, US). The alignment of the sequences was conducted using the MAFFT multiple alignment program version 7.388 plugin of the Geneious software (Biomatters Ltd., Auckland, New Zealand). Phylogenetic analyses were conducted using Maximum Likelihood method, Poisson model and supplying statistical support with bootstrapping of 1000 replicates, in MEGA X software (Kumar et al., 2018).
3. Results
By using diagnostic primer sets (Fahsbender et al., 2019), CaChPV DNA was detected in a total of 5 faecal samples, with an overall prevalence of 1.9 % (5/275). The detection rate in diarrhoeic dogs (subset A) was 1.9 % (3/155), whilst in the control group (subset B) was 1.6 % (2/120). Out of the three CaChPV-positive samples from subset A (Te/36/Ovud/19/ITA, Te/37/Ovud/19/ITA and Te/70/Ovud/19/ITA), CaChPV DNA was found in co-infection with CPV-2 (1/3), CPV-2 and CBuV (1/3) or in conjunction with CPV-2, CBuV and CCoV (1/3). Co-infections were not revealed in the two CaChPV-positive samples from subset B (Te/19A/Ovud/19/ITA and Te/58A/Ovud/19/ITA). Upon statistical analysis using the χ2 test, with Yates' correction (for continuity) for dichotomous variables and the Rule of Three (Tuyl et al., 2009), CaChPV prevalence did not significantly differ between diarrhoeic and healthy dogs. The viral loads in the CaChPV-positive samples ranged from 4.0 × 101 to 1.3 × 104 DNA copies/5 μl of template, with the highest titers in specimens from subset A (mean 6.5 × 103 DNA copies).
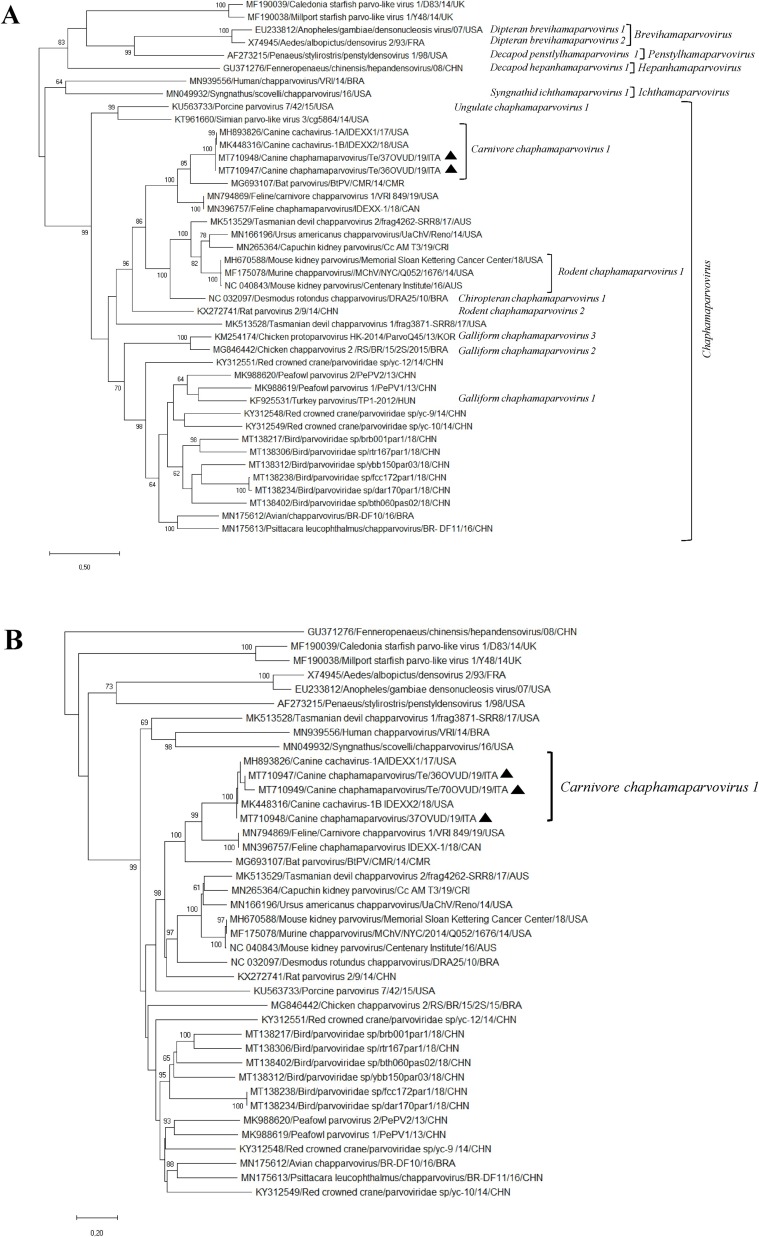
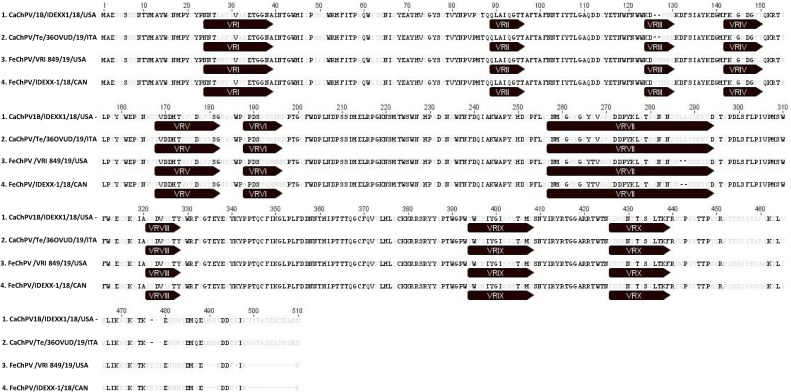
By sequence comparison in the short NS1 fragment, the five amplicons showed 99.1–100 % nt identities to each other and 99.6–100 % to the strains 1A-IDEXX1 and 1B-IDEXX2 (Fahsbender et al., 2019). The nearly complete genome sequence (4026 bp) of strains Te/36OVUD/19/ITA and Te/37OVUD/19/ITA, including a partial 5′ untranslated region (UTR) (419 bp), the complete NS1 sequence (663 aa), the structural protein (VP) (504 aa) and a partial 3′ UTR (108 bp), was generated (GenBank accessions no. MT710947 and MT710948). For one additional strain, Te/70OVUD/19/ITA (MT710949), the 5′ partial VP gene (360 aa) was obtained. Based on the full-genome sequences alignment, the two Italian CaChPVs were genetically closely related to the reference canine ChPV strains 1A-IDEXX1 and 1B-IDEXX2 (MH893826 and MK448316) (99.1–99.3 % nt identities), to ChPVs (MN396757 and MN794869) (71.8–71.9 %) recently detected in stool specimens of cats from an outbreak of gastroenteritis in shelters in Canada and to the ChPV strain BtPV/CMR/14 (MG693107) (67.3–67.4 %) detected in stools of a fruit bat in Cameroon (Yinda et al., 2018; Fahsbender et al., 2019; Li et al., 2020). Identity in the genome to the other members of the genus Chaphamaparvovirus ranged from 44.9 % to 58.0 % (Table 2 ). The genome coding sequence of strains Te/36OVUD/19/ITA and Te/37OVUD/19/ITA, excluding the terminal UTR regions, was 3497 nt in length, with 2 major open reading frames (ORFs) of 1992 nt and 1515 nt, coding respectively for the NS1 and for the VP capsid protein. A minor ORF of 564 nt in length, coding for a predicted 187 aa protein, overlapping the NS1 encoding gene, was located in a position (1367 → 1930 nt) equivalent to that of the nucleoprotein (NP) found in other members of the genus Chaphamaparvovirus (Pénzes et al., 2019). The NS1 of the two Italian strains was characterised by the putative start codon MQA located in a weak Kozak consensus sequence (ACAATGC) (Kozak, 2002) and contained two conserved replication initiator (endonuclease) motifs 99FHVHSMAL106 and 153SLIAYMC K 160 (Smith and Kotin, 2000). In addition, highly-conserved Walker motifs of the helicase domain, including Walker A (315 GPSNTGKS322), B (353IGVWEE 358), B′ (370 KQIFEGMECSIPV K 383) and C (395IIMTTN 400), were identified (Walker et al., 1982; James et al., 2003). The termination of the NS1 encoding gene overlapped the start of the VP encoding gene by 8 nt. Similar to other members of the subfamily Hamaparvovirinae (Pénzes et al., 2019), the conserved HDXXY and YXGXG motifs of the phospholipase A2 (PLA2) (Zádori et al., 2001) were absent in the VP protein of all the CaChPVs to date available, including the Italian strains. The complete genome from 34 ChPV strains were retrieved from the databases and used to perform phylogenetic analyses. Also, a selection of viruses representative of the genera Hepanhamaparvovirus, Penstylhamaparvovirus, Brevihamaparvovirus and Ichthamaparvovirus classified within the newly established subfamily Hamapaparvovirinae, was included in the analyses. On phylogenetic analysis based on the complete NS1 protein (Fig. 1 A), strains Te/36OVUD/19/ITA and Te/37OVUD/19/ITA segregated with CaChPVs 1A-IDEXX1 and 1B-IDEXX2 (bootstrap value 100 %) into the novel species Carnivore chaphamaparvovirus 1 (overall aa identity 98.6–99.8 %), that in turn fell into a well-defined cluster (bootstrap value 100 %), encompassing still unclassified strains represented by the Cameroonian fruit bat ChPV (Yinda et al., 2018) and the ChPVs of feline origin (Li et al., 2020). The aa identities within this group was 58.5–66.3 %. The high genetic conservation among all the CaChPVs identified to date (with an overall aa identity of 99.0–99.1 %) was also confirmed in the VP capsid based tree (Fig. 1B). Identities to the feline and bat ChPVs were 74.5–74.7 % and 71.1–71.2 %, respectively. In order to further investigate the relationship between the canine and the feline ChPVs, the variable regions (VRs) of the CaChPV strains 1B-IDEXX2 and Te/36OVUD/19/ITA and those of the feline ChPVs 1/VRI/849 and IDEXX-1 were mapped as described elsewhere (Pénzes et al., 2019) and compared to each other. Out of ten VRs (VR-I to VR-X), VR-II resulted more conserved, whilst the largest differences were located in VR-III and VR-VI to VR-X (Fig. 2 ).
Table 2.
Nucleotide identities comparison of the complete coding regions (NS1 and VP) of CaChPVs detected in this study with all the members of the genus Chaphamaparvovirus.
| Strain name | GenBank accession no. | Canine chaphamaparvovirus Te/36OVUD/19/ITA | Canine chaphamaparvovirus Te/37/OVUD/19/ITA |
|---|---|---|---|
| nt% | nt% | ||
| Canine chaphamaparvovirus/Te/36OVUD/19/ITA | MT710947 | – | 99.91 |
| Canine chaphamaparvovirus/Te/37OVUD/19/ITA | MT710948 | 99.91 | – |
| Canine cachavirus-1A/IDEXX1/17/USA | MH893826 | 99.25 | 99.28 |
| Canine cachavirus-1B/IDEXX1/18/USA | MK448316 | 99.14 | 99.17 |
| Feline/carnivore chapparvovirus 1/VRI 849/19/USA | MN794869 | 73.06 | 73.08 |
| Feline chaphamaparvovirus/IDEXX-1/18/CAN | MN396757 | 73.04 | 73.07 |
| Bat parvovirus/BtPV/CMR/14/CMR | MG693107 | 67.24 | 67.27 |
| Murine chapparvovirus/MChV/NYC/Q052/14/USA | MF175078 | 55.81 | 55.81 |
| Mouse kidney parvovirus/Centenary Institute/16/AUS | NC_040843 | 55.87 | 55.87 |
| Desmodus rotondus chapparvovirus/DRA25/10/BRA | NC_032097 | 58.02 | 57.99 |
| Rat parvovirus 2/9/2014/CHN | KX272741 | 54.84 | 54.84 |
| Porcine parvovirus 7/42/15/USA | KU563733 | 44.99 | 44.93 |
| Turkey parvovirus 1/TP1/12/HUN | KF925531 | 48.18 | 48.18 |
| Chicken protoparvovirus HK-2014/ParvoQ45/13/KOR | KM254174 | 46.73 | 46.73 |
| Chicken chapparvovirus 2/RS/BR/15/2S/15/BRA | MG846442 | 46.59 | 46.62 |
Fig. 1.
Phylogenetic analyses based on the aa sequence of the NS1 (A) and VP (B) of the CaChPVs identified in this study. The trees, constructed with a selection of ChPV strains representative of each species, were generated using Maximum Likelihood method based on the Poisson correction and supplying statistical support with bootstrapping of 1000 replicates. The scale bar indicates nucleotide substitutions per site. Black triangles indicate the CaChPV strains detected in this study. Evolutionary analyses were conducted in MEGA X (Kumar et al., 2018).
Fig. 2.
Alignment of the VP aa sequences of the canine strains, 1B-IDEXX2 (Fahsbender et al., 2019) and Te/36OVUD/19/ITA (identified in this study), and of the feline ChPVs 1/VRI/849 and IDEXX-1 (FeChPVs) (Li et al., 2020). Variable regions (VRs) are marked by the black bars. The alignment of the sequences was conducted using the MAFFT multiple alignment program version 7.388 plugin of the Geneious software (Biomatters Ltd., Auckland, New Zealand).
4. Discussion
The findings of this study, while providing firm evidence that CaChPV is a common component of canine faecal virome, do not allow to make any solid conclusions on the potential role of this novel parvovirus as a primary causative agent of gastrointestinal disease. In our analysis, CaChPV DNA was detected in animals with enteric signs only in co-infection with other canine viral pathogens. Similar results were also obtained in the Chinese study, in which two out of the five diarrhoeic CaChPV positive dogs were found in mixed infections with CPV-2 and CCoV or canine distemper virus (Hu et al., 2020). Whether CaChPV can elicit gastro-enteric signs in synergism with other pathogens exacerbating the clinical course of concurrent enteric infections, should be definitively demonstrated in experimental infections.
Although most ChPV strains identified to date in the various animal species have been discovered serendipitously in metagenomic studies of enteric virome, evidence for the pathogenic potential of these viruses have been reported. Horizontal transmission of the mouse kidney parvovirus (MKPV) (Rodent chaphamaparvoviruses 1 species) has been proved to induce inclusion body nephropathy (IBN) and kidney fibrosis in aged immunodeficient mice and, to lesser extent, in immunocompetent mice (Roediger et al., 2018; Ge et al., 2020; Lee et al., 2020). More recently, a newly identified ChPV was identified by qPCR and immunohistochemistry in the heart, intestine, liver, and lung of a dead peafowl suffering of enteritis and pneumonia (Liu et al., 2020).
Sequence analysis of the nearly complete genome of the strains Te/36OVUD/19/ITA and Te/37OVUD/19/ITA and of ∼ 1.1-kb long partial VP of the CaChPV strain Te/70OVUD/19/ITA revealed a strong sequence conservation with the viruses detected in diarrhoeic dogs in USA (Fahsbender et al., 2019), either in the NS1 (98.6–99.8 %) or in the VP (99.0–99.1 %) encoding genes. Upon phylogenetic analysis based on the NS1 aa sequence, all the strains of canine origin grouped tightly within the species Carnivore chaphamaparvovirus 1. Interestingly, this group segregated apart from but close to a ChPV identified in stools of a Cameroonian fruit bat (Yinda et al., 2018) and to ChPVs recently identified in cats with gastroenteritis (Li et al., 2020). The genetic relatedness among the CaChPVs and the strains detected in cats and bats (58.5–66.7 %) was consistent with the ranges established by ICTV for classification of parvoviruses into the same genus (cut-off > 35.0 % aa identity with a NS1 coverage > 80.0 %), whilst it did not match the criteria required to demark the same species (cut-off > 85.0 % aa identity) (Pénzes et al., 2019). The correlation between the canine and feline viruses (74.5–74.7 % nt identities in the nearly complete genome) was also confirmed in the VP-based tree. For parvoviruses, the capsid is the major determinant of host range (Hueffer and Parrish, 2003) and subject to antibody-mediated selection (Nelson et al., 2007). Minor genetic changes in these proteins are known to alter the host range and pathogenic potential of parvoviruses (Parrish and Kawaoka, 2005). When comparing the VP sequences of the canine strains 1B-IDEXX2 and Te/36OVUD/19/ITA with those of the feline ChPVs 1/VRI/849 and IDEXX-1, the highest divergence was observed in the predicted VRs of the capsid (Fig. 2), with several aa changes, including residue insertions and deletions, located at VR-III and VR-VII that have been involved in control of tissue tropism, antibody recognition and receptor attachment of several parvoviruses (Halder et al., 2012; Kailasan et al., 2015). The marked genetic heterogeneity observed between the CaChPVs and the feline strains indicates that at least two distinct groups of ChPVs, each characterised by peculiar genetic signatures, circulate in dogs and cats. Since some canine viruses can infect cats and vice versa (Martella et al., 2002; Matthijnssens et al., 2011; Di Martino et al., 2016, 2018; Diakoudi et al., 2019), it will be important to explore whether cross-species transmission of ChPVs might occur between the two carnivore species.
5. Conclusions
We gathered epidemiological information of CaChPV in a large dog population demonstrating that the circulation of this novel canine parvovirus is not geographically restricted to the American and Asiatic continents where the virus was so far detected (Fahsbender et al., 2019; Hu et al., 2020). This firmly demonstrates that CaChPV is a common component of canine enteric virome. The impact of these viruses on canine health remains to establish. In our study, we did not find any possible association between CaChPV and gastro-enteric disease in young dogs. Animal experiments or detailed observational studies would be required to address this thoroughly and to investigate whether there may be other possible implications for canine health.
Declaration of Competing Interest
All Authors declare that there are no financial or other relationships that might lead to a conflict of interest. All authors have seen and approved the manuscript and have contributed significantly to the work.
Acknowledgements
Funding: this work was financed by grants from Università degli Studi di Teramo.
References
- Appel M.J., Scott F.W., Carmichael L.E. Isolation and immunisation studies of a canine parvo-like virus from dogs with haemorrhagic enteritis. Vet. Rec. 1979;105:156–159. doi: 10.1136/vr.105.8.156. [DOI] [PubMed] [Google Scholar]
- Baker K.S., Leggett R.M., Bexfield N.H., Alston M., Daly G., Todd S., Tachedjian M., Holmes C.E., Crameri S., Wang L.F., Heeney J.L., Suu-Ire R., Kellam P., Cunningham A.A., Wood J.L., Caccamo M., Murcia P.R. Metagenomic study of the viruses of African straw-coloured fruit bats: detection of a chiropteran poxvirus and isolation of a novel adenovirus. Virology. 2013;441:95–106. doi: 10.1016/j.virol.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K.I., Parrish C.R. Parvoviridae. In: Knipe D.M., Howley P., editors. Fields Virology. 6th ed. Lippincott Williams &Wilkins; Philadelphia: 2013. pp. 1768–1791. [Google Scholar]
- Binn L.N., Lazar E.C., Eddy G.A., Kajima M. Recovery and characterization of a minute virus of canines. Infect. Immun. 1970;1:503–508. doi: 10.1128/iai.1.5.503-508.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia C., Martella V., Pratelli A., Tempesta M., Cavalli A., Buonavoglia D., Bozzo G., Elia G., Decaro N., Carmichael L. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 2001;82:3021–3025. doi: 10.1038/s41598-019-53422-9. [DOI] [PubMed] [Google Scholar]
- de Souza W.M., Romeiro M.F., Fumagalli M.J., Modha S., de Araujo J., Queiroz L.H., Durigon E.L., Figueiredo L.T.M., Murcia P.R., Gifford R.J. Chapparvoviruses occur in at least three vertebrate classes and have a broad biogeographic distribution. J. Gen. Virol. 2017;98:225–229. doi: 10.1099/jgv.0.000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Buonavoglia C. Canine parvovirus - a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 2012;155:1–12. doi: 10.1016/j.vetmic.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Carmichael L.E., Buonavoglia C. Viral reproductive pathogens of dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 2012;42:583–598. doi: 10.1016/j.cvsm.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B., Di Felice E., Ceci C., Di Profio F., Marsilio F. Canine kobuviruses in diarrhoeic dogs in Italy. Vet. Microbiol. 2013;166:246–249. doi: 10.1016/j.vetmic.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B., Di Profio F., Melegari I., Sarchese V., Cafiero M.A., Robetto S., Aste G., Lanave G., Marsilio F., Martella V. A novel feline norovirus in diarrheic cats. Infect. Genet. Evol. 2016;38:132–137. doi: 10.1016/j.meegid.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B., Di Profio F., Melegari I., Sarchese V., Massirio I., Luciani A., Lanave G., Marsilio F., Martella V. Serological and molecular investigation of 2117-like vesiviruses in cats. Arch. Virol. 2018;163:197–201. doi: 10.1007/s00705-017-3582-z. [DOI] [PubMed] [Google Scholar]
- Diakoudi G., Lanave G., Capozza P., Di Profio F., Melegari I., Di Martino B., Pennisi M.G., Elia G., Cavalli A., Tempesta M., Camero M., Buonavoglia C., Bányai K., Martella V. Identification of a novel parvovirus in domestic cats. Vet. Microbiol. 2019;228:246–251. doi: 10.1016/j.vetmic.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Fahsbender E., Altan E., Seguin M.A., Young P., Estrada M., Leutenegger C., Delwart E. Chapparvovirus DNA found in 4 % of dogs with diarrhea. Viruses. 2019;11:398. doi: 10.3390/v11050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z., Carrasco S.E., Feng Y., Bakthavatchalu V., Annamalai D., Kramer R., Muthupalani S., Fox J.G. Identification of a new strain of mouse kidney parvovirus associated with inclusion body nephropathy in immunocompromised laboratory mice. Emerg. Microbes Infect. 2020;20:1–33. doi: 10.1080/22221751.2020.1798288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder S., Ng R., Agbandje-McKenna M. Parvoviruses: structure and infection. Future Virol. 2012;7:253–278. doi: 10.2217/fvl.12.12. [DOI] [Google Scholar]
- Hu W., Liu Q., Chen Q., Ji J. Molecular characterization of Cachavirus firstly detected in dogs in China. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueffer K., Parrish C.R. Parvovirus host range, cell tropism and evolution. Curr. Opin. Microbiol. 2003;6:392–398. doi: 10.1016/s1369-5274(03)00083-3. [DOI] [PubMed] [Google Scholar]
- James J.A., Escalante C.R., Yoon-Robarts M., Edwards T.A., Linden R.M., Aggarwal A.K. Crystal structure of the SF3 helicase from adeno-associated virus type 2. Structure. 2003;11:1025–1035. doi: 10.1016/S0969-2126(03)00152-7. [DOI] [PubMed] [Google Scholar]
- Kailasan S., Halder S., Gurda B., Bladek H., Chipman P.R., McKenna R., Brown K., Agbandje-McKenna M. Structure of an enteric pathogen, bovine parvovirus. J. Virol. 2015;89:2603–2614. doi: 10.1128/JVI.03157-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Mehta N., Dubovi E.J., Simmonds P., Govindasamy L., Medina J.L., Street C., Shields S., Lipkin W.I. Characterization of novel canine bocaviruses and their association with respiratory disease. J. Gen. Virol. 2012;93:341–346. doi: 10.1099/vir.0.036624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Pushing the limits of the scanning mechanism for initiation of translation. Gene. 2002;299:1–34. doi: 10.1016/s0378-1119(02)01056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Q., Padula M.P., Pinello N., Williams S.H., O’Rourke M.B., Fumagalli M.J., Orkin J.D., Song R., Shaban B., Brenner O., Pimanda J.E., Weninger W., Souza W.M., Melin A.D., Wong J.J., Crim M.J., Monette S., Roediger B., Jolly C.J. Murine and related chapparvoviruses are nephro-tropic and produce novel accessory proteins in infected kidneys. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Pesavento P.A., Leutenegger C.M., Estrada M., Coffey L.L., Naccache S.N., Samayoa E., Chiu C., Qiu J., Wang C., Deng X., Delwart E. A novel bocavirus in canine liver. Virol. J. 2013;10:54. doi: 10.1186/1743-422X-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Gordon E., Idle A., Altan E., Seguin M.A., Estrada M., Deng X., Delwart E. Virome of a feline outbreak of diarrhea and vomiting includes bocaviruses and a novel chapparvovirus. Viruses. 2020;12:506. doi: 10.3390/v12050506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima D.A., Cibulski S.P., Tochetto C., Varela A.P.M., Finkler F., Teixeira T.F., Loiko M.R., Cerva C., Junqueira D.M., Mayer F.Q., Roehe P.M. The intestinal virome of malabsorption syndrome-affected and unaffected broilers through shotgun metagenomics. Virus Res. 2019;261:9–20. doi: 10.1016/j.virusres. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang H., Liu X., Li Y., Chen J., Zhang J., Wang X., Shen S., Wang H., Deng F., Wang M., Guan W., Hu Z. Genomic and transcriptional analyses of novel parvoviruses identified from dead peafowl. Virology. 2020;539:80–91. doi: 10.1016/j.virol.2019.10.013. [DOI] [PubMed] [Google Scholar]
- Martella V., Pratelli A., Gentile M., Buonavoglia D., Decaro N., Fiorente P., Buonavoglia C. Analysis of the capsid protein gene of a feline-like calicivirus isolated from a dog. Vet. Microbiol. 2002;85:315–322. doi: 10.1016/S0378-1135(01)00521-1. [DOI] [PubMed] [Google Scholar]
- Martella V., Lanave G., Mihalov-Kovács E., Marton S., Varga-Kugler R., Kaszab E., Di Martino B., Camero M., Decaro N., Buonavoglia C., Bányai K. Novel parvovirus related to primate bufaviruses in dogs. Emerg. Infect. Dis. 2018;24:1061–1068. doi: 10.3201/eid2406.171965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J., De Grazia S., Piessens J., Heylen E., Zeller M., Giammanco G.M., Bányai K., Buonavoglia C., Ciarlet M., Martella V., Van Ranst M. Multiple reassortment and interspecies transmission events contribute to the diversity of feline, canine and feline/canine-like human group A rotavirus strains. Infect. Genet. Evol. 2011;11:1396–1406. doi: 10.1016/j.meegid.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Nelson C.D., Palermo L.M., Hafenstein S.L., Parrish C.R. Different mechanisms of antibody-mediated neutralization of parvoviruses revealed using the fab fragments of monoclonal antibodies. Virology. 2007;361:283–293. doi: 10.1016/j.virol.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski R.M., Mitra N., Hause B.M. Discovery of a novel Parvovirinae virus, porcine parvovirus 7, by metagenomic sequencing of porcine rectal swabs. Virus Genes. 2016;52:564–567. doi: 10.1007/s11262-016-1322-1. [DOI] [PubMed] [Google Scholar]
- Parrish C.R., Kawaoka Y. The origins of new pandemic viruses: the acquisition of new host ranges by canine parvovirus and influenza A viruses. Annu. Rev. Microbiol. 2005;59:553–586. doi: 10.1146/annurev.micro.59.030804.121059. [DOI] [PubMed] [Google Scholar]
- Pénzes J.J., de Souza W.M., Agbandje-McKenna M., Gifford R.J. An ancient lineage of highly divergent parvoviruses infects both vertebrate and invertebrate hosts. Viruses. 2019;11:525. doi: 10.3390/v11060525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Tempesta M., Greco G., Martella V., Buonavoglia C. Development of a nested PCR assay for the detection of canine coronavirus. J. Virol. Methods. 1999;80:11–15. doi: 10.1016/s0166-0934(99)00017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Boros Á., Delwart E., Pankovics P. Novel circular single-stranded DNA virus from Turkey faeces. Arch. Virol. 2014;159:2161–2164. doi: 10.1007/s00705-014-2025-3. [DOI] [PubMed] [Google Scholar]
- Roediger B., Lee Q., Tikoo S., Cobbin J., Henderson J.M., Jormakka M., O’Rourke M.B., Padula M.P., Pinello N., Henry M., Wynne M., Santagostino S.F., Brayton C.F., Rasmussen L., Lisowski L., Tay S.S., Harris D.C., Bertram J.F., Dowling J.P., Bertolino P., Lai J.H., Wu W., Bachovchin W.W., Wong J.J., Gorrell M.D., Shaban B., Holmes E.C., Jolly C.J., Monette S., Weninger W. An atypical parvovirus drives chronic tubulointerstitial nephropathy and kidney fibrosis. Cell. 2018;175:530–543. doi: 10.1016/j.cell.2018.08.013. e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.H., Kotin R.M. An adeno-associated virus (AAV) initiator protein, Rep 78, catalyzes the cleavage and ligation of single-stranded AAV ori DNA. J. Virol. 2000;74:3122–3129. doi: 10.1128/jvi.74.7.3122-3129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyl F., Gerlach R., Mergersen K. The Rule of three, its variants and extensions. Int. Stat. Rev. 2009;77:266–275. doi: 10.1111/j.1751-5823.2009.00078.x. [DOI] [Google Scholar]
- Vennema H., de Bruin E., Koopmans M. Rational optimization of generic primers used for Norwalk-like virus detection by reverse transcriptase polymerase chain reaction. J. Clin. Virol. 2002;25:233–235. doi: 10.1016/s1386-6532(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Walker J.E., Saraste M., Runswick M.J., Gay N.J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.H., Che X., Garcia J.A., Klena J.D., Lee B., Muller D., Ulrich W., Corrigan R.M., Nichol S., Jain K., Lipkin W.I. Viral diversity of house mice in New York city. mBio. 2018;9:e01354–17. doi: 10.1128/mBio.01354-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Liu Z., Wang Y., Li W., Fu X., Lin Y., Shen Q., Wang X., Wang H., Zhang W. A novel rodent Chapparvovirus in feces of wild rats. Virol. J. 2016;13:133. doi: 10.1186/s12985-016-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinda C.K., Ghogomu S.M., Conceição-Neto N., Beller L., Deboutte W., Vanhulle E., Maes P., Van Ranst M., Matthijnssens J. Cameroonian fruit bats harbor divergent viruses, including rotavirus H, bastroviruses, and picobirnaviruses using an alternative genetic code. Virus Evol. 2018;4(1):vey008. doi: 10.1093/ve/vey008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zádori Z., Szelei J., Lacoste M.-C., Li Y., Garie!py S., Raymond P., Allaire M., Nabi I.R., Tijssen P. A viral phopholipase A2 is required for parvovirus infectivity. Dev. Cell. 2001;1:291–302. doi: 10.1016/s1534-5807(01)00031-4. [DOI] [PubMed] [Google Scholar]