Abstract
Rare or private, biallelic variants in the ABCA3 (ATP-binding cassette transporter A3) gene are the most common monogenic cause of lethal neonatal respiratory failure and childhood interstitial lung disease. Functional characterization of fewer than 10% of over 200 disease-associated ABCA3 variants (majority missense) suggests either disruption of ABCA3 protein trafficking (type I) or of ATPase-mediated phospholipid transport (type II). Therapies remain limited and nonspecific. A scalable platform is required for functional characterization of ABCA3 variants and discovery of pharmacologic correctors. To address this need, we first silenced the endogenous ABCA3 locus in A549 cells with CRISPR/Cas9 genome editing. Next, to generate a parent cell line (A549/ABCA3−/−) with a single recombination target site for genomic integration and stable expression of individual ABCA3 missense variant cDNAs, we used lentiviral-mediated integration of a LoxFAS cassette, FACS, and dilutional cloning. To assess the fidelity of this cell-based model, we compared functional characterization (ABCA3 protein processing, ABCA3 immunofluorescence colocalization with intracellular markers, ultrastructural vesicle phenotype) of two individual ABCA3 mutants (type I mutant, p.L101P; type II mutant, p.E292V) in A549/ABCA3−/− cells and in both A549 cells and primary, human alveolar type II cells that transiently express each cDNA after adenoviral-mediated transduction. We also confirmed pharmacologic rescue of ABCA3 variant–encoded mistrafficking and vesicle diameter in A549/ABCA3−/− cells that express p.G1421R (type I mutant). A549/ABCA3−/− cells provide a scalable, genetically versatile, physiologically relevant functional genomics platform for discovery of variant-specific mechanisms that disrupt ABCA3 function and for screening of potential ABCA3 pharmacologic correctors.
Keywords: ATP-binding cassette subfamily A member 3, ABCA3, surfactant, childhood interstitial lung disease, neonatal respiratory distress syndrome
Clinical Relevance
Rare, biallelic variants in the ABCA3 (ATP-binding cassette transporter A3) gene are the most common monogenic cause of lethal neonatal respiratory failure and childhood interstitial lung disease, and therapies remain limited and nonspecific. A scalable platform is required for functional characterization of ABCA3 variants and discovery of pharmacologic correctors. A549/ABCA3−/− cells provide a genetically versatile, physiologically relevant, functional genomics platform for discovery of variant-specific mechanisms that disrupt ABCA3 function and for screening of potential ABCA3 pharmacologic correctors.
The ABCA3 (ATP-binding cassette transporter A3) protein is a member of a superfamily of transporters with multiple membrane-spanning domains that hydrolyze ATP to transport different substrates across membranes (e.g., chloride ion by CFTR [cystic fibrosis transmembrane conductance regulator, also known as ABCC7]) (1, 2). ABCA3 is highly expressed in alveolar type II (ATII) cells, localizes to the lysosome-related, lamellar body–limiting membrane, is required for lamellar-body biogenesis, and transports phospholipids into the lamellar body, which assemble with SP-B (surfactant protein B) and SP-C to yield pulmonary surfactant (1, 3). Rare or private, biallelic variants in ABCA3 are the most common monogenic cause of severe neonatal respiratory distress syndrome in term infants and of childhood interstitial lung disease and may result in progressive, lethal respiratory failure (4–7). Ultrastructural evaluations of ATII cells from infants and children with biallelic ABCA3 variants demonstrate small, dense lamellar bodies with eccentrically placed inclusion bodies (4, 8). Current therapeutic strategies (e.g., glucocorticoids, hydroxychloroquine, and azithromycin) are nonspecific and largely ineffective, and lung transplant with an estimated 50% 5-year survival remains the definitive treatment for infants and children with progressive respiratory failure (6, 9–11).
Although more than 200 disease-associated ABCA3 variants (the majority missense variants) have been identified in symptomatic infants and children, fewer than 10% have been functionally classified as disrupting ABCA3 protein trafficking (type I) or ATPase-mediated phospholipid transport (type II) in cell-based systems (12–19). The mechanisms of disruption encoded by individual ABCA3 variants are difficult to predict on the basis of location in the gene or the mature protein, and the cell-based models used in these functional studies are not easily scalable for high-throughput screening. Similar to variant classification for CFTR (20), variants in ABCA3 can result in the absence of mature protein (nonsense, frameshift variants, ∼CFTR class I), disruption of intracellular trafficking (∼CFTR class II), or impaired phospholipid transport into the lamellar bodies (∼CFTR class III). Although infants with biallelic “null” (nonsense or frameshift) variants present with respiratory failure at birth and die during the first year of life without lung transplantation, the presentation, disease severity, and progression for infants and children with biallelic missense, in-frame insertions or deletions, or splicing variants are variable and difficult to predict (5, 21). The basis for this phenotypic variability is not known but may be associated with the specific type of ABCA3 mutant (e.g., disruption of intracellular ABCA3 trafficking or impaired lipid transport by decreased ATP-mediated hydrolysis) (12–19, 22), activation of intracellular stress or degradation pathways (15, 23), or other genetic or environmental modifiers.
Recent successful identification of correctors (e.g., lumacaftor, tezacaftor, and elexacaftor), which bind to mutant CFTR or potentiators (e.g., ivacaftor) of CFTR transport function, suggests that carefully characterized, cell-based, functional assays can be used for evaluating pharmacologic strategies to rescue ABCA3 function disrupted by rare or private variants when clinical trials or access to tissues are not feasible because of low pathogenic allele frequencies (24–28). We propose to apply similar strategies to develop personalized therapies for infants and children with ABCA3 deficiency.
To investigate variant-encoded mechanisms that disrupt ABCA3 function, inform clinical decision-making, and facilitate high-throughput screening of ABCA3 variant–specific pharmacologic correctors, we generated an A549 cell line with silenced endogenous ABCA3 expression and with integrated, asymmetric Lox sites (A549/ABCA3−/−) that permit Cre-recombinase-mediated, site-specific integration and stable expression of ABCA3 variants. To assess the fidelity of functional characterization in this human pulmonary epithelial cell model, we compared results of ABCA3-mutant function in A549/ABCA3−/− cells to both A549 cells and primary human ATII cells that each transiently expressed the same ABCA3 variants after adenoviral mediated transduction. We also replicated a previous report of rescue by pharmacologic correctors of variant-encoded mistrafficking of ABCA3 (24) in A549/ABCA3−/− cells.
Methods
Selection of ABCA3 Mutants
We selected two previously characterized ABCA3 mutants (p.L101P, type I mistrafficking mutant, and p.E292V, type II phospholipid transport mutant) for functional characterization in A549/ABCA3−/− cells (14–17, 19, 29). We selected the type I mistrafficking mutant p.G1421R, which was previously rescued by pharmacologic correctors C13 and C17 in A549 cells stably transfected with the Sleeping Beauty transposon system (24).
Cell Line Construction
A549/ABCA3−/− cells that stably express ABCA3 variants.
We adapted a “landing pad” strategy (30) for integration of individual ABCA3 variant cDNAs at a single intergenic site in the human pulmonary epithelial A549 cell line (31). Briefly, we used CRISPR/Cas9 (CRISPR/CRISPR-associated protein 9) genome editing to introduce biallelic, frameshift variants to silence endogenous ABCA3 expression in A549 cells. We extracted membrane and total protein from the A549/ABCA3−/− cells to confirm absence of ABCA3 protein by IB and protein mass spectrometry. We used lentiviral mediated integration of a LoxFAS/GFP/LoxP cassette, FACS, dilutional cloning, and inverse PCR (32) to generate a parent cell line (A549/ABCA3−/−) with a single recombination target site for genomic integration and stable expression of individual ABCA3 variant cDNAs. We cotransfected Cre-recombinase and individual lox-flanked ABCA3-mCherry constructs with cDNAs for ABCA3 wild type (WT) or three variants (p.L101P, p.E292V, or p.G1421R). We used FACS to select GFP-negative/mCherry-positive cells, performed Sanger sequencing to confirm the presence of ABCA3 WT and the variants, and expanded single-cell sorted clones. We used quantitative PCR to compare ABCA3 transcript expression for A549/ABCA3−/− cell lines and adenoviral transduced A549 cells that express ABCA3 WT or the variants. We performed karyotype analysis for the A549/ABCA3−/− cells and A549/ABCA3−/− cells that express ABCA3 WT, p.L101P, or p.E292V.
Methods of functional characterization of the different cell lines
We assessed IB, fluorescence-based colocalization, and vesicle ultrastructure of the A549/ABCA3−/− cells that stably express individual ABCA3 variants and the adenoviral transduced A549 and ATII cells, as previously described (14, 19) (see data supplement). We also assessed vesicle diameter and liposome uptake of the A549/ABCA3−/− cells that stably express individual ABCA3 variants (19, 29) (see data supplement).
Corrector rescue
To assess the fidelity of rescue by correctors in the A549/ABCA3−/− cell lines, we sought to replicate the observations of Kinting and colleagues (24). Briefly, we incubated A549/ABCA3−/− cells that express the ABCA3 type I mistrafficking mutant p.G1421R with 10 μM of C13 or C17 correctors (obtained from the Cystic Fibrosis Foundation), extracted protein, and performed IB. We determined the Pearson correlation coefficient (33) as an indicator of colocalization of ABCA3 mutant p.G1421R protein with lysosomal (anti-CD63, Active Biosciences) or endoplasmic-reticulum (ER) markers (SelectFX_ER, Thermo-Fisher) for 20 cells from each condition (p.G1421R untreated, p.G1421R+C13, p.G1421+C17). We measured vesicle diameter for 100 vesicles from 20 cells/condition and used ANOVA with the Tukey honestly significant difference post hoc test to compare colocalization and vesicle-diameter results from the three conditions (for additional details, see data supplement).
Results
Generation of A549/ABCA3−/− Cells Expressing Individual ABCA3 Constructs
Using extracted membrane and total protein from the A549/ABCA3−/− cells, we did not detect ABCA3 protein by IB or protein mass spectrometry. After lentiviral mediated integration of a LoxFAS/GFP/LoxP cassette, FACS, and dilutional cloning, we used inverse PCR to map the landing-pad location to an intergenic region of chromosome 12 (chr12:47693178+) in the A549/ABCA3−/− parent cell line. After cotransfection with ABCA3-mCherry constructs with cDNAs for ABCA3 WT, p.L101P, or p.E292V, we performed quantitative PCR and found similar amounts of ABCA3 transcript expression for A549/ABCA3−/− cell lines and adenoviral transduced A549 cells that express ABCA3 WT, p.L101P, or p.E292V (data not shown). We performed karyotype analysis for the A549/ABCA3−/− cells and A549/ABCA3−/− cells expressing ABCA3 WT, p.L101P, or p.E292V and found all cell lines had two copies of chromosome 16, the endogenous location of ABCA3.
ABCA3 Protein Processing
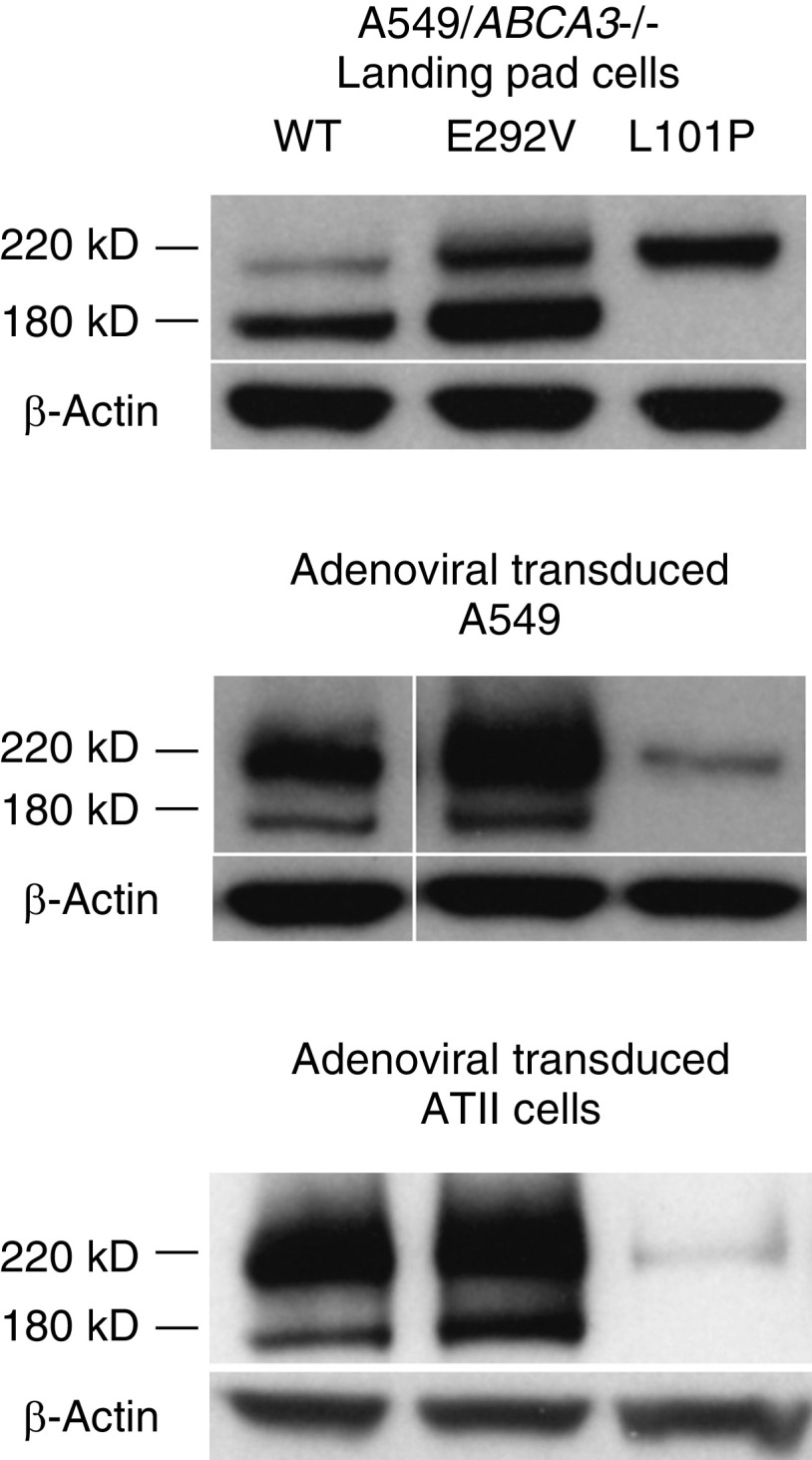
ABCA3 undergoes N-terminal cleavage from a primary translation product to a post-translationally modified product in the multivesicular bodies (34). Mistrafficked ABCA3 proteins that are retained in the endoplasmic reticulum fail to undergo this cleavage, and only the primary translation product is detected (1, 13–19). IB of mistrafficked ABCA3 mutant proteins demonstrates a single, uncleaved 220-kD band that results from the sum of mobilities of ABCA3 protein (190 kD) and mCherry or GFP (∼30 kD). Protein processing of ABCA3 WT, p.L101P, and p.E292V proteins was similar in A549/ABCA3−/− cells that stably express ABCA3 WT or variants and adenoviral transduced A549 and ATII cells (Figure 1). These results are consistent with those of previous reports that the p.L101P mutant is not trafficked to the lamellar bodies, whereas p.E292V is processed similarly to ABCA3 WT (1, 14–17, 19).
Figure 1.
ABCA3 (ATP-binding cassette transporter A3) protein processing results for A549/ABCA3−/− cells that express ABCA3 wild type (WT), p.E292V, or p.L101P are similar to results from adenoviral transduced A549 and alveolar type II (ATII) cells. IB of type I (mistrafficking) mutant p.L101P demonstrates a single, uncleaved 220-kD band in all three cell-model systems. Protein processing of type II (impaired phospholipid transport) mutant p.E292V is similar to that of ABCA3 WT. These experiments were performed three times with similar results.
Immunofluorescence Colocalization
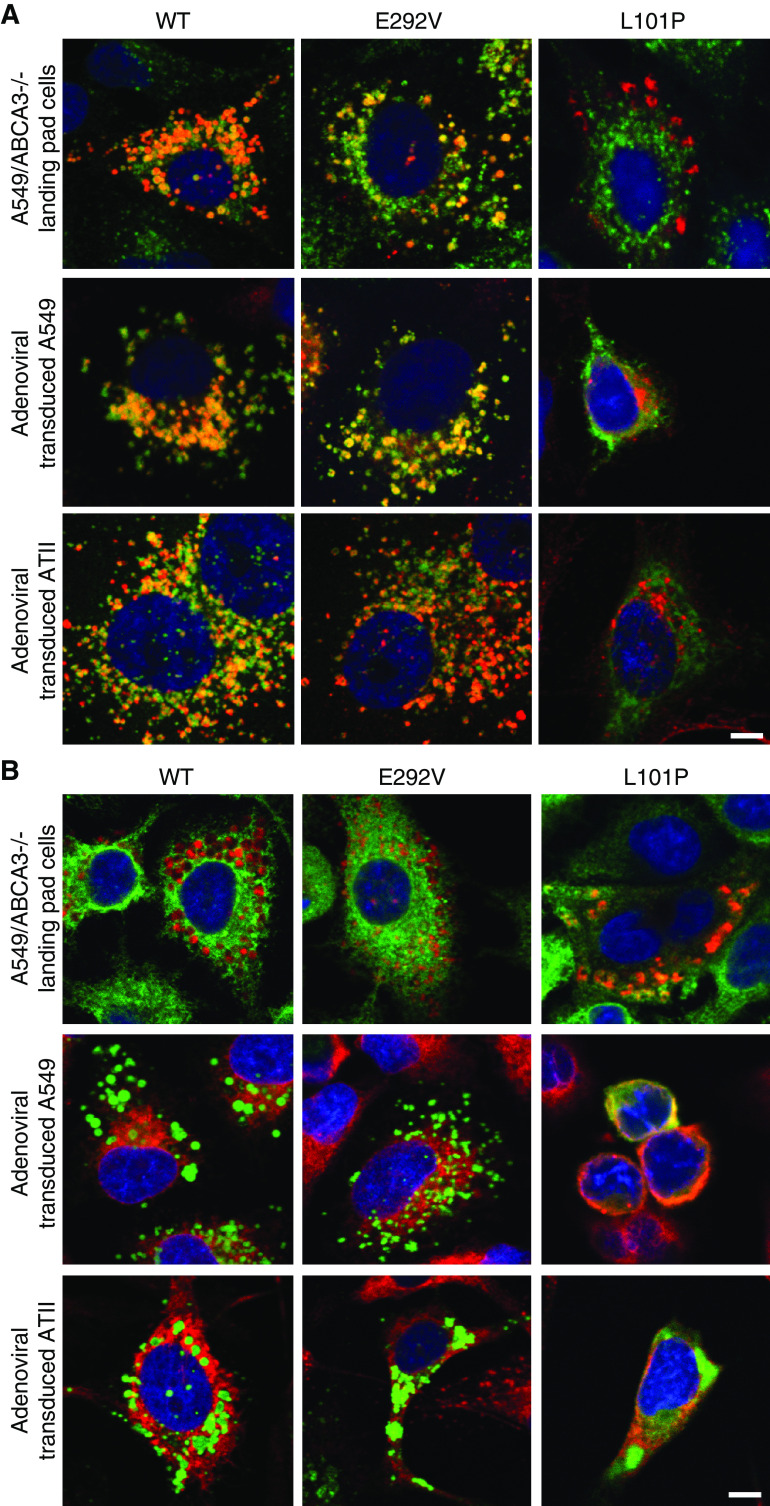
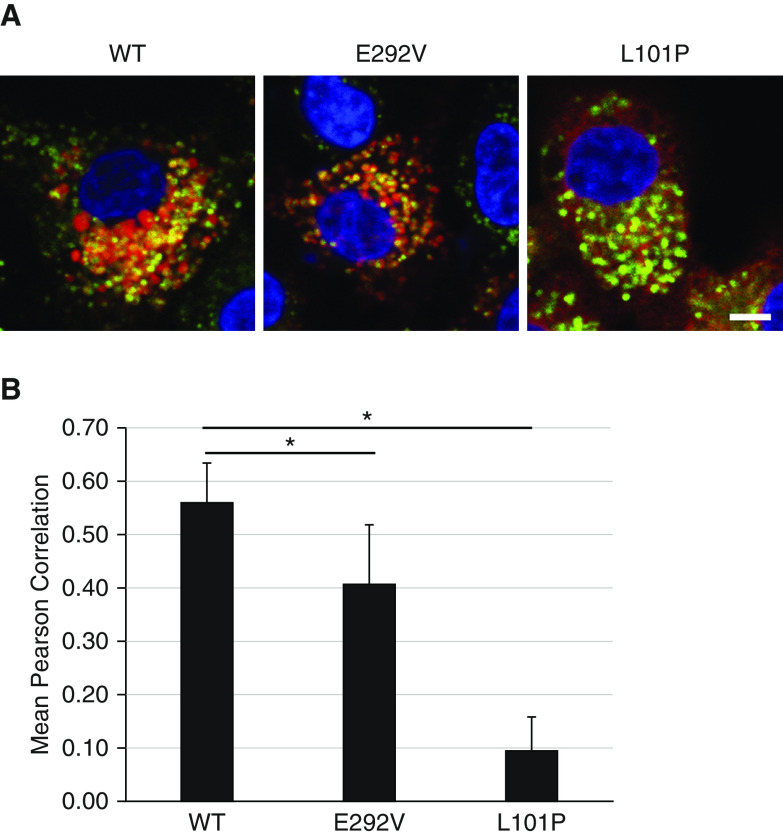
We compared immunofluorescence colocalization of mCherry-tagged A549/ABCA3−/− cells that express ABCA3 WT, p.L101P, or p.E292V mutants to results from adenoviral transduced A549 and ATII cells using the lysosomal marker anti-CD63 (Figure 2A) and the ER marker SelectFX_ER (Figure 2B). We observed similar immunofluorescence colocalization in all three cell systems. We used Volocity cellular imaging and analysis software (v6.3; GE Lifesciences) and the Pearson correlation to quantitate immunofluorescence colocalization for A549/ABCA3−/− cells that express ABCA3 WT, p.L101P, or p.E292V with lysosomal and ER markers (Figure 3). Consistent with prior studies that qualitatively showed disruption of trafficking to the lysosome-related, lamellar body–like vesicles by the p.L101P mutant protein (Figure 3A) (1, 14–17, 19), we found quantitative differences between p.L101P and ABCA3 WT colocalization with lysosome-related vesicles (anti-CD63) and the ER (SelectFX_ER) (Figure 3B). The colocalization profile of p.E292V was similar to ABCA3 WT.
Figure 2.
ABCA3 immunofluorescence colocalization results for A549/ABCA3−/− cells that express ABCA3 WT, p.E292V, or p.L101P are similar to results from adenoviral transduced A549 and ATII cells. Single x-y–plane confocal immunofluorescence colocalization demonstrates that ABCA3 WT and type II (impaired phospholipid transport) mutant p.E292V colocalize with (A) the lysosomal marker (anti-CD63), whereas type I (mistrafficking) mutant p.L101P colocalizes with (B) the endoplasmic-reticulum (ER) marker SelectFX_ER. Of note, in A549/ABCA3−/− cells, ABCA3 (or mutant) is labeled with mCherry (red), anti-CD63 (green), and SelectFX_ER (green). In adenoviral transduced A549 and ATII cells, ABCA3 (or mutant) is labeled with GFP (green), anti-CD63 (red), and SelectFX_ER (red). Scale bars, 10 μm. These experiments were performed three times with similar results.
Figure 3.
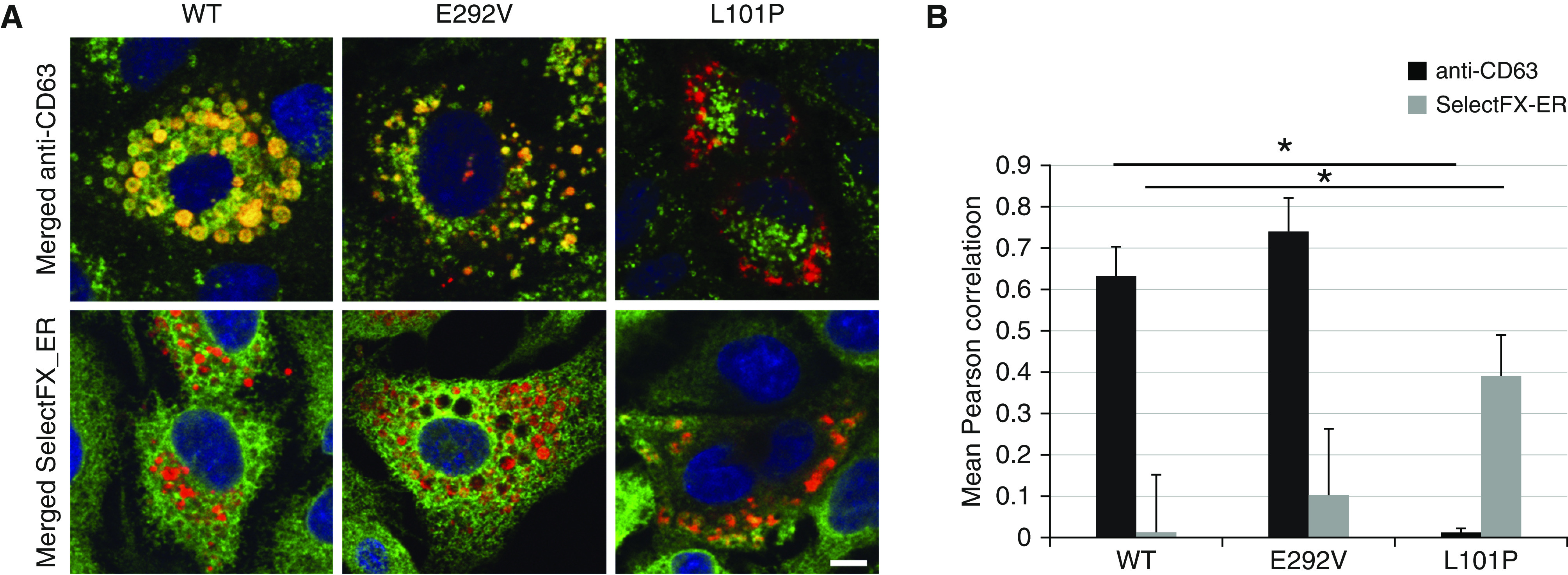
Qualitative and quantitative comparison of immunofluorescence colocalization with lysosomal and ER markers for A549/ABCA3−/− cells that express ABCA3 WT, p.E292V, or p.L101P. (A) Single x-y–plane confocal immunofluorescence colocalization with lysosomal (anti-CD63) and ER (SelectFX_ER) markers and A549/ABCA3−/− cells that express ABCA3 WT, p.E292V, or p.L101P. (B) Using the Pearson correlation for 20 cells/condition, we detected significant quantitative differences in colocalization for p.L101P (type I, mistrafficking mutant) as compared with ABCA3 WT. Immunofluorescence colocalization for p.E292V (type II, impaired phospholipid transport mutant) is similar to ABCA3 WT. *P < 0.001. Scale bar, 10 μm.
Vesicle Ultrastructure and Diameter
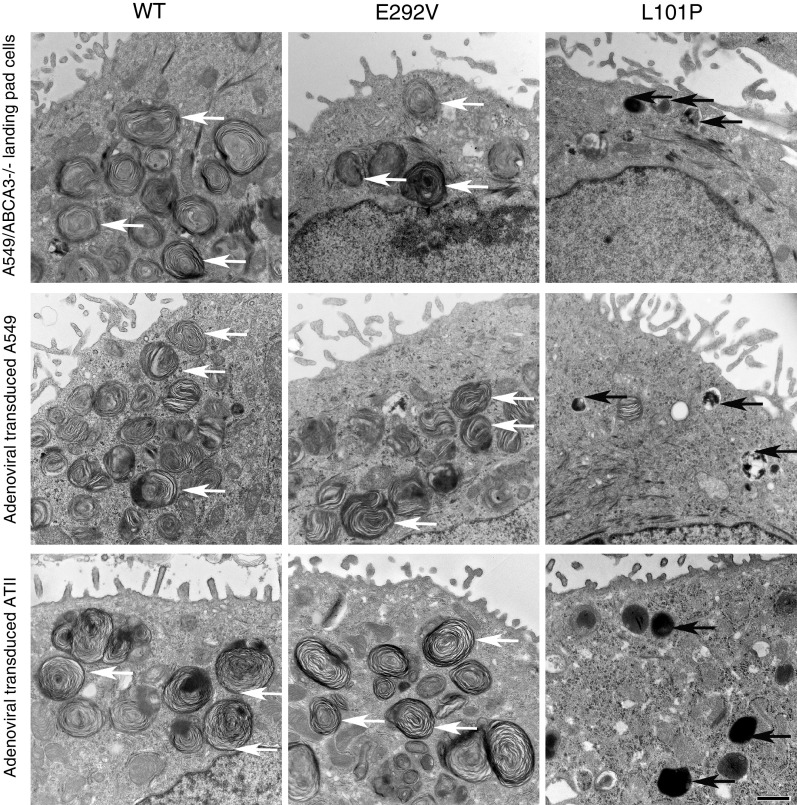
Vesicle ultrastructural results were similar in A549/ABCA3−/− cells that express individual ABCA3 WT, p.L101P, or p.E292V mutants and adenoviral transduced A549 and ATII cells (Figure 4). Specifically, the vesicle ultrastructure observed in cells that express p.E292V is similar to that of cells that express ABCA3 WT, whereas cells that express p.L101P exhibit smaller, dark vesicles similar to lamellar bodies observed in patients with ABCA3 deficiency (4, 8). We found that the mean mCherry-positive vesicle diameters of A549/ABCA3−/− cells that express either p.L101P (type I mutant) or p.E292V (type II mutant) were smaller as compared with cells that express ABCA3 WT (all P < 0.001) (Figure 5).
Figure 4.
EM of vesicle ultrastructure results for A549/ABCA3−/− cells that express ABCA3 WT, p.E292V, or p.L101P are similar to results from adenoviral transduced A549 and ATII cells. White arrows indicate normal-appearing lamellar body–like vesicles, and black arrows indicate abnormal-appearing lamellar body–like vesicles. Cells that express p.L101P demonstrate smaller, dark vesicles and few normal-appearing lamellar body–like vesicles in all three cell systems, whereas cells that express p.E292V have lamellar body–like vesicles similar to those of cells that express ABCA3 WT. Scale bar, 800 nm.
Figure 5.
Vesicle diameters of A549/ABCA3−/− cells that express p.L101P and p.E292V are smaller compared with A549/ABCA3−/− cells that express ABCA3 WT. (A) Single x-y–plane confocal immunofluorescence imaging and (B) ImageJ were used to measure vesicles and diameters of 100 ABCA3-mCherry–positive vesicles/condition. *P < 0.001. Scale bar, 10 μm.
Liposome Uptake
We used A549/ABCA3−/− cells that express ABCA3 WT, p.L101P, or p.E292V to compare colocalization of ABCA3 WT or mutant proteins (ABCA3-mCherry, red) with Top-Fluor-phosphatidylcholine (Avanti Polar Lipids)–containing liposomes (green). We found decreased colocalization of p.L101P and p.E292V with Top-Fluor-phosphatidylcholine as compared with ABCA3 WT, suggesting impaired liposome uptake for both type I (mistrafficking) and type II (impaired phospholipid transport) mutants (Figure 6).
Figure 6.
A549/ABCA3−/− cells that express p.E292V or p.L101P have decreased Top-Fluor-phosphatidylcholine (TopF-PC)–labeled liposome uptake compared with A549/ABCA3−/− cells that express ABCA3 WT. We used (A) single x-y–plane imaging and (B) Pearson correlation to measure immunofluorescence colocalization of ABCA3 WT or mutants (mCherry) with TopF-PC (green). Experiments were performed three times with similar results. *P < 0.001. Scale bar, 10 μm.
Corrector Rescue
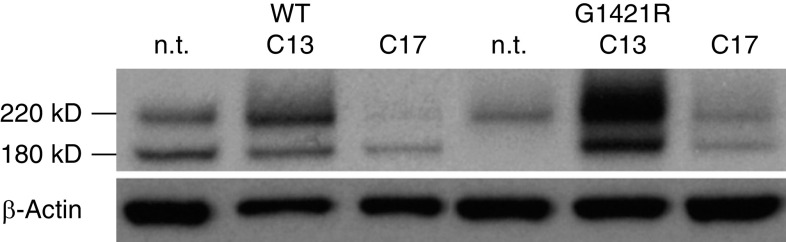
Similar to Kinting and colleagues (24), we found that incubation of A549/ABCA3−/− cells that express the type I mistrafficking mutant p.G1421R with C13 or C17 correctors increased the cleaved, mature 180-kD ABCA3 peptide (Figure 7), increased colocalization with lysosomal marker (anti-CD63), decreased colocalization with ER marker (SelectFX_ER), and increased ABCA3 positive vesicle diameter as compared with untreated A549/ABCA3−/− cells that express p.G1421R (Table 1). Interestingly, incubation with C13 or C17 did not rescue protein processing or colocalization for type I mistrafficking mutant p.L101P (data not shown), suggesting that the correctors are variant-specific. These results confirm the usefulness of the A549/ABCA3−/− cell lines for assessment of rescue by individual pharmacologic correctors.
Figure 7.
Exposure of A549/ABCA3−/− cells that express p.G1421R to C13 and C17 correctors increased the cleaved 180-kD ABCA3 peptide. We used IB to compare protein processing for A549/ABCA3−/− cells that express ABCA3 WT and the type I trafficking mutant p.G1421R after exposure to correctors C13 and C17. These experiments were performed twice with results similar to those of Kinting and colleagues (24). n.t. = not treated.
Table 1.
Exposure of A549/ABCA3−/− Cells That Express p.G1421 to C13 or C17 Correctors Increased Colocalization with Anti-CD63, Decreased Colocalization with SelectFX_ER, and Increased Mean Vesicle Diameter
| p.G1421R Untreated (Mean ± SD) | p.G1421+C13 (Mean ± SD) | p.G1421R+C17 (Mean ± SD) | |
|---|---|---|---|
| Colocalization with anti-CD63 | 0.71 ± 0.13 | 0.74 ± 0.09 | 0.80 ± 0.05* |
| Colocalization with SelectFX_ER | 0.30 ± 0.10 | 0.14 ± 0.09* | 0.10 ± 0.10* |
| ABCA3-positive mean vesicle diameter, μm | 0.98 ± 0.14 | 1.31 ± 0.29* | 1.09 ± 0.28* |
Definition of abbreviations: ABCA3 = ATP-binding cassette transporter A3; ER = endoplasmic-reticulum.
We compared colocalization with lysosomal marker anti-CD63 and ER marker SelectFX_ER and vesicle diameter for A549/ABCA3−/− cells that express type I (mistrafficking) mutant p.G1421R in the presence of C13 and C17 correctors to untreated using ANOVA with the Tukey honestly significant difference post hoc test.
P < 0.001.
Discussion
Although infants with biallelic frameshift or nonsense ABCA3 variants present with neonatal respiratory failure and die within the first of year life without lung transplantation, infants and children with missense, splice-site, or in-frame insertions or deletions have greater phenotypic diversity in presentation and disease course (5, 21). Development of precision medicine approaches for infants and children with ABCA3 deficiency that obviate the need for lung transplantation requires scalable, cell-based or model organism–based systems that permit investigation of disease mechanisms associated with each rare or private variant (35). The success of this approach for discovery of disease mechanisms and new therapies has been demonstrated by progress in CFTR variant–specific therapies for patients with cystic fibrosis (35, 36). A cell-based or model organism–based strategy is also critical when clinical trials are not feasible because of small, geographically dispersed patient cohorts and challenges of insurance coverage for medications that are not approved by the U.S. Food and Drug Administration (24, 26). Reproducibility of results, genetic versatility, and scalability are important for translation into clinical use. We have shown that functional characterization of individual ABCA3 variants in A549 cells with a single recombination target site for stable genomic integration and expression of individual ABCA3 variant cDNAs is similar to A549 cells or primary ATII cells that transiently express each cDNA after adenoviral mediated transduction. Prior studies have used transposon-mediated transfection into A549 cells to generate cell lines that stably express ABCA3 variants (12, 24, 29, 37). We extend these studies by using a strategy that integrates each ABCA3 variant at a single, characterized intergenic site in a parent A549 cell line with silenced endogenous ABCA3 expression, eliminates the need for chronic antibiotic selection, and reduces risk of disruption of normal cellular metabolism due to direct cytotoxic effects of transduction or transfection (38). In addition, pharmacologic corrector rescue of a known type I mistrafficking mutant p.G1421R in A549/ABCA3−/− cells replicated previous findings in A549 cells after transposon-mediated transfection (24).
Another advantage of this landing-pad system is the genetic versatility and efficiency of Cre-recombinase–mediated cassette integration. Although lentiviral transduction is initially required to integrate the LoxFAS/LoxP cassette, subsequent Cre-recombinase–mediated integration of ABCA3 variant constructs reduces the risk of direct cytotoxic effects by avoiding the need for viral transduction. This landing-pad strategy can also be adapted for functional characterization of variants associated with other monogenic disorders that disrupt surfactant metabolism.
Limitations of this cell-based model include its inability to synthesize and secrete functional surfactant and its two dimensional topography, which fails to reproduce spatial relationships of different cell types that regulate surfactant metabolism within the alveolus (39, 40). Although pulmonary surfactant is secreted exclusively by ATII cells (39), its surface tension–lowering function and its extracellular storage pool, tubular myelin, which contributes phospholipids to the surfactant monolayer at the air–liquid interface in the alveolus, are regulated not only by ATII cell surfactant production but also by reuptake of phospholipids and surfactant proteins by alveolar macrophages and ATII cells. In addition, some individuals may carry variants in other genes that modify the pulmonary phenotype (41), which would not be reproduced by this system but could be studied in a patient-derived induced pluripotent stem cell in vitro model (42). Finally, clinical disease in some individuals may be triggered by environmental exposures, including infection, which also would not be reproduced by this system.
Pathogenic variants in ABCA3 and other surfactant-associated genes have been identified in newborn infants, children, and adults with diverse pulmonary diseases (1, 5, 39). This phenotypic diversity suggests that pathogenicity of ABCA3 variants may result not only from disruption of surfactant metabolism but also from triggering other cellular pathways that disrupt ATII cell and alveolar epithelial-cell homeostasis. For example, in vitro ER stress induced by a nonsynonymous variant in SFTPC or by chemical agents (tunicamycin, thapsigargin) contributes to pathogenesis of fibrotic lung disease (43–45). These A549/ABCA3−/− cell lines could be adapted for systematic characterization of the mechanisms that disrupt ATII cell metabolism (e.g., ER stress or degradation pathways [e.g., autophagy]) for ABCA3 variants associated with fibrotic disease (15, 46). Although genetically engineered murine lineages have significantly advanced understanding of mechanisms of genetic disruption of surfactant function because of their preservation of pulmonary epithelial-cell spatial relationships (43, 47, 48), species–specific differences in pulmonary epithelial cell composition and organization and in surfactant metabolism make development of scalable, genetically versatile human-model systems important for clinical translation (42, 49). Despite its limitations, the availability of a human pulmonary epithelial cell line for the study of ABCA3 variants should serve as an efficient model for analysis of genetic mechanisms and for screening of pharmacologic correctors in a physiologically relevant cell type.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Hemangi Chaudhari, Ph.D.; Barak Cohen, Ph.D.; Susan Guttentag, M.D.; David Curiel, M.D., Ph.D.; and the Genome Engineering and iPSC Center at Washington University School of Medicine for their advice and assistance with this work.
Footnotes
Supported by U.S. National Institutes of Health grant R21/R33 HL120760 (F.S.C) and R01 HL149853 (J.A.W.), the Children’s Discovery Institute (J.A.W. and F.S.C.), and the Saigh Foundation (F.S.C.).
Author Contributions: Substantial contributions to the conception or design of the work: J.A.W., D.J.W., and F.S.C. Substantial contributions to the acquisition, analysis, or interpretation for the data for the work: J.A.W., P.Y., D.J.W., H.B.H., C.L., F.L., F.V.W., and F.S.C. Drafting or revising the work critically for important intellectual content: J.A.W., P.Y., D.J.W., and F.S.C. Final approval of the version to be published: J.A.W., P.Y., D.J.W., H.B.H., C.L., F.L., F.V.W., and F.S.C. Agreement to be accountable for all aspects of the work: J.A.W., P.Y., D.J.W., H.B.H., C.L., F.L., F.V.W., and F.S.C.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0034MA on July 21, 2020
References
- 1.Beers MF, Mulugeta S. The biology of the ABCA3 lipid transporter in lung health and disease. Cell Tissue Res. 2017;367:481–493. doi: 10.1007/s00441-016-2554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locher KP. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol. 2016;23:487–493. doi: 10.1038/nsmb.3216. [DOI] [PubMed] [Google Scholar]
- 3.Cheong N, Zhang H, Madesh M, Zhao M, Yu K, Dodia C, et al. ABCA3 is critical for lamellar body biogenesis in vivo. J Biol Chem. 2007;282:23811–23817. doi: 10.1074/jbc.M703927200. [DOI] [PubMed] [Google Scholar]
- 4.Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350:1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- 5.Wambach JA, Casey AM, Fishman MP, Wegner DJ, Wert SE, Cole FS, et al. Genotype-phenotype correlations for infants and children with ABCA3 deficiency. Am J Respir Crit Care Med. 2014;189:1538–1543. doi: 10.1164/rccm.201402-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldridge WB, Zhang Q, Faro A, Sweet SC, Eghtesady P, Hamvas A, et al. Outcomes of lung transplantation for infants and children with genetic disorders of surfactant metabolism. J Pediatr. 2017;184:157–164, e2. doi: 10.1016/j.jpeds.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wambach JA, Wegner DJ, Depass K, Heins H, Druley TE, Mitra RD, et al. Single ABCA3 mutations increase risk for neonatal respiratory distress syndrome. Pediatrics. 2012;130:e1575–e1582. doi: 10.1542/peds.2012-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doan ML, Guillerman RP, Dishop MK, Nogee LM, Langston C, Mallory GB, et al. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63:366–373. doi: 10.1136/thx.2007.083766. [DOI] [PubMed] [Google Scholar]
- 9.Williamson M, Wallis C. Ten-year follow up of hydroxychloroquine treatment for ABCA3 deficiency. Pediatr Pulmonol. 2014;49:299–301. doi: 10.1002/ppul.22811. [DOI] [PubMed] [Google Scholar]
- 10.Bush A, Cunningham S, de Blic J, Barbato A, Clement A, Epaud R, et al. chILD-EU Collaboration. European protocols for the diagnosis and initial treatment of interstitial lung disease in children. Thorax. 2015;70:1078–1084. doi: 10.1136/thoraxjnl-2015-207349. [DOI] [PubMed] [Google Scholar]
- 11.Klay D, Hoffman TW, Harmsze AM, Grutters JC, van Moorsel CHM. Systematic review of drug effects in humans and models with surfactant-processing disease. Eur Respir Rev. 2018;27:170135. doi: 10.1183/16000617.0135-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wittmann T, Schindlbeck U, Höppner S, Kinting S, Frixel S, Kröner C, et al. Tools to explore ABCA3 mutations causing interstitial lung disease. Pediatr Pulmonol. 2016;51:1284–1294. doi: 10.1002/ppul.23471. [DOI] [PubMed] [Google Scholar]
- 13.Cheong N, Madesh M, Gonzales LW, Zhao M, Yu K, Ballard PL, et al. Functional and trafficking defects in ATP binding cassette A3 mutants associated with respiratory distress syndrome. J Biol Chem. 2006;281:9791–9800. doi: 10.1074/jbc.M507515200. [DOI] [PubMed] [Google Scholar]
- 14.Wambach JA, Yang P, Wegner DJ, Heins HB, Kaliberova LN, Kaliberov SA, et al. Functional characterization of atp-binding cassette transporter a3 mutations from infants with respiratory distress syndrome. Am J Respir Cell Mol Biol. 2016;55:716–721. doi: 10.1165/rcmb.2016-0008OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weichert N, Kaltenborn E, Hector A, Woischnik M, Schams A, Holzinger A, et al. Some ABCA3 mutations elevate ER stress and initiate apoptosis of lung epithelial cells. Respir Res. 2011;12:4. doi: 10.1186/1465-9921-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumura Y, Ban N, Inagaki N. Aberrant catalytic cycle and impaired lipid transport into intracellular vesicles in ABCA3 mutants associated with nonfatal pediatric interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:L698–L707. doi: 10.1152/ajplung.90352.2008. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura Y, Ban N, Ueda K, Inagaki N. Characterization and classification of ATP-binding cassette transporter ABCA3 mutants in fatal surfactant deficiency. J Biol Chem. 2006;281:34503–34514. doi: 10.1074/jbc.M600071200. [DOI] [PubMed] [Google Scholar]
- 18.Flamein F, Riffault L, Muselet-Charlier C, Pernelle J, Feldmann D, Jonard L, et al. Molecular and cellular characteristics of ABCA3 mutations associated with diffuse parenchymal lung diseases in children. Hum Mol Genet. 2012;21:765–775. doi: 10.1093/hmg/ddr508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu JY, Yang P, Wegner DJ, Heins HB, Luke CJ, Li F, et al. Functional characterization of four ATP-binding cassette transporter A3 gene (ABCA3) variants. Hum Mutat. 2020;41:1298–1307. doi: 10.1002/humu.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med. 2016;4:662–674. doi: 10.1016/S2213-2600(16)00023-0. [DOI] [PubMed] [Google Scholar]
- 21.Kröner C, Wittmann T, Reu S, Teusch V, Klemme M, Rauch D, et al. Lung disease caused by ABCA3 mutations. Thorax. 2017;72:213–220. doi: 10.1136/thoraxjnl-2016-208649. [DOI] [PubMed] [Google Scholar]
- 22.Paolini A, Baldassarre A, Del Gaudio I, Masotti A. Structural features of the ATP-binding cassette (ABC) transporter ABCA3. Int J Mol Sci. 2015;16:19631–19644. doi: 10.3390/ijms160819631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beers MF, Zhao M, Tomer Y, Russo SJ, Zhang P, Gonzales LW, et al. Disruption of N-linked glycosylation promotes proteasomal degradation of the human ATP-binding cassette transporter ABCA3. Am J Physiol Lung Cell Mol Physiol. 2013;305:L970–L980. doi: 10.1152/ajplung.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinting S, Höppner S, Schindlbeck U, Forstner ME, Harfst J, Wittmann T, et al. Functional rescue of misfolding ABCA3 mutations by small molecular correctors. Hum Mol Genet. 2018;27:943–953. doi: 10.1093/hmg/ddy011. [DOI] [PubMed] [Google Scholar]
- 25.Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single phe508del allele. N Engl J Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han ST, Rab A, Pellicore MJ, Davis EF, McCague AF, Evans TA, et al. Residual function of cystic fibrosis mutants predicts response to small molecule CFTR modulators. JCI Insight. 2018;3:121159. doi: 10.1172/jci.insight.121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mall MA, Mayer-Hamblett N, Rowe SM. Cystic fibrosis: emergence of highly effective targeted therapeutics and potential clinical implications. Am J Respir Crit Care Med. 2019;201:1193–1208. doi: 10.1164/rccm.201910-1943SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottschalk LB, Vecchio-Pagan B, Sharma N, Han ST, Franca A, Wohler ES, et al. Creation and characterization of an airway epithelial cell line for stable expression of CFTR variants. J Cyst Fibros. 2016;15:285–294. doi: 10.1016/j.jcf.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Höppner S, Kinting S, Torrano AA, Schindlbeck U, Bräuchle C, Zarbock R, et al. Quantification of volume and lipid filling of intracellular vesicles carrying the ABCA3 transporter. Biochim Biophys Acta Mol Cell Res. 2017;1864:2330–2335. doi: 10.1016/j.bbamcr.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Maricque BB, Chaudhari HG, Cohen BA. A massively parallel reporter assay dissects the influence of chromatin structure on cis-regulatory activity. Nat Biotechnol. doi: 10.1038/nbt.4285. [online ahead of print] 19 Nov 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumura Y, Sakai H, Sasaki M, Ban N, Inagaki N. ABCA3-mediated choline-phospholipids uptake into intracellular vesicles in A549 cells. FEBS Lett. 2007;581:3139–3144. doi: 10.1016/j.febslet.2007.05.078. [DOI] [PubMed] [Google Scholar]
- 32.Pavlopoulos A. Identification of DNA sequences that flank a known region by inverse PCR. Methods Mol Biol. 2011;772:267–275. doi: 10.1007/978-1-61779-228-1_16. [DOI] [PubMed] [Google Scholar]
- 33.Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci. 1992;103:857–862. doi: 10.1242/jcs.103.3.857. [DOI] [PubMed] [Google Scholar]
- 34.Engelbrecht S, Kaltenborn E, Griese M, Kern S. The surfactant lipid transporter ABCA3 is N-terminally cleaved inside LAMP3-positive vesicles. FEBS Lett. 2010;584:4306–4312. doi: 10.1016/j.febslet.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 35.Musunuru K, Bernstein D, Cole FS, Khokha MK, Lee FS, Lin S, et al. Functional assays to screen and dissect genomic hits: doubling down on the national investment in genomic research. Circ Genom Precis Med. 2018;11:e002178. doi: 10.1161/CIRCGEN.118.002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliver KE, Han ST, Sorscher EJ, Cutting GR. Transformative therapies for rare CFTR missense alleles. Curr Opin Pharmacol. 2017;34:76–82. doi: 10.1016/j.coph.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schindlbeck U, Wittmann T, Höppner S, Kinting S, Liebisch G, Hegermann J, et al. ABCA3 missense mutations causing surfactant dysfunction disorders have distinct cellular phenotypes. Hum Mutat. 2018;39:841–850. doi: 10.1002/humu.23416. [DOI] [PubMed] [Google Scholar]
- 38.Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, et al. Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017;4:43–63. doi: 10.1016/j.gendis.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitsett JA, Wert SE, Weaver TE. Diseases of pulmonary surfactant homeostasis. Annu Rev Pathol. 2015;10:371–393. doi: 10.1146/annurev-pathol-012513-104644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Echaide M, Autilio C, Arroyo R, Perez-Gil J. Restoring pulmonary surfactant membranes and films at the respiratory surface. Biochim Biophys Acta Biomembr. 2017;1859:1725–1739. doi: 10.1016/j.bbamem.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Bullard JE, Nogee LM. Heterozygosity for ABCA3 mutations modifies the severity of lung disease associated with a surfactant protein C gene (SFTPC) mutation. Pediatr Res. 2007;62:176–179. doi: 10.1203/PDR.0b013e3180a72588. [DOI] [PubMed] [Google Scholar]
- 42.Jacob A, Morley M, Hawkins F, McCauley KB, Jean JC, Heins H, et al. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell. 2017;21:472–488, e10. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katzen J, Wagner BD, Venosa A, Kopp M, Tomer Y, Russo SJ, et al. An SFTPC BRICHOS mutant links epithelial ER stress and spontaneous lung fibrosis. JCI Insight. 2019;4:e126125. doi: 10.1172/jci.insight.126125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero F, Hong X, Shah D, Kallen CB, Rosas I, Guo Z, et al. Lipid synthesis is required to resolve endoplasmic reticulum stress and limit fibrotic responses in the lung. Am J Respir Cell Mol Biol. 2018;59:225–236. doi: 10.1165/rcmb.2017-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu SG, Villalba JA, Liang X, Xiong K, Tsoyi K, Ith B, et al. Palmitic acid-rich high-fat diet exacerbates experimental pulmonary fibrosis by modulating endoplasmic reticulum stress. Am J Respir Cell Mol Biol. 2019;61:737–746. doi: 10.1165/rcmb.2018-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero F, Summer R. Protein folding and the challenges of maintaining endoplasmic reticulum proteostasis in idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2017;14:S410–S413. doi: 10.1513/AnnalsATS.201703-207AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nureki SI, Tomer Y, Venosa A, Katzen J, Russo SJ, Jamil S, et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J Clin Invest. 2018;128:4008–4024. doi: 10.1172/JCI99287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rindler TN, Stockman CA, Filuta AL, Brown KM, Snowball JM, Zhou W, et al. Alveolar injury and regeneration following deletion of ABCA3. JCI Insight. 2017;2:e97381. doi: 10.1172/jci.insight.97381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basil MC, Katzen J, Engler AE, Guo M, Herriges MJ, Kathiriya JJ, et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.