Abstract
Respiratory depression is the main cause of morbidity and mortality associated with opioids. Obesity increases opioid-related mortality, which is mostly related to comorbid obstructive sleep apnea. Naloxone, a μ-opioid receptor blocker, is an effective antidote, but it reverses analgesia. Like humans with obesity, mice with diet-induced obesity hypoventilate during sleep and develop obstructive sleep apnea, which can be treated with intranasal leptin. We hypothesized that intranasal leptin reverses opioid-induced sleep-disordered breathing in obese mice without decreasing analgesia. To test this hypothesis, mice with diet-induced obesity were treated with morphine at 10 mg/kg subcutaneously and with leptin or placebo intranasally. Sleep and breathing were recorded by barometric plethysmography, and pain sensitivity was measured by the tail-flick test. Excitatory postsynaptic currents were recorded in vitro from hypoglossal motor neurons after the application of the μ-opioid receptor agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin and leptin. Morphine dramatically increased the frequency of apneas and greatly increased the severity of hypoventilation and obstructive sleep apnea. Leptin decreased the frequency of apneas, improved obstructive sleep apnea, and completely reversed hypoventilation, whereas morphine analgesia was enhanced. Our in vitro studies demonstrated that [D-Ala2, N-MePhe4, Gly-ol]-enkephalin reduced the frequency of excitatory postsynaptic currents in hypoglossal motoneurons and that application of leptin restored excitatory synaptic neurotransmission. Our findings suggest that intranasal leptin may prevent opioid respiratory depression during sleep in patients with obesity receiving opioids without reducing analgesia.
Keywords: morphine, sleep apnea syndromes, leptin, hypoventilation, opioid reversal agents
Clinical Relevance
Individuals with obesity are particularly susceptible to opioid-induced respiratory depression because of comorbid sleep-disordered breathing. Primary prevention of opioid-induced respiratory depression, which would not affect analgesia, is critical in patients with obesity. This translational study in obese mice identified intranasal leptin as a novel drug candidate that reverses morphine-induced apneas, hypoventilation, and upper-airway obstruction while enhancing analgesia.
Obesity increases the risk of opioid-induced respiratory depression (OIRD), especially during sleep, which is related to comorbid obstructive sleep apnea (OSA) (1, 2). Patients with obesity experience pain and need opioids more frequently than normal-weight individuals (3). Opioids remain an indispensable treatment for severe pain, and there is a critical need to develop pharmacotherapy, which would prevent OIRD without interference with analgesia.
Opioids decrease the respiratory rate and induce central apneas acting on μ-opioid receptors (MORs) in the brainstem, specifically in the preBőtzinger complex, Kőlliker-Fuse nucleus, and ventral respiratory group (4, 5). Opioids induce recurrent upper-airway obstruction indistinguishable from OSA (2, 6). The expression of MORs on hypoglossal motoneurons (HMNs) remains controversial (7, 8), but it is accepted that opioids directly or indirectly suppress HMN activity in vivo (9) and in vitro (8). Thus, OIRD is a heterogeneous condition that includes two independent respiratory phenotypes, impaired control of breathing and upper-airway obstruction.
The only treatment for life-threatening OIRD is naloxone, an opioid receptor antagonist (2). Naloxone antagonizes the analgesic effects of opioids and cannot be used to prevent OIRD in patients with OSA receiving opioids for pain control. Our goal is to develop preventive treatment of sleep-related OIRD, which would alleviate both control of breathing impairment and upper-airway obstruction without interfering with analgesia. Leptin, an adipocyte-produced hormone, acts as a powerful respiratory stimulant (10–14). Therefore, we examined effects of leptin on opioid-induced sleep disordered breathing in this study.
Leptin signaling occurs via the long isoform of leptin receptor LepRb, which is ubiquitous in the hypothalamus and in many areas of the medulla (15). We elected to use C57BL/6J mice as our model because this strain is susceptible to diet-induced obesity (DIO) and obesity-induced sleep disordered breathing with upper-airway obstruction, similar to human OSA (16). Furthermore, sleep disordered breathing in DIO C57BL/6J mice was associated with leptin resistance, like that of humans with obesity (17, 18). Humans with obesity and DIO C57BL/6J mice are resistant to the respiratory effects of leptin because of the limited permeability of the blood–brain barrier (12, 19). Our previous work has shown that intranasal (IN) administration of leptin circumvents leptin resistance at the blood–brain barrier and abolishes hypoventilation and OSA in DIO mice (12).
As in our previous papers (12–14, 16, 20), we examined the effects of leptin on control of breathing (nonobstructed breaths) and upper airway (obstructed breaths) separately based on the presence of inspiratory flow limitation (IFL). We hypothesized that in DIO C57BL/6J mice, opioids would suppress control of breathing, induce upper-airway obstruction during sleep, and inhibit HMNs and that IN leptin would prevent these abnormalities without attenuating analgesia. We chose the dose of IN leptin based on our previous study (12).
Methods
In total, 23 male C57BL/6J DIO mice were used in the study. Food and water were provided ad libitum except during sleep recording. Mice were housed in a standard laboratory environment at 24–26°C in the 12-hour light/dark cycle (9 am–9 pm lights-on). The study was approved by the Johns Hopkins and George Washington Universities animal use and care committees and complied with the American Physiological Society Guidelines. The study included three experimental protocols, which are described in detail in data supplement.
Polysomnographic recordings were performed in nine DIO mice. Each mouse underwent the following three studies with a 1-week interval between studies: 1) baseline (mice received IN vehicle, intraperitoneal saline, and a subcutaneous pump with saline); 2) morphine and vehicle (M+V) (mice received IN vehicle, intraperitoneal morphine [10 mg/kg], and a subcutaneous pump with morphine solution); and 3) morphine and leptin (M+L) (mice received IN leptin [0.8 mg/kg], intraperitoneal morphine [10 mg/kg], and a subcutaneous pump with morphine solution). The intraperitoneal bolus of morphine was injected 30 minutes after the IN administration of leptin. Morphine was infused via a subcutaneous osmotic pump to maintain steady morphine levels for the duration of the study (11 am–5 pm). The time course of our experiments is shown in Figure 1E.
Figure 1.
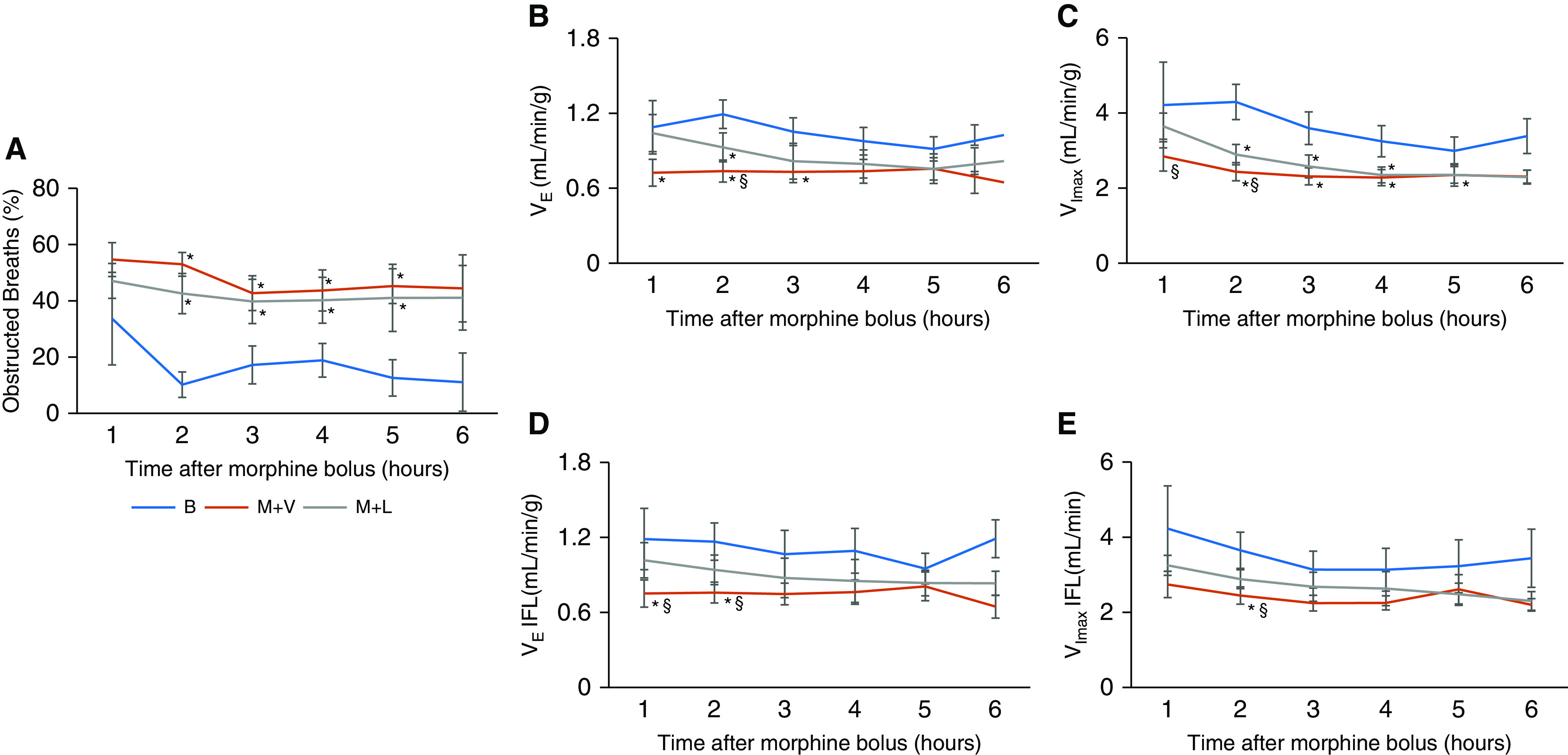
Time course of ventilation during non–rapid eye movement sleep. Time course of respiratory parameters during non–rapid eye movement sleep, including (A) frequency of inspiratory flow-limited breaths, (B) minute ventilation, (C) maximal inspiratory flow during non–flow limited breaths, severity of upper-airway obstruction measured by (D) minute ventilation, and by (E) maximal inspiratory flow during obstructed breathing. Means ± SEM. *Significant difference (P < 0.05) from baseline. §Significant difference (P < 0.05) from morpine and leptin. All comparisons were analyzed with a general linear model. B = baseline; IFL = inspiratory flow limitation; M+L = morphine and intranasal leptin; M+V = morphine and intranasal vehicle; VE = minute ventilation; VImax = maximal inspiratory flow.
Analgesia was tested with the tail-flick latency test after tail immersion in a hot water bath (50 ± 1°C) (21, 22). We performed tail-flick test in seven DIO mice and recorded tail-flick latencies at 15, 30, 60, and 120 minutes after morphine/saline administration. Analgesia experiments had the same design as sleep studies except that the subcutaneous pump was not inserted.
For electrophysiological recordings, the hindbrain was isolated from the brain tissue of seven mice (5–10 days old). Excitatory postsynaptic currents (EPSCs) were recorded in vitro from hypoglossal motor neurons as previously described (23), and changes in EPSC amplitude and frequency were examined after the application of the μ-opioid receptor agonist and leptin.
The effects of M+L treatments on ventilation, sleep architecture, and pain sensitivity were analyzed within the same mouse under three different conditions in a crossover manner (at baseline, after M+V treatment, and after M+L). The variances of the effects of the treatments on each parameter at the three time points were assessed by repeated measures analysis using a univariate general linear model. The variances of the differences within subjects in the repeated measures were evaluated by Mauchly’s sphericity test, which considered data spherical when P > 0.05. When the data did not meet the sphericity requirement for Mauchly’s test, F values, effect size (partial η2), and observed power were corrected using the Greenhouse-Geisser correction. To ensure the validity of the analysis with a small sample size, all the data are represented as means ± SEMs, including the F values and effect size (partial η2) for each parameter. Statistical analyses were conducted in SPSS version 20.0 (IBM SPSS Inc.).
For electrophysiology, comparisons between different [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) dosages (200 nM and 500 nM) and leptin (200 nm) administrations within subjects on each electrophysiological parameter were performed by a repeated measures test using one-way ANOVA and Dunnett’s post hoc analysis. Statistical analysis for electrophysiological data was performed using Graphpad Prism 5.0 (Graphpad Software). Statistical significance was considered at a level of P < 0.05.
Results
Leptin and Sleep Disordered Breathing in Morphine-treated DIO Mice
During wakefulness, morphine reduced minute ventilation (from 2.3 ± 0.6 ml/min/g to 1.6 ± 0.7 ml/min/g; F2,6 = 4.145; partial η2 = 0.34; P < 0.05). Continuous movement induced by morphine interfered with the respiratory analysis when mice were awake. IN leptin was administered 30 minutes before the morphine; animals were awake and moving after leptin instillation, and therefore the precise onset of leptin’s respiratory effects could not be established. Similarly, wakefulness in morphine-treated mice was characterized by constant activity, which masked the effects of leptin. In awake morphine-treated mice, minute ventilation was 1.6 ± 0.6 ml/min/g in the absence of leptin and 1.8 ± 0.6 ml/min/g in the presence of leptin (P > 0.05).
Morphine increased non–rapid eye movement (NREM) sleep and eliminated rapid eye movement (REM) sleep (see Table E1 in the data supplement). Leptin significantly increased sleep efficiency and consolidated NREM sleep in morphine-treated mice, increasing the duration of NREM bouts and decreasing the number of NREM bouts (Table E1).
The analysis of breathing during sleep showed that morphine induced upper-airway obstruction and suppressed both non–flow limited and flow-limited breathing throughout the entire 6-hour recording (Figure 1). IN leptin attenuated opioid-induced hypoventilation and obstructed breathing, but the effects of leptin were significant only during the first 2 hours of morphine infusion. Therefore, the main findings are reported for the first 2 hours of the study.
Morphine dramatically increased the number of apneic events during NREM sleep, from 13.9 ± 3.7 to 91.5 ± 20.0 (F2,6 = 12.365; partial η2 = 0.61; P = 0.006) (Figure 2). Apneas were classified as obstructive, in which they were characterized by the cessation of airflow in the presence of respiratory effort, or as central, in which the effort was absent. In some instances, however, effort could not be quantified, and therefore these events were labeled as unidentified (Table E1). The effect of morphine on control of breathing was evident during breathing in the absence of upper-airway obstruction (i.e., non–flow limited respiration). As expected, morphine suppressed minute ventilation (from 1.2 ± 0.1 ml/min/g to 0.7 ± 0.1 ml/min/g; F2,6 = 20.593; partial η2 = 0.72; P < 0.001) and decreased the respiratory rate (from 169.5 ± 7.2 to 100.3 ± 6.4 breaths per minute, F2,6 = 65.735; partial η2 = 0.89; P < 0.001) (Figures 3 and E2). The breath-by-breath analysis has also shown that morphine dramatically increased the frequency of upper-airway obstruction, which was defined by IFL with a plateau in early inspiration, from 11.9 ± 5.5% to 56.8 ± 4.8% of all breaths (F2,5 = 17.149; partial η2 = 0.71; P < 0.001) (Figures 3A, 3B, and 3E). In fact, three of nine mice did not have any obstructed breaths during NREM sleep at baseline, whereas all mice demonstrated markedly increased upper-airway obstruction during morphine treatment. The comparative analysis of obstructed breaths in five mice exhibiting IFL at baseline and during morphine treatment indicated that the severity of upper-airway obstruction increased. Morphine treatment significantly decreased the maximal inspiratory flow (from 3.4 ± 0.4 ml/min to 2.3 ± 0.5 ml/min; F2,2 = 7.805; partial η2 = 0.66; P < 0.05) and minute ventilation during obstructed breathing (Figures 3F and 3G and E3). The effect of morphine was present during the entire study (Figures 1 and E3).
Figure 2.
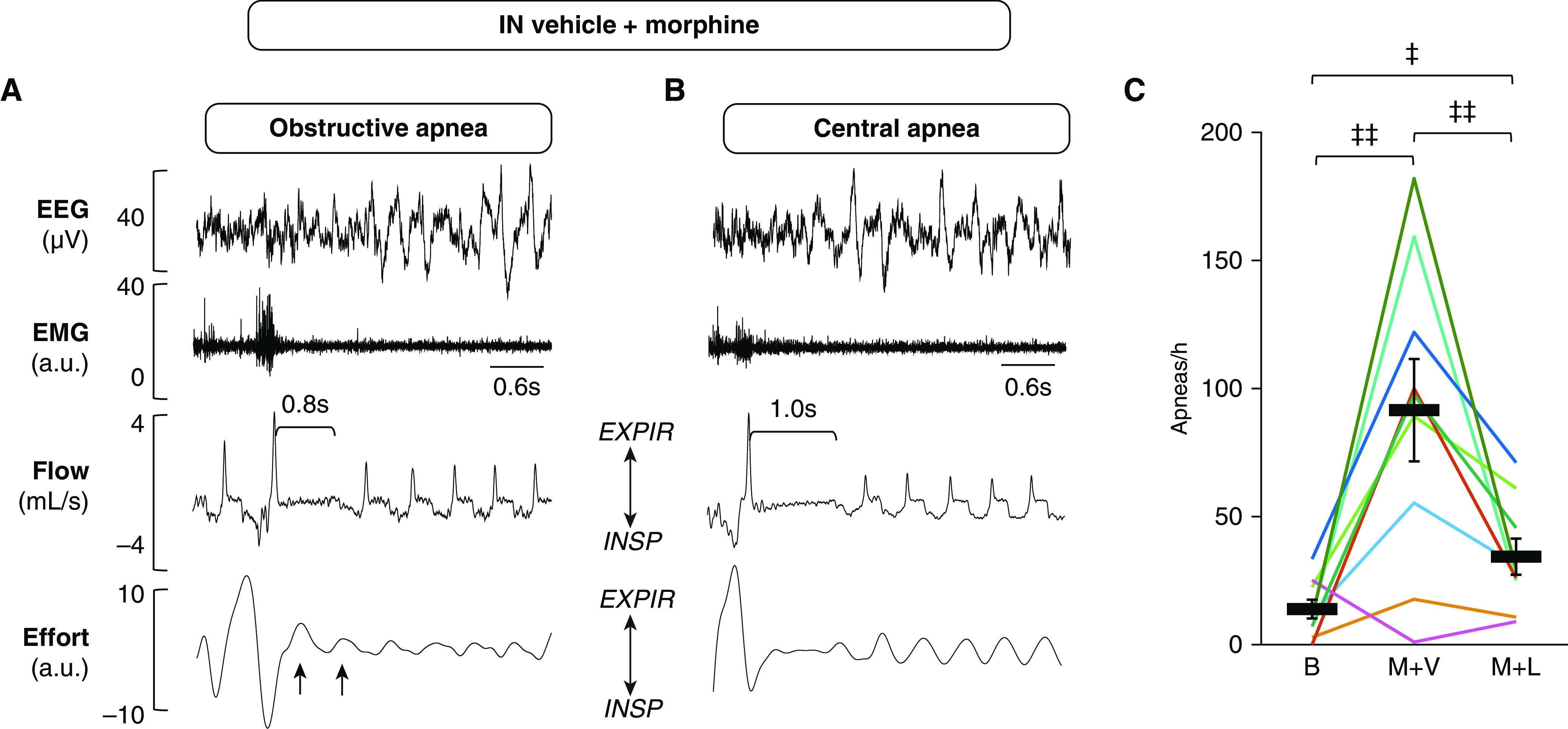
Intranasal leptin attenuated morphine-induced apneas in non–rapid eye movement sleep. (A and B) Representative recordings of apneas during non–rapid eye movement sleep after treatment with morphine at 10 mg/kg and intranasal vehicle. Electroencephalogram, nuchal electromyogram (arbitrary units), respiratory flow, and effort were measured continuously in freely moving mice. (A) An obstructive apnea; upper-airway obstruction was identified by continuous respiratory effort (black arrows) in the presence of apnea. (B) A central apnea identified by the absence of respiratory effort. (C) Leptin decreased the number of apneas per hour (n = 9). Bars show mean ± SE. ‡P < 0.05 and ‡‡P < 0.01. All comparisons were analyzed with a general linear model. a.u. = arbitrary units; EEG = electroencephalogram; EMG = electromyogram; EXPIR = expiration; IN = intranasal; INSP = inspiration.
Figure 3.
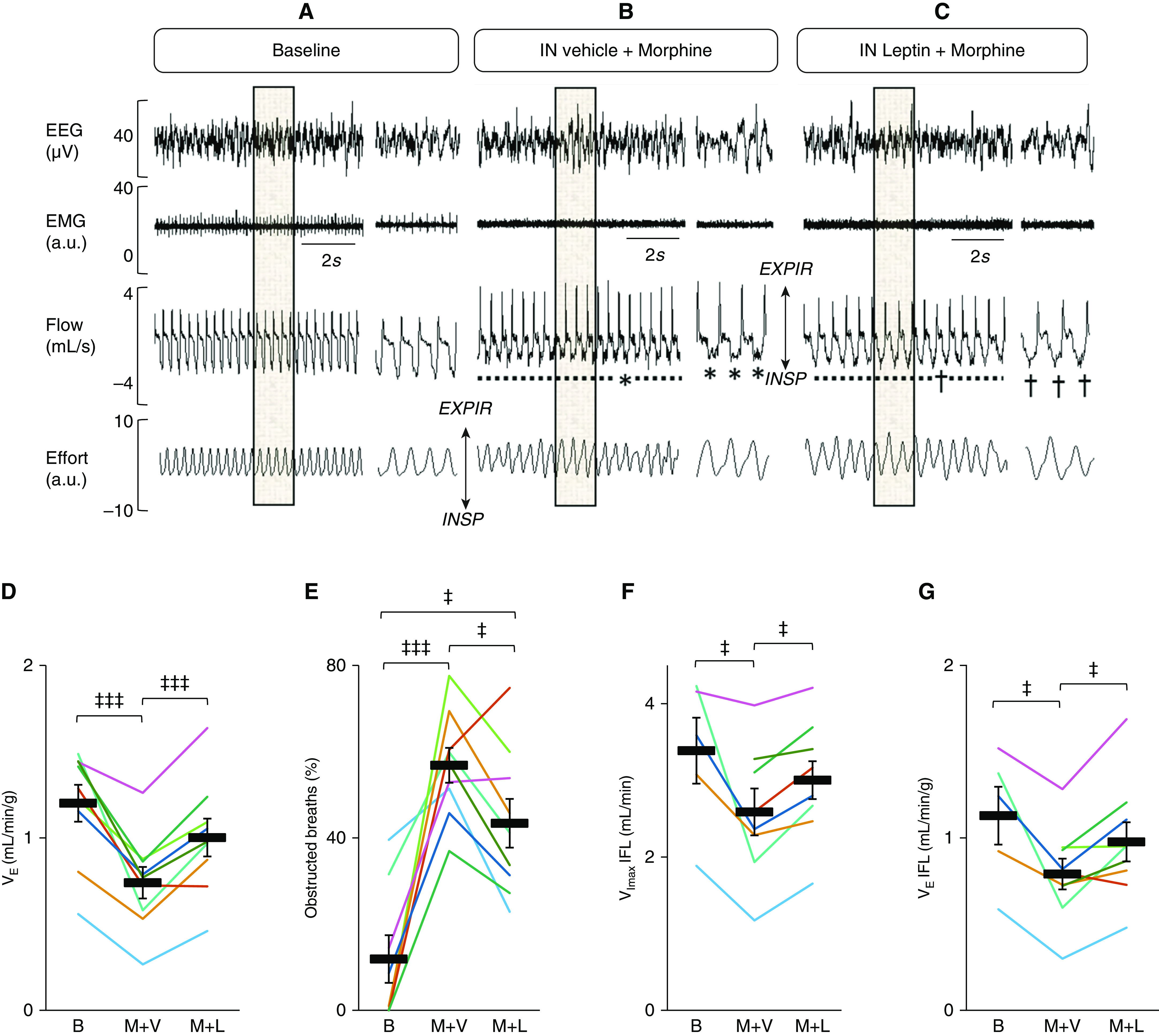
IN leptin reversed opioid-induced respiratory depression during non–rapid eye movement sleep in mice with diet-induced obesity. (A–C) Representative recordings during non–rapid eye movement sleep at baseline (B) and during morphine and vehicle treatment and morphine+leptin treatment. Electroencephalogram, nuchal electromyogram (arbitrary units), respiratory flow, and effort were measured continuously in freely moving mice from 11 am to 5 pm. The shaded areas are decompressed on the right side of each panel. (A) Baseline. (B) Severe IFL characterized by a plateau during early inspiration (*) after treatment with morphine and IN vehicle. (C) Residual IFL (†) remaining after IN leptin in a mouse treated with morphine. (D) Leptin abolished opioid-induced respiratory depression (evidenced by an increase in minute ventilation) (n = 9), (E) decreased the frequency of obstructed (IFL) breaths (n = 8), and decreased the severity of upper-airway obstruction (evidenced by reversals of morphine-induced reductions in maximal inspiratory flow) (n = 5) (F) and minute ventilation (G) during obstructed (IFL) breathing (n = 5). Each line corresponds with individual data for one mouse. Bars show mean and SEM. ‡P < 0.05 and ‡‡‡P < 0.001. All comparisons were analyzed with a general linear model.
The analysis of the first 2 hours of the recording showed that leptin decreased the number of apneas by 60% (Figure 2). Leptin stimulated ventilatory control, restoring minute ventilation to baseline (Figure 3D). In morphine-treated mice, IN leptin increased both the respiratory rate (from 100.3 ± 6.4 breaths per minute to 113.6 ± 4.4 breaths per minute; P < 0.001), and the tidal volume (from 0.32 ± 0.04 ml to 0.37 ± 0.03 ml; F2,6 = 3.659; partial η2 = 0.31; P < 0.05) (Figure E2). Leptin reduced the frequency of obstructed breaths by 21% (from 56.8 ± 4.8% to 44.6 ± 6.2%; F2,5 = 17.149; partial η2 = 0.71; P < 0.05) (Figure 3E). Leptin also increased the maximal inspiratory flow and minute ventilation during obstructed breathing from 2.3 ± 0.5 ml/min to 2.7 ± 0.4 ml/min (F2,2 = 7.805; partial η2 = 0.66; P < 0.05) and from 0.7 ± 0.2 ml/min/g to 1.0 ± 0.2 ml/min/g (F2,2 = 9.920; partial η2 = 0.71; P < 0.05), respectively, indicating that leptin alleviated morphine-induced upper-airway obstruction (Figures 3B, 3C, 3F, and 3G). The analyses of the full 6-hour recordings showed that the stimulating effects of leptin on the apnea rate, obstructed breathing, and minute ventilation were no longer significant, suggesting that the protective effects of leptin are relatively short (Figures 1 and E3).
Leptin and analgesia
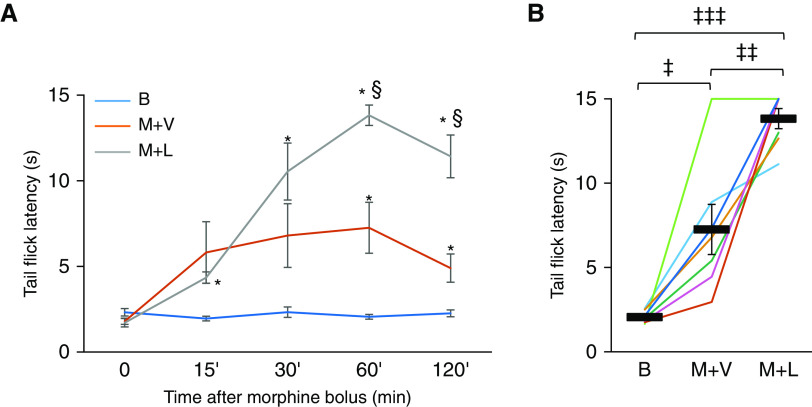
To examine whether leptin improves breathing without compromising analgesia, we performed the tail-flick test, a validated test of pain perception (21, 22), in a crossover, randomized manner. As expected, we observed an increase in tail-flick latency after morphine administration (Figure 4). IN leptin increased tail-flick latency measured 60 minutes and 120 minutes after morphine bolus (Figure 4A), and this observation was consistent in six of seven tested mice (Figure 4B).
Figure 4.
Leptin augmented the effect of morphine analgesia in the tail-flick test. Tail-flick latencies were recorded after immersion of the distal third of the tail in a 50 ± 1°C water bath (n = 7). A maximum of 15 seconds immersion time was used to avoid tissue damage. Intranasal administration of leptin or vehicle was followed by intraperitoneal morphine 30 minutes later. Tail-flick latencies were measured at baseline, before leptin/vehicle treatment (0), and at 15, 30, 60, and 120 minutes after morphine. (A) Time course of tail-flick latency for baseline (B), morphine and vehicle, and morphine and leptin, showing means ± SEM. *Significant difference (P < 0.05) from baseline. §Significant difference (P < 0.05) from morphine and vehicle. (B) Morphine increased tail flick latency at the peak of morphine analgesia, 60 minutes after morphine administration. Values are shown for individual mice (lines) and means ± SEM. ‡P < 0.05, ‡‡P < 0.01, and ‡‡‡P < 0.001. All comparisons were analyzed with a general linear model.
Leptin and Excitatory Neurotransmission to Hypoglossal Motoneurons
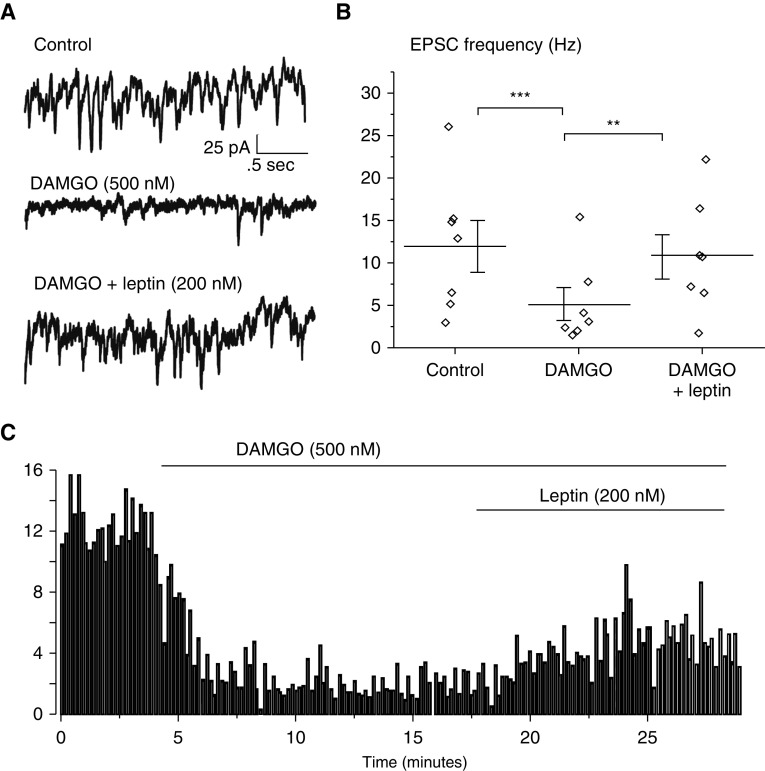
EPSCs were recorded in vitro in medullary slices of neonatal mice. HMNs were studied using patch-clamp techniques, and changes in EPSC amplitude and frequency were examined following the application of the μ-opioid receptor (MOR) agonist DAMGO and leptin. DAMGO significantly reduced EPSC frequency in HMNs, whereas the amplitude was not affected. Focal application of leptin restored EPSC frequency (Figure 5).
Figure 5.
A μ-opioid receptor agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) reduced excitatory postsynaptic current (EPSC) frequency in hypoglossal neurons (HMNs) and was reversed by the application of leptin. EPSCs were recorded in vitro from HMNs (n = 7). (A) Representative recording at baseline, after infusion of DAMGO, and after coinfusion of DAMGO and leptin. DAMGO (focally applied to HMNs via a puffer pipette) inhibited spontaneous EPSCs, whereas leptin application restored the frequency of EPSCs to near pre-DAMGO values. (B) EPSC frequency was significantly reduced after DAMGO infusion, and leptin infusion restored EPSC frequency to pre-DAMGO levels. (C) Time course of the experiment. Control EPSC frequency was quantified before and after the infusion of DAMGO, followed by a coinfusion of DAMGO and leptin. **P < 0.01 and ***P < 0.005. Comparisons were analyzed with a one-way ANOVA test.
Discussion
The main novel finding of our study was that IN leptin prevented both morphine-induced depression of control of breathing and upper-airway obstruction during sleep (i.e., OSA), whereas the analgesic effects of morphine were enhanced. In addition, we provided mechanistic insight into our findings, demonstrating that leptin reversed opioid-induced suppression of excitatory neurotransmission to HMNs. A dual effect of IN leptin on control of breathing and upper-airway patency in OIRD was demonstrated using our novel, extensively validated high-fidelity system of respiratory recording, which allows breath-by-breath analysis of inspiratory flow and effort (13, 14, 16, 24).
Intranasal Leptin Prevented Morphine-induced Suppression of Respiratory Control
Opioid-induced decrease in respiratory rate, irregular breathing pattern, and apneas have been extensively described in rodents (25–27). These effects of opioids are similar to those seen in humans and are attributable to inhibitory effects of MOR signaling in the medullary rhythm generators, respiratory premotoneurons, and impaired chemoreception throughout the respiratory network (4, 5, 28–31).
IN leptin prevented opioid-induced apneas and hypoventilation during sleep. A previous study from our laboratory showed that after IN leptin administration, carbon dioxide production increased by 5–6%, whereas minute ventilation increased by 40%. This out of proportion increase suggests that the metabolic effects of leptin have a minor role and that leptin is a powerful respiratory stimulant in DIO mice (12). Does leptin act on chemosensitivity or on the respiratory rhythm generator? The effect of leptin on control of breathing is consistent with our previous reports showing that leptin increases both hypercapnic and hypoxic chemosensitivity (10, 11, 32). Investigators localized effects of leptin on hypercapnic sensitivity to the nucleus of the solitary tract (33) or ventral respiratory group (34). Our group and others showed that leptin also acts in the carotid bodies (12, 32, 35, 36). Leptin may restore chemosensitivity in morphine-treated mice by acting on the carotid body directly or by activating “second carotid body neurons” in the nucleus of the solitary tract. In contrast, there are very little data to suggest that leptin activates the respiratory rhythm generator, although leptin signaling at other sites with an abundant presence of MORs, such as the preBőtzinger complex and Kőlliker-Fuse nucleus, may contribute to this effect (4, 5, 15). The effects of leptin on respirations were relatively short-acting, suggesting that although IN leptin may be sufficient for short-acting opioids such as morphine, it should be redosed for longer-acting opioids such as transdermal fentanyl (31).
IN Leptin Prevented Morphine-induced Upper-Airway Obstruction (OSA) During Sleep
Morphine exacerbated upper-airway obstruction in a mouse model of sleep disordered breathing. At baseline, DIO mice show OSA during REM sleep but show only modest inspiratory flow limitation during NREM sleep (16). Morphine treatment resulted in severe upper-airway obstruction during NREM sleep. Upper-airway obstruction can be attributed to dysfunction of the pharyngeal dilator, the genioglossus muscle of the tongue innervated by the hypoglossal nerve (20, 37, 38). Our data are consistent with previous reports that showed the inhibition of hypoglossal motoneurons and suppression of tongue muscle activity by opioids (8, 9). This suggests that the morphine-induced impairment of pharyngeal patency is caused by the decreased excitatory neurotransmission to HMNs demonstrated after MOR agonist administration.
IN leptin dramatically attenuated morphine-induced OSA. Our in vitro studies in medullary slices of neonatal mice showed that leptin reversed the opioid-induced suppression of excitatory synaptic neurotransmission to HMNs. We have previously shown that HMNs do not express LepRb but are synaptically connected to LepRb-expressing neurons (12). Our study demonstrates that IN leptin protects the upper airway by acting on HMNs presynaptically. The effects of leptin on HMNs may protect not only against opioid-induced OSA but also against hypoventilation due to prolonged IFL without frank apneas or hypopneas (39).
Leptin, Sleep, and Pain
We observed an increase in NREM sleep and the absence of REM sleep after morphine administration. These findings were consistent with previous reports of morphine-induced sedation in rodents (40) and with opioid effects on sleep in humans (41). Leptin-treated mice had a greater sleep efficiency and increased the duration of NREM bouts. We have previously reported that intracerebroventricular leptin increased NREM sleep in mice (13).
Morphine increased tail-flick latency, and this increase was further prolonged by IN leptin. The tail-flick test relies on a spinal-mediated withdrawal reflex to measure nociception. The mechanism for the leptin potentiation of morphine analgesia is unclear. Intranasal leptin induces leptin receptor signaling in the brain without changing plasma leptin concentrations. Therefore, leptin probably acts in the central nervous system (12). Previous studies have shown that leptin signaling might have a role in modulating pain sensitivity and response to opioids (21, 22, 42). Wang and colleagues suggested that leptin modulates cholinergic function to restore analgesia induced by a microinjection of neostigmine to the pontine reticular formation. However, in the same study, morphine microinjection had no analgesic effect (21). A possible mechanism would be the increased production of β-endorphin because of the activation of proopiomelanocortin neurons by leptin (31, 43). Of note, leptin receptors are present in the dorsal horn of the spinal cord, the first pain-processing synapse (43). In addition, leptin may interfere with the metabolism of morphine (44). Future studies should investigate whether leptin modifies the analgesic properties of morphine by modulating sensory-discriminative and/or affective-motivational pain responses and whether IN leptin affects morphine pharmacokinetics (31).
Limitations
Our study had several limitations. First, mice in general are relatively resistant to opioids (45). The animals rapidly develop tolerance to morphine, which may influence results during repeated morphine administration. To address this possibility, we randomized treatment order in a crossover fashion with 1-week intervals between tests, as described in Methods. Second, effort detection was not always optimal, and therefore many apneic events could not be classified as obstructive or central. Third, the study design (IN leptin was administered before morphine) and variability in mouse activity after leptin and morphine administrations did not allow us to determine the time of the onset of leptin’s effect or to reliably estimate the effect of leptin during wakefulness. Fourth, although we have shown that leptin protects against opioid-mediated upper-airway obstructions by restoring excitatory input to HMNs, the localization of leptin’s action on respiratory drive remains unknown. Finally, the efficacy of IN leptin has been studied only in obese male C57BL/6J mice during the light phase at a single dose, and the dose–response was not evaluated. This mouse strain shows respiratory instability and frequent apneas during wakefulness (46–48), with relative resistance to the respiratory effects of opioids (49). However, our previous study (16) and current report demonstrate that obese C57BL/6J mice had a stable breathing pattern with infrequent apneas during sleep at baseline, which was significantly impaired by opioids. Sex (11), strain (49, 50), and obesity status can modify respiratory responses to leptin and opioids.
Conclusions and Implications
Our report provides the first evidence that leptin markedly attenuates morphine-induced sleep disordered breathing without reducing analgesia. Our translational data suggest that IN leptin may prevent opioid-induced apneas, hypoventilation, and upper-airway obstruction during sleep in patients with obesity and OSA.
Supplementary Material
Footnotes
Supported by the U.S. National Institutes of Health (grants R01HL 128970, R01 133100, and R01HL 138932), the American Heart Association (grant AHA 19CDA34660245), the American Thoracic Society (unrestricted award), São Paulo Research Foundation (FAPESP) (grant 2018/08758–3), and National Council for Scientific and Technological Development (CNPq) (grant 142073/2017–2).
Author Contributions: C.F., H.P., D.M., and V.Y.P. conceptualized and designed the experiments. C.F., H.P., X.W., and J.D. performed the experiments. C.F., H.P., X.W., J.D., S.R.S., and T.F.-C. analyzed data. L.J.K. did the statistical analysis. C.F., L.U.S., D.M., and V.Y.P. wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0117OC on June 30, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nagappa M, Weingarten TN, Montandon G, Sprung J, Chung F. Opioids, respiratory depression, and sleep-disordered breathing. Best Pract Res Clin Anaesthesiol. 2017;31:469–485. doi: 10.1016/j.bpa.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Montandon G, Slutsky AS. Solving the opioid crisis: respiratory depression by opioids as critical end point. Chest. 2019;156:653–658. doi: 10.1016/j.chest.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Stokes A, Berry KM, Collins JM, Hsiao C-W, Waggoner JR, Johnston SS, et al. The contribution of obesity to prescription opioid use in the United States. Pain. 2019;160:2255–2262. doi: 10.1097/j.pain.0000000000001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31:1292–1301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levitt ES, Abdala AP, Paton JFR, Bissonnette JM, Williams JT. μ opioid receptor activation hyperpolarizes respiratory-controlling Kölliker-Fuse neurons and suppresses post-inspiratory drive. J Physiol. 2015;593:4453–4469. doi: 10.1113/JP270822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Ryswyk E, Antic NA. Opioids and sleep-disordered breathing. Chest. 2016;150:934–944. doi: 10.1016/j.chest.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Kivell BM, Day DJ, McDonald FJ, Miller JH. Developmental expression of μ and δ opioid receptors in the rat brainstem: evidence for a postnatal switch in μ isoform expression. Brain Res Dev Brain Res. 2004;148:185–196. doi: 10.1016/j.devbrainres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Lorier AR, Funk GD, Greer JJ. Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PLoS One. 2010;5:e8766. doi: 10.1371/journal.pone.0008766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajiha M, DuBord M-A, Liu H, Horner RL. Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo. J Physiol. 2009;587:2677–2692. doi: 10.1113/jphysiol.2009.171678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, et al. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med. 1999;159:1477–1484. doi: 10.1164/ajrccm.159.5.9809025. [DOI] [PubMed] [Google Scholar]
- 11.Polotsky VY, Wilson JA, Smaldone MC, Haines AS, Hurn PD, Tankersley CG, et al. Female gender exacerbates respiratory depression in leptin-deficient obesity. Am J Respir Crit Care Med. 2001;164:1470–1475. doi: 10.1164/ajrccm.164.8.2101100. [DOI] [PubMed] [Google Scholar]
- 12.Berger S, Pho H, Fleury-Curado T, Bevans-Fonti S, Younas H, Shin M-K, et al. Intranasal leptin relieves sleep-disordered breathing in mice with diet-induced obesity. Am J Respir Crit Care Med. 2019;199:773–783. doi: 10.1164/rccm.201805-0879OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Q, Pho H, Kirkness J, Ladenheim EE, Bi S, Moran TH, et al. Localizing effects of leptin on upper airway and respiratory control during sleep. Sleep (Basel) 2016;39:1097–1106. doi: 10.5665/sleep.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pho H, Hernandez AB, Arias RS, Leitner EB, Van Kooten S, Kirkness JP, et al. The effect of leptin replacement on sleep-disordered breathing in the leptin-deficient ob/ob mouse. J Appl Physiol (1985) 2016;120:78–86. doi: 10.1152/japplphysiol.00494.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleury Curado T, Pho H, Berger S, Caballero-Eraso C, Shin M-K, Sennes LU, et al. Sleep-disordered breathing in C57BL/6J mice with diet-induced obesity. Sleep (Basel) 2018;41:zsy089. doi: 10.1093/sleep/zsy089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phipps PR, Starritt E, Caterson I, Grunstein RR. Association of serum leptin with hypoventilation in human obesity. Thorax. 2002;57:75–76. doi: 10.1136/thorax.57.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ip MSM, Lam KSL, Ho C, Tsang KWT, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 19.Banks WA. Role of the blood-brain barrier in the evolution of feeding and cognition. Ann N Y Acad Sci. 2012;1264:13–19. doi: 10.1111/j.1749-6632.2012.06568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleury Curado TA, Pho H, Dergacheva O, Berger S, Lee R, Freire C, et al. Silencing of hypoglossal motoneurons leads to sleep disordered breathing in lean mice. Front Neurol. 2018;9:962. doi: 10.3389/fneur.2018.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Baghdoyan HA, Lydic R. Leptin replacement restores supraspinal cholinergic antinociception in leptin-deficient obese mice. J Pain. 2009;10:836–843. doi: 10.1016/j.jpain.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glovak Z, Mihalko S, Baghdoyan HA, Lydic R. Leptin status alters buprenorphine-induced antinociception in obese mice with dysfunctional leptin receptors. Neurosci Lett. 2017;660:29–33. doi: 10.1016/j.neulet.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Bryan C, LaMantia A-S, Mendelowitz D. Altered neurobiological function of brainstem hypoglossal neurons in DiGeorge/22q11.2 deletion syndrome. Neuroscience. 2017;359:1–7. doi: 10.1016/j.neuroscience.2017.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez AB, Kirkness JP, Smith PL, Schneider H, Polotsky M, Richardson RA, et al. Novel whole body plethysmography system for the continuous characterization of sleep and breathing in a mouse. J Appl Physiol (1985) 2012;112:671–680. doi: 10.1152/japplphysiol.00818.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren J, Ding X, Funk GD, Greer JJ. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology. 2009;110:1364–1370. doi: 10.1097/ALN.0b013e31819faa2a. [DOI] [PubMed] [Google Scholar]
- 26.Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, et al. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D. 5-Hydroxytryptamine 1A/7 and 4α receptors differentially prevent opioid-induced inhibition of brain stem cardiorespiratory function. Hypertension. 2007;50:368–376. doi: 10.1161/HYPERTENSIONAHA.107.091033. [DOI] [PubMed] [Google Scholar]
- 28.Weil JV, McCullough RE, Kline JS, Sodal IE. Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N Engl J Med. 1975;292:1103–1106. doi: 10.1056/NEJM197505222922106. [DOI] [PubMed] [Google Scholar]
- 29.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuel IO, Clarke RSJ, Dundee JW. Some circulatory and respiratory effects of morphine in patients without pre-existing cardiac disease. Br J Anaesth. 1977;49:927–933. doi: 10.1093/bja/49.9.927. [DOI] [PubMed] [Google Scholar]
- 31.Yaksh T, Wallace M. Opioids, analgesia, and pain management. In: Brunton LL, Hilal-Dandan R, Knollmann BC, editors. Goodman gilmans pharmacol basis therapeutics, 13e. New York, NY: McGraw-Hill Education; 2017. [Google Scholar]
- 32.Caballero-Eraso C, Shin M-K, Pho H, Kim LJ, Pichard LE, Wu Z-J, et al. Leptin acts in the carotid bodies to increase minute ventilation during wakefulness and sleep and augment the hypoxic ventilatory response. J Physiol. 2019;597:151–172. doi: 10.1113/JP276900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inyushkina EM, Merkulova NA, Inyushkin AN. Mechanisms of the respiratory activity of leptin at the level of the solitary tract nucleus. Neurosci Behav Physiol. 2010;40:707–713. doi: 10.1007/s11055-010-9316-2. [DOI] [PubMed] [Google Scholar]
- 34.Bassi M, Furuya WI, Zoccal DB, Menani JV, Colombari DSA, Mulkey DK, et al. Facilitation of breathing by leptin effects in the central nervous system. J Physiol. 2016;594:1617–1625. doi: 10.1113/JP270308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan F, Wang H, Feng J, Wei Z, Yu H, Zhang X, et al. Leptin signaling in the carotid body regulates a hypoxic ventilatory response through altering TASK channel expression. Front Physiol. 2018;9:249. doi: 10.3389/fphys.2018.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin M-K, Kim LJ, Caballero-Eraso C, Polotsky VY. Experimental approach to examine leptin signaling in the carotid bodies and its effects on control of breathing. J Vis Exp. 2019:10.3791/60298. doi: 10.3791/60298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 38.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger KI, Ayappa I, Chatr-Amontri B, Marfatia A, Sorkin IB, Rapoport DM, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest. 2001;120:1231–1238. doi: 10.1378/chest.120.4.1231. [DOI] [PubMed] [Google Scholar]
- 40.Toyama S, Shimoyama N, Tagaito Y, Nagase H, Saitoh T, Yanagisawa M, et al. Nonpeptide orexin-2 receptor agonist attenuates morphine-induced sedative effects in rats. Anesthesiology. 2018;128:992–1003. doi: 10.1097/ALN.0000000000002161. [DOI] [PubMed] [Google Scholar]
- 41.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3:33–36. [PubMed] [Google Scholar]
- 42.Younger J, Kapphahn K, Brennan K, Sullivan SD, Stefanick ML. Association of leptin with body pain in women. J Womens Health (Larchmt) 2016;25:752–760. doi: 10.1089/jwh.2015.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodgers HM, Liban S, Wilson LM. Attenuated pain response of obese mice (B6.Cg-lep(ob)) is affected by aging and leptin but not sex. Physiol Behav. 2014;123:80–85. doi: 10.1016/j.physbeh.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Dalesio NM, Hendrix CW, McMichael DH, Thompson CB, Lee CKK, Pho H, et al. Effects of obesity and leptin deficiency on morphine pharmacokinetics in a mouse model. Anesth Analg. 2016;123:1611–1617. doi: 10.1213/ANE.0000000000001578. [DOI] [PubMed] [Google Scholar]
- 45.Moskowitz AS, Terman GW, Carter KR, Morgan MJ, Liebeskind JC. Analgesic, locomotor and lethal effects of morphine in the mouse: strain comparisons. Brain Res. 1985;361:46–51. doi: 10.1016/0006-8993(85)91273-9. [DOI] [PubMed] [Google Scholar]
- 46.Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol (1985) 2002;92:1133–1140. doi: 10.1152/japplphysiol.00785.2001. [DOI] [PubMed] [Google Scholar]
- 47.Stettner GM, Zanella S, Huppke P, Gärtner J, Hilaire G, Dutschmann M. Spontaneous central apneas occur in the C57BL/6J mouse strain. Respir Physiol Neurobiol. 2008;160:21–27. doi: 10.1016/j.resp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, Strohl KP. Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir Physiol Neurobiol. 2008;162:117–125. doi: 10.1016/j.resp.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fechtner L, El Ali M, Sattar A, Moore M, Strohl KP. Fentanyl effects on breath generation in C57BL/6J and A/J mouse strains. Respir Physiol Neurobiol. 2015;215:20–29. doi: 10.1016/j.resp.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gillombardo CB, Darrah R, Dick TE, Moore M, Kong N, Decker MJ, et al. C57BL/6J mouse apolipoprotein A2 gene is deterministic for apnea. Respir Physiol Neurobiol. 2017;235:88–94. doi: 10.1016/j.resp.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.