Abstract
Aims
While pulmonary embolism (PE) appears to be a major issue in COVID-19, data remain sparse. We aimed to describe the risk factors and baseline characteristics of patients with PE in a cohort of COVID-19 patients.
Methods and results
In a retrospective multicentre observational study, we included consecutive patients hospitalized for COVID-19. Patients without computed tomography pulmonary angiography (CTPA)-proven PE diagnosis and those who were directly admitted to an intensive care unit (ICU) were excluded. Among 1240 patients (58.1% men, mean age 64 ± 17 years), 103 (8.3%) patients had PE confirmed by CTPA. The ICU transfer and mechanical ventilation were significantly higher in the PE group (for both P < 0.001). In an univariable analysis, traditional venous thrombo-embolic risk factors were not associated with PE (P > 0.05), while patients under therapeutic dose anticoagulation before hospitalization or prophylactic dose anticoagulation introduced during hospitalization had lower PE occurrence [odds ratio (OR) 0.40, 95% confidence interval (CI) 0.14–0.91, P = 0.04; and OR 0.11, 95% CI 0.06–0.18, P < 0.001, respectively]. In a multivariable analysis, the following variables, also statistically significant in univariable analysis, were associated with PE: male gender (OR 1.03, 95% CI 1.003–1.069, P = 0.04), anticoagulation with a prophylactic dose (OR 0.83, 95% CI 0.79–0.85, P < 0.001) or a therapeutic dose (OR 0.87, 95% CI 0.82–0.92, P < 0.001), C-reactive protein (OR 1.03, 95% CI 1.01–1.04, P = 0.001), and time from symptom onset to hospitalization (OR 1.02, 95% CI 1.006–1.038, P = 0.002).
Conclusion
PE risk factors in the COVID-19 context do not include traditional thrombo-embolic risk factors but rather independent clinical and biological findings at admission, including a major contribution to inflammation.
Keywords: COVID-19, Pulmonary embolism, Computed tomography angiography, Intensive care unit, Risk factors
Graphical Abstract
Graphical Abstract.
Introduction
Since December 2019, the severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection has caused a global pandemic and a public health crisis of unprecedented magnitude.1 The clinical picture of coronavirus disease (COVID-19) ranges from completely asymptomatic to rapidly devastating courses with acute respiratory distress syndrome associated with high fatality rates.2,3
In addition to pulmonary symptoms, cardiac injuries appear to be a prominent feature of COVID-19; they occur in 20–30% of hospitalized patients and contribute to 30–40% of deaths.4–6 SARS-CoV-2 infection is also accompanied by pulmonary vascular complications, such as pulmonary embolism (PE). Reports of PE associated with COVID-19, which indicate higher PE prevalence in COVID-19 than usually encountered in non-infected critically ill patients, have emerged in the literature.7–10 COVID-19 complicated by acute PE may mark a turning point in patient prognosis by the additional hypoxaemia or haemodynamic collapse, which lead to intensive care unit (ICU) admission and mechanical ventilation.7,11 Several pieces of evidence suggest that COVID-19 is associated with abnormal haemostasis,12 severe inflammation, endothelial dysfunction, and disseminated intravascular coagulation, and that COVID-19 is predisposing to venous thrombo-embolic events.13,14
Current guidelines recommend considering anticoagulation at prophylactic dose for all patients admitted with COVID-1915,16 and following ESC guidelines if PE is confirmed.17 However, the risk of PE in COVID-19 patients under prophylactic antithrombotic treatment remains high.9 In this regard, several empirical anticoagulation strategies have been proposed by clinicians and national societies, namely intermediate dose (double prophylaxis) of parenteral anticoagulation for routine treatment.8,13
In the COVID-19 context, (i) PE might be associated with several previously unknown risk factors; and (ii) PE is a major issue, and the failure to identify and correctly manage this complication with optimal prophylactic anticoagulation could aggravate patient prognosis.
This study aimed to describe the baseline characteristics of COVID-19 patients with PE and the associated risk factors in a multicentre cohort.
Methods
Study settings and population
From 26 February to 20 April 2020, all consecutive adult patients admitted to hospital with a diagnosis of SARS-CoV-2 infection were included in a retrospective multicentre (24 centres, Supplementary material online, File 1) observational study, called the Critical Covid-19 France (CCF) study and initiated by the French Society of Cardiology (NCT04344327).
Following World Health Organization criteria, SARS-CoV-2 infection was determined by positive results from real-time reverse transcription–PCR (RT–PCR) of nasal and pharyngeal swabs or lower respiratory tract aspirates (confirmed case) or was determined by typical imaging characteristics on chest computed tomography (CT) when laboratory testing was inconclusive (probable case). Patients without computed tomography pulmonary angiography (CTPA) to diagnose PE, those who were directly admitted to the ICU on their arrival, and those who were still hospitalized and had not experienced PE at study completion were excluded. In accordance with guidelines, CTPA was performed to rule out PE if supplementary oxygen was needed in COVID-19 patients with limited disease extension,18 or when unenhanced CT findings could not explain the severity of respiratory failure.15
The CCF study was declared and authorized by the French data protection committee (CNIL, authorization 2207326v0) and conducted in accordance with the 1964 Declaration of Helsinki.
Data collection
All data were collected by local investigators in an electronic case report form via the REDCap software (Research Electronic Data Capture, Vanderbilt University) hosted by a secured server from the French Institute of Health and Medical Research at the Paris Cardiovascular Research Centre. Patient baseline information included demographic characteristics, co-existing medical conditions, and medications. Clinical parameters and biological findings were recorded at admission. On the chest CT scan, the degree of pulmonary lesions with ground-glass opacities and areas of consolidation was categorized as low to moderate (<50% involvement) or severe (>50% involvement). Data on pharmacological therapies, mode of respiratory, complications, and final vital status were also gathered during the hospitalization.
The anticoagulation regimen prescribed before PE occurrence was categorized into three groups: (i) prophylactic dose (daily low molecular weight heparin or twice daily subcutaneous unfractionated heparin); (ii) intermediate dose (double the preventive dose); and (iii) therapeutic dose before hospitalization. All medical interventions, including anticoagulation and pharmacological treatments for COVID-19, were performed at the discretion of the medical team.
CT protocol and analysis
The CTPA protocol was performed using a multidetector scanner after intravenous injection of 50–75 mL of high concentration iodinated contrast agent at a flow rate of 3–4 mL/s, which was triggered on the main pulmonary artery.18 The CT scan patterns of COVID-19 and the presence of PE were analysed locally by a senior radiologist of the centre.
Outcomes
The primary outcome was PE confirmed by CTPA.7,11 Death, admission to the ICU, invasive mechanical ventilation, and non-invasive ventilation were also considered to assess the association between PE and these outcomes in patients with COVID-19. The PE severity was assessed by systolic blood pressure, simplified PE severity index (sPESI), troponin level, and presence of right ventricular dysfunction on transthoracic echocardiogram, following the ESC guidelines.17
Statistical analysis
Continuous data were reported as the mean ± standard deviation (SD) for normally distributed data or the median and interquartile range (IQR) for non-normally distributed data. Categorical data were reported as counts and percentages. Comparisons employed the χ2 or Fisher’s exact test for categorical variables and the Student’s t-test or Mann–Whitney–Wilcoxon test, as appropriate, for continuous variables.
Complete-cases multiple logistic regression was employed to identify PE predictors in the context of COVID-19, with final selection based on the most favourable goodness-of-fit measures (Akaike information criterion). Missing data are shown in Table 1 and were handled using multiple random forest imputation utilizing chained equations (mice R package, 20 sets of imputations) before multivariable analysis. Non-parametric bootstrap with 2000 replicates was used to calculate 95% confidence intervals (CIs) for PE occurrence. A two-tailed P-value <0.05 was considered statistically significant. All data were analysed using R software, version 3.6.3 (R Project for Statistical Computing).
Table 1.
Baseline characteristics of the study population (n = 1240)
| Variables | Overall (n = 1240) | No. of patients with data available |
|---|---|---|
| Demographics | ||
| Age, years | 64 ± 17 | 1240 |
| Male, n (%) | 721 (58.1) | 1240 |
| BMI, kg/m2 | 28.1 ± 6.3 | 1128 |
| Time from illness onset to hospitalization*, days | 7.2 ± 4.7 | 1206 |
| Cardiovascular risk factors, n (%) | ||
| Smoking | 181 (14.9) | 1215 |
| Hypertension | 559 (45.4) | 1230 |
| Diabetes | 268 (21.7) | 1235 |
| Dyslipidaemia | 316 (25.6) | 1234 |
| Familial premature CVD | 19 (1.6) | 1180 |
| Comorbidities, n (%) | ||
| COPD | 77 (6.2) | 1240 |
| Chronic kidney disease | 126 (10.3) | 1225 |
| Stroke | 94 (7.7) | 1226 |
| Peripheral arterial disease | 60 (4.9) | 1226 |
| Atrial fibrillation | 117 (9.5) | 1231 |
| Chronic heart failure | 117 (9.5) | 1230 |
| Coronary artery disease | 133 (10.7) | 1238 |
| Malignancy | 167 (13.5) | 1240 |
| Venous thrombo-embolic disease | 98 (7.9) | 1240 |
| Immunodeficiency | 63 (5.1) | 1240 |
| Treatment before hospitalization, n (%) | ||
| Therapeutic dose anticoagulation | 136 (11.0) | 1240 |
| VKA | 47 (3.8) | |
| NOAC | 78 (6.3) | |
| Heparin | 11 (0.9) | |
| ACEi | 218 (17.6) | 1240 |
| ARB | 177 (14.3) | 1240 |
| Clinical characteristics | ||
| NYHA functional class, n (%) | 1097 | |
| I–II | 534 (48.7) | |
| III–IV | 563 (51.3) | |
| Heart rate, b.p.m. | 86 ± 18 | 1125 |
| Systolic pressure, mmHg | 131 ± 21 | 1223 |
| Diastolic pressure, mmHg | 75 ± 13 | 1223 |
| Respiratory frequency, breaths/min | 23 ± 7 | 852 |
| Chest pain, n (%) | 132 (10.6) | 1240 |
| O2 saturation, % | 95 ± 3 | 1229 |
| Temperature, °C | 37.6 ± 1.0 | 1223 |
| FiO2, % | 28 ± 11 | 1196 |
| Glasgow score <15, n (%) | 60 (4.9) | 1227 |
| HF signs, n (%) | 67 (5.5) | 1221 |
| SIC score >4, n (%) | 446 (67.0) | 666 |
| qSOFA score = 1, n (%) | 526 (61.7) | 852 |
| Electrocardiogram, n (%) | 998 | |
| Sinus rhythm | 912 (91.4) | |
| Atrial fibrillation | 86 (8.6) | |
| Laboratory, mean ± SD | ||
| PaO2, mmHg | 82 ± 29 | 935 |
| pH | 7.45 ± 0.06 | 927 |
| PaO2/FiO2 ratio <150, n (%) | 53 (5.8) | 906 |
| Lactates, mmol/L | 1.4 ± 1.0 | 788 |
| NT-proBNP, pg/mL | 2517 ± 6608 | 361 |
| BNP, pg/mL | 199 ± 621 | 368 |
| Leucocytes†, 109/L | 7.3 ± 5.6 | 1224 |
| Lymphocytes, 109/L | 1.3 ± 3.6 | 1208 |
| Haemoglobin, g/dL | 13.2 ± 1.9 | 1228 |
| Platelets‡, 109/L | 225 ± 97 | 1214 |
| C-reactive protein§, mg/L | 91 ± 77 | 1201 |
| Prothrombin rate, % | 85 ± 18 | 958 |
| APTT, ratio | 1.1 ± 0.3 | 884 |
| Creatinine, μmol/L | 89 ± 67 | 1224 |
| GFR, mL/min/m2 | 86 ± 28 | 1222 |
| Aspartate aminotransferase, UI/L | 55 ± 70 | 1130 |
| Alanine aminotransferase, UI/L | 51 ± 94 | 1132 |
| Albumin, g/L | 32 ± 7 | 674 |
| D-dimer¶, μg/L | 1,642 ± 4,211 | 508 |
| Fibrinogen, g/L | 6.1 ± 1.7 | 536 |
| Ferritin, μg/L | 1235 ± 2483 | 301 |
| Lactate dehydrogenase, UI/L | 368 ± 213 | 295 |
| Troponin elevation, n (%) | 202 (27.2) | 743 |
| SARS-CoV-2-positive RT–PCR, n (%) | 1124 (90.6) | 1240 |
| Abnormalities on chest CT, n (%) | ||
| Parenchymal involvement | 1240 | |
| Low or moderate (<50%) | 1028 (82.9) | |
| Severe (>50%) | 212 (17.1) | |
| Treatment introduced during hospitalization, before PE occurrence, n (%) | ||
| Anticoagulation | 837 (71.4) | 1172 |
| Prophylactic dose | 738 (63.0) | |
| Intermediate dose | 99 (8.4) | |
| Antibiotics | 939 (75.7) | 1240 |
| Antiviral | 154 (12.4) | 1240 |
| Chloroquine | 205 (16.5) | 1240 |
| Corticosteroids | 73 (5.9) | 1240 |
| Outcomes, n (%) | ||
| PE | 103 (8.3) | 1240 |
| Death | 151 (12.2) | 1240 |
| Stroke | 6 (0.5) | 1240 |
| Deep vein thrombosis | 18 (1.5) | 1240 |
| ICU hospitalization | 185 (14.9) | 1240 |
| Invasive mechanical ventilation | 108 (8.7) | 1240 |
| Non-invasive ventilation | 31 (2.5) | 1240 |
| High flow nasal canula | 56 (4.5) | 1240 |
Values are n (%) or mean ± SD.
PE, pulmonary embolism; BMI, body mass index; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; VKA, vitamin K antagonist; NOAC, non-vitamin K antagonist oral anticoagulant; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptors blocker; NYHA, New York Heart Association; FiO2, fraction of inspired oxygen; HF, heart failure; SIC score, sepsis-induced coagulopathy score; qSOFA score, quick sequential organ failure assessment score; PaO2, partial pressure of oxygen; SD, standard deviation; NT-proBNP, N-terminal probrain natriuretic peptide; BNP, brain natriuretic peptide; APTT, activated partial thromboplastin time; GFR, glomerular filtration rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT–PCR, reverse transcription–PCR; CTPA, computed tomography pulmonary angiogram; ICU, intensive care unit.
Increment of 5 units;
increment of 50 units;
increment of 2 units;
increment of 1 SD (77 units);
increment of 500 units.
Results
Overall population
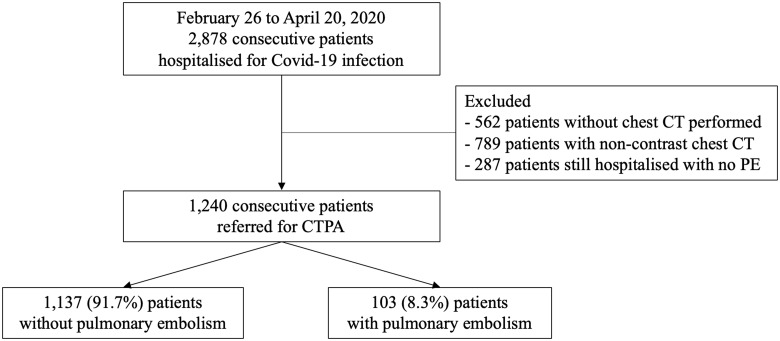
Among 2878 consecutive patients who were hospitalized for SARS-CoV-2 infection (Supplementary material online, File 2), 1240 patients with CTPA, across 24 French hospitals (58.1% men, mean age 64 ± 17 years) between 26 February 2020 and 20 April 2020, were included (Figure 1). Of these 1240 patients (Table 1), 103 (8.3%, bootstraped 95% CI 6.69–9.84%) patients were diagnosed with PE (Figure 2). Among those, there were 52 (50.5%) low risk, 12 (11.6%) intermediate to low risk, 7 (6.8%) intermediate to high risk, and 32 (31.1%) high risk of PE severity. Of the 103 patients with PE, the PE diagnosis was performed in the first 48 h after admission for 80 (77.7%) patients and after 48 h for 23 (22.3%) patients. The median delay between admission and ICU transfer was 2 days (IQR 1–4), and the median delay between admission and death without transfer to the ICU was 6.4 days (IQR 3–10). The median duration of hospitalization was 6.8 days (IQR 5–12).
Figure 1.
Flowchart for the Critical Covid-19 France Study. CT, computed tomography; CTPA, computed tomography pulmonary angiography.
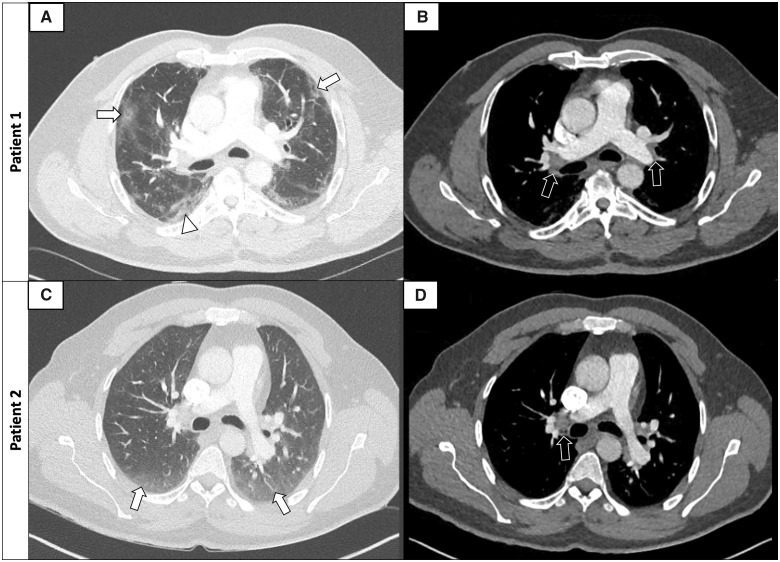
Figure 2.
Examples of axial CTPA images of PE in COVID-19 patients (lung windows: A–C; mediastinum windows: B–D). Patient 1: 42-year-old male with peripheral ground-glass opacities of 25–50% (A, arrows) with small areas of consolidation (arrowhead) and bilateral proximal PE (B, arrows). Patient 2: 57-year-old male with peripheral ground-glass opacities <25% (C, arrows) and right proximal PE (D, arrow). CTPA, computed tomography pulmonary angiography; PE, pulmonary embolism.
Risk factors for PE in patients with COVID-19
In the univariable analysis (Table 2), male gender [odds ratio (OR) 1.83, 95% CI 1.19–2.89, P = 0.009], history of stroke (OR 0.24, 95% CI 0.04–0.77, P = 0.037), history of atrial fibrillation (OR 0.10, 95% CI 0.01–0.44, P = 0.004), and the presence of chest pain (OR 2.21, 95% CI 1.28–3.69, P = 0.004) or dyspnoea (OR 1.73, 95% CI 1.11–2.75, P = 0.022) were significantly associated with PE occurrence. Patients with PE had longer delay from symptoms onset to hospitalization than patients without PE (OR 1.38, 95% CI 1.13–1.68, P = 0.009). The presence of systemic inflammation was associated with PE, as evidenced by a higher leucocyte level, C-reactive protein, and platelets in the case of PE (OR 1.09, 95% CI 1.02–1.16, P = 0.028; OR 1.33, 95% CI 1.11–1.59, P = 0.013, and OR 1.11, 95% CI 1.01–1.22, P = 0.022, respectively). A higher D-dimer level was associated with a higher risk of PE (OR 1.06, 95% CI 1.02–1.10, P < 0.001). Severe pulmonary lesions in CT (OR 1.71, 95% CI 1.05–2.71, P = 0.036) and high FiO2 (OR 1.02, 95% CI 1.00–1.03, P = 0.044) were associated with PE occurrence.
Table 2.
Univariable analysis for the comparison of PE occurrence (n = 1240)
| Variables | Diagnosis of PE |
OR (95% CI) | P-value | |
|---|---|---|---|---|
| No (n = 1137) | Yes (n = 103) | |||
| Demographics | ||||
| Age, years | 64 ± 17 | 63 ± 16 | 0.99 (0.98–1.01) | 0.387 |
| Male, n (%) | 648 (57.0) | 73 (70.9) | 1.83 (1.19–2.89) | 0.009 |
| BMI, kg/m2 | 28.2 ± 6.3 | 27.3 ± 5.6 | 0.98 (0.94–1.01) | 0.135 |
| Time from illness onset to hospitalization*, days | 7.0 ± 4.5 | 8.6 ± 5.7 | 1.38 (1.13–1.68) | 0.009 |
| Cardiovascular risk factors, n (%) | ||||
| Smoking | 172 (15.4) | 9 (8.9) | 0.54 (0.25–1.04) | 0.106 |
| Hypertension | 515 (45.7) | 44 (42.7) | 0.89 (0.59–1.33) | 0.633 |
| Diabetes | 249 (22.0) | 19 (18.4) | 0.81 (0.47–1.33) | 0.477 |
| Dyslipidaemia | 294 (26.0) | 22 (21.4) | 0.78 (0.47–1.25) | 0.361 |
| Familial premature CVD | 17 (1.6) | 2 (2.0) | 1.39 (0.20–5.00) | 0.667 |
| Comorbidities, n (%) | ||||
| COPD | 69 (6.1) | 8 (7.8) | 1.32 (0.57–2.69) | 0.638 |
| Chronic kidney disease | 117 (10.4) | 9 (9.0) | 0.87 (0.39–1.68) | 0.787 |
| Stroke | 92 (8.2) | 2 (1.9) | 0.24 (0.04–0.77) | 0.037 |
| Peripheral arterial disease | 54 (4.8) | 6 (5.8) | 1.25 (0.47–2.78) | 0.827 |
| Atrial fibrillation | 116 (10.3) | 1 (1.0) | 0.10 (0.01–0.44) | 0.004 |
| Chronic heart failure | 105 (9.3) | 12 (11.8) | 1.31 (0.66–2.39) | 0.526 |
| Coronary artery disease | 124 (10.9) | 9 (8.7) | 0.74 (0.34–1.41) | 0.460 |
| Malignancy | 159 (14.0) | 8 (7.8) | 0.53 (0.23–1.04) | 0.105 |
| Venous thrombo-embolic disease | 88 (7.7) | 10 (9.7) | 1.30 (0.61–2.48) | 0.604 |
| Immunodeficiency | 58 (5.1) | 5 (4.9) | 0.98 (0.33–2.27) | 1.000 |
| Treatment before hospitalization, n (%) | ||||
| Therapeutic dose anticoagulation | 131 (11.5) | 5 (4.9) | 0.40 (0.14–0.91) | 0.044 |
| VKA | 46 (4.0) | 1 (1.0) | 0.27 (0.01–1.22) | 0.173 |
| NOAC | 77 (6.8) | 1 (1.0) | 0.15 (0.01–0.70) | 0.035 |
| Heparin | 8 (0.7) | 3 (2.9) | 4.36 (0.89–15.7) | 0.056 |
| ACEi | 203 (17.9) | 15 (14.6) | 0.79 (0.43–1.36) | 0.481 |
| ARB | 166 (14.6) | 11 (10.7) | 0.71 (0.35–1.30) | 0.346 |
| Clinical characteristics | ||||
| NYHA functional class, n (%) | 0.022 | |||
| I–II | 502 (49.8) | 32 (36.4) | Ref. | |
| III–IV | 507 (50.2) | 56 (63.6) | 1.73 (1.11–2.75) | |
| Heart rate, b.p.m. | 86 ± 18 | 90 ± 20 | 1.01 (1.00–1.02) | 0.100 |
| Systolic pressure, mmHg | 131 ± 21 | 131 ± 22.0 | 1.00 (0.99–1.01) | 0.842 |
| Diastolic pressure, mmHg | 74 ± 12.9 | 77 ± 13.7 | 1.01 (1.00–1.03) | 0.112 |
| Respiratory frequency, breaths/min | 23 ± 7 | 24 ± 6 | 1.00 (0.97–1.04) | 0.953 |
| Chest pain, n (%) | 112 (9.9) | 20 (19.4) | 2.21 (1.28–3.69) | 0.004 |
| O2 saturation, % | 95 ± 4 | 95 ± 3 | 0.98 (0.92–1.03) | 0.385 |
| Temperature, °C | 37.6 ± 1.0 | 37.6 ± 0.9 | 1.02 (0.83–1.25) | 0.840 |
| FiO2, % | 28 ± 11 | 31 ± 15 | 1.02 (1.00–1.03) | 0.044 |
| Glasgow score <15, n (%) | 54 (4.8) | 6 (5.9) | 1.28 (0.48–2.84) | 0.627 |
| HF signs, n (%) | 60 (5.4) | 7 (6.9) | 1.32 (0.53–2.80) | 0.682 |
| SIC score >4, n (%) | 401 (66.3) | 45 (73.8) | 1.42 (0.80–2.66) | 0.297 |
| qSOFA score = 1, n (%) | 477 (61.1) | 49 (69.0) | 1.41 (0.85–2.43) | 0.234 |
| Electrocardiogram, n (%) | 0.254 | |||
| Sinus rhythm | 831 (91.0) | 81 (95.3) | Ref. | |
| Atrial fibrillation | 82 (9.0) | 4 (4.7) | 0.52 (0.15–1.29) | |
| Laboratory, mean ± SD | ||||
| PaO2, mmHg | 82 ± 30 | 77 ± 20 | 0.99 (0.98–1.00) | 0.045 |
| pH | 7.5 ± 0.1 | 7.5 ± 0.1 | 5.82 (0.08–438) | 0.364 |
| PaO2/FiO2 ratio <150, n (%) | 44 (5.3) | 9 (11.2) | 2.28 (1.00–4.68) | 0.043 |
| Lactates, mmol/L | 1.4 ± 1.0 | 1.3 ± 0.6 | 0.88 (0.64–1.21) | 0.252 |
| NT-proBNP, pg/mL | 2336 ± 6324 | 4101 ± 8656 | 1.00 (1.00–1.00) | 0.235 |
| BNP, pg/mL | 177 ± 505 | 365 ± 1,182 | 1.00 (1.00–1.00) | 0.316 |
| Leucocytes‡, 109/L | 7.1 ± 4.8 | 9.6 ± 10.9 | 1.09 (1.02–1.16) | 0.028 |
| Lymphocytes, 109/L | 1.3 ± 3.4 | 1.3 ± 1.2 | 0.99 (0.93–1.07) | 0.688 |
| Haemoglobin, g/dL | 13.2 ± 1.9 | 13.2 ± 2.2 | 1.00 (0.90–1.11) | 0.998 |
| Platelets†, 109/L | 223 ± 97 | 246 ± 94 | 1.11 (1.01–1.22) | 0.022 |
| C-reactive protein§, mg/L | 89 ± 75 | 114 ± 95 | 1.33 (1.11–1.59) | 0.013 |
| Prothrombin rate, % | 85 ± 18 | 83 ± 16 | 0.99 (0.98–1.00) | 0.121 |
| APTT ratio | 1.2 ± 0.3 | 1.1 ± 0.2 | 0.68 (0.25–1.86) | 0.379 |
| Creatinine, μmol/L | 88 ± 67 | 93 ± 71 | 1.00 (1.00–1.00) | 0.526 |
| GFR, mL/min/m2 | 86 ± 28 | 85 ± 28 | 1.00 (0.99–1.01) | 0.693 |
| Aspartate aminotransferase, UI/L | 55 ± 70 | 54 ± 69 | 1.00 (1.00–1.00) | 0.859 |
| Alanine aminotransferase, UI/L | 51 ± 94 | 53 ± 91 | 1.00 (1.00–1.00) | 0.818 |
| Albumin, g/L | 32 ± 6 | 31 ± 8 | 0.97 (0.94–1.01) | 0.251 |
| D-dimer¶ μg/L | 1371 ± 4,120 | 3519 ± 4385 | 1.06 (1.02–1.10) | <0.001 |
| Fibrinogen, g/L | 6.1 ± 1.6 | 6.3 ± 2.0 | 1.09 (0.93–1.29) | 0.383 |
| Ferritin, μg/L | 1229 ± 2511 | 1301 ± 2212 | 1.00 (1.00–1.00) | 0.877 |
| Lactate dehydrogenase, UI/L | 364 ± 192 | 397 ± 335 | 1.00 (1.00–1.00) | 0.570 |
| Troponin elevation, n (%) | 176 (26.3) | 26 (35.1) | 1.52 (0.90–2.51) | 0.138 |
| SARS-CoV-2 positive RT–PCR, n (%) | 1036 (91.1) | 88 (85.4) | 0.57 (0.32–1.06) | 0.086 |
| Abnormalities on chest CT, n (%) | ||||
| Parenchymal involvement | 0.036 | |||
| Low or moderate (<50%) | 922 (83.3) | 76 (74.5) | Ref. | |
| Severe (>50%) | 185 (16.7) | 26 (25.5) | 1.71 (1.05–2.71) | |
| Treatment introduced during hospitalization, before PE occurrence, n (%) | ||||
| Anticoagulation | ||||
| Prophylactic dose | 720 (67.0) | 18 (18.4) | 0.11 (0.06–0.18) | <0.001 |
| Intermediate dose | 94 (8.8) | 5 (5.1) | 0.58 (0.20–1.32) | 0.292 |
| Antibiotics | 853 (75.0) | 86 (83.5) | 1.67 (1.00–2.96) | 0.072 |
| Antiviral | 140 (12.3) | 14 (13.6) | 1.13 (0.60–1.98) | 0.825 |
| Hydroxychloroquine | 182 (16.0) | 23 (22.3) | 1.51 (0.91–2.44) | 0.130 |
| Corticosteroids | 63 (5.5) | 10 (9.7) | 1.85 (0.87–3.59) | 0.133 |
| Outcomes, n (%) | ||||
| Death | 142 (12.5) | 9 (8.7) | 0.68 (0.31–1.31) | 0.338 |
| Stroke | 4 (0.4) | 2 (1.9) | 5.79 (0.71–31.9) | 0.082 |
| Deep vein thrombosis | 6 (0.5) | 12 (11.7) | 24.4 (9.13–72.8) | <0.001 |
| ICU hospitalization | 153 (13.5) | 32 (31.1) | 2.90 (1.83–4.53) | <0.001 |
| Invasive mechanical ventilation | 83 (7.3) | 25 (24.3) | 4.08 (2.46–6.68) | <0.001 |
| Non-invasive ventilation | 29 (2.6) | 2 (1.9) | 0.81 (0.12–2.75) | 1.000 |
| High flow nasal canula | 48 (4.2) | 8 (7.8) | 1.94 (0.82–4.02) | 0.129 |
Values are n (%) or mean ± SD.
PE, pulmonary embolism; OR, odds ratio; 95% CI, 95% confidence interval; BMI, body mass index; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; VKA, vitamin K antagonist; NOAC, non-vitamin K antagonist oral anticoagulant; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptors blocker; NYHA, New York Heart Association; FiO2, fraction of inspired oxygen; HF, heart failure; SIC score, sepsis-induced coagulopathy score; qSOFA score, quick sequential organ failure assessment score; PaO2, partial pressure of oxygen; NT-proBNP, N-terminal probrain natriuretic peptide; BNP, brain natriuretic peptide; APTT, activated partial thromboplastin time; GFR, glomerular filtration rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CT, computed tomography; RT–PCR, reverse transcription–PCR; ICU, intensive care unit; SD, standard deviation.
Increment of 5 units;
increment of 50 units;
increment of 2 units;
increment of 1 SD (77 units);
increment of 500 units.
Patients who received anticoagulation with a therapeutic dose before admission (OR 0.40, 95% CI 0.14–0.91, P = 0.044) or anticoagulation with a prophylactic dose introduced during hospitalization (OR 0.11, 95% CI 0.06–0.18, P < 0.001) had a lower PE incidence rate.
Higher age, history of malignancy, history of venous thrombo-embolic disease, smoking, and obesity were not associated with PE occurrence in our study (for all P > 0.05). Cardiovascular comorbidities, such as diabetes, hypertension, chronic heart failure, or coronary artery disease, were not associated with a higher risk of PE (for all P > 0.05).
In the multivariable analysis (Table 3), the following variables remained significantly associated with PE: male gender (OR 1.03, 95% CI 1.003–1.069, P = 0.04), anticoagulation with a prophylactic dose (OR 0.83, 95% CI 0.79–0.85, P < 0.001) or a therapeutic dose (OR 0.87, 95% CI 0.82–0.92, P < 0.001), C-reactive protein (OR 1.03, 95% CI 1.01–1.04, P = 0.001), and time from symptom onset to hospitalization (OR 1.02, 95% CI 1.006–1.038, P = 0.002).
Table 3.
Multivariable analysis for prediction of PE occurrence
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
| Male | 1.03 | 1.003–1.069 | 0.04 |
| Age | 1.00 | 1.00–1.00 | 0.52 |
| Smoking | 0.96 | 0.91–1.00 | 0.08 |
| Malignancy | 0.98 | 0.93–1.03 | 0.46 |
| Venous thrombo-embolic disease | 1.00 | 1.00–1.01 | 0.52 |
| Time from illness onset to hospitalization*, days | 1.02 | 1.006–1.038 | 0.002 |
| C-reactive protein† | 1.03 | 1.01–1.04 | 0.001 |
| Anticoagulation prophylactic dose introduced during the hospitalization | 0.83 | 0.79–0.85 | <0.001 |
| Anticoagulation therapeutic dose before the hospitalization | 0.87 | 0.82–0.92 | <0.001 |
Increment of 5 units;
increment of 1 SD (77 units).
CI, confidence interval; PE, pulmonary embolism.
Prognostic consequences of PE in patients with COVID-19
ICU transfer [32 (31.1%) vs. 153 (13.5%) patients; P < 0.001] and mechanical ventilation [25 (24.3%) vs. 83 (7.3%) patients; P < 0.001] were more frequent for PE patients than for patients without PE. The PE occurrence was not associated with mortality (P = 0.338) (Table 2; Supplementary material online, File 3).
Discussion
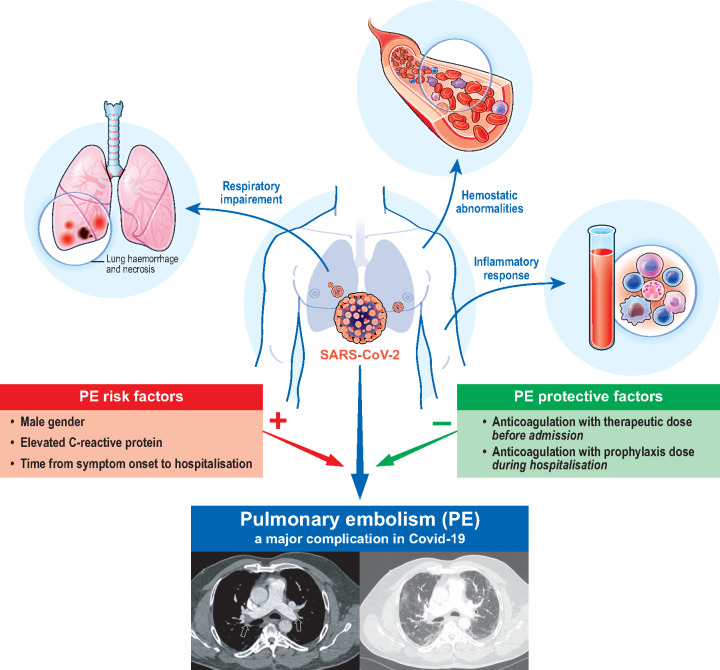
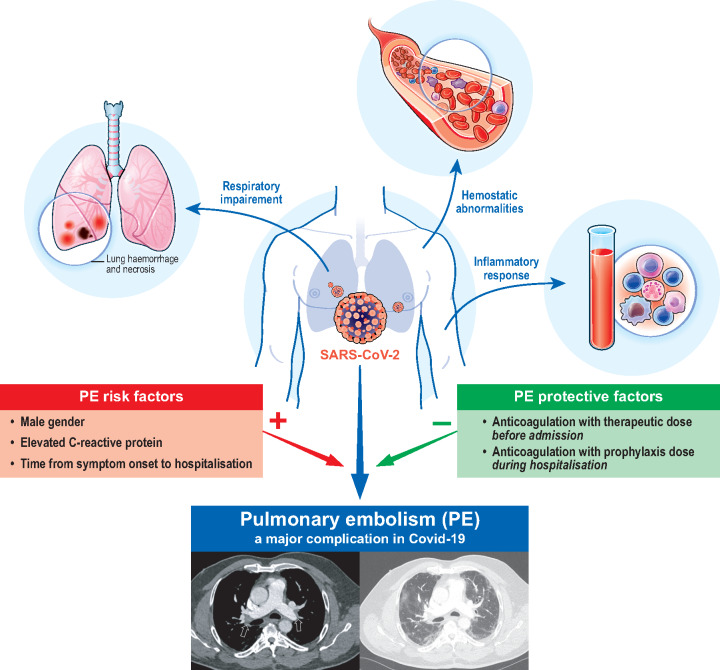
Using a multicentre study of patients who are sequentially hospitalized for COVID-19 with CTPA across 24 French centres, we described the baseline characteristics of COVID-19 patients with PE, their evolution during hospitalization, the associated risk factors of PE, and the role of the groups of anticoagulant regimens (Take home figure).
Take home figure.
PE risk and protective factors in COVID-19 patients.
The mortality rate observed in this series (12.2%) was comparable with that of other published series despite the heterogeneity among healthcare systems and populations studied.2,3 In this study, the PE incidence was 8.3%, which is lower than observed in previously reported case series. This difference could be explained by a large majority of non-severe patients compared with only patients in ICU or only severe COVID-19 patients in the first publications.9,11 In smaller case series, the PE occurrence in COVID-19 patients clearly marked a turning point in patient prognosis, with a higher probability of ICU admission, an increased use of invasive ventilation, and a longer hospital stay;7,11 findings comparable with our study. The PE occurrence was not associated with mortality, which raises the question of whether PE diagnosis was motivating enough for ICU transfer despite similar clinical severity.
Despite reports of the main characteristics from small case series of COVID-19 patients with PE, epidemiological and prognostic data from larger series are limited.7–9,11 The patient characteristics reported in this study confirmed that male gender and longer delay from onset of symptoms to hospitalization were associated with an increased risk of PE. As previously described,11 the traditional risk factors of venous thrombo-embolic disease were not associated with the occurrence of PE in our study. However, a lack of statistical power cannot be excluded, and further investigations will be necessary to confirm these findings. In accordance with the literature and as presented on Figure 2, there were no specific radiological signs of PE in COVID-19 patients.9,11,18 While severe forms of COVID-19 are more frequent in patients with pre-existing cardiovascular disease and cardiovascular-associated comorbidities compared with the general population,5,19 our study suggests that pre-existing cardiovascular diseases are not associated with a higher PE risk in COVID-19 patients.
PE pathophysiology in the COVID-19 context may be different from that described in other circumstances.13 In agreement with the literature, our study shows that COVID-19 is associated with abnormal haemostasis,12 increased inflammation reflected by high C-reactive protein levels, and increased leucocyte counts.13,14,20
Autopsy series showing diffuse alveolar damage and inflammation revealed pulmonary intravascular coagulopathy (PIC) as the pathogenic mechanism of PE.21 Indeed, CoV-2 infection-induced down-regulation of angiotensin-converting enzyme 2 (ACE2) receptors negatively regulates lymphocyte function, contributing to PIC.22 In addition, pulmonary endothelial cell dysfunction triggered by interleukin (IL)-1, IL-6, and tumour necrosis factor is thought to play an important role in the thrombo-inflammatory processes.22 These data reinforce the idea that inflammation and coagulopathy are two essential elements of the PE pathophysiology in the COVID-19 context. They both play a role as factors predisposing to and consequences of PE, causing a prothrombogenic vicious circle.23 These data should make us change our paradigm regarding the risk stratification of venous thrombo-embolic disease, such as PE, in the COVID-19 context.24
This study suggests that anticoagulation therapy with a therapeutic dose administered before admission or anticoagulation therapy with a prophylactic dose introduced during hospitalization could reduce the PE occurrence in accordance with the guidelines.15,16 This result, together with the protective role of a short delay between onset of symptoms and hospitalization, reflects the importance of preventive treatment administered at the earliest opportunity. During the 2009 swine flu pandemic, higher doses of empirical systemic heparin anticoagulation in patients with acute respiratory distress syndrome were reported as significantly reducing PE incidence without increased haemorrhagic complications.25
Limitations
This study has some limitations. First, the data collection was retrospective. However, the relatively short time between a patient’s hospitalization and the gathering of his data (15 days, IQR 10–20) allowed investigators to easily recover a large amount of data of interest. The mean burden of missing data on the variables of the multivariable model was only 2.2%. Secondly, we chose to restrict the study population to patients who underwent CTPA, as CTPA is the gold standard test to confirm or eliminate the diagnosis of PE. Thirdly, the variables analysed to assess the risk factors were collected at admission. Consequently, this approach does not take into account the evolution of these items or other events that occurred during hospitalization and are likely to increase the risk of PE. Fourthly, the lack of statistical power prevents us from (i) concluding on the potential interest of the intermediate dose anticoagulation protocol; (ii) concluding on the association of PE with complications, such as death; and (iii) performing subgroup analysis. Nevertheless, our study was not designed to appropriately conclude on the efficacy of anticoagulation in patients with COVID-19 or to assess bleeding complications due to anticoagulation. Although a recent study suggested that there is no difference in terms of bleeding events,26 further studies are required to support this observation.
In conclusion, through a multicentre case series of patients hospitalized for COVID-19 with CTPA-proven PE, we identified independent risk factors associated with the occurrence of PE in COVID-19 patients, including clinical and biological variables at admission. These risk factors do not include traditional thrombo-embolic risk factors, which reflect the major part of inflammation and coagulopathy in PE linked with COVID-19.
Conflict of interest: A.C. acknowledges the following without any relationship to the current manuscript: research grant from RESICARD (research nurses); and consultant and lecture fees from the companies Amgen, AstraZeneca, Bayer Pharma, Alliance BMS-Pfizer, Novartis, and Sanofi-Aventis. The other authors have nothing to declare.
Supplementary Material
References
- 1. Carter P, Anderson M, Mossialos E.. Health system, public health, and economic implications of managing COVID-19 from a cardiovascular perspective. Eur Heart J 2020;doi: 10.1093/eurheartj/ehaa342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, for the COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KWthe Northwell COVID-19 Research ConsortiumBarnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP.. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim I-C, Kim JY, Kim HA, Han S.. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J 2020;41:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C.. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leonard-Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O, Schneider F, Labani A, Bilbault P, Moliere S, Leyendecker P, Roy C, Ohana M.. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology 2020;doi: 10.1148/radiol.2020201561.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H.. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;doi:10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S.. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation 2020; doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 10. Danzi GB, Loffi M, Galeazzi G, Gherbesi E.. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 2020;41:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E.. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology 2020;doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian CD, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carier Piazza G, Becjman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH.. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020;doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Bondi-Zoccai G, Brown TS, Nigoghossian CD, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA.. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol 2020;75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance#p01.
- 16.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected. Interim guidance 13 March 2020. https://www.who.int/docs/default-source/coronaviruse /clinical-management-of-novel-cov.pdf.
- 17. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL, ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- 18. Revel M-P, Parkar AP, Prosch H, Silva M, Sverzellati N, Gleeson F, Brady A.. COVID-19 patients and the radiology department—advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur Radiol 2020;doi: 10.1007/s00330-020-06865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L, Fan Y, Lu Z.. Experiences and lesson strategies for cardiology from the COVID-19 outbreak in Wuhan, China, by ‘on the scene’ cardiologists. Eur Heart J 2020;41:1788–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes P-M, Meziani F, CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carsana L, Sonzogni A, Nasr A, Rossi R, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M.. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. Infect Dis (except HIV/AIDS); 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C.. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol 2020;S2665991320301211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N.. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt J-D, Sacco C, Alexia B, Sandri MT, Barco S, Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obi AT, Tignanelli CJ, Jacobs BN, Arya S, Park PK, Wakefield TW, Henke PK, Napolitano LM.. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord 2019;7:317–324. [DOI] [PubMed] [Google Scholar]
- 26. Paranjpe I, Fuster V, Lala A, Russak A, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN.. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 2020;doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.