Abstract
Analysing wastewater can be used to track infectious disease agents that are shed via stool and urine. Sewage surveillance of SARS-CoV-2 has been suggested as a tool to determine the extent of COVID-19 in cities and serve as an early warning for (re-)emergence of SARS-CoV-2 circulation in communities. The focus of this review is on the strength of evidence, opportunities and challenges for the application of sewage surveillance to inform public health decision making. Considerations for undertaking sampling programs are reviewed including sampling sites, strategies, sample transport, storage and quantification methods; together with the approach and evidence base for quantifying prevalence of infection from measured wastewater concentration. Published SARS-CoV-2 sewage surveillance studies (11 peer reviewed and 10 preprints) were reviewed to demonstrate the current status of implementation to support public health decisions. Although being very promising, a number of areas were identified requiring additional research to further strengthen this approach and take full advantage of its potential. In particular, design of adequate sampling strategies, spatial and temporal resolution of sampling, sample storage, replicate sampling and analysis, controls for the molecular methods used for the quantification of SARS-CoV-2 RNA in wastewater. The use of appropriate prevalence data and methods to correlate or even translate SARS-CoV-2 concentrations in wastewater to prevalence of virus shedders in the population is discussed.
Keywords: SARS-CoV-2, Wastewater, COVID-19, SSewage surveillance, Wastewater-based epidemiology
Graphical abstract
Highlights
-
•
Use of 24 h composite samples, replicates, controls and normalization improve applicability of SARS-CoV-2 wastewater data.
-
•
Virus concentrations can be normalized with wastewater flows and population or faecal markers.
-
•
Correlation between wastewater SARS-CoV-2 data and community prevalence can be improved.
-
•
Trend analysis and early warning provide the most value for sewage surveillance.
-
•
Inferring prevalence from wastewater concentration requires combination with seroprevalence data and epidemiological models.
Introduction: the concept of sewage surveillance
Analysing wastewater can be used to track infectious disease agents that are shed via stool and urine. Such environmental surveillance has been in use for poliovirus, the causative agent of acute flaccid paralysis (AFP), in the United Nation's Polio Eradication Program since 1988 [1]. Although health surveillance focuses on monitoring AFP and screening stool samples for poliovirus, the rationale for sewage surveillance of poliovirus is derived from the characteristics of poliovirus infections. More than 95% of individuals infected with poliovirus develop no or mild gastrointestinal symptoms, but do excrete large numbers of poliovirus in faeces for periods of up to several weeks, and the virus particles remain detectable and infectious in (waste)water for days to weeks, depending on the environmental conditions [2]. Therefore, surveillance of wastewater for poliovirus can be used to determine the extent of a poliovirus outbreak and can even be used as early warning of poliovirus circulation in a community before cases of AFP are noted [3]. Sewage surveillance of poliovirus has played a key role in documenting the eradication of polio from India and Egypt, and served to detect outbreaks of poliovirus infections in polio-free countries, such as The Netherlands, Finland, Israel, Brazil, China and others [4]. In the current ‘endgame’ of the Global Polio Eradication Program, sewage surveillance is used to detect any residual poliovirus circulation in endemic countries and provide early warning of import of poliovirus. Sewage surveillance has been studied for other enteric viruses [5,6] and for antimicrobial resistance [7].
In the current pandemic of COVID-19, sewage surveillance of SARS-CoV-2 has been suggested as a tool to determine the extent of COVID-19 in cities and serve as an early warning for (re-)emergence of SARS-CoV-2 circulation in communities [8]. Again, the rationale for sewage surveillance is based on the characteristics of the virus. The majority of individuals infected with SARS-CoV-2 develop no or mild symptoms of respiratory illness, but do excrete large numbers of SARS-CoV-2, particularly in nasal fluids [9], but also in stools and, less frequently, in urine. SARS-CoV-2 RNA remains detectable in wastewater for days to weeks, depending on the conditions [10]. So, the suggested use cases of sewage surveillance of SARS-CoV-2, such as early warning of virus (re-)emergence, determining the size of the infected population and demonstration of absence of virus circulation, show a high similarity with those of poliovirus. O'Reilly et al. [11] suggested that the lessons learned in the sewage surveillance in the Global Polio Eradication Initiative can support successful implementation of SARS-CoV-2 sewage surveillance to support public health decisions. The objective of this paper is to review the current state-of-knowledge on SARS-CoV-2 sewage surveillance. Recent reviews have focused on several aspects of SARS-CoV-2 sewage surveillance: SARS-CoV-2 concentration and detection methods, shedding of SARS-CoV-2 by COVID-19 cases, longevity and infectivity of SARS-CoV-2 in wastewater and assessment of potential health risks via wastewater [12, 13, 14, 15, 16, 17, 18] as well as the economics of sewage surveillance of SARS-CoV-2 [19]. A global collaborative [20] generated a repository of sewage surveillance studies and their methods (www.covid19wbec.org). The focus of this review is on the strength of evidence, opportunities and challenges for the application of sewage surveillance to inform public health decision making.
Monitoring SARS-CoV-2 RNA in wastewater as health surveillance tool
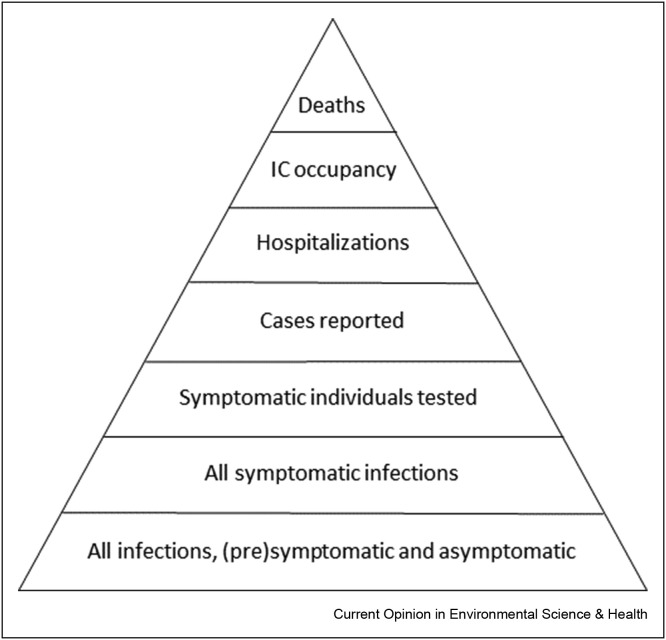
To evaluate the feasibility of monitoring SARS-CoV-2 RNA in wastewater as health surveillance tool, it is important to look at the potential place and added value of sewage surveillance in the COVID-19 surveillance efforts by health agencies. Such communicable disease surveillance is the systematic collection, analysis and interpretation of disease-specific data for use in planning, implementing and evaluating public health policies and practices and serves two key functions: early warning of threats to public health and monitoring of trends of endemic diseases and progress towards disease control objectives [21]. The new disease COVID-19 was first recognized through surveillance of disease symptoms, but the wide range of symptoms and overlap of COVID-19 symptoms with many other disease agents rendered this type of surveillance insufficiently specific. qPCR for SARS-CoV-2 RNA in rhinopharyngeal swabs was introduced in January 2020 as a tool to specifically monitor COVID-19 [22]. Individuals with symptoms that are suggestive of COVID-19 are tested and national and global surveillance systems [23] are reporting the data on laboratory-confirmed COVID-19 cases and interpret them as basis for decision making on COVID-19 control policies, such as quarantine and social lockdowns. Testing policies have evolved over time; in the early stages of the pandemic, testing was restricted to individuals with a relatively strict set of symptoms, but knowledge evolved about (1) the wide spectrum from very mild to very severe symptoms, (2) presymptomatic and asymptomatic carriage and transmission. Testing policies broadened to include individuals with mild symptoms and testing facilities became more widely available [24,25] resulting in more (a larger fraction of) cases being recognised and reported. Other health surveillance data that are widely used are hospitalizations and deaths of COVID-19 patients [26]. Another type of surveillance that is used is serological surveillance; testing the blood of individuals for antibodies against SARS-CoV-2 [27]. This is done to monitor the build-up of immunity in the population and to understand how many individuals in a population have been infected with SARS-CoV-2. As the development of an immune response and detectable antibody levels in blood require several weeks, serosurveillance is a retrospective tool. Seroprevalence surveys have been conducted in many countries and almost invariably demonstrate that many more individuals have been infected than seen through the ‘rhinopharyngeal swab surveillance’ [27,28]. Arora et al. [29] have published an overview of these studies, and provided a snapshot of the cumulative reported prevalence of COVID-19 and the seroprevalence with the data from March 1 to July 23 in 12 countries that showed that on the average seroprevalence was 8.5 times (standard deviation 6.1) higher than the cumulative prevalence of COVID-19 using the test data. These authors generated a Serotracker dashboard (https://serotracker.com/Dashboard) that now contains almost 500 seroprevalence estimates from national and regional data from 16 countries. This implies that the current health surveillance systems only see the tip of the iceberg, and that the surveillance pyramid [30] for COVID-19 has a wide base (Figure 1 ).
Figure 1.
The reporting pyramid of COVID-19. The base holds all infections with COVID-19 in the population, both symptomatic and asymptomatic. This base contributes to the SARS-CoV-2 discharged into the sewerage network. Moving upwards the numbers shrink, since not everybody with an infection is symptomatic, and not every symptomatic individual gets tested and there may be some delay or loss of reporting the local test data to national public health agencies. Ascending further, the numbers shrink by the nature of COVID-19: only a fraction of the reported COVID-19 cases end up in hospital, IC or die as a result of the disease.
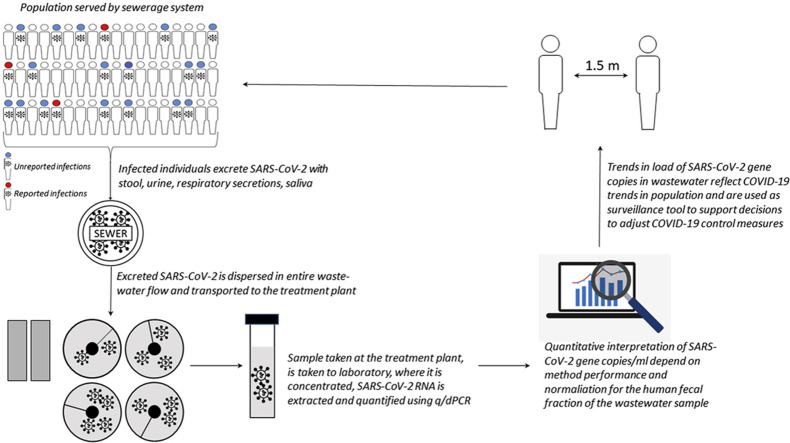
So, only a fraction of infected individuals are tested. But everybody uses the toilet. A large proportion of infected individuals shed SARS-CoV-2 in their stool [9]. Also, SARS-CoV-2 RNA from urine [31] and respiratory secretions (from handwashing, showering, nasal lavages, tissues) may contribute to the load of SARS-CoV-2 into the sewer system, as indicated by the detection of SARS-CoV-2 RNA in washbasin and shower siphons [32]. So, the sewerage network collects excreta with SARS-CoV-2 from all individuals, including all infected individuals, and transports it to the wastewater treatment plant (WWTP). Sampling wastewater is therefore sampling a mix of the total population in the sewershed (Figure 2 ), and encompasses the large proportion of infected individuals that do not get tested, because they are not or only mildly symptomatic, are reluctant to get tested, are not included in the testing policy or have less access to testing. As a consequence, sewage surveillance better represents the true SARS-CoV-2 circulation in the population. Sewage surveillance is the systematic collection, analysis and interpretation of data on SARS-CoV-2 RNA in wastewater to observe (and provide an early warning of) trends in COVID-19 in the communities. Sewage surveillance has been shown to be sensitive and fast enough to provide an early warning of increasing SARS-CoV-2 in cities [33,34]. This is important information to support stratified lockdown decisions. Sewage surveillance is aimed to monitor the circulation of SARS-CoV-2 communities; large communities such as inhabitants of a large city, or smaller communities, such as nursing homes, university or office campuses, prisons, and so on. It is a very cost-efficient tool [19] for population surveillance, and also a noninvasive tool, which does not require repeated sampling of individuals (such as nursing home inhabitants) to survey for (absence of) SARS-CoV-2 circulation in that community. Sewage surveillance has also been suggested for areas with limited access to health or testing facilities, low healthcare-seeking behaviour, such as informal settlements [35]. However, such settings are also unlikely to have adequate sewerage or central (faecal) waste collection, posing challenges for such a surveillance program.
Figure 2.
The concept of sewage surveillance of SARS-CoV-2.
Quantification of SARS-CoV-2 RNA in wastewater
Sewage surveillance relies on the collection and quantification of the SARS-CoV-2 RNA concentration in wastewater. To obtain meaningful information for surveillance, many elements are relevant: sampling site, procedure and strategy; sample transport, storage and preservation; concentration and quantification method and contextual information about the amount of human faecal input in the sample. Although reliable quantitative data of SARS-CoV-2 RNA in wastewater is the basis, sewage surveillance programs need sufficient spatial and temporal resolution to be able to give timely warning of increasing virus circulation in the population. Each will be addressed in the following paragraphs.
Sampling
The key for population surveillance is to obtain a representative sample of that population. For populations connected to a sewer system, samples are generally collected at the influent of WWTP treating domestic (but also industrial) sewage. WWTPs are the preferred sampling locations because (i) the infrastructure required for sampling (e.g., autosamplers and flow meters) is generally already available, and (ii) collected samples represent the population in the sewershed [33,36,37]. But also sewer pumping stations have been used or samples have been taken via manholes in the street [38,39] or the sewer pipes leaving a building or campus. Both grab [40,41] and composite sampling over 24 h using autosamplers have been used [33,36,37]. The latter allow to collect discrete time- (fixed sampling frequency and volume), volume- (frequency proportional to flow but fixed volume) and flow-proportional (fixed frequency but sampled volume proportional to flow). Owing to the complexity and variability of wastewater and SARS-CoV-2 shedding, composite samples, in particular flow-composite, are more representative than grab samples, as demonstrated for other markers of human input in sewer systems [42]. Grab samples provide only a snapshot of wastewater composition and can thus not be used to obtain a reliable picture of pathogen (or any other (bio)marker) circulation in a given population [43]. Furthermore, the frequency at which sample aliquots are collected is also a crucial parameter of composite sampling. Experience from monitoring pollutants and drugs in sewers has shown that if only few relevant water pulses (e.g., toilet flushes) are expected to contain the markers of interest, because of a small population or low prevalence, the likelihood of missing such pulses is high if low sampling frequencies are being used [44,45]. In particular, if small communities with expected low prevalence of infected people are being monitored, care should be taken to guarantee that adequate sampling modes and frequencies are used. For sampling of pollutants in wastewater, precautionary high sampling frequencies (≤5 min) are advised if important fluctuations are expected or if no information about sewers dynamics is available [42] and this likely holds true also for pathogens surveillance. This is particularly important when sampling in settings with low or intermittent wastewater flows (e.g., office buildings, campuses, nursing homes). The idea of focusing sampling to specific parts of the day, in particular morning hours, has been used in several SARS-CoV-2 studies [37,46]. The reasoning behind this approach is that people are more likely to defecate in the morning [47], and more concentrated samples can be collected if sampling occurs only during the morning. However, frequency of bowel movements has been shown to vary greatly both inter- (from once in 4 days to 3 times a day) [48] and intra-individual (due to episodes of constipation, menstrual cycle and use of oral contraceptives) [49]. Furthermore, the onset of virus shedding, not only through faeces but also oral fluids, to detectable levels might happen at a later stage during the day as the infection develops in individuals. Consequently, highly relevant pulses might be missed by focusing only on specific hours of the day. Residence time of wastewater in sewers should also not be neglected since, depending on the size and architecture of the sewer system (i.e., gravity and/or pressurized sewers), wastewater pulses might reach the sampling point (e.g., influent of a WWTP) only hours after input [50]. Although sampling only during the morning might be appealing to reduce non-human inputs and collect concentrated samples, there is a substantial risk of missing potentially relevant wastewater pulses and 24 h composite sampling should be preferred.
Sewage surveillance studies have used sample volumes of 50–200 ml of wastewater, but generally only an aliquot of that volume is analysed in the reverse transcription quantitative polymerase chain reaction (RT-qPCR) [33,36,39,51, 52, 53]. In low prevalence regions or times, larger sample volumes may be needed for the quantification of SARS-CoV-2 RNA.
A review of COVID-19 studies by Parasa et al. [54]indicated that SARS-CoV-2 RNA has been detected in faecal samples from 40.5% (95% confidence interval [CI] 27.4%–55.1%) of COVID-19 patients, whereas 7.4% (95% CI, 4.3%–12.2%) reported diarrhoea, implying the majority of virus shedders shed SARS-CoV-2 in formed stool. Residence time, turbulence and flows between toilet and WWTP influents can be considered sufficient to breakdown and disperse stool into suspended solids [55], this is not necessarily the case when moving upstream and sampling from smaller/individual sewers. Although there is no data about the physical state of SARS-CoV-2 virus particles in stools, Ye et al. (2016) showed that 26% of spiked murine hepatitis virus, a coronavirus used as model for enveloped viruses, attached to wastewater particles, whereas only 6% of spiked MS2, a F+ coliphage used as model for nonenveloped model viruses, attached to these particles. Some authors [34,56] have used primary sludge from wastewater as sample matrix, arguing this is a well-mixed matrix. No data were provided to support this, but these authors did find that the trend in the SARS-CoV-2 concentration in primary sludge matched with the trends in daily recorded COVID-19 cases [34,56] 14 d cumulative number of recorded cases, 7 d moving average of percentage positive COVID-19 tests of people [56] and hospitalizations [34] in the population served by the wastewater treatment.
Sample transport, preservation and conservation
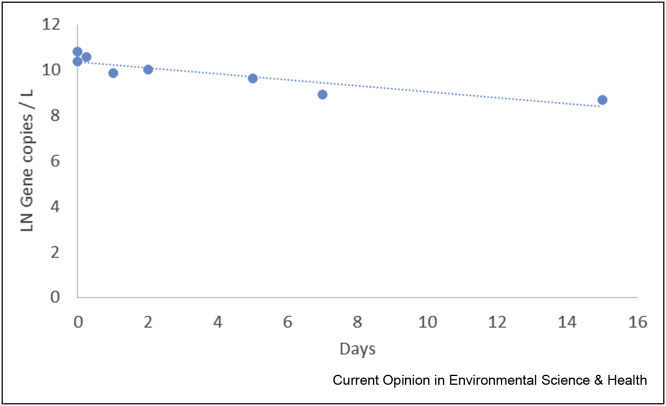
During composite sampling, samples are usually stored at 4 °C and transferred to a transport container and transported to the laboratory at 4 °C. Data from our laboratory indicate that SARS-CoV-2 RNA is stable in wastewater when stored at 5 °C (Figure 3 ).
Figure 3.
Decay of the SARS-CoV-2 RNA concentration in raw wastewater, stored at 5 °C, as determined with the RT-qPCR for the N2 gene fragment (methods see Medema et al., 2020).
Owing to shortage of supplies, lockdowns and/or long travel distances between sampling site and laboratory, some authors have frozen wastewater samples at −80 °C [34,57]. Although virus concentrates in lysis buffer can be stored frozen for years without affecting the PCR signal strength (C. Gerba, pers. comm.), only limited data are available on the effect of freezing of wastewater samples. Exploratory experiments conducted in our laboratory indicated freezing of wastewater samples at −80 °C yielded a loss of 1–3 Ct in the qPCR of the N1, N2 and N3 gene fragments. Weidhaas et al. [38] report 90% loss of qPCR signal after storage of pasteurized wastewater samples from three different sites for 1 week at −80 °C. We observed similar losses when wastewater samples were pasteurized for 90 min at 60 °C (data not shown). Pastorino et al. [58] report around 50% loss of RNA signal by pasteurization for 30 min at 56 °C or 60 min at 60 °C. Pasteurization is suggested as biosafety measure to inactivate most pathogens in wastewater before sample processing, but is not required when samples are processed with sufficient biosafety precautions (working in flow cabinet, gloves, closed centrifuge tubes).
Concentration and quantification method
Quite a number of recent reviews have addressed the methods [13,15,17]and longevity of the SARS-CoV-2 RNA signal in wastewater, and the first studies to compare the efficiency of different concentration methods to recover SARS-CoV-2 RNA from wastewater have been published [59,60]. As discussed in these reviews, a range of different protocols, reagents, standards and PCR-targets are used for (1) the concentration of the SARS-CoV-2 virus particles/RNA from wastewater or sludge, (2) the extraction of RNA from the concentrate and (3) the RT and qPCR. Although comparability between studies would benefit from a standard protocol, there is insufficient evidence at present to justify the selection of particular methods, reagents and targets. Moreover, several laboratories have reported delays in supplies. Nevertheless, to be useful for public health policy decisions, the data produced by sewage surveillance studies need to be reliable. To establish correlations with the prevalence of COVID-19 in the community, good quality quantitative data on SARS-CoV-2 RNA in wastewater (or sludge) are key. This implies that sewage surveillance studies:
-
1.
Need to incorporate adequate replicates to address the variability in recovery efficiency of the concentration method, the extraction method and the RT-qPCR. Most studies report technical replicates of the RT-qPCR reaction, but only D'Aoust et al. [56] used concentration replicates.
-
2.
Use test standards to evaluate the efficiency of the concentration, extraction and RT-qPCR steps using model viruses that resemble SARS-CoV-2. Many different model viruses have been used as matrix spike and/or positive extraction and/or RT-qPCR/dPCR control. Given the differences between viruses in attachment to particles [61], and in the efficiency of methods to extract nucleic acids from different viruses [62], we argue that a model coronavirus, that can be distinguished from SARS-CoV-2, would be most appropriate as control.
-
3
.Test the quality of quantified reference standards used to transform qPCR results into gene copies per ml. The use of droplet digital RT-PCR (RT-ddPCR) enables quantification, which is independent from the availability and quality of quantified reference standards, which makes RT-ddPCR quantifications potentially more accurate. In addition, RT-ddPCR appears to be more sensitive and less variable, especially for low concentrations [63]. The use of RT-ddPCR on COVID-19 samples from rhinopharyngeal swabs demonstrated the higher sensitivity of RT-ddPCR [64,65]. However, the first report on the use of RT-ddPCR for SARS-CoV-2 sewage surveillance on a limited number of samples demonstrated lower sensitivity, due to inhibition, of the RT-ddPCR reactions [56] demonstrating the need for optimization of RT-ddPCR before it can be implemented in sewage surveillance programs.
Contextual information
The SARS-CoV-2 sewage surveillance studies reported gene copies (or Ct-value) per unit volume [33,36,57,66]. Although these results are informative about the presence (or absence) of SARS-CoV-2 in a given community, they are not ideal for trend monitoring. In fact, urban sewers have substantial and variable non-human inputs, such as household appliances, industrial wastewater or, in combined sewers, stormwater, all of which can affect the measured concentrations. To compensate for this variability, gene copies per ml can be multiplied by flows measured at the time and site of sampling to convert concentrations into viral loads (expressed as gene copies per day). Weidhaas et al. [38] and Westhaus et al. [67] have used virus loads, and we urge nation-wide monitoring programs to include wastewater flows in their interpretation. Close collaboration with wastewater professionals is crucial to achieve good interpretation of the collected samples. Another aspect, which has been generally overlooked in recently published SARS-CoV-2 sewage surveillance studies, is the actual size of the population contributing to the sampled wastewater (i.e., a combination of residents, commuters and occasional visitors), also referred to as de facto population (in contrast to static figures about registered inhabitants, referred to as de jure population) [68]. In wastewater-based epidemiology (WBE, a term used to refer to surveillance studies focusing on chemical biomarkers, such as drug metabolites or exposure biomarkers, in wastewater [69] literature, this aspect has been discussed thoroughly [68,70,71]. In fact, the de facto and de jure population can differ greatly, mainly due to commuters entering or leaving a catchment, but also because of holidays, tourisms and major public events [72]. Population figures are generally considered in sewage surveillance/WBE studies when comparing catchments with different population sizes. Absolute mass (or viral) loads will obviously be higher in catchments with larger populations, but this does not mean that the per-capita loads are also higher. When the goal is to monitor the evolution (trends) of a given wastewater marker over time in one location, it can be assumed that, except for major public events, population dynamics will follow relatively constant patterns over time and its influence can thus be neglected [71]. However, in the current situation, population dynamics have deviated significantly from normal patterns because of the introduction of restrictions to control the spread of SARS-CoV-2. Decreased mobility has been observed during ‘lockdowns’ (e.g., shelter in place, working from home), whereas the opposite occurred as measures were being (partly) relieved (e.g., partial return to office, tourism) [73]. This would mean that while strict lockdowns are in place, the de facto population can be assumed to be close to the de jure. Yet, changes can be expected as restrictions ease and these should ideally be taken into account while interpreting wastewater data. Mobile phone data have been proposed as an ideal proxy to obtain accurate information about population dynamics in studied catchment [74], yet access to these data is not straightforward because of high costs and/or privacy regulations. Alternatively, a whole range of markers that can be measured directly in wastewater samples have been suggested. However, some show promising results, such as ammonium, certain pharmaceuticals (e.g. gabapentin, carbamazepine) or food additives (e.g., caffeine, artificial sweeteners) [67,69, 70, 71, 72∗,75]. It should be noted that these chemicals are mostly present in the dissolved fraction and are being used to normalize for biomarkers released via urine (e.g., drug metabolites, exposure biomarkers). Other parameters, such as (drinking) water and electricity consumption have also been considered but did not show good correlations with supposedly more accurate indicators such as mobile phone data [72]. Currently, there is no consensus about the ideal indicator and, in most cases, registered inhabitants (de jure) or estimates of population equivalents provided by the WWTP are still being used to calculate population-normalized mass loads. However, in the specific case of pathogens that interact with the gastrointestinal tract, such as SARS-CoV-2, alternative approaches could be considered to normalize measured viral loads to account for population dynamics and non-human inputs. For instance, faecal sterols such as coprostanol have been suggested [76,77] or, alternatively, other viruses that are ubiquitous in human intestinal tracts (such as CrAssphage [78,79] or pepper mild mottle virus (PMMoV: [56]) can be quantified alongside SARS-CoV-2 and used to normalize the SARS-CoV-2 signal for the amount of human faecal input in each individual wastewater sample. D'Aoust et al. [56] found PMMoV to be superior to HF183 Bacteroides 16 S ribosomal rRNA and eukaryotic 18 S rRNA, as PMMoV showed more reproducibility within and between WWTP. The advantage of these parameters is that, as they are shed by all individuals, they are unaffected by cultural or seasonal effects. Differences in use or prescription of pharmaceuticals and personal care products (e.g. gabapentin, caffeine and artificial sweeteners) between countries and seasons, can in fact introduce biases when comparing across seasons and/or locations. Furthermore, these anthropogenic markers provide an indication of the faecal load in measured samples and can hence be used to normalize figures without having to estimate of the actual number of inhabitants. Given the important disruption that current and future containment measures have on population dynamics, there is a need to further investigate how specific anthropogenic markers or other proxies can be integrated in monitoring programs to improve interpretation of sewage data and strengthen our ability to quickly detect significant changes in pathogen circulation.
Temporal resolution and scale
Studies published so far and national monitoring programs have often reported about SARS-CoV-2 trends in wastewater per week, as they generally rely on the collection and analysis of one 24 h composite sample per week and location [33,34,38,67]. Although this appears to be a reasonable scale to follow trends with respect to the development of COVID-19 symptoms, and also to reflect the weekly publication of updated epidemiological data from other indicators (e.g., rhinopharyngeal swabs, serology), it assumes that, within 1 week, there is little or no day-to-day variation in viral loads. This is, however, not necessarily the case and depends on the size of the catchment and the type of pollutant/marker being monitored [45,80,81]. For instance, a study about antibiotic mass loads at the influent of a medium-size WWTP (approximately 220,000), reported limited day-to-day variations [82]. On the other hand, mass loads of cocaine and its main metabolite benzoylecgonine measured in a small catchment (approximately 7200 inhabitants) exhibited important day-to-day variations [83]. To the best of our knowledge, day-to-day variations of SARS-CoV-2 viral loads in wastewater have not been investigated yet. However, should SARS-CoV-2 viral loads in wastewater also exhibit substantial day-to-day variations, sampling once a week might not be sufficient to rapidly and unequivocally detect changing trends. Although collecting and analysing samples on a daily basis is likely unnecessary, besides being logistically and financially unbearable, certainly if the goal is to implement nation-wide monitoring programs, there is an urgent need to gather more data about short-term variation in SARS-CoV-2 loads in wastewater and determine which is the minimum number of samples that needs to be collected.
Monitoring the circulation of SARS-CoV-2 through sewage surveillance is not limited to WWTP influents and large communities. There is in fact a growing interest to extend its application to a smaller scale, targeting specific communities, such as neighbourhoods, nursing homes, campuses, industrial complexes, airplanes and cruise ships. These are particularly interesting case studies as they either involve potentially vulnerable populations (e.g., nursing homes) or situations in which physical distancing is more difficult to implement (e.g., airplanes and cruise ships). However, the smaller the community and, consequently, the size of the catchment, the more difficult it becomes to obtain representative samples, in particular if the number of relevant pulses decreases, flows are intermittent and/or there is insufficient dispersion and mixing in sewers (i.e., homogenization) before collection [42,45,84]. Using commercially available discrete autosamplers, even if flow-proportional mode, there will most likely always be sampling errors [84] making data interpretation challenging. Although positive results would imply that the virus is circulating in the community, the opposite conclusion can hardly be drawn from a negative result, as this could simply be due to sampling errors (i.e., missing a relevant wastewater pulse). Continuous flow sampling (i.e., diverting a fraction of the flow to a container) would be necessary to obtain truly representative samples, yet this is hardly standardisable or implementable on a large scale, as no commercial system is available and ad-hoc solutions would need to be developed for each location. Going upstream in a sewer system to monitor neighbourhoods might be less problematic, provided conditions resemble those of the influent of a WWTP (i.e., sufficient flow, number of relevant pulses, dispersion and mixing). Sampling might for instance be possible directly from manholes, provided an autosampler and flow measurement device can be placed. Alternatively, pumping stations could also be suitable, although care should be taken to collect representative samples (i.e., sufficient mixing to avoid formation of gradients in wet wells) and sampling should be adapted to pump cycles. The latter case resembles the situation which is likely found in transport vessels such as airplanes or cruise ships, equipped with either collection tanks or dedicated treatment reactors. Ahmed et al. [85] recently reported the positive detection of SARS-CoV-2 RNA from samples collected from the sanitation systems of airplanes and cruise ships, showing that sewage surveillance can be successfully implemented also to screen transport vessels. Specifically, airplane samples were collected using a valve at the bottom of vacuum trucks which empty the tanks. Yet, a certain degree of stratification can be expected within the truck, thus making it difficult to obtain a representative sample. This would lead to the same situation mentioned previously, namely that a positive result would indicate presence of the virus but its absence cannot be guaranteed from a negative one. In their study, Ahmed et al. also reported results from cruise ships. In particular, samples were collected from the influent and effluent of the membrane bioreactor on board the ship [85]. Because of the design of cruise ships’ sewer system, sampling in this setting resembles more the case of a small catchment and should hence be less prone to sampling uncertainties discussed earlier. Still, adequate sampling strategies should be implemented to make sure that representative samples are being collected. For instance, changes in hydraulic retention times, which are known to occur during the day [86], should be taken into account when defining sampling frequencies. Although the implementation of sewage surveillance on a smaller scale, or at specific-sites, can certainly be appealing, the points raised above highlight the risks of misinterpretation due to suboptimal sampling strategies that these applications bear. If sewage surveillance is to be implemented in such settings, a thorough analysis of uncertainties and a transparent communication of its limitations are of utmost importance.
Quantitative relation between SARS-CoV-2 circulation in the population and SARS-CoV-2 RNA in wastewater—theory
Sewage surveillance relies on the assumption that there is a, at least semi-, quantitative relation between the circulation of SARS-CoV-2 in the population and the concentration of SARS-CoV-2 RNA in wastewater. The following paragraphs discuss the available knowledge on SARS-CoV-2 circulation in the population, shedding of SARS-CoV-2 by infected individuals and the challenges of translating SARS-CoV-2 information from the population to wastewater and vice versa.
Number of infected persons
As indicated in par. 2, the number of reported COVID-19 cases is an underestimation of the true number of infected individuals, as not everybody is tested. Hence, the reported laboratory-confirmed case numbers are an underestimate of the number of virus shedders in the population. The number of people shedding virus in the population will depend on the number of infections and the duration (number of days) over which each individual sheds RNA. Seroprevalence studies provide more complete data on the number of infected individuals and demonstrate that reported case numbers based on rhinopharyngeal swab testing can, depending on the testing policy, vastly underestimate the true percentage of the population that is infected with SARS-CoV-2. Infectious disease models have been generated to estimate the number of infectious individuals in a population, extrapolating via the case fatality ratio or infection fatality ratio (https://ourworldindata.org/covid-models). Seroprevalence data and epidemiological model prevalence estimates have not been used in sewage surveillance studies to relate to SARS-CoV-2 wastewater concentrations, but would provide better estimates of the true number of infectious individuals in a population. In future modelling approaches, these prevalence data can be used as priors, which can be updated using wastewater-based estimates, or vice versa.
SARS-CoV-2 RNA shedding loads
The presence of SARS-CoV-2 RNA in the faeces of infected patients was identified in some of the earliest observational studies [87, 88, 89, 90, 91, 92, 93, 94∗, 95]. Since then a large number of studies has been published, and several research groups have aimed to review and summarise what is known about the frequency, magnitude and duration of RNA shedding in asymptomatic, presymptomatic and symptomatic COVID-19 [9,14,96, 97, 98, 99, 100∗]. Although these reviews vary in their scope and specific purpose, some key findings are in common. There is a high degree of variability both between studies and within studies regarding the shedding of RNA, both in terms of the magnitude and duration. Walsh et al. [100] performed a systematic review including 113 studies to assess the detection pattern and viral load of SARS-CoV-2 over the course of an infection, and included upper respiratory tract (URT), lower respiratory tract and stool samples. The duration of virus RNA shedding in the URT lasted just over 2 weeks (median = 14.5 days); however, some patients shed for considerably longer, with the maximum recorded duration (URT) of 84 days. Shedding in stool in general was reported to be longer and more erratic in comparison with URT samples; however, the quantification of detection time was not estimated across studies as the values were thought to be truncated by the maximum duration of follow-up rather than the true duration of virus detection.
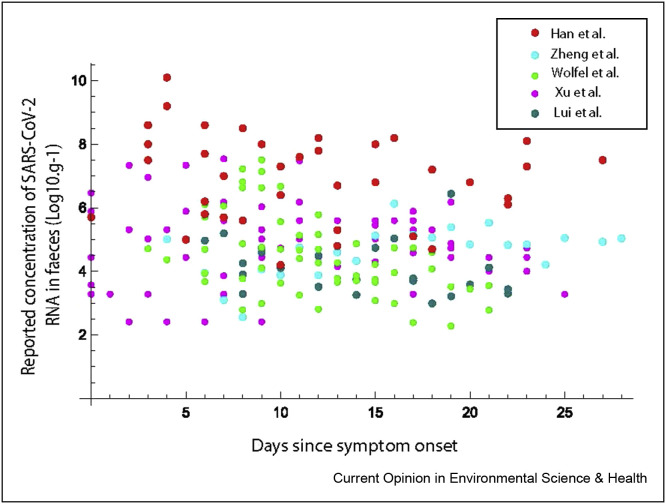
Although some studies have reported a higher viral load in patients with severe illness in comparison to mild, this finding was not consistent, or always significant. Furthermore, Walsh et al. [100] reported that seven studies measured the viral loads between presymptomatic, asymptomatic and symptomatic patients to have little or no difference. Data from six studies that reported quantitative concentrations of RNA in faecal samples over the course of an infection were extracted for further analysis. These studies are summarised in Table 1 and illustrated in Figure 4 . Although there is some evidence from these reported concentrations that higher loads occur earlier in infection, the data show a high variability in concentration supporting the erratic pattern observed by Walsh et al. [100].
Table 1.
Studies reporting quantitative concentrations of RNA in faecal samples extracted for further analysis.
|
Reference |
Number of patients (n) | Adults/children | Country | Reported metric | Concentrations of positive results. Median (range) | Method of data extraction from original publication |
|---|---|---|---|---|---|---|
| Xu et al., 2020 | 10 | Children (2 months–15 years) | China | Ct per swab | 5.02 (2.4–7.5) | Estimated from reported Cts on days from admission to hospital |
| Han et al., 2020 | 12 | Children (27 days–16 years) | Korea | Log10 viral RNA load per ml | 7.63 (<4.10–10.27) | Digitised from Figure B in reference |
| Lui et al., 2020 | 11 | Adults | Hong Kong | Log10 virus copies per ml | 3.90 (2.99, 6.44) | Concentrations reported in supplementary material |
| Wölfel et al., 2020 | 9 | Adults | Germany | Log10 copies per swab | 4.27 (2.28, 7.51) | Digitised from Figure 1c in reference |
| Zheng et al., 2020 | 55 | Adults | China | Log10 virus copies/ml | 4.46 (1.99–8.13) | Digitised from Figure 2 in reference |
Figure 4.
Summary of reported concentrations of SARS-CoV-2 in faecal samples by day (noting data from Xu et al., 2020 is reported in days from hospitalisation).
It is not clear when the virus begins being shed by infected individuals. Most observational studies from analysis of clinical samples begin some days following symptom onset (Fig. 4). Obtaining presymptomatic samples presents a clear logistical challenge. Nevertheless, epidemiological studies indicate that transmission (and hence shedding) occurs before [101,102], and possibly for 3–5 days before symptom onset. Some authors have suggested that this presymptomatic time may indeed represent the peak of shedding, with postsymptomatic samples representing a declining trajectory.
Not all stool samples from infected patients are positive for SARS-CoV-2 RNA. In a meta-analysis of 60 studies including 4243 patients, the pooled prevalence of stool samples that were positive for virus RNA was 48.1% (95% CI, 38.3%–57.9%), and 70.3% of those collected after loss of virus from respiratory specimens tested positive. It is not clear the extent to which this prevalence represents the portion faecal shedders in the population (perhaps only 50% of infected people shed), or if this relates to the erratic pattern of shedding referred to by Walsh et al. [100] and illustrated in Figure 3 (perhaps a greater proportion if not all infected individuals shed with faeces; however, some samples will yield a negative PCR due to low fluctuations in concentration). Nonetheless, all infected people will shed virus RNA, and loads from URT secretions (generated at 1.5–2 L per day) [103,104] and saliva (generated at 0.5–1.5 L per day) [105,106] will no doubt contribute to the wastewater load either by passive transport via the gastrointestinal tract or the alternative pathways of showering, bathing and hand washing [32].
Estimating prevalence of SARS-CoV-2 infections from wastewater data
To illustrate the theoretical relationship between the number of shedders in the contributing population and the expected concentration of SARS-CoV-2 in wastewater, assuming no decay of the RNA in the sewer signal and complete hydraulic mixing, a Monte Carlo simulation was undertaken. The concentration of RNA in sewage is given by:
where N is the number of people shedding; fl is the faecal load (g. person−1 day−1); Cfaeces is the concentration of RNA in the faeces of infected people; and Q is the total flow to sewer per day.
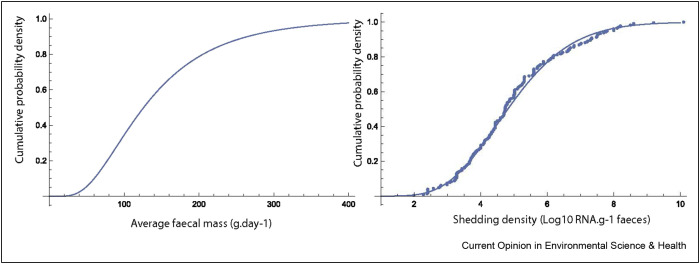
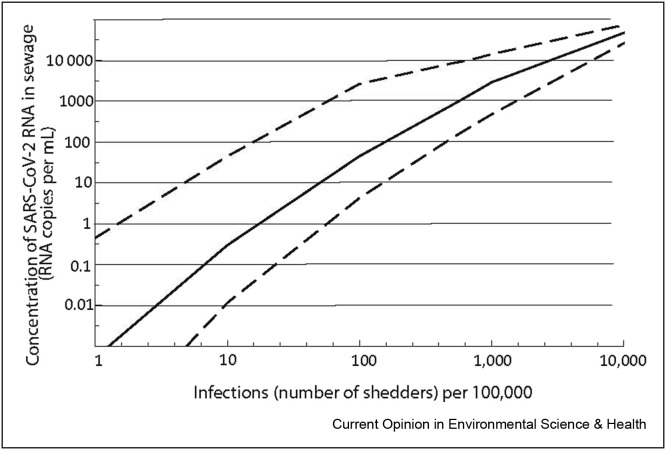
The amount of faeces generated per person per day was described by a Lognormal distribution, defined using the reported mean (149 = ) and median (126 = of faecal generation from the review undertaken by Rose et al. [107]. The concentration of RNA in faeces was described by a gamma distribution (shape = 10.78; scale = 0.46) fitted to the combined data set of reported concentrations (Figure 5 ) using the method of maximum likelihood (Gamma distribution provided the best fit in comparison to Normal and Lognormal distributions). A Monte Carlo simulation (100,000 iterations) was used to obtain a distribution of concentration of RNA in sewage assuming a wastewater volume of 150 L per person per day. The median and 95% quantiles of the relationship are illustrated in Figure 6 . It should be noted that this ignores (variability in) non-human flow input into the sewer network. The uncertainty of the estimated concentration is dominated by the virus shedding data. At high concentrations in wastewater, this uncertainty around is relatively small, but at concentrations of 1–10 per ml, the uncertainty spans over more than an order of magnitude. In future prevalence estimates from wastewater, the use of Bayesian hierarchical models would allow to take into account existing knowledge (i.e., priors) about virus circulation in communities, based on seroprevalence data or epidemiological models, which could then be updated using wastewater data to obtain new (i.e., posterior) estimates about prevalence, as has been used in WBE of drug use [108,109]. This would strengthen not only the sewage surveillance, but also the epidemiological models and seroprevalence information.
Figure 5.
Lognormal distribution fitted to the load of faecal mass shed per person per day (left) and gamma distribution (solid line) fitted to reported concentrations (dots – data from Figure 1) of RNA in faeces from reviewed studies.
Figure 6.
Modelled relationship (Median (solid line), 5th and 95th quantiles) between the number of infected people in the population and concentration of RNA in sewage.
Quantitative relation between SARS-CoV-2 circulation in the population and SARS-CoV-2 RNA in wastewater—practice
Published SARS-CoV-2 sewage surveillance studies describe how SARS-CoV-2 RNA data in wastewater or primary sludge are collected and how they are connected to data on the prevalence of COVID-19 in the community. PubMed, Scopus, Google and medRxiv were searched with the keywords ‘SARS-CoV-2’ AND ‘wastewater’. Reviews were excluded, and the abstracts of the peer-reviewed publications and preprints found were scanned for sewage surveillance studies containing quantitative data on SARS-CoV-2 RNA in concentrations wastewater and on COVID-19 prevalence (or number of reported cases). Publications containing this combination of data were selected for more in-depth review and extraction of (1) the place and period of study, (2) the prevalence of COVID-19 prevalence in the population and the number, type and source of prevalence data collected, (3) the concentration of SARS-CoV-2 RNA in wastewater (or sludge) and the number of sites, samples, type of samples, methods, (4) how wastewater data and prevalence data were spatially and temporally aligned and (5) how the wastewater concentrations were normalized and analysed to correlate or translate them to COVID-19 prevalence in the population.
Eleven peer-reviewed publications and 10 preprints were reviewed. Table 2 presents the extracted information. The countries spanned Europe, North and South America, Asia and Australia and studies were conducted in medium to large urban areas. The most frequently used prevalence data are the reports of public health agencies or hospitals about the number of laboratory-confirmed COVID-19 cases. We found several publications in which no (detailed) source of these data was reported and also in which number of cases was reported but the population denominator was missing. Several publications provided a detailed description of the data, whether point or cumulative prevalence was reported and for what period cases were accumulated. Some publications complemented reported prevalence/case numbers with data on hospitalizations. Prevalence data and sewer data are ideally drawn from the same population. In several studies, these populations were not aligned and sewer data were taken at a WWTP of a city and prevalence data were taken from the region. Other studies aligned the populations via the cities covered by the public health surveillance data and one or more WWTP. Two studies aligned the population using ZIP code information. This requires access to the health data to locate cases by ZIP code, which is the Best fit’, but requires information about the ZIP codes served by a sewer network and WWTP.
Table 2.
Studies reporting and combining COVID-19 prevalence data and SARS-CoV-2 RNA concentrations in wastewater.
| Reference | Area | Country | COVID-19 prevalence (per 100,000) | Prevalence data | SARS-CoV-2 RNA concentration in wastewater (gene copies/ml) | Sewer data (only data used to compare to prevalence) | Sewer data analysis | How are prevalence and wastewater data compared? |
|---|---|---|---|---|---|---|---|---|
| Peer reviewed | ||||||||
| Wu et al., 2020 |
Massachusetts |
USA |
26 |
Cumulative number of reported cases in Massachusetts as confirmed by laboratory diagnosis and population |
57–303 |
10 samples, 2 catchment areas of 1 WWTP, 1 week, composite samples, pasteurized |
SARS-CoV-2 concentration N1, N2, M3 averaged and presented with and without normalization with PMMoV concentration per sample |
Observed percentage (0.026%) of cases vs expected percentage (5%) of cases, calculated from SARS-CoV-2 shedding (600,000 per ml stool), average wastewater (591 L) and stool ( × 1 × 200 g/d) production per capita, no loss in sewer, total suspension |
| Ahmed et al., 2020a |
Brisbane |
Australia |
8.3–42 |
28-day cumulative reported number of cases in city and at home population |
0.019–0.12 |
2 samples, 1 WWTP, 1 week, composite grab samples |
SARS-CoV-2 RNA concentration of N-gene with 2 methods |
Prevalence and wastewater data presented. Monte Carlo analysis of prevalence based on wastewater concentration, combined with wastewater production per capita per day, at home population in city, normal distribution of daily stool mass (log gram avg 2.11, std 0.25), shedding rate of SARS-CoV-2 RNA copies/g of faeces was modelled as a log-uniform distribution from 2.56 to 7.67 |
| Medema et al., 2020 |
Netherlands |
0.1–100 |
Cumulative number of reported cases in cities in study period as confirmed by laboratory diagnosis |
<1--2200 |
25 samples, 6 WWTP, 3 months, 24 -h flow composite samples |
SARS-CoV-2 concentration of N1, N2, N3 gene and Ct value of E-gene |
Significant correlation between cumulative number of reported cases and concentration in wastewater |
|
| Kumar et al., 2020 |
Ahmedabad |
India |
1000–2700 |
Cumulative (?) reported number of cases in city as confirmed by laboratory diagnosis |
Up to 0.35 |
2 samples, 1 WWTP, 3 weeks, grab samples |
Prevalence and wastewater data presented |
|
| Randazzo et al., 2020 |
Murcia |
Spain |
8.5–129 |
Cumulative number of reported cases in cities in study period as confirmed by laboratory diagnosis |
Approx 100--1000 |
42 samples, 6 WWTP, 1 month, morning grab samples |
SARS-CoV-2 concentration of N1, N2, N3 gene |
Prevalence and wastewater data presented |
| Haramoto et al., 2020 |
Yamanashi |
Japan |
4.4 |
Reported cases in prefecture divided by population as confirmed by laboratory diagnosis. |
<4 |
5 samples, 1 WWTP, 2 months, grab samples |
SARS-CoV-2 RNA concentration of using a range pf qPCR assays |
Prevalence and wastewater data presented |
| Westhaus et al., 2020 |
Nordrhein Westfalen |
Germany |
30-174 (acute); 72-220 (cumulative) |
Reported cases in cities as confirmed by laboratory diagnosis |
3–20 |
9 WWTP, 1 day, 24 -h flow composite samples |
SARS-CoV-2 load, calculated from the concentration of M gene and the daily flow on the day of sampling. With and without normalization with creatinine |
Correlation between SARS-CoV-2 load in wastewater (both with and without creatinine normalization) and acute and cumulative prevalence. SARS-CoV-2 concentration in wastewater and prevalence were not significantly correlated. |
| Prado et al., 2020 |
Niteroi |
Brazil |
51 |
Reported cases in cities as confirmed by laboratory diagnosis and population |
Ct 36--40 |
12 samples, 1 sewer network, 10 -h composite samples, pasteurized |
Prevalence and wastewater data presented |
|
| Peccia et al., 2020 |
Newhaven |
USA |
0--50 (daily in study period); 14.5–1304 (cumulative in study period) |
Daily number of reported cases in New Haven (and hospitalizations in New Haven hospital) in study period, as confirmed by laboratory diagnosis |
1700–460,000 |
>40 Primary sludge samples, 1 WWTP, 1.5 months, morning grab samples, frozen at −80 °C |
SARS-CoV-2 N1 and N2 gene concentration, normalized for total RNA extracted for each sample |
Parallel time series in reported number of cases and hospitalizations and normalized SARS-CoV-2 gene copies/ml of sludge with the maximized correlation coefficient if the wastewater concentration were moved forward for 7 days and 3 days with confirmed cases and hospitalizations respectively |
| Trottier et al., 2020 |
Montpellier |
France |
8 |
Daily reported cases and hospitalizations in Herault Province, confirmed by laboratory diagnosis. Population data. |
1–78 |
6 samples, 1 WWTP, 1.5 months, composite samples |
SARS-CoV-2 concentration of N1, N3 |
Trends in prevalence and SARS-CoV-2 concentration in wastewater, which were inversed |
| Guerrero-Latorre et al., 2020 |
Quito |
Peru |
358--2077 (cumulative case numbers); 81-579 (acute case numbers) |
Case numbers in areas draining into the river, population not specified |
207–3190 |
3 river samples at 3 sites in Quito, where 97% of wastewater is discharged untreated. 1 day, grab samples |
SARS-CoV-2 concentration of N1, N2 gene |
Prevalence and river water data presented |
| Not (yet) peer-reviewed | ||||||||
| Bar Or et al., 2020 |
Bnei Brak |
Israel |
366–1001 |
Reported cases in Bnei Brak as confirmed by laboratory diagnosis |
Ct 33--37 |
3 samples, 1 city network, 1.5 week |
SARS-CoV-2 qPCR Ct value |
Correlation between Ct-value in wastewater and reported cases |
| Weidhaas et al., 2020 |
Utah |
USA |
2.4–16 |
Average of daily new cases in two months period per sewershed. GIS Census link of reported cases as confirmed by laboratory diagnosis and total population in sewerheds. |
0.023–-1.04 |
126, 10 WWTP, sampled weekly for 2 months, 24 -h flow composite samples, |
Use flow to calculate gene copies/capita/d |
correlate two-month cumulative reported cases to two month cumulative estimated cases calculated from gene copies/capita per day, corrected for recovery of method (26%), using estimate on g faeces per capita per day (500) and virus shedding 10ˆ4.7/ml faeces |
| correlate weekly reported cases to gene copies per capita per day for each WWTP | ||||||||
| Zhao et al., 2020 |
Wuhan |
China |
No data |
7.4 |
3 samples, 1 WWTP in medium risk period (2 weeks), type unspecified |
SARS-CoV-2 qPCR of ORF1 and RDB2 genes, not specified |
Few quantitative wastewater data, medium risk 1/3 samples WWTP +, low risk period not detected in city WWTP inlet, only in hospital wastewater |
|
| Hemalatha et al., 2020 |
Hyderabad |
India |
No data |
31–532 |
9 samples, 7 WWTP, 1 month, grab samples |
SARS-CoV-2 qPCR concentration calculated from Ct value, using generic information but without use of standard curve |
Calculation of prevalence based on wastewater concentration, combined with daily stool mass (128 g), shedding rate of 10ˆ7 SARS-CoV-2 RNA copies/g of faeces |
|
| Chavarria-Miro et al., 2020 |
Barcelona |
Spain |
0--8000 (cumulative estimated shedders) |
Shedder estimation not defined, no population data |
Approx 0.1–10 |
14 samples, 2 WWTP, 1.5 months, 24 -h composite samples |
SARS-CoV-2 concentration of IP2, IP4, E, N1 and N2 gene |
Decline in wastewater concentration parallels decline in estimated shedders |
| Wurtzer et al., 2020 |
Paris |
France |
0-2000 (daily reported case numbers) |
Laboratory-confirmed emergence department visitors, hospitalizations in Paris hospitals |
50–3000 |
>20 samples, 3 WWTP, 1.5 months, sampling not specified |
SARS-CoV-2 concentration of E gene |
Parallel trend in prevalence (estimated shedders, laboratory confirmed emergence department visitors, hospitalizations) and wastewater data presented. Estimation of daily virus shedders using emergency department visitors that were laboratory confirmed COVID-19 cases, virus shedding (not defined) and 2 day lag between symptom onset and diagnosis |
| Green et al., 2020 |
Onondaga County, New York |
USA |
<70--349 |
Laboratory-confirmed cases reported in one hospital, reflecting 40% of population, and total population data, both matched to sewershed via ZIP code |
<LOQ; 7.5–112 |
22 samples, sewer network and WWTP, 1 week, composite samples |
SARS-CoV-2 IP2 IP4 gene and CrAssphage concentration, ratio calculated |
Spatial correlation between incidence and SARS-CoV-2 to CrAssphage ratio in wastewater |
| Kaplan et al., 2020 |
Newhaven |
USA |
0--50 (daily in study period); 14.5–1304 (cumulative in study period) |
Daily number of reported cases in New Haven (and hospitalizations in New Haven hospital) in study period, as confirmed by laboratory diagnosis |
1700–460,000 |
>40 Primary sludge samples, 1 WWTP, 1.5 months, morning grab samples, frozen at −80 °C |
SARS-CoV-2 N1 and N2 gene concentration, normalized for total RNA extracted for each sample |
Used the Peccia et al., 2020 data in a model to estimate and compare that to observed hospital admissions |
| Hata et al., 2020 |
Ishikawa and Toyama |
Japan |
0–18.9 |
Reported cases in cities as confirmed by laboratory diagnosis and population |
14–44 |
27 samples, 4 WWTP, 2.5 weeks, morning grab samples |
SARS-CoV-2 concentration of N1, N2, NIID |
Prevalence and wastewater data presented. SARS-CoV-2 PCR in wastewater more frequently positive when prevalence exceeded 10/100,000 |
| D'Aoust et al., 2020 | Ottawa and Gatineau | Canada | 4.8–57.3 | Reported cases in cities as confirmed by laboratory diagnosis and population | 1.7–3800 | 14 primary sludge samples, grab samples first 55 days, composite (grab sample every 6 h, composited over 24 h) samples day 56 onward, 3 months | Average SARS-CoV-2 concentration of technical triplicates and several extraction replicates with N1 and N2, normalized for PMMoV concentration | Significant correlation between PMMoV normalized SARS-CoV-2 concentrations (N1 and N2) with daily new cases, 14d-cumulative cases, 7d rolling average of daily percentage of positive COVID-19 tests. Normalization through daily solids mass flux through WWTP did not yield significant correlat ion. |
For the wastewater data, we extracted only data that were used to combine with prevalence data, so for instance samples from WWTP effluent were ignored. The inlet of the WWTP was the most frequently reported site for sample collection. Other sampling sites were primary sludge and sampling sites ‘upstream’ in the sewer network, at pumping stations or via manholes. The number of wastewater samples per study was low to moderate: 10 studies presented results from 2 to 10 samples, 8 studies reported between 10 and 100 samples and only one study reported on more than 100 samples.
Two main approaches have been used to connect SARS-CoV-2 concentrations in wastewater (or primary sludge) with the COVID-19 prevalence data. The first is to look for temporal or spatial correlations between the two types of data (see Figure 7 ). Several studies simply described similar trends in wastewater concentrations and COVID-19 prevalence, but others analysed the correlation between these data. Studies differed in the data that were used for the correlation analysis. Medema et al. [33] correlated SARS-CoV-2 concentrations in wastewater with the reported 4 weeks cumulative COVID-19 prevalence in the city and found significant correlations. D'Aoust et al. [56] observed this only after normalization of the wastewater concentrations for PMMoV concentration, for acute and 2-week cumulative prevalence and percentage of positive COVID-19 tests. Westhaus et al. [67] did not observe a significant correlation between cumulative or acute prevalence and wastewater concentrations, but did find a significant correlation with virus loads in wastewater. Weidhaas et al. [38] observed a correlation with virus loads in wastewater and 2-week cumulative prevalence data with only 2 of 10 WWTP. The second approach used is to estimate the prevalence of COVID-19 in the community from wastewater concentrations or loads. To translate wastewater concentrations into prevalence, Ahmed et al. [36], Wu et al. [39], Weidhaas et al. [38], Wurtzer et al. [53] and Hemalatha et al. [110] used (different) estimates of concentration of the virus in stools of individuals infected with SARS-CoV-2, of stool mass or volume shed per day and wastewater volumes produced per capita per day. A main difference between the approaches used can be drawn between studies that carried out point estimates of incidence or prevalence [38,39] and those which implemented a (at least partly) stochastic approach [36,110]. In the former case, authors used average or median shedding rates in stool and daily per capita stool mass, combined with measured RNA concentrations in wastewater, estimates of catchment population size and wastewater flows. Wu et al. [39,52] calculated prevalence estimates using the lowest gene concentration measured in wastewater, two point-estimates of viral genomes per ml/grams of stool (i.e., 6 × 105 and 30 × 106), wastewater flows and size of the served populations. Prevalence estimates ranged from 5% to 0.1%, versus a reported prevalence of 0.026% (based on positive test results at the time of the study). In their study, Weidhaas et al. [38] used a similar approach to calculate the incidence of infections in the populations and plotted these against confirmed cases. Instead of using point estimates for virus shedding and daily stool mass, Ahmed et al. [36] used probability distributions and computed prevalence estimates using Monte Carlo simulations. However, instead of using measured flows, these authors estimated the latter from population figures (residents) and an estimate of per capita wastewater production of 250 L/day [111]. Over a 4-day period, the estimated infection prevalence ranged from 0.064% to 0.142% (95% CI). A similar method was implemented also by Hemalatha et al. [110] yet it is not clear which parameters were used (i.e., population, flow or daily per capita wastewater production) or if Monte Carlo simulations were used. The authors also reported a second method, based on a previously reported approach by Hellmér et al. [5], which compared actual number of RNA copies per ml wastewater with the number of RNA particles expected to be present in wastewater per infected person. The two approaches provided very similar results, likely because most of the variables (point estimates) used were the same, and a prevalence of 6.6% was estimated.
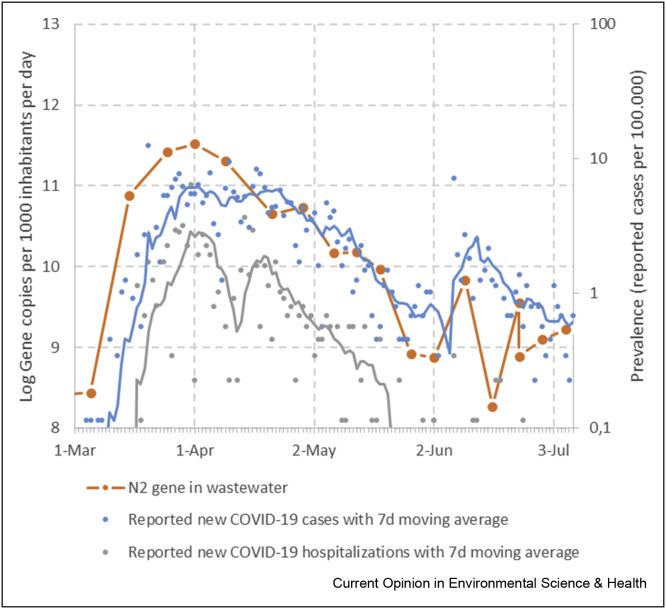
Figure 7.
Comparison of sewage surveillance data and prevalence data for Amsterdam, The Netherlands, from Mar 1 to Jul 8, 2020. Load of SARS-CoV-2 RNA (N2 gene assay) in wastewater at the inlet of the Amsterdam WWTP (orange line and points; methods see Medema et al., 2020). Prevalence of laboratory-confirmed COVID-19 cases (blue points, data: National Institute of Public Health and the Environment, Netherlands), with 7d moving average (blue line) and of COVID-19 hospitalizations (grey points, data: National Institute of Public Health and the Environment, Netherlands), with 7d moving average (grey line).
Ahmed et al. [36] indicated that the estimated prevalence matched with the observed prevalence, whereas the wastewater-based prevalence estimates of Wu et al. [39] and Kumar et al. [41] where considerably higher than the observed prevalence. The limited data on, and the variability of virus shedding over the course of the infection and between individuals make such estimates highly uncertain. But, as indicated, the observed prevalence data used in these studies, based on reported, laboratory-confirmed COVID-19 cases, is not an accurate reflection of the true prevalence of SARS-CoV-2 infections and seroprevalence data or prevalence estimates by epidemiological models would be more appropriate to compare against sewage surveillance data.
Outlook: application for public health decision making
Sewage surveillance of SARS-CoV-2 has grown very rapidly. Initially, studies have shown ‘proof-of-concept’ of sewage surveillance as sensitive early warning tool for COVID-19 surveillance in (sewered) cities [33,36,39,53,112]. This has triggered many follow-up studies, a global summit about sewage surveillance organised by the Water Research Foundation [113] and a lot of attention in the press. In the European Union, an EU-wide feasibility study has been started. WWTP in 52 cities of 17 countries are being tested for SARS-CoV-2 and SARS-CoV-2 has been detected in 28 WWTP in concentrations 1 - 946 gene (N2) copies per ml. This study also aims to connect the national and regional sewage surveillance initiatives in EU Member States by engaging in comparative testing and proficiency studies [114]. Several countries (Australia, Finland, France, Italy, Netherlands, Portugal, Spain) have engaged in national sewage surveillance programs, of which the program of the Netherlands is most ambitious (monitor all >300 WWTP in the country, every day). This review discusses of the strength of the current surveillance strategies and methods and of the correlation between wastewater data and prevalence data, with reference to the knowledge generated by earlier sewage surveillance of poliovirus and WBE of drugs and other chemical compounds, and provides several recommendations for improving design, methods and interpretation of sewage surveillance programs for SARS-CoV-2. As discussed, application of sewage surveillance to generate prevalence estimates is still uncertain and would need more data on virus RNA shedding, particularly by a- or presymptomatic individuals, good quality assurance of the methods to detect virus RNA in wastewater (sludge) and appropriate normalization of virus RNA loads in wastewater. Comparing sewage surveillance data with seroprevalence data and prevalence estimates from epidemiological models can yield mutual improvements in the certainty of the prevalence estimates, and hence in the value of sewage surveillance. But the largest added value of sewage surveillance lies in the application as cost-effective early warning tool for (re-)emergence of SARS-CoV-2. Table 3 illustrates how using sewage surveillance alongside individual testing can support public health decisions. When trends in individual case reporting and sewage numbers are consistent, added confidence is provided to public health authorities in their current course of action. When the sewage numbers are inconsistent with reported cases, then the sewage numbers can provide the valuable early warning that has been discussed. A few days advance warning of a new outbreak in a small community can improve preparedness (particularly in more remote settings) and limit transmission by raising awareness for increased prevention measures in the small population sooner.
Table 3.
Illustration of the value of sewage surveillance alongside individual case testing for early warning and support of public health decisions.
| Scenario | Surveillance | Interpretation | Possible Action | |
|---|---|---|---|---|
| Reported cases from individual testing | Sewage testing (e.g. weekly testing program) | |||
| Large city, following a COVID-19 wave. | Low number of daily reported cases | Low numbers in sewage (or not detected) | Consistent |
|
| An increase in load by around an order of magnitude |
Inconsistent
|
|
||
| Large city, in the midst of a COVID-19 wave | High numbers (increasing or plateauing) of daily reported cases | Load increasing or plateauing |
|
|
| Load decreasing |
|
|
||
| Small community, believed to be free of COVID-19 | Zero reported cases | Not detected | Consistent |
|
| Detected | Inconsistent
|
|
||
On a broader scale, several studies have demonstrated that sewage surveillance could detect increases in virus circulation before this was observed in cases reported through the health surveillance [33,34]. Our continued surveillance of SARS-CoV-2 RNA in wastewater of Amsterdam shows that the virus loads (as measured with the N2 qPCR assay) match the trends in reported laboratory-confirmed COVID-19 cases and hospitalizations in the Amsterdam population (Fig. 6), which provides confidence in the validity of the wastewater data to reflect the virus circulation in the population. In addition, trend analysis requires relative rather than absolute data. As long as the wastewater data from a WWTP are generated by the same laboratory with the same protocol, the data are suited for trend analysis. Given the current multitude of methods that are used in the different sewage surveillance laboratories, a use case that require relative data (early warning) is more valid than a use case that requires more absolute data (prevalence estimate).
Specific cases may however benefit from the more absolute quantitative approach. For example, in a small community (Table 3) where SARS-CoV-2 is detected in the wastewater occurs when there are zero known cases in the population. Assessing the magnitude of the concentration would be helpful to ask: How likely is it that the concentration represents one or more shedders in the population? How many infected people could there be? Was the magnitude of shedding consistent with an individual in the early stage of infection (and hence infectious) or in the later prolonged shedding phase? Even though there is a great deal of uncertainty in the relationship, when used with care for investigating specific questions it may prove to be a very valuable tool.
Given the current challenges, to maximise the opportunities of wastewater surveillance for supporting public health decisions, the following factors are emphasized:
-
•
Many aspects of wastewater sampling and analysis affect the measured SARS-CoV-2 concentration in wastewater: representative site selection, type of sampling, sample storage, concentration and quantification methods. We argue for the use of 24 -h composite sampling, use of replicates and controls to determine the recovery efficiency of each step of the method.
-
•
Wastewater analysis results should be reported as viral loads and/or normalized using specific population or faecal markers, rather than virus concentrations which are susceptible to dilution effects.
-
•
To improve the correlation between wastewater SARS-CoV-2 data and prevalence data, the use of normalized viral load data from wastewater, data on acute and cumulative prevalence and prevalence estimates from serosurveys and epidemiological models, and matching of the population these data are collected on is recommended.
-
•
Trend analysis of viral loads in wastewater or primary sludge currently provides the most value for wastewater monitoring programs in the short term and may provide an early warning of (re-)emergence of COVID-19 in cities.
-
•
Inference about prevalence from wastewater concentrations will require the development of comprehensive mathematical frameworks and combination with seroprevalence data and epidemiological models.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Ronald Italiaander and Goffe Elsinga for the SARS-CoV-2 RNA decay data. The research was supported by the Dutch Government (RVO: TKI Watertechnology).
This review comes from a themed issue on Environmental Health: COVID-19
Edited by Avelino Núñez-Delgado
References
- 1.Asghar H., Diop O.M., Weldegebriel G., Malik F., Shetty S., Bassioni L El, Akande A.O., Maamoun E Al, Zaidi S., Adeniji A.J. Environmental surveillance for polioviruses in the global polio eradication initiative. J Infect Dis. 2014;210:S294–S303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betancourt W., Shulman L. Global water pathogen project. Michigan State University; 2019. Polioviruses and other enteroviruses. [Google Scholar]
- 3.Van Der Avoort H.G.A.M., Reimerink J.H.J., Ras A., Mulders M.N., Van Loon A.M. Isolation of epidemic poliovirus from sewage during the 1992–3 type 3 outbreak in The Netherlands. Epidemiol Infect. 1995;114:481–491. doi: 10.1017/s0950268800052195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GPEI . 2019. Eradication, integration, certification and containment. [Google Scholar]
- 5.Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl Environ Microbiol. 2014;80:6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaconelli M., Ferraro G.B., Mancini P., Suffredini E., Veneri C., Ciccaglione A.R., Bruni R., Libera S. Della, Bignami F., Brambilla M. Nine-year nationwide environmental surveillance of hepatitis E virus in urban wastewaters in Italy (2011–2019) Int J Environ Res Publ Health. 2020:17. doi: 10.3390/ijerph17062059. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large-scale investigation into Hepatitis E virus in urban sewage over 9 years (2011–2019), collecting 1374 sewage samples from 48 wastewater treatment plants located in all the 20 regions of Italy. Study demonstrates use of sewage surveillance to show considerable circulation of Hepatitis E virus in the Italian population, despite a relatively small number of notified cases, geographical distribution and, using sequencing, predominance of G3 strains.
- 7.Hendriksen R.S., Munk P., Njage P., van Bunnik B., McNally L., Lukjancenko O., Röder T., Nieuwenhuijse D., Pedersen S.K., Kjeldgaard J. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat Commun. 2019;10:1–12. doi: 10.1038/s41467-019-08853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580:176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- 9.Weiss A., Jellingsø M., Sommer M.O.A. Spatial and temporal dynamics of SARS-CoV-2 in COVID-19 patients: a systematic review and meta-analysis. EBioMedicine. 2020;58:102916. doi: 10.1016/j.ebiom.2020.102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ Res. 2020;191:110092. doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]; Well-designed study into decay of the SARS-CoV-2 RNA signal in wastewater at different temperatures, indicating the signal is persisting long enough in wastewater for sewage surveillance. Decay rate constants can be used in sewer models.
- 11.O'Reilly K.M., Allen D.J., Fine P., Asghar H. The challenges of informative wastewater sampling for SARS-CoV-2 must be met: lessons from polio eradication. The Lancet Microbe. 2020;1:e189–e190. doi: 10.1016/S2666-5247(20)30100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carducci A., Federigi I., Dasheng L., Julian R.T., Marco V. Making waves: coronavirus detection, presence and persistence in the water environment: state of the art and knowledge needs for public health. Water Res. 2020;179:115907. doi: 10.1016/j.watres.2020.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179:115899. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci Total Environ. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson J.R., Nancharaiah Y.V., Gu X., Lee W.L., Rajal V.B., Haines M.B., Girones R., Ng L.C., Alm E.J., Wuertz S. Making waves: wastewater surveillance of SARS-CoV-2 for population-based health management. Water Res. 2020;184:116181. doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corpuz M.V.A., Buonerba A., Vigliotta G., Zarra T., Ballesteros F., Campiglia P., Belgiorno V., Korshin G., Naddeo V. Viruses in wastewater: occurrence, abundance and detection methods. Sci Total Environ. 2020;745:140910. doi: 10.1016/j.scitotenv.2020.140910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amoah I.D., Kumari S., Bux F. Coronaviruses in wastewater processes: source, fate and potential risks. Environ Int. 2020;143:105962. doi: 10.1016/j.envint.2020.105962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]; Modeling study to examine the feasibility, economy, opportunities and challenges of enumerating active coronavirus infections locally and globally using sewage surveillance. Study indicates that the sensitivity is around one symptomatic/asymptomatic infected case per 100 to 2,000,000 non-infected people. Sewage surveillance is shown to be orders of magnitude cheaper and faster than clinical screening for COVID-19 in the population, yet cannot fully replace it.
- 20.Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ Sci Technol. 2020:54. doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- 21.WHO . 2006. Communicable disease surveillance and response systems Guide to monitoring and evaluating. [Google Scholar]
- 22.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO coronavirus disease (COVID-19) dashboard | WHO coronavirus disease (COVID-19) dashboard. [accessed Oct 19, 2020],
- 24.CDC: Updated guidance on testing persons for coronavirus disease 2019 (COVID-19). [accessed Oct 19, 2020],
- 25.ECDC: Diagnostic testing and screening for SARS-CoV-2. [accessed Oct 19, 2020],
- 26.COVID-19 map - johns hopkins coronavirus resource center. [accessed Oct 19, 2020],
- 27.Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J., Fry A.M., Cannon D.L., Chiang C.F., Gibbons A. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-may 12, 2020. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4130. [DOI] [PubMed] [Google Scholar]
- 28.ECDC: Immune responses and immunity to SARS-CoV-2. [accessed Oct 19, 2020],
- Arora R.K., Joseph A., Van Wyk J., Rocco S., Atmaja A., May E., Yan T., Bobrovitz N., Chevrier J., Cheng M.P. SeroTracker: a global SARS-CoV-2 seroprevalence dashboard. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; A dashboard that systematically monitors and synthesises findings from hundreds of global SARS-CoV-2 serological studies. The dashboard allows users to visualise seroprevalence estimates on a world map and compare estimates between regions, population groups, and testing modalities. Important reference data for sewage surveillance studies.
- 30.Gibbons C.L., MJJ Mangen, Plass D., Havelaar A.H., Brooke R.J., Kramarz P., Peterson K.L., Stuurman A.L., Cassini A., Fèvre E.M. Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Publ Health. 2014;14:147. doi: 10.1186/1471-2458-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan V.W.-S., Chiu P.K.-F., Yee C.-H., Yuan Y., Ng C.-F., Teoh J.Y.-C. A systematic review on COVID-19: urological manifestations, viral RNA detection and special considerations in urological conditions. World J Urol. 2020 doi: 10.1007/s00345-020-03246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Döhla M., Wilbring G., Schulte B., Kümmerer B.M., Diegmann C., Sib E., Richter E., Haag A., Engelhart S., Eis-Hübinger A.M. SARS-CoV-2 in environmental samples of quarantined households. medRxiv. 2020;49:1–19. doi: 10.3390/v14051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ Sci Technol Lett. 2020:7. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat Biotechnol. 2020 doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sewage surveillance of SARS-CoV-2 showing parallel time series between normalized SARS-CoV-2 gene copies/ml of sludge and reported number of cases and hospitalizations. Correlation coefficient at maximum if the wastewater concentration were moved forward for 7 days and 3 days with confirmed cases and hospitalizations respectively, showing early warning potential of sewage surveillance.
- WHO: Status of environmental surveillance for SARS-CoV-2 virus. [accessed Sep 24, 2020]; This brief explores potential use cases, considerations, and research needs for this emerging tool for SARS-CoV-2 detection that may be explored in close coordination with established public health surveillance for COVID-19.
- 36.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weidhaas J., Aanderud Z., Roper D., VanDerslice J., Gaddis E., Ostermiller J., Hoffman K., Jamal R., Heck P., Zhang Y. 2020. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5 doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ort C., Lawrence M.G., Reungoat J., Mueller J.F. Sampling for PPCPs in wastewater systems: comparison of different sampling modes and optimization strategies. Environ Sci Technol. 2010;44:6289–6296. doi: 10.1021/es100778d. [DOI] [PubMed] [Google Scholar]
- 43.Ort C., Lawrence M.G., Rieckermann J., Joss A. Sampling for pharmaceuticals and personal care products (PPCPs) and illicit drugs in wastewater systems: are your conclusions valid? A critical review. Environ Sci Technol. 2010;44:6024–6035. doi: 10.1021/es100779n. [DOI] [PubMed] [Google Scholar]
- 44.Ort C., Gujer W. Sampling for representative micropollutant loads in sewer systems. Water Sci Technol. 2006;54:169–176. doi: 10.2166/wst.2006.591. [DOI] [PubMed] [Google Scholar]
- 45.Teerlink J., Hering A.S., Higgins C.P., Drewes J.E. Variability of trace organic chemical concentrations in raw wastewater at three distinct sewershed scales. Water Res. 2012;46:3261–3271. doi: 10.1016/j.watres.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Hata A., Honda R., Hara-Yamamura, Meuchi Y. Detection of SARS-CoV-2 in wastewater in Japan by multiple molecular assays-implication for wastewater-based epidemiology (WBE) medRxiv. 2020 doi: 10.1101/2020.06.09.20126417. [DOI] [Google Scholar]
- 47.Heaton K.W., Radvan J., Cripps H., Mountford R.A., Braddon F.E.M., Hughes A.O. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818–824. doi: 10.1136/gut.33.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bassotti G., Bellini M., Pucciani F., Bocchini R., Bove A., Alduini P., Battaglia E., Bruzzi P., Chistolini F., Morelli A. An extended assessment of bowel habits in a general population. World J Gastroenterol. 2004;10:713–716. doi: 10.3748/wjg.v10.i5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Judkins T.C., Dennis-Wall J.C., Sims S.M., Colee J., Langkamp-Henken B. Stool frequency and form and gastrointestinal symptoms differ by day of the menstrual cycle in healthy adult women taking oral contraceptives: a prospective observational study. BMC Wom Health. 2020;20 doi: 10.1186/s12905-020-01000-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapo K.E., Paschka M., Vamshi R., Sebasky M., McDonough K. Estimation of U.S. sewer residence time distributions for national-scale risk assessment of down-the-drain chemicals. Sci Total Environ. 2017;603–604:445–452. doi: 10.1016/j.scitotenv.2017.06.075. [DOI] [PubMed] [Google Scholar]
- 51.Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Reports Med. 2020;1:100098. doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., Bonneau R., Brown M.A., Bushman M., Chai P.R. SARS-CoV-2 titers in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. medRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.06.15.20117747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wurtzer S., Marechal V., Mouchel J.-M., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. medRxiv. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parasa S., Desai M., Thoguluva Chandrasekar V., Patel H.K., Kennedy K.F., Roesch T., Spadaccini M., Colombo M., Gabbiadini R., Artifon E.L.A. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larrarte F., Pons M.N. Suspended solids concentration in wastewater: influence of sampling conditions. Urban Water J. 2011;8:397–404. [Google Scholar]
- D'Aoust P.M., Mercier É., Montpetit D., Jia J.-J., Alexandrov I., Tariq Baig A., Mayne J., Zhang X., Alain T., Servos M.R. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities 1 with low COVID-19 incidence and prevalence 2. medRxiv. 2020 doi: 10.1101/2020.08.11.20173062. [DOI] [PMC free article] [PubMed] [Google Scholar]; Well-designed sewage surveillance study, matching the population that was monitored via the sewershed and via nasopharyngeal swabs via ZIP code and used Pepper Mild Mottle Virus to normalize the SARS-CoV-2 RNA concentration in wastewater.
- 57.La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R. Evaluation of heating and chemical protocols for inactivating SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.04.11.036855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rusiñol M., Martínez-Puchol S., Forés E., Itarte M., Girones R., Bofill-Mas S. Concentration methods for the quantification of coronavirus and other potentially pandemic enveloped virus from wastewater. Curr Opin Environ Sci Heal. 2020;17:21–28. doi: 10.1016/j.coesh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ Sci Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- 62.Hjelmsø M.H., Hellmér M., Fernandez-Cassi X., Timoneda N., Lukjancenko O., Seidel M., Elsässer D., Aarestrup F.M., Löfström C., Bofill-Mas S. Evaluation of methods for the concentration and extraction of viruses from sewage in the context of metagenomic sequencing. PloS One. 2017;12 doi: 10.1371/journal.pone.0170199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huggett J.F., Cowen S., Foy C.A. Considerations for digital PCR as an accurate molecular diagnostic tool. Clin Chem. 2015;61:79–88. doi: 10.1373/clinchem.2014.221366. [DOI] [PubMed] [Google Scholar]
- 64.Falzone L., Musso N., Gattuso G., Bongiorno D., Palermo C.I., Scalia G., Libra M., Stefani S. Sensitivity assessment of droplet digital PCR for SARS-CoV-2 detection. Int J Mol Med. 2020;46:957–964. doi: 10.3892/ijmm.2020.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suo T., Liu X., Feng J., Guo M., Hu W., Guo D., Ullah H., Yang Y., Zhang Q., Wang X. ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg Microb Infect. 2020;9:1259–1268. doi: 10.1080/22221751.2020.1772678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chavarria-Miró G., Anfruns-Estrada E., Guix S., Paraira M., Galofré B., Sáanchez G., Pintó R., Bosch A. Sentinel surveillance of SARS-CoV-2 in wastewater anticipates the occurrence of COVID-19 cases. medRxiv. 2020 doi: 10.1101/2020.06.13.20129627. [DOI] [Google Scholar]
- Westhaus S., Weber F.A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T. Detection of SARS-CoV-2 in raw and treated wastewater in Germany – suitability for COVID-19 surveillance and potential transmission risks. Sci Total Environ. 2021;751:141750. doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sewage surveillance study showing a correlation between SARS-CoV-2 load in wastewater (both with and without creatinine normalization) and acute and cumulative prevalence, but not with SARS-CoV-2 concentration in wastewater. Also highlight use of sequencing to confirm SARS-CoV-2 signal in wastewater and absence of infectious SARS-CoV-2 in wastewater.
- 68.Daughton C.G. Real-time estimation of small-area populations with human biomarkers in sewage. Sci Total Environ. 2012;414:6–21. doi: 10.1016/j.scitotenv.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 69.Gracia-Lor E., Castiglioni S., Bade R., Been F., Castrignanò E., Covaci A., González-Mariño I., Hapeshi E., Kasprzyk-Hordern B., Kinyua J. Measuring biomarkers in wastewater as a new source of epidemiological information: current state and future perspectives. Environ Int. 2017;99:131–150. doi: 10.1016/j.envint.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 70.O'Brien J.W., Thai P.K., Eaglesham G., Ort C., Scheidegger A., Carter S., Lai F.Y., Mueller J.F. A model to estimate the population contributing to the wastewater using samples collected on census day. Environ Sci Technol. 2014;48:517–525. doi: 10.1021/es403251g. [DOI] [PubMed] [Google Scholar]
- 71.Been F., Rossi L., Ort C., Rudaz S., Delémont O., Esseiva P. Population normalization with ammonium in wastewater-based epidemiology: application to illicit drug monitoring. Environ Sci Technol. 2014;48:8162–8169. doi: 10.1021/es5008388. [DOI] [PubMed] [Google Scholar]
- Baz-Lomba J.A., Di Ruscio F., Amador A., Reid M., Thomas K.V. Assessing alternative population size proxies in a wastewater catchment area using mobile device data. Environ Sci Technol. 2019;53:1994–2001. doi: 10.1021/acs.est.8b05389. [DOI] [PubMed] [Google Scholar]; Innovative use of mobile phone data for population mobility and population estimates in combination with wastewater-based epidemiology for illicit drugs, which could work in (post-) lockdown settings to determine population mobility.
- 73.COVID-19 - mobility trends reports - apple. [accessed Oct 19, 2020],
- 74.Thomas K.V., Bijlsma L., Castiglioni S., Covaci A., Emke E., Grabic R., Hernández F., Karolak S., Kasprzyk-Hordern B., Lindberg R.H. Comparing illicit drug use in 19 European cities through sewage analysis. Sci Total Environ. 2012;432:432–439. doi: 10.1016/j.scitotenv.2012.06.069. [DOI] [PubMed] [Google Scholar]
- 75.Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daughton C.G. Using biomarkers in sewage to monitor community-wide human health: isoprostanes as conceptual prototype. Sci Total Environ. 2012;424:16–38. doi: 10.1016/j.scitotenv.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 77.Chen C., Kostakis C., Gerber J.P., Tscharke B.J., Irvine R.J., White J.M. Towards finding a population biomarker for wastewater epidemiology studies. Sci Total Environ. 2014;487:621–628. doi: 10.1016/j.scitotenv.2013.11.075. [DOI] [PubMed] [Google Scholar]
- 78.Crank K., Li X., North D., Ferraro G.B., Iaconelli M., Mancini P., La Rosa G., Bibby K. CrAssphage abundance and correlation with molecular viral markers in Italian wastewater. Water Res. 2020;184:116161. doi: 10.1016/j.watres.2020.116161. [DOI] [PubMed] [Google Scholar]
- 79.Green H., Wilder M., Middleton F.A., Collins M., Fenty A., Gentile K., Kmush B., Zeng T., Larsen D.A. Quantification of SARS-CoV-2 and cross-assembly phage (crAssphage) from wastewater to monitor coronavirus transmission within communities. medRxiv. 2020;2 2020.05.21.20109181. [Google Scholar]
- 80.Pouzol T., Lévi Y., Bertrand-Krajewski J.L. Modelling daily and hourly loads of pharmaceuticals in urban wastewater. Int J Hyg Environ Health. 2020;229:113552. doi: 10.1016/j.ijheh.2020.113552. [DOI] [PubMed] [Google Scholar]
- 81.Plósz B.G., Leknes H., Liltved H., Thomas K.V. Diurnal variations in the occurrence and the fate of hormones and antibiotics in activated sludge wastewater treatment in Oslo, Norway. Sci Total Environ. 2010;408:1915–1924. doi: 10.1016/j.scitotenv.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 82.Coutu S., Wyrsch V., Wynn H.K., Rossi L., Barry D.A. Temporal dynamics of antibiotics in wastewater treatment plant influent. Sci Total Environ. 2013;458–460:20–26. doi: 10.1016/j.scitotenv.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 83.Ort C., van Nuijs A.L.N., Berset J.D., Bijlsma L., Castiglioni S., Covaci A., de Voogt P., Emke E., Fatta-Kassinos D., Griffiths P. Spatial differences and temporal changes in illicit drug use in Europe quantified by wastewater analysis. Addiction. 2014;109:1338–1352. doi: 10.1111/add.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harman C., Reid M., Thomas K.V. Concerning the viewpoint; “an anti-doping sampling strategy utilizing the sewerage systems of sport villages. Environ Sci Technol. 2011;45:4191. doi: 10.1021/es200839n. [DOI] [PubMed] [Google Scholar]
- 85.Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., Edson J., Ehret J., Gyawali P., Hamilton K.A. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. J Trav Med. 2020;27:1–11. doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai Y., Li X., Zaidi A.A., Shi Y., Zhang K., Feng R., Lin A., Liu C. Effect of hydraulic retention time on pollutants removal from real ship sewage treatment via a pilot-scale air-lift multilevel circulation membrane bioreactor. Chemosphere. 2019;236:124338. doi: 10.1016/j.chemosphere.2019.07.069. [DOI] [PubMed] [Google Scholar]
- 87.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA, J Am Med Assoc. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen C., Gao G., Xu Y., Pu L., Wang Q., Wang L., Wang W., Song Y., Chen M., Wang L. SARS-CoV-2-Positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann Intern Med. 2020;172:832–834. doi: 10.7326/M20-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]; Detailed investigation of SARS-CoV-2 RNA shedding in faeces of COVID-19 patients with time series and quantitative shedding data.
- 95.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amirian E.S. Potential fecal transmission of SARS-CoV-2: current evidence and implications for public health. Int J Infect Dis. 2020;95:363–370. doi: 10.1016/j.ijid.2020.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. SARS-CoV-2 viral load dynamics, duration of viral shedding and infectiousness: a living systematic review and meta-analysis. medRxiv. 2020 doi: 10.1016/S2666-5247(20)30172-5. pre-print:2020.07.25.20162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W.H., Tam A.R. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones Ab ✉ D.L., Quintela Baluja M., Graham D.W., Corbishley A., Mcdonald J.E., Malham S.K., Hillary L.S., Connor T.R., Gaze W.H., Moura I.B. 2020. Fecal shedding of SARS-CoV-2 and its potential role in person-to-person transmission and the environment-based spread of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K.A., Jordan K., Clyne B., Rohde D., Drummond L., Byrne P., Ahern S., Carty P.G., O'Brien K.K., O'Murchu E. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81:357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detailed investigation of SARS-CoV-2 RNA shedding in faeces of COVID-19 patients with time series and quantitative shedding data.
- 101.Furukawa N.W., Furukawa N.W., Brooks J.T., Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26:E1–E6. doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tindale L.C., Stockdale J.E., Coombe M., Garlock E.S., Lau W.Y.V., Saraswat M., Zhang L., Chen D., Wallinga J., Colijn C. Evidence for transmission of covid-19 prior to symptom onset. Elife. 2020;9:1–34. doi: 10.7554/eLife.57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beule A.G. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr Top Otorhinolaryngol, Head Neck Surg. 2010;9:Doc07. doi: 10.3205/cto000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taherali F., Varum F., Basit A.W. A slippery slope: on the origin, role and physiology of mucus. Adv Drug Deliv Rev. 2018;124:16–33. doi: 10.1016/j.addr.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 105.Humphrey S.P., Williamson R.T. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 106.Iorgulescu G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J Med Life. 2009;2:303–307. [PMC free article] [PubMed] [Google Scholar]
- 107.Rose C., Parker A., Jefferson B., Cartmell E. The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit Rev Environ Sci Technol. 2015;45:1827–1879. doi: 10.1080/10643389.2014.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones H.E., Hickman M., Kasprzyk-Hordern B., Welton N.J., Baker D.R., Ades A.E. Illicit and pharmaceutical drug consumption estimated via wastewater analysis. Part B: placing back-calculations in a formal statistical framework. Sci Total Environ. 2014;487:642–650. doi: 10.1016/j.scitotenv.2014.02.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Been F., Schneider C., Zobel F., Delémont O., Esseiva P. Integrating environmental and self-report data to refine cannabis prevalence estimates in a major urban area of Switzerland. Int J Drug Pol. 2016;36:33–42. doi: 10.1016/j.drugpo.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 110.Hemalatha M., Kiran U., Kumar Kuncha S., Kopperi H., Gokulan C.G., Mohan S.V., Mishra R.K. Comprehensive surveillance of SARS-CoV-2 spread using wastewater-based epidemiology studies. medRxiv. 2020 doi: 10.1101/2020.08.18.20177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tscharke B.J., O'Brien J.W., Ort C., Grant S., Gerber C., Bade R., Thai P.K., Thomas K.V., Mueller J.F. Harnessing the power of the census: characterizing wastewater treatment plant catchment populations for wastewater-based epidemiology. Environ Sci Technol. 2019;53:10303–10311. doi: 10.1021/acs.est.9b03447. [DOI] [PubMed] [Google Scholar]
- 112.Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol Hepatol. 2020;5:533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Water Research Foundation . 2020. T: Wastewater surveillance of the COVID-19 genetic signal in sewersheds: recommendations from global experts. [Google Scholar]
- 114.EU Joint Research Centre: EU creates a pan-European Umbrella study for early coronavirus detection in wastewater. [accessed Oct 19, 2020].