Abstract
We collect the nasopharyngeal and oropharyngeal swabs of 63 subjects with severe symptoms or contacts with COVID-19 confirmed cases to perform a pilot-study aimed to verify the “in situ” expression of SARS-CoV-2 host invasion genes (ACE2, TMPRSS2, PCSK3, EMILIN1, EMILIN2, MMRN1, MMRN2, DPP4). ACE2 (FC = +1.88, p ≤ 0.05) and DPP4 (FC = +3, p < 0.01) genes showed a significant overexpression in COVID-19 patients. ACE2 and DPP4 expression levels had a good performance (AUC = 0.75; p < 0.001) in distinguishing COVID-19 patients from negative subjects. Interestingly, we found a significant positive association of ACE2 mRNA and PCSK3, EMILIN1, MMRN1 and MMRN2 expression and of DPP4 mRNA and EMILIN2 expression only in COVID-19 patients. Noteworthy, a subgroup of severe COVID-19 (n = 7) patients, showed significant high level of ACE2 mRNA and another subgroup of less severe COVID-19 patients (n = 6) significant raised DPP4 levels.
These results indicate that a group of SARS-CoV-2 host invasion genes are functionally related in COVID-19 patients and suggests that ACE2 and DPP4 expression level could act as genomic biomarkers. Moreover, at the best of our knowledge, this is the first study that shows an elevated DPP4 expression in naso- and oropharyngeal swabs of COVID-19 patient thus suggesting a functional role of DPP4 in SARS-CoV-2 infections.
Keywords: Genetics, Biochemistry, Molecular biology, Clinical genetics, Virology, ACE2, DPP4, SARS-CoV-2, Covid-19, Host invasion genes, Genomic biomarkers
Genetics; Biochemistry; Molecular Biology; Clinical Genetics; Virology; ACE2; DPP4; SARS-CoV-2; Covid-19; Host invasion genes; Genomic Biomarkers
1. Introduction
SARS-CoV-2 betacoronavirus is the cause of worldwide disease COVID-19 [1]. COVID-19 ranges from asymptomatic or mild sub-clinical symptoms to acute respiratory distress syndrome (ARSD). COVID-19 manifests differently depending on patients age, underlying health conditions, and gender [2]. A growing body of evidence indicates sex differences in the clinical outcome of COVID-19 [3,4]. It is known that although men and women have a similar sensitivity to SARS-CoV-2 infection, men are more prone to have higher severity and mortality than women, regardless of age [5]. However, phenotypic variability of COVID-19 is a consequence also of the individual genetic variability, that can make certain subjects more susceptible or more resistant to viral infection and disease progression [6, 7]. Currently, no suitable vaccines are available for SARS-CoV-2, although there are ongoing clinical trials and also studies of treatments with monoclonal antibodies [8].
The identification of the molecular mechanisms underlying the development and progression of COVID-19 is crucial for identifying appropriate medical therapies to combat viral infection. The main mechanism of the entire process of infection include the action of different human proteins. ACE2 is the host receptor for the novel SARS-CoV-2 [9]. A recent expression profile of ACE2 RNA in lung at a single cell resolution suggested that ACE2 expression is concentrated in alveolar type II (AT2) cells [10]. SARS-CoV-2 entry also depends on the activity of the TMPRSS2 protease previously detected in the nasal and bronchial epithelium [4, 6] where TMPRSS2 is co-expressed together to ACE2 [9,11]. PCSK3/furin is one of the important proteases that facilitates viral invasion. In fact, it cleaves viral S protein and helps SARS-CoV-2 interaction with ACE2 receptor [12]. A main regulator of extracellular PCSK3/furin activity is EMILIN1 and, in particular, its N-terminal EMI domain [13]. EMILIN1 belongs to EMILIN/Multimerin family that includes also EMILIN2, Multimerin1 (MMRN1 or EMILIN4) and Multimerin2 (MMRN2 or EMILIN3), all containing an N-terminal EMI domain. EMILIN/Multimerin family is responsible for the activation of different precursor proteins as growth factors, hormones, receptors and adhesion molecules, as well as cell surface glycoproteins of infectious viruses [14]. Given their role in the activation of cell surface proteins, this protease family might be implicated in viral infections. Recently, another interaction between virus and host cell besides ACE2 receptor was suggested. DPP4, also known as cluster of differentiation 26 (CD26), may interact with the viral S glycoprotein [15]. DPP4 is a type II transmembrane glycoprotein expressed in alveolar epithelial cells, endothelial cells and in bronchiolar epithelial cells [16], where it performs different functions as post-translational cleavage of hormones and chemokines, T-cell activation, cell adhesion, and apoptosis [17].
The combination of binding and activation allows the virus to enter the host cell, so the expression and distribution of these key proteins decides the path of infection by the virus and this is important for understanding pathogenesis and designing therapeutic strategies. In this pilot-study, we investigate the expression pattern and level of SARS-CoV-2 host invasion genes (ACE2, TMPRSS2, PCSK3, EMILIN1, EMILIN2, MMRN1, MMRN2, DPP4) in nasopharyngeal and oropharyngeal swabs of negative subjects and severely affected COVID-19 patients. In fact, we collected naso- and oropharyngeal swabs in the period 20th March-20th April 2020, that was the period with raised numbers of affected patients and also with worse prognosis in Italy [18].
ACE2 and DPP4 genes resulted over-expressed in nasopharyngeal and oropharyngeal swabs of COVID-19 vs negative subjects. Noteworthy, a positive correlation among ACE2 mRNA and PCSK3, EMILIN1, MMRN1 and MMRN2 expression and between DPP4 mRNA and EMILIN2 expression only in COVID-19 patients suggest that a coordinated expression pattern of these genes is crucial for SARS-CoV-2 infection and these expression patterns may be useful for predictive diagnosis, prognosis and pathophysiology of COVID-19.
2. Materials and methods
2.1. Patients’ recruitment and sample collection
The nasopharyngeal and oropharyngeal swabs of 63 cases with acute respiratory symptoms or contacts with COVID-19 confirmed cases were collected from 20 March to 20 April 2020, during triage at the Emergency Room (ER) of Policlinico Tor Vergata, PTV (Rome, Italy). Patients’ swabs, collected in viral transport media (VTM), were referred to the Virology Unit of PTV for diagnosis. Residual positive (n = 35) and negative (n = 28) samples for SARS-CoV-2 were used for RNA expression analysis. The ethical committee of Tor Vergata University/Hospital (protocol no. 50/20) approved this study. Informed written consent was obtained from each patient.
2.2. Diagnostic test of SARS-CoV-2
To detect the qualitative presence of SARS-CoV-2 viral nucleic acids, we used the Allplex™ 2019-nCoV Assay (Seegene Inc.) (http://www.seegene.com/upload/product/Allplex_2019_nCoV_performance_data.pdf). Nucleic acids were isolated and purified from 300 μL of specimen using an automated nucleic acid extraction system. The assay is designed to detect SARS-CoV-2 RdRP and N genes and E gene of all Sarbecovirus including SARS-CoV-2. Briefly, first line screening was done for E and RNase P (internal control) genes. Clinical samples positive for E gene (Ct ≤ 35.0) were subjected to confirmatory test with primers specific for RdRp and HKU ORF (HKU-orf1-nsp14). Positive Control and No Template Control were run for all genes. A specimen was considered confirmed positive for SARS-CoV-2 if reaction growth curves crossed the threshold line within 35 cycles (Ct cut off ≤ 35.0) for E gene, and both RdRp, ORF or either RdRp or ORF.
2.3. Gene expression studies
RNA extracted from nasopharyngeal and oropharyngeal swabs was evaluated by NanoDrop DS-11 (DeNovix) (5–40 ng/μl and 5–30 ng/μl, respectively for DNA and RNA) with a 260/280 ratio of ~1.8 for DNA and of ~2.0 for RNA. 100 ng of total RNA has been retrotranscribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). We analyzed the expression of ACE2, TMPRSS2, PCSK3, EMILIN1, EMILIN2, MMRN1, MMRN2 and DPP4 genes. Real time PCR (qRT-PCR) has been performed using ABI7500 Fast Real-time PCR System (Life Technologies) and specific primer pairs (Table 1).
Table 1.
Real-Time PCR primer sequences and amplification settings.
| Genes | Accession number | Sequence (5′-3′) | Annealing temperature (C°) | Size (bp) | |
|---|---|---|---|---|---|
| ACE2 | NM_001371415 | Fw | GGTGGGAGATGAAGCGAGAG | 60 | 150 |
| Rev | CTTGAAACTGGAATTGGTAAAGGGT | ||||
| TMPRSS2 | NM_005656 | Fw | GGGACATGGGCTATAAGAAT | 53 | 137 |
| Rev | ACAGGCATCACTGTGGTAC | ||||
| PCSK3 | NM_002569 | Fw | GCATTGGAGGGGTGCGCAT | 61 | 106 |
| Rev | CCCCAGCTGGCACTGTAGAT | ||||
| EMILIN1 | NM_007046 | Fw | CCAAAGCATCATGTACCGCC | 58 | 110 |
| Rev | CACAGTCATCGCCCCCATAA | ||||
| MMRN1 | NM_007351 | Fw | TAGCTCCAGATTTTTCCAAAGGA | 57 | 112 |
| Rev | TCCAAGTTATTAAACAGGATAGGAC | ||||
| EMILIN2 | NM_032048 | Fw | TGTCTGGGCGGGGTCTG | 62 | 102 |
| Rev | GTGACTTCGGGTATCCTGGTG | ||||
| MMRN2 | NM_024756 | Fw | CCAGGCTTCCAGTACTAGCC | 59 | 111 |
| Rev | GGGCACCAGTTACGTCCTAC | ||||
| DPP4 | NM_001935 | Fw | GAAAGGTGTCAGTACTATTCTGTGT | 59 | 130 |
| Rev | CCAGGACTCTCAGCCCTTTATC | ||||
| GAPDH | NM_002046 | Fw | AAGGTCGGAGTCAACGGATTT | 59 | 100 |
| Rev | TGAAGGGGTCATTGATGGCA | ||||
| RPLP0 | NM_001002 | Fw | ACCCAGCTCTGGAGAAACT | 57 | 198 |
| Rev | AAAAGGAGGTCTTCTCGGG | ||||
| ACTB | NM_001101 | Fw | ATTGCCGACAGGATGCAGAA | 59 | 150 |
| Rev | GCTGATCCACATCTGCTGGAA |
ACE2 (Angiotensin-converting enzyme 2), TMPRSS2 (Transmembrane protease serine 2), PCSK3 (proprotein convertase subtilisin/kexin 3), EMILIN1 (elastin microfibrils interface located protein-1), MMRN1 (multimerin-1), EMILIN2 (elastin microfibrils interface located protein-2), MMRN2 (multimerin-2), DPP4 (Dipeptidyl peptidase-4), GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase), RPLP0 (ribosomal protein, large, P0), ACTB (β-Actin). Fw (Forward), Rev (Reverse). PCR (Polymerase Chain Reaction).
Primers have been designed and evaluated by using two different software: Primer BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and Tm Calculator (www.thermofisher.com). Primers were designed according the following criteria: 1. At least one primer must be designed at an exon-exon junction; 2. Primer pair must be separated by at least one intron on the corresponding genomic DNA; 3. PCR product size must be about 100–200 bp; 4. Common SNPs in the primers have been excluded after evaluation on Ensembl Genome Browser (https://www.ensembl.org/index.html); 5. Primers must amplify all the known isoform of each gene, when present. GAPDH, ACTB and RPLP0 genes were used for data normalization. The qRT-PCR expression analyses were performed in triplicate. Data analysis was performed using the comparative threshold cycle (Ct) method quantification (2−ΔCt method) (https://www.protocols.io/view/comparative-ct-method-quantification-2-ct-method-zp7f5rn).
2.4. Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software, USA) and SPSS program, version 19 (IBM Corp, USA). Kolmogorov-Smirnov test was used to analyze the distribution of expression data from qRT-PCR assays. Mann-Whitney test and Kruskal-Wallis test were used for data analysis as appropriate. For parametric and non-parametric distribution, expression data are represented as mean and range. Clinical details are represented as mean and standard deviation (SD). For all analysis, significance was set at p ≤ 0.05.
3. Results
3.1. Clinical study
The 63 naso- and oropharyngeal swabs analysed in this pilot-study were collected in the period 20th March-20th April 2020 that was the most critical for SARS-CoV-2 infection in Italy. Thirty-five patients showed symptoms (dry cough, fever and dyspnea) referable to COVID-19; others (n = 28) had contacts with SARS-CoV-2 positive patients but resulted negative after the diagnostic test. All COVID-19 patients (n = 35) were hospitalized at Policlinico Tor Vergata, PTV (Rome, Italy), while most of the negative subjects (19/28) returned home or were kept under brief observation. Clinical data of the 63 subjects studied are summarized and reported in Table 2.
Table 2.
Clinical data of the 63 subjects studied.
| Characteristic | SARS-CoV-2 diagnostic test |
|
|---|---|---|
| Negative | Positive | |
| No. | 28 | 35 |
| Age | 64.0 ± 17.0 | 59.0 ± 16.0 |
| Male (%) | 71.4 | 72.0 |
| Female (%) | 28.6 | 28.0 |
| Dry Cough (%) | N.A. | 31.4 |
| Fever (%) | N.A. | 65.7 |
| Dyspnea (%) | N.A. | 57.1 |
| Gastrointestinal disorders (%) | N.A. | 8.6 |
| FiO2 range (n, %) | 24–31 | 21 (n = 3, 8.6) |
| 35–50 (n = 12, 34.3) | ||
| 35–80 (n = 2, 5.7) | ||
| 50–60 (n = 5, 14.3) | ||
| 50–80 (n = 2, 5.7) | ||
| 60–80 (n = 3, 8.6) | ||
| 80–100 (n = 2, 5.7) | ||
| N.A. (n = 7, 20.0) | ||
| Hypertension, (%) | N.A. | 31.4 |
| Neu (103/μL) | 7.8 ± 4.2 | 5.2 ± 3.3 (∗neutrophilia in 7/35 patients: 9.9 ± 4.6) |
| Lym (103/μL) | 2.8 ± 5.2 | 1.3 ± 0.7 (∗important lymphopenia in 23/35 patients: 0.9 ± 0.4) |
| CRP (mg/L) | 107.3 ± 90.4 | 67.2 ± 75.2(∗elevated values in 25/35 patients: 93.1 ± 74.5) |
| LDH (U/L) | 737.8 ± 1652.5 | 364.1 ± 192.2 (∗elevated values in 25/35 patients: 407.8 ± 189.0) |
| TNF-α (pg/ml) | N.A. | 31.8 ± 36.7 |
| IL-6 (gr/dl) | N.A. | 40.9 ± 46.6 |
| Trop I hs (ng/L) | N.A. | 117.9 ± 339.9 |
| Fibrinogen (mean mg/dl) | 551.4 ± 272.7 | 573.7 ± 198.4 |
| D-dimer (mean ng/mL) | 1811.2 ± 1875.8 | 3083.4 ± 7088.3 |
Continuous data are expressed as mean ± standard deviation (SD); categorical data are expressed as percentage (%), n (number of cases). FiO2 (Fraction of inspired oxygen). Neu (neutrophils; normal values 1,5–7 103/mL). Lym (lymphocyte; normal values 1,5-5 103/mL). CRP (C-Reactive Protein; normal values 0–5 mg/L). LDH (Lactate dehydrogenase; normal values 125–220 U/L). TNF-α (Tumor Necrosis Factor alfa; normal values < 50 pg/ml). IL-6 (Interleukin-6; normal range values = 4.6–12.4; low <4.6; high> 12.4). Fibrinogen (normal values 200–400 mg/dl). D-dimer (normal values 0–500 ng/ml). N.A. (Not Available).
Most of the enrolled subjects were male (46/63; 73%). Age ranged between 20 and 92 years old (median age 61 years old). Twenty-seven of them are over 65 (18 men and 9 women). We clustered the 63 individuals in two groups: SARS-CoV-2 positive (COVID-19 patients, n = 35; 56%; 26 men and 9 women) and SARS-CoV-2 negative (negative subjects, n = 28; 44%; 20 men and 8 women). Age in COVID-19 patients ranged from 20 and 92 years old (median age 59 ± 16). Thirty-three out of 35 patients have been subjected to serological analysis, resulting positive for IgG only (94.3%). Eighteen out of 35 were hospitalized in the Infectious Diseases ward and thirteen out of 35 were hospitalized in the Respiratory Diseases ward. Four out of 35 patients needed Intensive Care Unit (ICU) and, after clinical stabilization, two of them went back to the Infectious Diseases ward. Four out of 35 patients (all men, 11.4%) died of concurrent causes associated with SARS-CoV-2, on average, two weeks after hospitalization. The average length of hospitalization was 48 days; the most serious patient was hospitalized for just over two months in ICU.
Twenty-six out of 35 patients (74.3%) were subjected to oxygen therapy at medium-low flows (FiO2 range 35–50%) with cycles of CPAP and BiPAP (FiO2 50–80%) for an average of 30 days of stay; two patients underwent invasive maneuveurs and therefore were intubated for an average of 14 days (FiO2 range 80–100%), while for seven subjects (20%) we were unable to find useful information about their ventilation (Table 2).
For the serum-blood analysis at the entrance, seven patients presented a dramatic neutrophilic condition (9.9 103/μL ± 4.6, Table 2), twenty-three patients presented an important lymphopenia (0.9 103/μL ± 0.4, Table 2). All subject presented no significant clinical CRP, LDH, IL-6 and TNF-α. One patient, later died, showed BNP value of 581 pg/mL (normal value < 100 pg/mL) associated with a Troponin I hs value of 1543.4 ng/L (normal value < 34.2). In all the patients, we found an elevation of both fibrinogen (mean: 573.7 mg/dl, Table 2) and D-dimer (mean: 3083.4 ng/mL, Table 2).
Age in SARS-CoV-2 negative group ranged from 27 and 84 years old (median age 64 ± 17). All these subjects resulted negative for SARS-CoV-2 diagnostic test. Twenty-four patients (n = 24/28; 85.7%) passed through the ER, eight of which (33.3%) were kept under brief observation and subsequently discharged; four (16.7%) have been transferred to competent departments responsible for further treatments and assessments. None of these patients undergone oxygen-therapy at medium-high flows, except for cases of respiratory support via Venturi Mask (VMK; FiO2 range 24–31%).
3.2. Gene expression level in SARS-CoV-2 positive and negative nasopharyngeal and oropharyngeal swabs
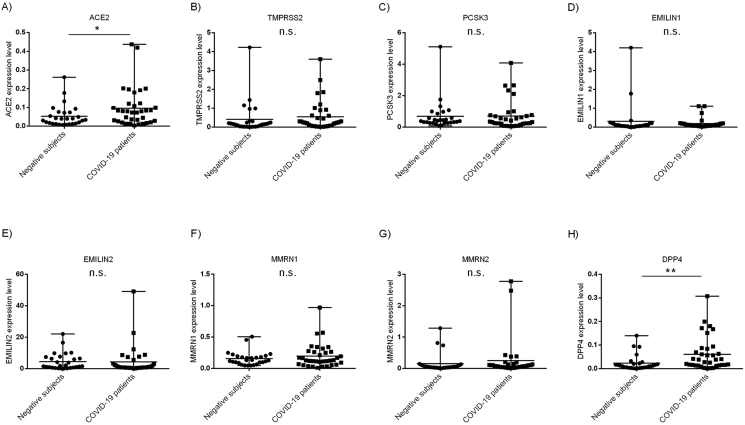
We analyzed the expression level of the eight SARS-CoV-2 host invasion genes by qRT-PCR using specific primer pairs (Table 1) on cDNA from residual unidentified nasopharyngeal and oropharyngeal swabs of 35 COVID-19 patients and 28 patients negative for SARS-CoV-2 virus presence. ACE2 (FC = +1.88, p ≤ 0.05) and DPP4 (FC = +3.0; p < 0.01) showed a significant overexpression in nasopharyngeal and oropharyngeal swabs of COVID-19 vs negative patients (Figure 1, A,H). No significant differences were observed in the other genes, even if most of them resulted overexpressed in COVID-19 patients’ swabs (Figure 1, B-G).
Figure 1.
Expression level of the eight genes involved in the SARS-CoV-2 entry into the host cell and infection. Kolmogorov-Smirnov test was used to analyze the distribution of expression data. Mann-Whitney test was used for data analysis. Expression data (2−ΔCt) are represented as mean and range. A) Expression level of ACE2 gene in nasopharyngeal and oropharyngeal swabs of COVID-19 vs negative patients (FC = +1.88, p ≤ 0.05); H) Expression level of DPP4 gene in nasopharyngeal and oropharyngeal swabs of COVID-19 vs negative patients (FC = +3.0; p < 0.01); B-G) Expression level of TMPRSS2, PCSK3, EMILIN1, EMILIN2, MMRN1, MMRN2 genes in nasopharyngeal and oropharyngeal swabs of COVID-19 vs negative patients.
Interestingly, seven COVID-19 patients (TP6, TP22, TP23, TP27, TP43, TP52, TP64; hereafter named as “ACE2h-COVID-19” showed higher ACE2 mRNA level in nasopharyngeal and oropharyngeal swabs (Figure 2A). Accordingly, the COVID-19 patients’ group without these seven subjects show no difference in nasopharyngeal and oropharyngeal ACE2 expression when compared with negative individuals (Figure 2A).
Figure 2.
Expression level of ACE2 and DPP4 mRNA in COVID-19 patients, negative subjects, ACE2h-COVID-19 and DPP4h-COVID-19 patients. Panel A (left) ACE2 expression level in negative subjects, COVID-19 patients and ACE2h-COVID-19 patients; Panel A (right) DPP4 expression level in negative subjects, COVID-19 patients and DPP4h-COVID-19 patients. Panel B (left) ACE2 expression level in negative subjects, COVID-19 patients and DPP4h-COVID-19 patients; Panel B (right) DPP4 expression level in negative subjects, COVID-19 patients and ACE2h-COVID-19 patients. Kolmogorov-Smirnov test was used. Kruskal-Wallis test was used for data analysis. Expression data (2−ΔCt) are represented as mean and range. Negative subjects n = 28; COVID-19 patients n = 28–29; ACE2h-COVID-19 patients n = 7; DPP4h-COVID-19 patients n = 6.
Moreover, six COVID-19 patients (TP57, TP59, TP60, TP61, TP64, TP66; hereinafter referred to as "DPP4h-COVID-19″) showed a higher DPP4 expression level in nasopharyngeal and oropharyngeal swabs when compared to negative subjects and other COVID-19 patients (Figure 2A). Furthermore, the COVID-19 patients’ group, without DPP4h-COVID-19 patients, shows no difference in nasopharyngeal and oropharyngeal DPP4 expression when compared to negative subjects (Figure 2A). Noteworthy, ACE2h-COVID-19 patients show DPP4 levels comparable to the other COVID-19 patients and to negative subjects (Figure 2B) and the same pattern is observed for DPP4h-COVID-19 patients (Figure 2B).
3.3. Clinical study of ACE2h-COVID-19 patients
ACE2h-COVID-19 patients showed comorbidities that included cardiovascular complications as smoking habits or high blood pressure and nephrolithiasis or chronic renal failure. Clinical data of ACE2h-COVID-19 patients are reported in Table 3.
Table 3.
Clinical symptoms of the seven ACE2h-COVID-19 patients.
| TP43 | TP27 | TP6 | TP22 | TP23 | TP52 | TP64 | |
|---|---|---|---|---|---|---|---|
| Swab result | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| Gender | M | M | M | M | M | M | F |
| Age | 57 | 57 | 52 | 92 | 61 | 40 | 52 |
| Dry cough | + | - | + | + | + | - | - |
| Fever | + | + | + | + | + | + | + |
| Dyspnea | + | + | + | + | + | + | - |
| Gastrointestinal disorders | - | + | + | - | - | - | - |
| FiO2 (range %) | 50–80 | 50–60 | 50–60 | 80–100 | 50–60 | 50–60 | 21 |
| Hypertension | - | - | - | + | - | + | + |
| Neu (103/μL) | 2.7 | 2.8 | 3.5 | 3.8 | 5.0 | 1.8 | 3.3 |
| Lym (103/μL) | 1.4 | 0.6 | 0.9 | 0.8 | 0.6 | 0.3 | 1.0 |
| LDH (U/L) | 221.0 | 363.0 | 254.0 | 463.0 | 405.0 | 341.0 | 231.0 |
| CRP (mg/L) | 44.5 | 55.3 | 51.9 | 139.2 | 117.6 | 101.3 | 2.7 |
| TNF-α (pg/ml) | 51.7 | 4.2 | 22.2 | N.A. | 13.7 | 15.1 | 84.5 |
| IL-6 (gr/dl) | 103.0 | 49.9 | 12.7 | N.A. | 27.7 | 5.0 | 7.4 |
| Trop I hs (ng/L) | 2.8 | 7.8 | 16.3 | 106.3 | 6.7 | 665.0 | <2 |
| Fibrinogen (mg/dl) | 579.0 | 713.0 | 444.0 | 671.0 | 904.0 | 516.0 | 293.0 |
| D-dimer (ng/mL) | 1699.0 | 408.0 | 1752.0 | 1311.0 | 868.0 | 1222.0 | 184.0 |
When available, categorical data are reported as range percentage (%) or value. FiO2 (Fraction of inspired oxygen). Neu (neutrophils; normal values 1,5–7 103/mL). Lym (lymphocyte; normal values 1,5-5 103/mL). CRP (C-Reactive Protein; normal values 0–5 mg/L). LDH (Lactate dehydrogenase; normal values 125–220 U/L). TNF-α (Tumor Necrosis Factor alfa; normal values < 50 pg/ml). IL-6 (Interleukin-6; normal range values = 4.6–12.4; low <4.6; high> 12.4). Fibrinogen (normal values 200–400 mg/dl). D-dimer (normal values 0–500 ng/ml).
One patient was diagnosed with laryngeal and prostate cancer (TP22). The youngest one had a chronic renal failure (TP52) and the woman an history of osteopenia. Three patients were hospitalized for about a month, one patient was hospitalized in Infectious Disease Unit then in ICU for 71 days, and the oldest one (TP22) died after 14 days of hospitalization. The average length of hospitalization was 35 days. Six out of patients were males (n = 6/7; 85.7%), age ranged from 40 and 92 years old (median age: 56 ± 16) (Table 3).
Clinical manifestations at the onset included fever (7/7), dry cough (4/7) and dyspnea (6/7); two patients referred also gastrointestinal disorders as vomiting, diarrhea and abdominal discomfort (TP27 and TP6). Computed tomography of the chest (CT) showed abnormal images and the typical signs of pneumonia as ground-glass opacity and local patchy shadowing. At the entrance, laboratory results showed a relative neutrophilia condition (3.3 103/mL ± 0.9, Table 3). All seven patients presented low lymphocytes values (0.8 103/mL ± 0.3, Table 3), indicating a lymphopenia. Six out seven patients showed elevated CRP (84.9 mg/L ± 39.7, Table 3) and higher LDH value (342.8 U/L ± 88.4, Table 3); three out of 7 patients higher levels of IL-6 (60.2 g/dl ± 38.7); and only two showed a TNF-α > 50 pg/ml (68.1 pg/ml ± 203.2). In all the patients we found an elevation of fibrinogen (588.6 mg/dl ± 198.1, Table 3), and all but two presented high D-dimer values (1370.4 ng/mL ± 364.5, Table 3). The oldest patient (TP22), later died, showed Troponin I value of 106.3 ng/L (normal values < 34.2 ng/L).
Due to a severe deficiency of oxygen in the blood (70.7mmHg ±11.8), all patients but one (TP64) were treated with oxygen therapy, in particular mechanical with medium-high flows (FiO2 range 50–60%) with cycles of CPAP (FiO2 60%) and BLB (FiO2 80%) for an average of 10 and 19 days of stay, respectively. Two patients were intubated. According to the clinical records, six out of 7 patients compared to the other COVID-19 patients, had a more severe onset clinical course. The clinical course has been characterized by a worse respiratory outcome. As a matter of fact, all these patients have been treated with medium high flows oxygen therapy: four of them with a FiO2% range between 50-60% (TP27, TP6, TP23 and TP52), one with a FiO2% range between 50-80% (TP43), and the oldest one with a range of FiO2% between 80-100% (TP22).
3.4. Clinical study of DPP4h-COVID-19 patients
DPP4h-COVID-19 patients showed comorbidities that included high blood pressure (TP57, TP64 and TP66) and osteopenia (TP64). Their clinical data are reported in Table 4.
Table 4.
Clinical symptoms of the six DPP4h-COVID-19 patients.
| TP57 | TP59 | TP60 | TP61 | TP64 | TP66 | |
|---|---|---|---|---|---|---|
| Swab result | Positive | Positive | Positive | Positive | Positive | Positive |
| Gender | M | M | F | M | F | F |
| Age | 76 | 54 | 72 | 82 | 52 | 84 |
| Dry cough | - | - | - | - | - | - |
| Fever | + | + | + | + | + | + |
| Dyspnea | - | - | - | - | - | - |
| Gastrointestinal disorders | - | - | - | - | - | - |
| FiO2 (range %) | 21 | 35–50 | 35–50 | 35–50 | 21 | 35–50 |
| Hypertension | + | - | - | - | + | + |
| Neu (103/μL) | 8.3 | 3.3 | 3.6 | 3.3 | 3.3 | 4.0 |
| Lym (103/μL) | 2.0 | 1.8 | 1.7 | 0.4 | 1.0 | 1.4 |
| LDH (U/L) | 240.0 | N.A. | 164.0 | 320.0 | 231.0 | 329.0 |
| CRP (mg/L) | 18.8 | 3.6 | 0.6 | 62.8 | 2.7 | 13.9 |
| TNF-α (pg/ml) | 0.9 | N.A. | N.A. | 11.5 | 84.5 | N.A. |
| IL-6 (gr/dl) | 13.4 | N.A. | 6.3 | 22.0 | 7.4 | N.A. |
| Trop I hs (ng/L) | 3.7 | N.A. | <2.0 | 11.1 | <2.0 | 9.0 |
| Fibrinogen (mg/dl) | 719.0 | 352.0 | 257.0 | 872.0 | 293.0 | 579.0 |
| D-dimer (ng/mL) | 4572.0 | 699.0 | 156.0 | 901.0 | 184.0 | 551.0 |
When available, categorical data are reported as range percentage (%) or value. FiO2 (Fraction of inspired oxygen). It is the assumed percentage of oxygen concentration participating in gas exchange in the alveoli. Natural air includes 21% oxygen. Oxygen-enriched air has higher FiO2 than 21%, up to 100%. Neu (neutrophils; normal values 1,5–7 103/mL). Lym (lymphocyte; normal values 1,5-5 103/mL). CRP (C-Reactive Protein; normal values 0–5 mg/L). LDH (Lactate dehydrogenase; normal values 125–220 U/L). TNF-α (Tumor Necrosis Factor alfa; normal values < 50 pg/ml). IL-6 (Interleukin-6; normal range values = 4.6–12.4; low <4.6; high> 12.4). Fibrinogen (normal values 200–400 mg/dl). D-dimer (normal values 0–500 ng/ml). N.A. (Not Available).
The outcome of these six patients was characterized by a better course than those of the ACE2h-COVID-19 patients group. Five were hospitalized in the Infectious Diseases Unit ward, only one in the Respiratory Diseases ward. Half of DPP4h-COVID-19 patients were male (median age: 71 ± 15). The women age ranged from 52 and 84 years old (median age: 68 ± 16). Interestingly, one of them (TP64) also showed higher ACE2 mRNA level in nasopharyngeal and oropharyngeal swabs (Table 3 and Table 4). Clinical manifestation at the onset include only fever (6/6). No one presented dry cough, dyspnea and gastrointestinal disorders. However, their chest CT showed the typical signs of pneumonia as ground-glass opacity. All patients but two (TP57 and TP64) were treated with oxygen therapy at medium-low flows (FiO2 range 35–50%) for an average of 10 days of stay. Nobody passed away.
At the entrance, laboratory results showed a neutrophilia condition in one patient (TP57; 8.3 103/mL, Table 4). Three individuals (TP61, TP64 and TP66) presented a relative lymphopenia (1.0 103/mL ± 0.5, Table 4); three patients (TP57, TP61 and TP66) showed a slight rise in CRP levels (32.0 mg/L ± 26.9, Table 4) and an elevation of fibrinogen (723.0 mg/dl ± 146.5, Table 4). All but two (TP60 and TP64) presented high D-dimer values (mean 1681 ng/mL, Table 4).
3.5. ROC curve analysis
We conducted a ROC curve analysis to evaluate the capacity of overexpressed genes to identify COVID-19 patients from negative individuals. We tested a predictive model, combining the expression levels of ACE2 and DPP4 genes. Analysis of ROC curves revealed that the area under the ROC curve (AUC) was 0.75 with 95% confidence interval 0.63 to 0.87 (p-value <0.001) (Figure 3).
Figure 3.
Receiver operator characteristic (ROC) curves. Receiver operator characteristic (ROC) curves between COVID-19 patients and negative subjects (set as control group). Area under the ROC curve (AUC) 0.75, 95% confidence interval 0.63 to 0.87, p-value < 0.001.
3.6. Correlation analysis
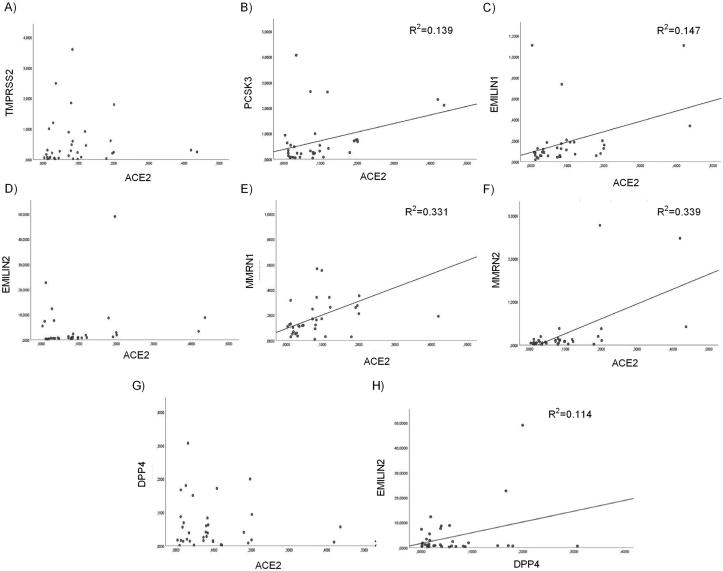
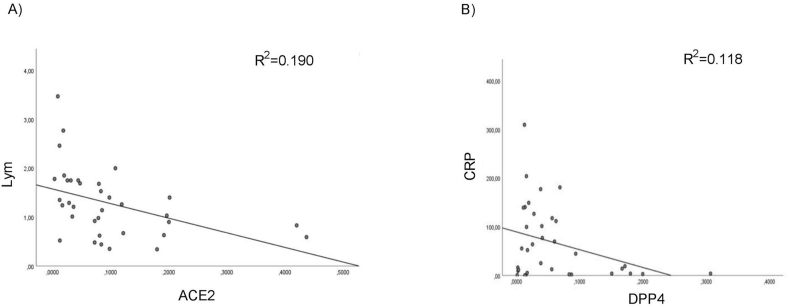
No correlation was found among the expression data of the eight SARS-CoV-2 host invasion genes and the age of our 63 patients. We analyze, by Pearson correlation test, the relationship between the expression profile of the eight SARS-CoV-2 host invasion genes respectively in COVID-19 patients (n = 35) and negative subjects (n = 28) and we found a significant positive association among ACE2 mRNA and PCSK3, EMILIN1, MMRN1 and MMRN2 expression and between DPP4 mRNA and EMILIN2 expression only in COVID-19 patients (Figure 4). Moreover, we evaluate by Pearson correlation test, the relationship among the SARS-CoV-2 entry genes expression values and the clinical data of COVID-19 patients. A significant negative correlation was found among ACE2 expression level and lymphopenia in COVID-19 patients (Figure 5A) and also a significant negative association between DPP4 mRNA and CRP levels (Figure 5B).
Figure 4.
Correlation analysis of ACE2 and DPP4 expression in nasopharyngeal and oropharyngeal swabs. Scatter plots depicting the relationship between ACE2 and (A) PCSK3 mRNA expression (R2 = 0.139, p < 0.05, Pearson r = 0.372); (B) EMILIN1 mRNA expression (R2 = 0.147, p < 0.05, Pearson r = 0.383); (C) MMRN1 mRNA expression (R2 = 0.331, p < 0.0005, Pearson r = 0.575); (D) MMRN2 mRNA expression (R2 = 0.339, p < 0.0005, Pearson r = 0.582). Scatter plot depicting the relationship between DPP4 and (E) EMILIN2 mRNA expression (R2 = 0.114, p < 0.05, Pearson r = 0.338).
Figure 5.
Correlation analysis of ACE2 and DPP4 m-RNA levels with clinical data in nasopharyngeal and oropharyngeal swabs of COVID-19 patients. Scatter plots depicting the relationship between (A) ACE2 mRNA and number of lymphocytes (Lym) (R2 = 0.190, p < 0.005, Pearson r = -0.436; (B) DPP4 mRNA and CRP levels (R2 = 0.118, p < 0.05, Pearson r = -0.344).
Regression analyses performed in ACE2h-COVID-19 patients, indicate a significant positive correlation among ACE2 expression level and the number of neutrophils and LDH values (Figure 6). No correlation among DPP4 expression level and clinical data was found in DPP4h-COVID-19 patients.
Figure 6.
Correlation analysis of ACE2 m-RNA expressions with clinical data in nasopharyngeal and oropharyngeal swabs of ACE2h-COVID-19 patients. Scatter plots depicting the relationship between (A) ACE2 and number of neutrophils (neu) (R2 = 0.645, p < 0.05, Pearson r = -0.803); (B) ACE2 and LDH levels (R2 = 0.578, p < 0.05, Pearson r = -0.760).
4. Discussion
Several differences in clinical manifestations and complications of COVID-19 patients have been observed suggesting variability in the disease process [19]. Genetic variants in the host human genome, can in part explain the broad inter-individual variation of disease susceptibility and/or severity [6, 19, 20, 21]. Moreover, the expression levels of human genes encoding the main proteins involved in this mechanism could be critical for the susceptibility, symptoms and outcome of SARS-CoV-2 infection. Notably, elevated ACE2 expression promoted in vitro the susceptibility to SARS-CoV infection [22, 23]. Moreover, ACE2 is up-regulated in response to SARS-CoV-2 infection [24]. Interestingly, conditions like old age, obesity, chronic kidney disease (CKD) and chronic obstructive pulmonary disease (COPD) in which was reported DPP4 upregulation are associated with severe COVID-19 [25]. It was shown that DPP4 acted for Coronavirus (CoV) co-receptor, thus suggesting a potential similar mechanism of SARS–CoV-2 entry [26]. A recent study clearly reported a correlation between DPP4 and ACE2 expression, suggesting that both membrane proteins are relevant in the pathogenesis of virus entry [27].
The respiratory tract can be considered as a vulnerable target to SARS-CoV-2 infection [28, 29]. Therefore, we analyzed the expression level of eight genes involved in the virus entry in the upper respiratory tract, in particular nose and pharynx. We selected naso- and oropharyngeal swabs in the critical epidemic period 20th March-20th April 2020, during which a lot of seriously affected patients referred to Policlinico Tor Vergata of Rome. Our results indicate a significant nasal and oropharyngeal overexpression of ACE2 and DPP4 genes in COVID-19 patients and that this overexpression is not correlated with patients’ age. Moreover, to evaluate the ability of ACE2 and DPP4 levels to discriminate COVID-19 patients from negative subjects, we performed a ROC curve analysis. We evaluated the discriminating potential of the combined gene expression levels and we observed that they allow us to identify about 75% of COVID-19 patients (Figure 3). These results support the hypothesis that ACE2 and DPP4 expression level are altered in COVID-19 patients and that their combined evaluation could be a potential good discriminatory genomic biomarker. Interestingly, the expression level of ACE2 and DPP4 genes is significant higher respectively in a subgroup of seven (ACE2h-COVID-19) and six (DPP4h-COVID-19) COVID-19 patients (Figure 2).
All seven ACE2h-COVID-19 patients except one presented dyspnea; two patients were intubated. CT showed abnormal images and the typical signs of pneumonia as ground-glass opacity. ACE2h-COVID-19 patients showed, at the entrance, a worse lymphopenia condition, a reduced cytokine pattern and lower levels of both fibrinogen and D-dimer (Table 2 vs Table 3). Accordingly, a recent meta-analysis demonstrated that admission lymphopenia and neutrophilia are associated with poor outcomes in patients with COVID-19 [30]. Lymphocytes express ACE2 on their surface and may represent a direct target of the virus. SARS-CoV-2 might directly infect them, resulting in lymphopenia that in turns might be related to lymphocytic dysfunction. Interestingly, our regression analysis demonstrated a significant negative correlation between ACE2 expression and the number of lymphocytes in COVID-19 patients (Figure 5A).
Based on these results we can hypothesize that subjects with higher expression level of ACE2 in nasopharyngeal and oropharyngeal cells are more vulnerable to develop more severe complications of COVID-19. The main function of ACE2 receptor is the downregulation of the renin-angiotensin-system (RAS), balancing the overdrive of RAS mediated response and the renal, gastrointestinal absorption of amino acids. However, it also acts as a means of clathrin-mediated internalization of viruses such as SARS coronavirus [31]. By now, the functional role of the surface angiotensin-converting enzyme 2 (ACE2) as a receptor protein for viral entry is well known. This protein is widely found in different organs such as the lung, kidney, heart, and endothelial tissue. According to our results, other study report that in symptomatic patients affected by COVID-19 ACE2 expression is higher in alveolar epithelial cells [24, 29, 32]. Since an important therapeutic approach is to impair the fusion of SARS-CoV-2 with the type II pneumocytes ACE2 receptor, thus inhibiting and preventing any type of destruction of the type II pneumocytes, its overexpression in the nasopharyngeal and oropharyngeal swabs of severe COVID-19 patients when compared to negative subjects and to other positive patients, suggest that this approach may be useful also in the first step of infection, when ACE2 expression in nasopharyngeal and oropharyngeal tissue is high.
The six DPP4h-COVID-19 patients manifest a better clinical course than ACE2h-COVID-19 patients. In fact, their clinical symptom at onset was only fever, only four out six were treated with oxygen therapy at medium-low flows and no one of them died (Table 4). Laboratory analyses at the entrance were in the normal range for the most of them. To the best of our knowledge, none of these patients showed comorbidities associated to DPP4 over-expression as obesity and metabolic syndromes, thus suggesting a functional role of this receptor in the early phase of virus entry besides that of modifier gene of COVID-19 severity [33].
Dipeptidyl peptidase 4 (DPP4), also known as CD26, is a 110 kDa transmembrane glycoprotein expressed on the surface of a wide variety of epithelial cells and some lymphocytes. DPP4 is an important protease that is widely expressed on the surface of human cells and plays a key role in immune-regulation, inflammation, oxidative stress, cell adhesion, and apoptosis by targeting different substrates. DPP4 is also the main receptor for MERS-COV [34]. In docking analysis that examined the SARS-CoV-2 S protein and DDP4, a significant interaction between these proteins was found [15]. Interestingly, COVID-19 patients with COPD comorbidity show serious adverse outcomes and subjects with COPD express higher rates of DPP4 [35].
Recent studies have shown that the inhibition of DPP4 can exert antihypertensive effects by interfering with the function of the RAS system. Moreover, both ACE2 and DPP4 are proteins dysregulated in diabetes [36].
It might be possible that diabetic patients might be more affected by COVID-19 due to increased ACE2 and DPP4 levels that in turns mediate infection and contribute to a compromised vasculature [36].
Finally, our regression analysis found a positive association only in COVID-19 patients, among ACE2 expression level and PCSK3, EMILIN1, MMRN1, and MMRN2 ones and also between DPP4 and EMILIN2 mRNAs (Figure 4). This result suggests that a coordinated nasopharyngeal and oropharyngeal expression level of these genes contribute to the virus entry and progression. Interestingly, regression analyses indicate also a significant negative correlation between ACE2 expression and the number of lymphocytes (Figure 5A) and between DPP4 mRNA and C-reactive protein (CRP) levels at the entrance in COVID-19 patients (Figure 5B). Accordingly, ACE2h-COVID-19 patients display a worse clinical outcome of the disease respect to DPP4h-COVID-19 ones.
Anyway, we only examined the expression of SARS-CoV-2 host invasion genes at the RNA level; consequently, we cannot explicitly conclude that these proteins are downregulated in COVID-19 patients.
The significant upregulation of ACE2 and DPP4 in COVID-19 patients suggest that their joint expression level might be a good discriminator of clinical outcome in COVID-19 patients and that both these receptors may play a complementary role in the virus entry and in disease progression. Moreover, our data suggest the therapeutic potential of known drugs targeted against ACE2 and DPP4 to treat or prevent COVID-19 symptoms in particular, derived vascular complications.
At the best of our knowledge, this is the first study that explores DPP4 expression in naso- and oropharyngeal swabs of COVID-19 patient demonstrating a significant upregulation of this receptor and suggesting its potential functional role in binding SARS-CoV-2 virus.
Declarations
Author contribution statement
F. Amati and G. Novelli: Conceived and designed the experiments; Wrote the paper.
C. Vancheri: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
A. Latini: Performed the experiments; Analyzed and interpreted the data.
V. Colona, S. Loddo, A. Di Lorenzo, P. Rogliani and M. Andreoni: Analyzed and interpreted the data.
S. Grelli: Performed the experiments; Contributed reagents, materials, analysis tools or data.
M. D'Apice, E. Balestrieri, C. Passarelli and A. Minutolo: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Rotary Club Sybaris.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This work is part of the GEFACOVID2.0 Consortium (http://www.lorenzinifoundation.org/wp-content/uploads/2020/06/NEWS-GEFACOVID2.0_final-Consortium-News-GN-vEF.pdf).
References
- 1.World Health Organization . 2020. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes it.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it [Google Scholar]
- 2.Qin L., Li X., Shi J. Gendered effects on inflammation reaction and outcome of COVID-19 patients in wuhan. J. Med. Virol. 2020;4 doi: 10.1002/jmv.26137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng Y., Wu P., Lu W. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020;25(11):29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin J.M., Bai P., He W. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Publ. Health. 2020;29(8):152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casanova J.L., Su H.C. COVID human genetic effort. A global effort to define the human genetics of protective immunity to SARS-CoV-2 infection. Cell. 2020;181:1194–1199. doi: 10.1016/j.cell.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latini A., Agolini E., Novelli A. COVID-19 and genetic variants of protein involved in the SARS-CoV-2 entry into the host cells. Genes (Basel) 2020;27(11):E1010. doi: 10.3390/genes11091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marovich M., Mascola J.R., Cohen M.S. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA. 2020;14(324):131–132. doi: 10.1001/jama.2020.10245. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin Z., Chen K., Zou J. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertram S., Heurich A., Lavender H. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PloS One. 2012;7 doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coutard B., Valle C., de Lamballerie X. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furinlike cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zacchigna L., Vecchione C., Notte A. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell. 2006;124:929–942. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Seidah N.G., Chretien M. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999;848:45–62. doi: 10.1016/s0006-8993(99)01909-5. [DOI] [PubMed] [Google Scholar]
- 15.Vankadari N., Wilce J.A. Emerging Wuhan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microb. Infect. 2020;17(9):601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widagdo W., Raj V.S., Schipper D. Differential expression of the Middle East respiratory syndrome coronavirus receptor in the upper respiratory tracts of humans and dromedary camels. J. Virol. 2016;14(90):4838–4842. doi: 10.1128/JVI.02994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boonacker E., Van Noorden C.J. The multifunctional or moonlighting protein CD26/DPPIV. Eur. J. Cell Biol. 2003;82:53–73. doi: 10.1078/0171-9335-00302. [DOI] [PubMed] [Google Scholar]
- 18.De Flora S., La Maestra S. Growth and decline of the COVID-19 epidemic wave in Italy from March to June 2020. J. Med. Virol. 2020 doi: 10.1002/jmv.26499. [DOI] [PubMed] [Google Scholar]
- 19.Harb J.G., Noureldine H.A., Chedid G. SARS, MERS, COVID-19: clinical manifestations and organ-system complications: a mini review. Pathog. Dis. 2020;78(4):ftaa033. doi: 10.1093/femspd/ftaa033. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strafella C., Caputo V., Termine A. Analysis of ACE2 genetic variability among populations highlights a possible link with COVID-19-related neurological complications. Genes. 2020;3(11):741. doi: 10.3390/genes11070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godri Pollitt K.J., Peccia J., Ko A.L. COVID-19 vulnerability: the potential impact of genetic susceptibility and airborne transmission. Hum. Genom. 2020;12(14):17. doi: 10.1186/s40246-020-00267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Zhou W., Yang L. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol. Res. 2020;157:104833. doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sungnak W., Huang N., Bécavin C. HCA Lung Biological Network. SARS-CoV-2 entry factors are highly express in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziegler C.G.K., Allon S.J., Nyquist S.K. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;28(181):1016–1035. doi: 10.1016/j.cell.2020.04.035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S., Mazumder T., Banik S. The association of cardiovascular diseases and diabetes mellitus with COVID-19 (SARS-CoV-2) and their possible mechanisms. SN Compr. Clin. Med. 2020;25:1–6. doi: 10.1007/s42399-020-00376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Zhang Z., Yang L. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience. 2020;21(23):1014002020. doi: 10.1016/j.isci.2020.101400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi F., Qian S., Zhang S. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020;526(1):135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou X., Chen K., Zou J. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M.Y., Li L., Zhang Y. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect .Dis. Poverty. 2020;28(9):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry B., Cheruiyot I., Vikse J. Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: a meta-analysis. Acta Biomed. 2020;7(91) doi: 10.23750/abm.v91i3.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuba K., Imai Y., Ohto-Nakanishi T. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chua R.L., Lukassen S., Trump S. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;38:970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 33.Bassendine M.F., Bridge H.S., McCaughan G.W. A role for dipeptidyl peptidase 4 (DPP4) in disease severity? J. Diabetes. 2020;12:649–658. doi: 10.1111/1753-0407.13052. [DOI] [PubMed] [Google Scholar]
- 34.Lu G., Hu Y., Wang Q. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500(7461):227–231. doi: 10.1038/nature12328. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seys L.J.M., Widagdo W., Verhamme F.M. DPP4, the Middle East respiratory syndrome coronavirus receptor, is Upregulated in lungs of Smokers and chronic obstructive pulmonary disease patients. Clin. Infect. Dis. 2018;6(66):45–53. doi: 10.1093/cid/cix741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valencia I., Peiró C., Lorenzo Ó. DPP4 and ACE2 in diabetes and COVID-19: therapeutic targets for cardiovascular complications? Front. Pharmacol. 2020;7(11):1161. doi: 10.3389/fphar.2020.01161. [DOI] [PMC free article] [PubMed] [Google Scholar]