Abstract
Background and Objectives:
EUS-guided biliary drainage (EUS-BD) is used as a rescue method after failed endoscopic retrograde cholangiography. However, it is considered a technically challenging procedure. Phantoms and ex vivo biliary dilatation models have been used to enhance the learning of EUS-BD, but they provide a limited level of realism. The aim of this study was to develop a swine biliary dilatation model that uses temperature-controlled endobiliary radiofrequency ablation (EB-RFA) for training in EUS-BD.
Materials and Methods:
Target temperature-controlled (80°C at 7 or 10 W for 60–120 s) EB-RFA was performed on seven pigs. Two weeks after the EB-RFA, EUS examination was performed to confirm biliary dilatation, and EUS-BD was then attempted by performing a hepaticogastrostomy (HGS) or cholecystogastrostomy (CGS).
Results:
Twelve sessions of EB-RFA (11 at the distal common bile duct [CBD] and one at the proximal CBD) were successfully performed on the seven pigs. There were no immediate postprocedural adverse events. Two weeks after the procedures, clinical signs of jaundice were observed in all the pigs. EUS examination revealed massive intrahepatic and extrahepatic biliary dilatations in all the pigs, and EUS-BD was attempted in the same session. HGS was performed on six pigs. Technical success was achieved in five of the six pigs (83.3%). Technical failure in HGS occurred during the stent deployment, and CGS was successfully performed on one pig.
Conclusions:
Our study shows that EB-RFA is an effective minimally invasive method for creating biliary dilatation models. It may be considered suitable for training in EUS-BD.
Keywords: animal experimentation, benign stricture, radiofrequency ablation
INTRODUCTION
EUS-BD has been shown to be an effective therapeutic option for patients with biliary obstruction for whom endoscopic retrograde cholangiography (ERC) was unsuccessful.[1] However, EUS-BD is technically challenging and requires a substantial learning curve. Furthermore, the overall number of patients who undergo EUS-BD is low even in centers with a high volume of biliary interventions, and this may negatively impact training.[2,3]
Phantoms and ex vivo biliary dilatation models have been used to enhance the learning in this field, but they provide a limited level of realism.[4,5] The swine model has been described as a realistic tool for EUS training that approximates human anatomy and provides excellent haptic sensations.[6] Some in vivo swine biliary dilatation models have been described, but they provide irregular biliary dilatation and can be technically cumbersome.[7,8,9,10,11,12,13]
Radiofrequency ablation (RFA) is a safe and effective treatment option for several gastrointestinal diseases including pancreatobiliary disorders.[14,15,16] RFA can deliver heat energy to tissues, which causes necrosis around the RFA probe and eventually induces strictures around the tissues that damaged by the thermal energy.[12,17]
A new endobiliary RFA (EB-RFA) probe with a sensor that controls the temperature at the tissue-electrode interface was recently introduced. It has been shown to limit the charring of the surface of the electrode, thereby improving the accuracy of the ablation and reducing the adverse event rate.[18,19]
This study aimed to develop a swine biliary dilatation model that uses temperature-controlled EB-RFA for training in EUS-BD.
MATERIALS AND METHODS
The first World Endoscopy Organization (WEO) International School of EUS (WISE) was established in 2018. WISE is a training program focused on teaching a specialized group of young doctors with intermediate EUS experience by means of lectures, live case observations, web conferences sharing routine cases and difficulties, and hands-on training sessions using phantoms and live pigs.[6] Six trainees had < 2 years of experience in EUS and EUS-FNA and had performed <20 interventional EUS procedures. These trainees performed EUS-BD on swine biliary dilatation models.
Animal models
Seven 14-month-old mini pigs (Sus Scrofa) with a mean weight of 30.5 kg were used in this in vivo experimental study. All the pigs ingested only water for 24 h and fasted overnight before EB-RFA. The pigs were sedated with general anesthesia. Intramuscular injection of atropine sulfate (0.04 mg/kg) was administered as pre-medication. Induction was achieved with a combination of tiletamine/zolazepam (Zoletil 50; Virbac, Korea; 5 mg/kg, intramuscular) and xylazine hydrochloride (Rompun; Bayer, Korea; 2 mg/kg, intramuscular). The animals were then intubated, and 2%–2.5% isoflurane (Forane; JW Pharmaceutical, Korea) was administered throughout the procedure to maintain general anesthesia. Cardiopulmonary parameters were monitored, and the animals were placed in the left lateral decubitus position on a fluoroscopy table.
Equipment
A dedicated biliary radiofrequency (RF) catheter with a distal temperature sensor (ELRA RF catheter, STARmend, Goyang, Korea) was used to deliver energy to the common bile duct (CBD). The catheter had a diameter of 7 Fr, a length of 175 cm, and an active distal part available in two different lengths (18 or 33 mm) according to the area to be ablated. In our study, we only used the catheter with an 18-mm active part, which has a 9-mm leading tip proximal to a 7-mm insulated segment. This active segment of the catheter is made of four stainless circumferential ring electrodes (width 3 mm) spaced 2 mm apart [Figure 1]. The electrodes provide a bipolar conduction pattern, and no ground pads are needed. The catheter is connected to a VIVA combo RF generator (VCS 10, STARmed, Goyang, Korea), which delivers energy in a target temperature-controlled mode. Due to the presence of a temperature sensor at the distal end of the catheter, the energy delivery automatically stops as soon as the pre-set temperature is reached.
Figure 1.

ELRA radiofrequency catheter with an 18 mm active segment made of four stainless circumferential ring electrodes (width 3 mm) spaced 2 mm apart
Radiofrequency ablation-based biliary stricture creation
ERC was performed on all pigs using a duodenoscope (TJF-240, Olympus, Tokyo, Japan) dedicated to exclusive use in the swine model. Following intubation of the duodenal bulb, the major duodenal papilla was visualized just after the pylorus, and biliary cannulation was performed using a standard cannula (Contour ERCP Cannula, Boston Scientific, Natick, MA, USA). Contrast medium was injected for cholangiography, and a 0.025-inch guidewire (VisiGlide; Olympus, Japan, Tokyo) was inserted. An ELRA probe was then advanced over the wire into the CBD under fluoroscopic guidance with no need for sphincterotomy [Figure 2]. Since swine do not have the sphincter of Oddi, the major duodenal papilla is wide and easily accessible. As the endpoint was to achieve biliary stricture, energy for EB-RFA was then delivered in a target temperature-controlled mode (80°C at 7 or 10 W for 60–120 s).[13] Parameters such as the number of sessions and session duration were empirically set at the discretion of the endoscopist.
Figure 2.
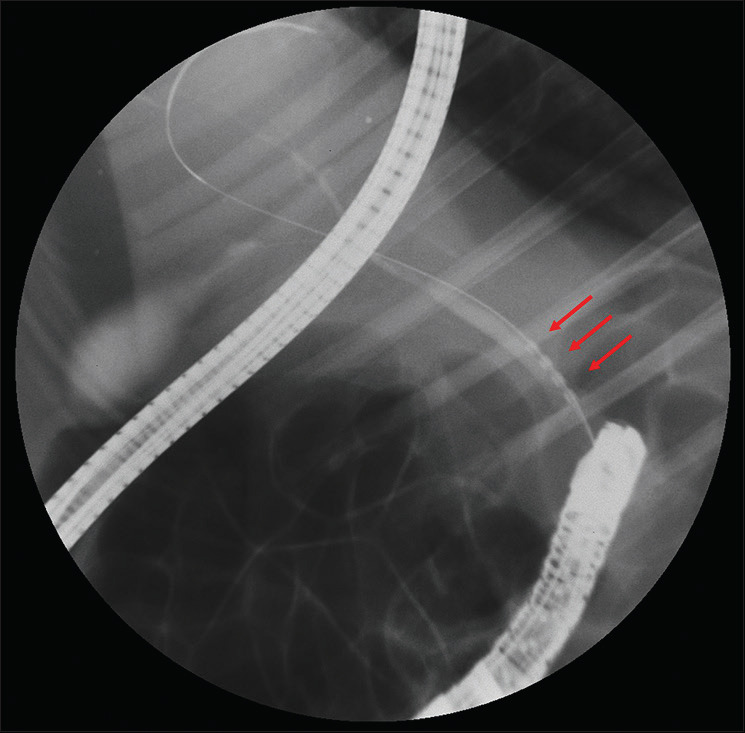
Cholangiography showing a radiofrequency catheter (arrows) in the distal common bile duct
This animal study included pilot (single session of RFA) and confirmation groups. In the pilot groups, a single session of EB-RFA was performed on the first two pigs for safety purposes. After confirmation of safety, two sessions of EB-RFA were performed on five pigs to confirm the efficacy and safety of CBD dilatation.
EUS-biliary drainage procedures
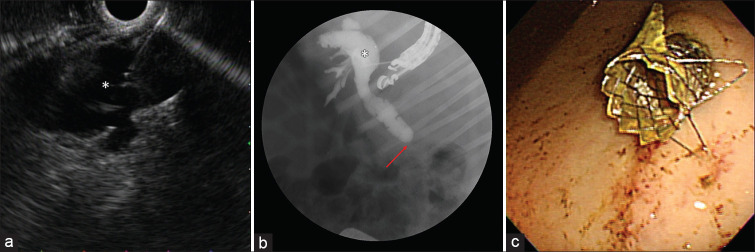
Two weeks after the EB-RFA, the pigs were re-scoped using a linear EUS scope (GF-UCT260, Olympus, Tokyo, Japan) and an EU-ME1 echo processor (Olympus, Tokyo, Japan). During the EUS examination, a hyperechoic lesion was seen in the CBD at the site of the previous EB-RFA [Figure 3a] with associated massive upstream dilatation of the biliary tree [Figure 3b]. EUS-BD was then attempted by performing a hepaticogastrostomy (HGS) or a cholecystogastrostomy (CGS). During HGS, the dilated left hepatic duct was accessed from the stomach with a 19 G needle (Expect Needle; Boston Scientific, Natick, MA, USA) [Figure 4a]. After the puncture, bile was aspirated to confirm the intraductal position, and then, contrast medium was injected to confirm the presence of a distal biliary stricture [Figure 4b]. A 0.025-inch guidewire was then inserted through the needle and advanced across the major papilla or placed in the CBD. After successful guidewire manipulation and withdrawal of the needle, a hepatogastric fistula was created using a wire-guided needle knife (MicroKnife, Boston Scientific, Natick, MA, USA). The fistulous tract was then dilated with a 4-mm balloon (Hurricane, Boston Scientific, Natick, MA, USA). An uncovered self-expandable metal stent (SEMS) (6 mm × 40 mm; Bonastent, Standard Sci Tech, Seoul, Korea) was released into the intrahepatic bile duct to prevent migration. After the successful deployment of the uncovered SEMS, a fully covered SEMS (8 mm × 70 mm or 8 mm × 90 mm; Bonastent, Standard Sci Tech, Seoul, Korea) was released coaxially into the uncovered SEMS, thereby bridging the bile duct and the gastric lumen [Figure 4c].
Figure 3.
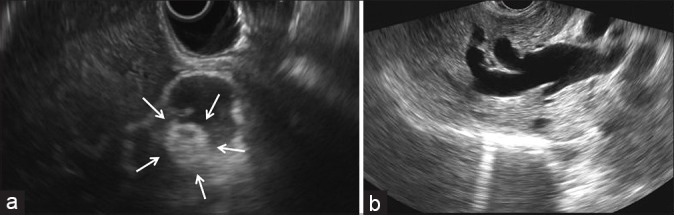
EUS view showing (a) a hyperechoic round lesion (arrows) in the common bile duct at the site of the previous radiofrequency ablation with (b) associated upstream biliary dilatation
Figure 4.
EUS-guided hepaticogastrostomy: (a) EUS showing dilated left hepatic duct (*) accessed with a 19 G needle, (b) fluoroscopic view showing the 19 G needle in the dilated left hepatic duct (*) and subsequent cholangiography showing dilated common bile duct with distal biliary stricture (arrow), (c) endoscopic view from the stomach showing the distal end of a fully covered self-expandable metal stent
During the CGS, the dilated gallbladder was accessed using the same technique described above, and a 10 mm × 20 mm lumen-apposing metal stent (Niti-S Spaxus, Taewoong, Ilsan, South Korea) was inserted under combined EUS and fluoroscopic guidance [Figure 5].
Figure 5.
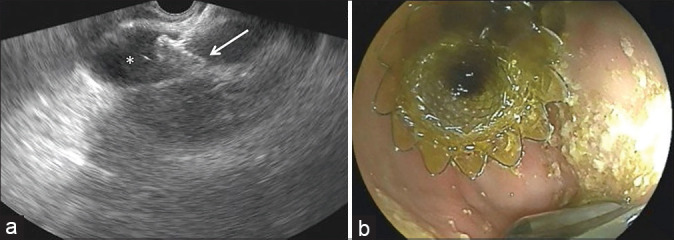
EUS-guided cholecystogastrostomy: (a) EUS view showing the proximal end of the lumen-apposing metal stent in the distended gallbladder (*), (b) endoscopic view from the stomach showing the distal end of the lumen-apposing metal stent correctly in place
Ethical approval
Approval was obtained from the Institutional Animal Care and Use Committee (IACUC) before the initiation of the study (IACUC number: 2017-14-285).
RESULTS
Baseline characteristics and outcomes are summarized in Table 1. EB-RFA was successfully performed on all seven pigs. One session of RFA was performed on two pigs in the pilot group and two sessions of RFA were performed on five pigs in the confirmation group. In a total of 12 sessions of RFA, EB-RFA was performed on the distal CBD in 11 sessions, and one session of RFA was performed on the proximal CBD. Three sessions of RFA were performed for 60 s, two sessions for 90 s, and seven sessions for 120 s. No technical issues or intraprocedural adverse events occurred.
Table 1.
Swine characteristics and results of endobiliary radiofrequency ablation and EUS-guided biliary drainage
| Case | Rounds | Ablation mode | Ablation site | Biliary dilation | EUS-BD | Type of stent | Technical success of EUS-BD | Adverse events | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Power (W) | Temperature | Time | Uncovered SEMS | Covered SEMS | LAMS | |||||||
| 1 | Single | 7 | 75 | 120 | Distal CBD | Yes | HGS | 6 mm×40 mm | 8 mm×70 mm | Yes | No | |
| 2 | Single | 7 | 80 | 120 | Distal CBD | Yes | HGS | 6 mm×40 mm | 8 mm×90 mm | Yes | No | |
| 3 | First | 10 | 80 | 120 | Proximal CBD | Yes | HGS | 6 mm×40 mm | 8 mm×70 mm | Yes | No | |
| Second | 10 | 80 | 60 | Distal CBD | ||||||||
| 4 | First | 7 | 80 | 120 | Distal CBD | Yes | HGS | 6 mm×40 mm | 8 mm×70 mm | No | No | |
| Second | 10 | 80 | 60 | |||||||||
| 5 | First | 7 | 80 | 120 | Distal CBD | Yes | HGS | 6 mm×40 mm | 8 mm×90 mm | Yes | No | |
| Second | 10 | 80 | 60 | |||||||||
| 6 | First | 7 | 80 | 120 | Distal CBD | Yes | HGS | 6 mm×40 mm | 8 mm×70 mm | Yes | No | |
| Second | 7 | 80 | 120 | |||||||||
| 7 | First | 10 | 80 | 90 | Distal CBD | Yes | CGS | 10×20 mm | Yes | No | ||
| Second | 10 | 80 | 90 | |||||||||
EB-RFA, EUS-BD, SEMS, LAMS: Lumen-apposing metal stent, CBD, HGS, CGS
Two weeks after the EB-RFA, clinical signs of jaundice were observed in all the pigs [Figure 6]. There were no EB-RFA-related delayed adverse events. Intrahepatic and extrahepatic bile duct dilatations and gallbladder distension were observed in all the pigs during the EUS examination; therefore, the same procedure of EUS-BD was attempted by six endosonographers.
Figure 6.
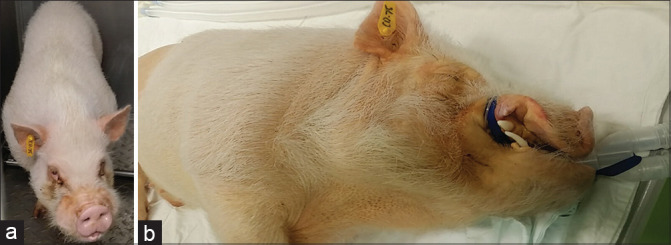
(a) Normal swine before EB-RFA, (b) jaundiced swine 2 weeks after EB-RFA
Six endosonographers who had performed < 20 interventional EUS procedures attempted EUS-BD. HGS was performed on six pigs. Technical success was obtained in five of the six pigs (83.3%). Needle puncture into the dilated intrahepatic bile duct was performed successfully in all the pigs using a 19 G needle. Guidewire manipulation and fistula dilation were also successful. In one of the six pigs (17%), the uncovered metal stent was correctly deployed, but the deployment of the fully covered metal stent failed. CGS was successfully performed in one pig without adverse events.
DISCUSSION
Our study shows that EB-RFA is an effective and safe minimally invasive method for creating a critical biliary stricture with associated upstream biliary dilatation in a swine model for training in EUS-BD.
The mechanism for creating an RFA-induced biliary stricture entails the destruction of the normal bile duct wall near the electrode. The RF waves passing through the electrode agitate tissue ions, thereby causing a rise in temperature due to frictional heat, which leads to protein denaturation, cell desiccation, and coagulative necrosis. Thereafter, biliary stricture develops during the healing process.[12] EB-RFA is a technically easy procedure in swine models. The major duodenal papilla in swine is patulous because swine do not have the sphincter of Oddi. Furthermore, the main pancreatic duct drains separately into the minor papilla, which is located about 20 cm distally in the descending duodenum.[20] Therefore, the CBD can be easily accessed with no sphincterotomy or risk of pancreatitis.
Several biliary dilatation phantoms or models based on ex vivo and in vivo designs for training in EUS-BD have been described.[4,5,7,11,21] In a study by Qian et al.,[21] a porcine bile duct dilatation model based on the laparoscopic placement of a double-balloon occlusion device was used. Although biliary dilatation was achieved 1 week after the surgical procedure, the endoscopic approach of the biliary dilatation model is simpler and safer. The endoscopic approach is less invasive and more convenient than the surgical approach for creating biliary dilatation models. In addition, it simplifies postoperative care and reduces the morbidity and mortality associated with the surgical approach.
The ex vivo biliary dilatation model was recently introduced for training in EUS-BD.[4,5] This “hybrid” three-dimensional printing/ex vivo model appears to be effective for teaching and training in the various steps of EUS-BD. However, endosonographers can hardly feel needle penetration or encounter adverse events when working with these models, as ex vivo biliary dilatation models do not provide realistic simulation. Therefore, in vivo models may be better for training in various EUS-BD techniques than ex vivo models.
There are several non-EB-RFA endoscopic approaches for producing animal biliary dilatation models. In a recent study, biliary dilatation models were developed using endoclips or by the placement of a detachable snare on the major duodenal papilla under cap-assisted endoscopy.[11] Although biliary stricture was identified in all cases 2 weeks after the endoscopic procedures, gallbladder dilatation was not observed with this model. Furthermore, in a study by Lee et al.,[9] endoscopic clipping or endoscopic band ligation (EBL) of the major papilla and subsequent argon plasma coagulation (APC) were performed on nine pigs. Successful biliary dilatation was observed in four pigs treated with EBL and APC 2 weeks after the procedure. However, biliary dilatation was observed in only two of five pigs treated with endoscopic clipping and APC after two sessions of treatment. Although non-EB-RFA endoscopic methods may be technically easy, biliary stricture and the associated biliary dilatation are not consistently created.
Biliary dilatation models that use EB-RFA for thermal energy delivery are effective for creating biliary stricture and are potentially reproducible.[7,12,13] In a study by Park et al.,[12] EB-RFA was performed on 12 pigs, and a monopolar catheter was used to deliver 60 W, 80 W, or 100 W for 60 s. Although bile duct stricture associated with biliary dilatation was observed in all the pigs, bile duct perforation occurred in only two pigs. In a recent study by Cho et al.,[13] temperature-controlled EB-RFA was performed on six pigs. Successful biliary dilatation was achieved in all the pigs 4 weeks after the EB-RFA with no adverse events, such as perforation and hemorrhage.
In this study, temperature-controlled EB-RFA was performed on seven pigs with the successful creation of biliary dilatation and no early or delayed adverse events. The temperature control system at the ablation site automatically stops energy delivery, thereby limiting the charring process and preventing adverse events. Furthermore, the bipolar power supply provides a localized ablation pattern that prevents dispersion and increases safety. Based on previous reports and our experience, temperature-controlled EB-RFA with the same bipolar catheter that we used in this study (ELRA probe) may be considered effective and safe.
EUS examination performed 2 weeks after RFA revealed a round hyperechoic nonshadowing endoluminal image that may represent fibrinopurulent debris and granulation tissue at the same level as that in the previous EB-RFA. The degree of biliary dilatation in all cases was suitable for EUS-BD. Technical success was achieved in six of the seven pigs (86%). Even though the first uncovered metal stent was correctly deployed in the one pig on which we performed HGS, the release of the second covered metal stent failed. The swine biliary dilatation model described in our study provides excellent haptic sensations that we consider to be of primary importance for training, especially for a therapeutic procedure such as EUS-BD that has a high complexity index.
There are several limitations in this study. First, the study included a small number of experimental animals. In addition, compared to porcine ex vivo models that can be re-used several times, the disadvantage this swine model is its high cost. However, ex vivo models do not provide adequate levels of realism necessary for training in EUS-BD; therefore, they may be considered in the initial phase of training.
CONCLUSIONS
Temperature-controlled EB-RFA is a reliable technique for creating critical biliary stricture with associated upstream biliary dilatation (including gallbladder) and is a safe and effective procedure. This biliary dilatation model has become ideal for training in EUS-BD. Further studies with large sample sizes are necessary to confirm the promising findings of this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hindryckx P, Degroote H, Tate DJ, et al. Endoscopic ultrasound-guided drainage of the biliary system: Techniques, indications and future perspectives. World J Gastrointest Endosc. 2019;11:103–14. doi: 10.4253/wjge.v11.i2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonozuka R, Itoi T, Tsuchiya T, et al. EUS-guided biliary drainage is infrequently used even in high-volume centers of interventional EUS. Gastrointest Endosc. 2016;84:206–7. doi: 10.1016/j.gie.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Yoon WJ, Park DH, Choi JH, et al. The underutilization of EUS-guided biliary drainage: Perception of endoscopists in the East and West. Endosc Ultrasound. 2019;8:188–93. doi: 10.4103/eus.eus_57_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhir V, Itoi T, Fockens P, et al. Novel ex vivo model for hands-on teaching of and training in EUS-guided biliary drainage: Creation of “Mumbai EUS” stereolithography/3D printing bile duct prototype (with videos) Gastrointest Endosc. 2015;81:440–6. doi: 10.1016/j.gie.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Dhir V, Itoi T, Pausawasdi N, et al. Evaluation of a novel, hybrid model (Mumbai EUS II) for stepwise teaching and training in EUS-guided biliary drainage and rendezvous procedures. Endosc Int Open. 2017;5:E1087–95. doi: 10.1055/s-0043-118097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ligresti D, Kuo YT, Baraldo S, et al. EUS anatomy of the pancreatobiliary system in a swine model: The WISE experience. Endosc Ultrasound. 2019;8:249–54. doi: 10.4103/eus.eus_10_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rumalla A, Petersen BT, Baron TH, et al. Development of a swine model for benign stenosis of the bile duct by endoscopic application of intraluminal thermal injury. Gastrointest Endosc. 2003;57:73–7. doi: 10.1067/mge.2003.27. [DOI] [PubMed] [Google Scholar]
- 8.Alcaide N, Lorenzo-Pelayo S, Ruiz-Zorrilla R, et al. Su1353 endoscopic porcine model of biliary obstruction using over-the-scope clips: Feasibility and applicability to training in EUS-guided drainage procedures. Gastrointestinal endoscopy. 2013;77:AB294–5. [Google Scholar]
- 9.Lee TH, Choi JH, Lee SS, Cho HD, et al. A pilot proof-of-concept study of a modified device for one-step endoscopic ultrasound-guided biliary drainage in a new experimental biliary dilatation animal model. World J Gastroenterol. 2014;20:5859–66. doi: 10.3748/wjg.v20.i19.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minaga K, Kitano M, Itonaga M, et al. Endoscopic ultrasound-guided biliary drainage using a newly designed metal stent with a thin delivery system: A preclinical study in phantom and porcine models. J Med Ultrason (2001) 2018;45:391–7. doi: 10.1007/s10396-017-0850-1. [DOI] [PubMed] [Google Scholar]
- 11.Park JS, Kwon CI, Jeong S, et al. Development of a swine bile duct dilation model using endoclips or a detachable snare under cap-assisted endoscopy. Gastrointest Endosc. 2014;80:325–9. doi: 10.1016/j.gie.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Park JS, Jeong S, Kim JM, et al. Development of a swine benign biliary stricture model using endoscopic biliary radiofrequency ablation. J Korean Med Sci. 2016;31:1438–44. doi: 10.3346/jkms.2016.31.9.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JH, Jeong S, Kim EJ, et al. Long-term results of temperature-controlled endobiliary radiofrequency ablation in a normal swine model. Gastrointest Endosc. 2018;87:1147–50. doi: 10.1016/j.gie.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Huang GL, Xu M, et al. Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: A comparison of local therapeutic efficacy. Int J Hyperthermia. 2017;33:446–53. doi: 10.1080/02656736.2017.1278622. [DOI] [PubMed] [Google Scholar]
- 15.Auriemma F, De Luca L, Bianchetti M, et al. Radiofrequency and malignant biliary strictures: An update. World J Gastrointest Endosc. 2019;11:95–102. doi: 10.4253/wjge.v11.i2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rustagi T, Chhoda A. Endoscopic Radiofrequency Ablation of the Pancreas. Dig Dis Sci. 2017;62:843–50. doi: 10.1007/s10620-017-4452-y. [DOI] [PubMed] [Google Scholar]
- 17.Ligresti D, Baraldo S, Chavan R, et al. Endoscopic ultrasound-guided biliary drainage in a novel radiofrequency ablation-based swine biliary dilatation model. Endoscopy. 2019;51:E162–3. doi: 10.1055/a-0867-9348. [DOI] [PubMed] [Google Scholar]
- 18.Cho JH, Lee KH, Kim JM, et al. Su1612 safety and efficacy of a novel endobiliary radiofrequency ablation catheter (Elra®) in a swine model. Gastrointest Endosc. 2015;81:AB350–1. [Google Scholar]
- 19.Nayar MK, Oppong KW, Bekkali NL, et al. Novel temperature-controlled RFA probe for treatment of blocked metal biliary stents in patients with pancreaticobiliary cancers: Initial experience. Endosc Int Open. 2018;6:E513–7. doi: 10.1055/s-0044-102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrer J, Scott WE, 3rd, Weegman BP, et al. Pig pancreas anatomy: Implications for pancreas procurement, preservation, and islet isolation. Transplantation. 2008;86:1503–10. doi: 10.1097/TP.0b013e31818bfda1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian Z, Maynar M, Usón-Garallo J, et al. Animal model of bile duct dilatation created with minimally invasive surgery. Acad Radiol. 1999;6:317–20. doi: 10.1016/s1076-6332(99)80457-7. [DOI] [PubMed] [Google Scholar]