Abstract
EUS-guided tissue acquisition (EUS-TA) has made rapid development since its introduction in the early 1990s. The technique is widely accepted and invaluable for staging and diagnosis of a variety of upper gastrointestinal and mediastinal lesions. Fine-needle aspiration (FNA) has long been the gold standard, but due to its limitations such as the inability to retain stroma and associated cellular architecture, novel biopsy needles (FNB) were designed. Overall, FNA and FNB needles perform seemingly equally in terms of diagnostic accuracy, however, the second-generation FNB needles require less passes. The third-generation FNB needles (crown-cut needle types) seem to be preferable to FNA needles as well as to the second-generation FNB needles, when larger histological specimens and preserved tissue architecture are required. EUS-TA is constantly under development, and new applications of this technique include tumor risk stratification according to its genetic profile as well as minimally invasive creation of patient-derived organoids, hallmarks of personized medicine. It remains yet to be shown, whether these applications will lead to a decisive shift from aspiration to biopsy, i.e., from A to B.
Keywords: EUS, FNA, fine-needle biopsy, pancreas
INTRODUCTION
The idea of coupling an ultrasound transducer with an endoscope came in the early 1980s when the first EUS examinations were performed.[1,2] Early echoendoscopes were equipped with radial scanning mechanical transducers, providing good imaging resolution of the gastrointestinal wall as well as of the neighboring organs. However, EUS did not become universally accepted until the development of echoendoscopes with a linear array transducer, providing a possibility for ultrasonic guidance of a biopsy needle during the scanning procedure. The first dedicated biopsy instrument for EUS-FNA was developed by our group in the early 1990s [Figure 1], allowing for a precise diagnosis of even diminutive lesions, which was previously impossible unless surgery was performed.[3,4,5,6,7,8] During this pioneering era, indications of EUS-guided tissue sampling were defined and include today staging of upper gastrointestinal and lung cancer, as well as investigation of lymph nodes, submucosal tumors, adrenals, pancreas, and the biliary tract.[9] EUS-guided tissue acquisition (EUS-TA) was also proven to have a major clinical impact in aforementioned indications, sparing the patients for invasive diagnostic procedures, such as thoracoscopy, mediastinoscopy, laparoscopy, or open surgery. Today, EUS-TA is widely accepted and is the cornerstone of the diagnostic process both in gastroenterology and in pulmonology when combined with endobronchial ultrasound-guided transbronchial needle aspiration biopsy.[10] Since the early 1990s, design of conventional needles has been further improved with the aim of harvesting more tissue for histology, challenging standard aspiration techniques. The aim of this review is to discuss new trends and directions in EUS-TA while reviewing data from previous studies with EUS-FNA and discuss results from recent studies with these novel fine-needle biopsy (FNB) needles.
Figure 1.
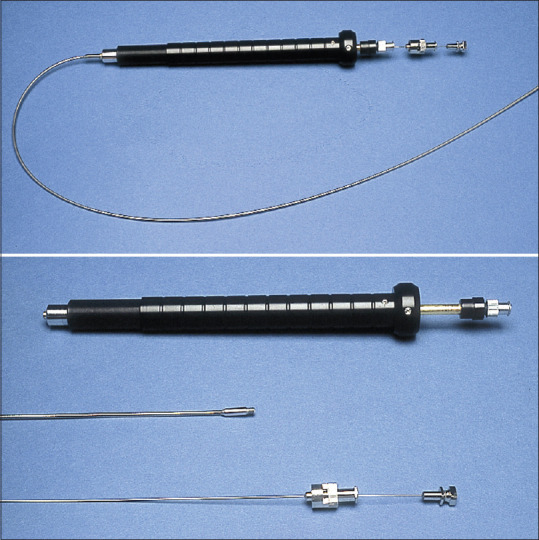
Prototype biopsy instrument (GIP Medizin Technique/MediGlobe), type Hancke/Vilmann
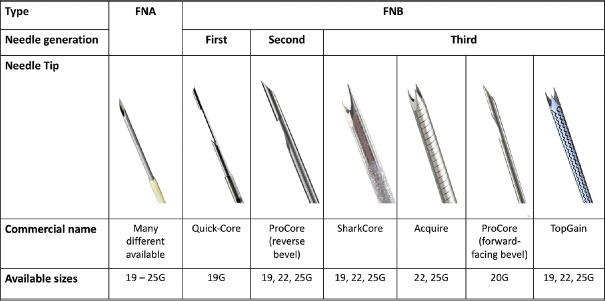
NEEDLE DESIGN
The first needles developed for EUS-TA were aspiration needles (FNA) with a simple tip bevel.[8] Studies evaluating these devices demonstrated a high overall diagnostic accuracy (87%–92%).[9,11] However, while EUS-FNA performed very well in case of lymph nodes and extraluminal masses, the diagnostic accuracy in case of gastrointestinal wall lesions was moderate (67%–84%).[9,11] This, together with the need for specimens with preserved architecture (in case of lymphoma, autoimmune pancreatitis, neuroendocrine tumors, or other), led to the development of biopsy needles (FNB) with a beveled side-slot (Quick-Core® and ProCore®, Cook Medical, Bloomington, IN, USA), considered to be te first and second generation of FNB needles, respectively. The beveled side-slot design was modified to be forward-facing (20G ProCore®, Cook Medical, Bloomington, IN, USA) in an attempt to improve TA, but no data supporting this are currently available. The third-generation FNB needles incorporate various designs but may be uniformly characterized as crown-cut needles. The cutting tip of one of these needles is shaped as a fork-tip, with two opposite cutting edges, and became available in 2014 (SharkCore™, Medtronic, Minneapolis, MN, USA). Another needle has three cutting edges symmetrically distributed at the tip – a Franseen needle type (Acquire™, Boston Scientific, Marlborough, MA, USA). Recently, another Franseen-type needle has become available with a slight adjustment of the angle of the cutting edges of the spikes compared to the Acquire™ needle (TopGain®, Mediglobe, Achenmühle, Germany). An overview of different needle designs is presented in Figure 2.
Figure 2.
Overview over different needle designs
EUS needles are composed of different materials, including aluminum, stainless steel, chromium-cobalt, and nitinol, offering various degrees of hardness and tensile properties. Nitinol needles, for example, are more flexible and preferred in cases where the echoendoscope is fully angulated, for example, with lesions in the uncinate process. Furthermore, all needles undergo postproductional modifications (by polymer coating, laser etching, mechanical dimpling, or sandblasting) in order to enhance the visualization of the needle tip during TA. The echogenicity of the currently available needles is dissimilar, but the difference has not been shown to impact neither tissue quality nor quantity.[12]
Performance of different needle designs has been tested in several randomized controlled trials (RCT) and meta-analyses as well as numerous low-quality studies. The reversed bevel design has been most thoroughly examined in several RCTs and two meta-analyses evaluating needle performance in various solid lesions and lymph nodes.[13,14] Compared to the FNA-needle, no overall difference was observed in sample adequacy, histologic core procurement rate, and adverse event rate.[13,14] However, in order to reach a diagnostic sample, fewer passes were needed with the reversed bevel FNB needle compared to FNA needle.[13,14] Subgroup analysis of nonpancreatic lesions, for example, submucosal tumors, are lacking in the aforementioned studies, and FNB needles may be useful in these cases.[15] A meta-analysis by Facciorusso et al. evaluating diagnostic accuracy in subepithelial lesions, further strengthened the superiority of the FNB sampling in these lesions.[16] In an interesting cost-analysis model originating from an RCT, FNB sampling was shown to have an overall lower cost compared to FNA, mainly caused by a higher diagnostic yield of these needles and lack of rapid on-site evaluation (ROSE) in the FNB group.[15] As ROSE is not systematically utilized outside US, results from this study may not apply to other countries.
As for the third-generation FNB needles, no difference between the fork-tip and Franseen needles was observed in terms of diagnostic accuracy and histologic core procurement rate.[17] Furthermore, comparison of the two needles with the standard FNA needle was only reported for sample diagnostic adequacy, an outcome with no clearly established clinical significance, where both needles outperformed the FNA needle.[17] A retrospective comparative study found the second-generation ProCore® needle to be inferior to the third-generation SharkCore™ needle regarding sample adequacy for histological analysis.[18] Facciorusso et al. evaluated currently available needle designs and sizes in a recent large network meta-analysis including results from 2711 patients.[19] This study, restricted to solid pancreatic lesions, failed to show any difference between FNA and FNB needles in terms of diagnostic accuracy, sample adequacy, or histologic procurement rate. Evidence regarding the newly developed 20G ProCore® needle with forward bevel and Franseen-type TopGain® needle is scarce, with no comparative studies currently available.[20] Even though many studies have shown that different needle designs seem to have similar rates of diagnostic accuracy and histologic procurement, only a few studies have evaluated the actual size of the tissue obtained. Bang et al. were the first to measure the size of the specimen obtained by the Acquire™ needle (median size 2.9 mm) as well as median tumor percentage in the tissue.[21] This quality parameter was followed by Karsenti et al. who, in a prospective setting, compared the new 20G Procore® with the 22G Acquire™ needle.[22] The length of tissue core biopsies per needle pass was significantly higher with the Acquire™ needle compared to the ProCore® needle (mean 8.2 vs. 4.2 mm, respectively).
NEEDLE SIZE
EUS needles are available in different sizes, ranging from 25G (0.46 mm) to 19G (0.91 mm). The standard needle is considered to be a 22G (0.64 mm) FNA needle. The effect of the needle size on diagnostic performance has been evaluated in several studies. Whereas a single older meta-analysis found a trend toward higher sample adequacy for the 25G needle, other more recent meta-analyses failed to replicate this finding.[19,23,24] Most studies compared 25G and 22G needles, and comparisons between 19G and thinner needles are sparse. In terms of diagnostic accuracy, no difference between 19G and 22G needles was observed,[25,26] and the only RCT comparing the 19G with 25G needle was retracted from publication.[27,28] It should be underlined that even though diagnostic accuracy as well as sample adequacy may be similar between the different needle sizes, no comparison of the size of the procured micro-cores has been reported.
TECHNICAL ASPECTS OF TISSUE SAMPLING
Rapid on-site evaluation (ROSE) involves immediate assessment of the procured tissue by a cytopathologist or a cytotechnician present during the procedure. Three available RCTs failed to show an advantage of ROSE in terms of diagnostic accuracy, sample adequacy, or quality.[29,30,31] However, fewer FNA passes are required when ROSE is utilized. Results from meta-analyses are conflicting: whereas older studies showed increased diagnostic accuracy when using ROSE, newer studies reported no difference.[19,32,33,34,35] Availability of ROSE service is variable, and ROSE is more utilized in US compared to Europe and Asia.[36]
Application of negative pressure during TA seems to improve diagnostic accuracy.[37] Standard suction technique implies application of negative pressure with a 10 or 20 mL syringe, and seems to increase diagnostic accuracy and sensitivity when compared to no suction.[38,39,40,41] Increasing the negative pressure (50 mL) does not seem to improve accuracy for malignancy.[42,43] In a single RCT, preflushing of the needle with saline (wet-suction) improved sample adequacy and quality compared to standard suction.[44] However, diagnostic accuracy was not reported. Effect of slow removal of the stylet (slow-pull technique) during sampling has been evaluated in several RCTs, and found comparable to standard suction.[45,46,47] At the end of the sampling procedure, negative pressure persists in the needle and may lead to increased tissue contamination from the puncture site during needle removal.[48] Neutralizing residual negative pressure is, therefore, recommended prior to the removal of the needle.[37]
Fanning technique involves continuous back-and-forth movements of the needle in different areas of the lesion of interest. In one RCT, the fanning technique is shown to decrease the number of passes required to establish a diagnosis, but overall difference in diagnostic accuracy was not statistically significant due to a small sample size.[49] Another prospective, nonrandomized trial showed superiority of the fanning technique combined with “slow-pull” in terms of diagnostic accuracy and blood contamination.[50] Similarly, the effect of the needle stylet has been evaluated in several RCTs and two meta-analyses.[51,52] However, the use of the stylet does not increase diagnostic accuracy, adequacy nor yield, and current ESGE guidelines do not recommend for or against its use.[37]
SAMPLE PREPARATION AND PROCESSING
Samples obtained during EUS-TA contain free cells, blood contaminants, and/or tissue micro-cores, and several different methods of sample processing exist which vary between different centers. Direct smears are performed by a majority of respondents in an international survey, and glass slides can be left to air-dry, immersed in alcohol, or fixated by a spray-based fixative.[36] An alternative to the aforementioned is the liquid-based cytology, where the sample is transported in a liquid medium (saline, alcohol, or Cytolyt®). However, if no fixative is used (in case of saline), the sample should be promptly transferred to the cytology laboratory in order to minimize cellular degeneration. Creation of cell blocks involves utilization of the centrifuged pellet, usually by adding plasma and thrombin in order to form a clot (cell block), which is then processed as histology. This method is shown to be superior to the conventional smear cytology in several prospective, nonrandomized trials.[53,54,55] Nonetheless, the difference between the two methods was not statistically significant in a recent meta-analysis including only subepithelial tumors.[56] Micro-cores (visible tissue fragments) are usually processed separately and fixated in formalin and embedded in paraffin. Histological preparation methods, including the cell block technique and direct preparation of micro-cores, are optimized for subsequent immunohistochemistry and downstream molecular analyses, which are increasingly utilized.
ADVERSE EVENTS
EUS-TA is an established and safe procedure with an overall risk of adverse events as low as 0.29%.[57] Adverse events described include hemorrhage (0.15%–3.7%),[57,58,59,60] acute pancreatitis (0.29%–2.0%),[60,61,62,63,64] and infection (0.4%–3.9%).[60,63,65,66,67,68] Observed hemorrhage is in most cases self-limiting, and EUS-TA can be safely performed even in patients treated with aspirin and nonsteroidal anti-inflammatory drugs.[37] However, P2Y12 receptor antagonists and oral anticoagulants should be discontinued prior to EUS-TA in order to minimize the risk of hemorrhage.[37] Risk of infection is considered generally low when performing EUS-TA of solid lesions, even in transrectal and/or transcolonic approach,[65] and current guidelines do not recommend routine prophylactic antibiotic treatment when biopsy of solid lesions is performed.[37] In case of cystic lesions, however, the risk of infection is higher, and administration of prophylactic antibiotics is recommended.[37] The risk of tumor cell seeding along the needle-tract, otherwise seen in percutaneous biopsy, is considered negligible in the setting of EUS-TA of solid lesions.[69] Similarly, in a meta-analysis of 5124 cases of EUS-FNA in pancreatic cystic lesions, no peritoneal seeding was observed.[60]
NOVEL APPLICATIONS AND FUTURE ASPECTS
Advances in DNA sequencing techniques in recent years have led to significantly lower costs and higher availability of genetic analyses. Mutations in the KRAS gene are frequently observed in case of pancreatic cancer (up to 90%) and have been proposed as a biomarker. Combination of cytology, and KRAS mutation, has been shown to increase sensitivity, especially in indeterminate cases.[70,71,72] Furthermore, the expression of several different biomarkers in FNA samples has been shown to correlate with survival. Itoi et al. reported correlation between mRNA expression of ribonucleotide reductase subunit M2 (RRM2) and survival in 35 patients receiving gemcitabine.[73] Similarly, Ma et al. found that S100A4 mRNA levels in FNA samples correlate with survival.[74] Levels of hENT1 and HSP27 in FNA tissue also seem to predict sensitivity to gemcitabine in patients with unresectable pancreatic cancer.[75,76] Only a few studies addressed the question whether FNA or FNB is superior in terms of sample adequacy for next-generation sequencing (NGS). Larson et al. observed no difference between the two needle types,[77] but in a larger trial by Elhanafi et al. including 167 patients, the proportion of samples sufficient for NGS was higher in the FNB group.[78] However, both the abovementioned studies are retrospective in design, and prospective comparative studies are lacking.
Recently, novel tumor culture models were developed, where tumor cells are embedded into a three-dimensional matrix with which the cells can interact (organoids). These models are superior to conventional expensive and time-consuming monolayer cultures or xenograft models that have been extensively used for in-depth insight and understanding of the carcinogenesis and cancer–environment interactions of different tumors. In case of pancreatic cancer, it was recently shown that organoids can be successfully and rapidly generated from tumor cells, not only from resected specimens, but also from EUS-FNB samples obtained at the time of initial diagnosis and before the initiation of oncological treatment.[79] In a second study, patient-derived pancreatic cancer organoids were exposed to different chemotherapeutic agents, and their response to chemotherapy was measured (”pharmacotyping”).[80] The study showed a strong correlation between patient response and organoid susceptibility to chemotherapeutics. Although still in development, EUS-guided organoid creation and propagation is a new and fascinating application of EUS-TA and may open new ways to precision medicine.
CONCLUSION
EUS-TA is invaluable for staging and diagnosis of a variety of upper gastrointestinal and mediastinal lesions. Overall, FNA and FNB needles perform seemingly equally in terms of diagnostic accuracy, however, the second-generation FNB needles require less passes. The third-generation FNB needles (crown-cut needle types) seem to be preferable to FNA needles as well as to the second-generation FNB needles, when larger histological specimens and preserved tissue architecture are required. All future studies comparing histological procurement between different needles types should preferably be randomized and as a minimum also evaluate the size and quality of the specimen. EUS-TA is constantly under development, and new applications of this technique include tumor risk stratification according to its genetic profile as well as minimally invasive creation of patient-derived organoids, hallmarks of personized medicine. It remains yet to be shown, whether these applications will lead to a decisive shift from aspiration to biopsy, i.e., from A to B.
Financial support and sponsorship
The authors received financial support from Danish Cancer Society, Arvid Nilsson Foundation, Ingrid Munkholm Foundation, Toyota Foundation, Novo Nordisk Foundation, Tømrermester Holm Foundation, Harboefonden, and Herlev Hospital Research Foundation.
Conflicts of interest
Peter Vilmann is member of Advisory Board for Medi-Globe.
Acknowledgments
The authors wish to thank Danish Cancer Society, Arvid Nilsson Foundation, Ingrid Munkholm Foundation, Toyota Foundation, Novo Nordisk Foundation, Tømrermester Holm Foundation, Harboefonden, and Herlev Hospital Research Foundation for their financial support.
REFERENCES
- 1.DiMagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet. 1980;1:629–31. doi: 10.1016/s0140-6736(80)91122-8. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda M, Nakano Y, Saito K, et al. Endoscopic ultrasonography in the diagnosis of pancreatic carcinoma.The use of a liquid-filled stomach method. Scand J Gastroenterol Suppl. 1984;94:65–76. [PubMed] [Google Scholar]
- 3.Vilmann P. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of lymph nodes. Gastrointest Endosc. 1996;43:S24–9. doi: 10.1016/s0016-5107(96)81510-0. [DOI] [PubMed] [Google Scholar]
- 4.Vilmann P, Hancke S. Endoscopic ultrasound scanning of the upper gastrointestinal tract using a curved linear array transducer: The linear anatomy. Gastrointest Endosc Clin N Am. 1995;5:507–21. [PubMed] [Google Scholar]
- 5.Vilmann P, Hancke S. A new biopsy handle instrument for endoscopic ultrasound-guided fine-needle aspiration biopsy. Gastrointest Endosc. 1996;43:238–42. doi: 10.1016/s0016-5107(96)70324-3. [DOI] [PubMed] [Google Scholar]
- 6.Vilmann P, Hancke S, Henriksen FW, et al. Endosonographically-guided fine needle aspiration biopsy of malignant lesions in the upper gastrointestinal tract. Endoscopy. 1993;25:523–7. doi: 10.1055/s-2007-1010389. [DOI] [PubMed] [Google Scholar]
- 7.Vilmann P, Hancke S, Henriksen FW, et al. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of lesions in the upper gastrointestinal tract. Gastrointest Endosc. 1995;41:230–5. doi: 10.1016/s0016-5107(95)70343-8. [DOI] [PubMed] [Google Scholar]
- 8.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 9.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–95. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 10.Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) Endoscopy. 2015;47:545–59. doi: 10.1055/s-0034-1392040. [DOI] [PubMed] [Google Scholar]
- 11.Gress FG, Hawes RH, Savides TJ, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc. 1997;45:243–50. doi: 10.1016/s0016-5107(97)70266-9. [DOI] [PubMed] [Google Scholar]
- 12.Tang SJ, Vilmann AS, Saftoiu A, et al. EUS needle identification comparison and evaluation study (with videos) Gastrointest Endosc. 2016;84:424–3300. doi: 10.1016/j.gie.2016.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh HC, Kang H, Lee JY, et al. Diagnostic accuracy of 22/25-gauge core needle in endoscopic ultrasound-guided sampling: Systematic review and meta-analysis. Korean J Intern Med. 2016;31:1073–83. doi: 10.3904/kjim.2016.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 15.Aadam AA, Wani S, Amick A, et al. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016;4:E497–505. doi: 10.1055/s-0042-106958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Facciorusso A, Sunny SP, Del Prete V, et al. Comparison between fine-needle biopsy and fine-needle aspiration for EUS-guided sampling of subepithelial lesions: A meta-analysis. Gastrointest Endosc. 2020;91:14–22. doi: 10.1016/j.gie.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Facciorusso A, Del Prete V, Buccino VR, et al. Diagnostic yield of Fran seen and fork-tip biopsy needles for endoscopic ultrasound-guided tissue acquisition: A meta-analysis. Endosc Int Open. 2019;7:E1221–30. doi: 10.1055/a-0982-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nayar MK, Paranandi B, Dawwas MF, et al. Comparison of the diagnostic performance of 2 core biopsy needles for EUS-guided tissue acquisition from solid pancreatic lesions. Gastrointest Endosc. 2017;85:1017–24. doi: 10.1016/j.gie.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 19.Facciorusso A, Wani S, Triantafyllou K, et al. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest Endosc. 2019;90:893–903. doi: 10.1016/j.gie.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri C, Fornelli A, Fuccio L, et al. High diagnostic adequacy and accuracy of the new 20G procore needle for EUS-guided tissue acquisition: Results of a large multicentre retrospective study. Endosc Ultrasound. 2019;8:261–8. doi: 10.4103/eus.eus_14_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a Fran seen needle design: Initial assessment. Dig Endosc. 2017;29:338–46. doi: 10.1111/den.12769. [DOI] [PubMed] [Google Scholar]
- 22.Karsenti D, Tharsis G, Zeitoun JD, et al. Comparison of 20-gauge Procore® and 22-gauge Acquire® needles for EUS-FNB of solid pancreatic masses: An observational study. Scand J Gastroenterol. 2019;54:499–505. doi: 10.1080/00365521.2019.1599418. [DOI] [PubMed] [Google Scholar]
- 23.Affolter KE, Schmidt RL, Matynia AP, et al. Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: A systematic review and meta-analysis. Dig Dis Sci. 2013;58:1026–34. doi: 10.1007/s10620-012-2439-2. [DOI] [PubMed] [Google Scholar]
- 24.Facciorusso A, Stasi E, Di Maso M, et al. Endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions with 22 versus 25 Gauge needles: A meta-analysis. United Eur Gastroenterol J. 2017;5:846–53. doi: 10.1177/2050640616680972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Songür N, Songür Y, Bırcan S, et al. Comparison of 19- and 22-gauge needles in EUS-guided fine needle aspiration in patients with mediastinal masses and lymph nodes. Turk J Gastroenterol. 2011;22:472–8. doi: 10.4318/tjg.2011.0322. [DOI] [PubMed] [Google Scholar]
- 26.Song TJ, Kim JH, Lee SS, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–45. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 27.Ramesh J, Bang JY, Hebert-Magee S, et al. Randomized trial comparing the flexible 19G and 25G needles for endoscopic ultrasound-guided fine needle aspiration of solid pancreatic mass lesions. Pancreas. 2015;44:128–33. doi: 10.1097/MPA.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Ramesh J, Bang JY, Hebert-Magee S, et al. Randomized trial comparing the flexible 19g and 25G needles for endoscopic ultrasound-guided fine needle aspiration of solid pancreatic mass lesions. Pancreas. 2015;44:128–33. doi: 10.1097/MPA.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Lee LS, Nieto J, Watson RR, et al. Randomized noninferiority trial comparing diagnostic yield of cytopathologist-guided versus 7 passes for EUS-FNA of pancreatic masses. Dig Endosc. 2016;28:469–75. doi: 10.1111/den.12594. [DOI] [PubMed] [Google Scholar]
- 30.Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: A prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429–39. doi: 10.1038/ajg.2015.262. [DOI] [PubMed] [Google Scholar]
- 31.Kappelle WF, Van Leerdam ME, Schwartz MP, et al. Rapid on-site evaluation during endoscopic ultrasound-guided fine-needle aspiration of lymph nodes does not increase diagnostic yield: A randomized, multicenter trial. Am J Gastroenterol. 2018;113:677–85. doi: 10.1038/s41395-018-0025-8. [DOI] [PubMed] [Google Scholar]
- 32.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong F, Zhu J, Kong X, et al. Rapid on-site evaluation does not improve endoscopic ultrasound-guided fine needle aspiration adequacy in pancreatic masses: A meta-analysis and systematic review. PLoS One. 2016;11:E0163056. doi: 10.1371/journal.pone.0163056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matynia AP, Schmidt RL, Barraza G, et al. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697–705. doi: 10.1111/jgh.12431. [DOI] [PubMed] [Google Scholar]
- 35.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 36.van Riet PA, Cahen DL, Poley JW, et al. Mapping international practice patterns in EUS-guided tissue sampling: Outcome of a global survey. Endosc Int Open. 2016;4:E360–70. doi: 10.1055/s-0042-101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline-March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 38.Wallace MB, Kennedy T, Durkalski V, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441–7. doi: 10.1067/mge.2001.117764. [DOI] [PubMed] [Google Scholar]
- 39.Lee JK, Choi JH, Lee KH, et al. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–51. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Tarantino I, Di Mitri R, Fabbri C, et al. Is diagnostic accuracy of fine needle aspiration on solid pancreatic lesions aspiration-related.A multicentre randomised trial? Dig Liver Dis. 2014;46:523–6. doi: 10.1016/j.dld.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 41.Puri R, Vilmann P, Săftoiu A, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 42.Kudo T, Kawakami H, Hayashi T, et al. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: A multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030–70. doi: 10.1016/j.gie.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Sato J, Ishiwatari H, Sasaki K, et al. Benefit of high negative pressure during endoscopic ultrasound-guided fine-needle aspiration with standard 22-gauge needles for pancreatic lesions: A retrospective comparative study. Scand J Gastroenterol. 2019;54:108–13. doi: 10.1080/00365521.2018.1564788. [DOI] [PubMed] [Google Scholar]
- 44.Attam R, Arain MA, Bloechl SJ, et al. Wet suction technique (WEST): A novel way to enhance the quality of EUS-FNA aspirate.Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401–7. doi: 10.1016/j.gie.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 45.Cheng S, Brunaldi VO, Minata MK, et al. Suction versus slow-pull for endoscopic ultrasound-guided fine-needle aspiration of pancreatic tumors: A prospective randomized trial. HPB (Oxford) 2019 Oct 30; doi: 10.1016/j.hpb.2019.10.007. S1365-182X(19)30738-5. [DOI] [PubMed] [Google Scholar]
- 46.Di Mitri R, Mocciaro F, Antonini F, et al. Stylet slow-pull vs. standard suction technique for endoscopic ultrasound-guided fine needle biopsy in pancreatic solid lesions using 20 Gauge Procore needle: A multicenter randomized trial. Dig Liver Dis. 2020 Feb;52(2):178–184. doi: 10.1016/j.dld.2019.08.023. [doi: 10.1016/j.dld. 2019.08.023] [DOI] [PubMed] [Google Scholar]
- 47.Saxena P, El Zein M, Stevens T, et al. Stylet slow-pull versus standard suction for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic lesions: A multicenter randomized trial. Endoscopy. 2018;50:497–504. doi: 10.1055/s-0043-122381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aadam AA, Oh YS, Shidham VB, et al. Eliminating the residual negative pressure in the endoscopic ultrasound aspirating needle enhances cytology yield of pancreas masses. Dig Dis Sci. 2016;61:890–9. doi: 10.1007/s10620-015-3860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bang JY, Magee SH, Ramesh J, et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–50. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JM, Lee HS, Hyun JJ, et al. Slow-pull using a fanning technique is more useful than the standard suction technique in EUS-guided fine needle aspiration in pancreatic masses. Gut Liver. 2018;12:360–6. doi: 10.5009/gnl17140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JH, Park SW, Kim MK, et al. Meta-analysis for cyto-pathological outcomes in endoscopic ultrasonography-guided fine-needle aspiration with and without the stylet. Dig Dis Sci. 2016;61:2175–84. doi: 10.1007/s10620-016-4130-5. [DOI] [PubMed] [Google Scholar]
- 52.Lai A, Davis-Yadley A, Lipka S, et al. The use of a stylet in endoscopic ultrasound with fine-needle aspiration: A systematic review and meta-analysis. J Clin Gastroenterol. 2019;53:1–8. doi: 10.1097/MCG.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 53.Qin SY, Zhou Y, Li P, et al. Diagnostic efficacy of cell block immunohistochemistry, smear cytology, and liquid-based cytology in endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions: A single-institution experience. PLoS One. 2014;9:e108762. doi: 10.1371/journal.pone.0108762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010;45:868–75. doi: 10.1007/s00535-010-0217-5. [DOI] [PubMed] [Google Scholar]
- 55.Kim JH, Lee SJ, Moon SH, et al. Incremental value of cell block preparations over conventional smears alone in the evaluation of EUS-FNA for pancreatic masses. Hepatogastroenterology. 2014;61:2117–22. [PubMed] [Google Scholar]
- 56.Zhang XC, Li QL, Yu YF, et al. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: A meta-analysis. Surg Endosc. 2016;30:2431–41. doi: 10.1007/s00464-015-4494-1. [DOI] [PubMed] [Google Scholar]
- 57.Jenssen C, Faiss S, Nürnberg D. Complications of endoscopic ultrasound and endoscopic ultrasound-guided interventions – Results of a survey among German centers. Z Gastroenterol. 2008;46:1177–84. doi: 10.1055/s-2008-1027334. [DOI] [PubMed] [Google Scholar]
- 58.Affi A, Vazquez-Sequeiros E, Norton ID, et al. Acute extraluminal hemorrhage associated with EUS-guided fine needle aspiration: Frequency and clinical significance. Gastrointest Endosc. 2001;53:221–5. doi: 10.1067/mge.2001.111391. [DOI] [PubMed] [Google Scholar]
- 59.Varadarajulu S, Eloubeidi MA. Frequency and significance of acute intracystic hemorrhage during EUS-FNA of cystic lesions of the pancreas. Gastrointest Endosc. 2004;60:631–5. doi: 10.1016/s0016-5107(04)01891-7. [DOI] [PubMed] [Google Scholar]
- 60.Zhu H, Jiang F, Zhu J, et al. Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: A systematic review and meta-analysis. Dig Endosc. 2017;29:667–75. doi: 10.1111/den.12851. [DOI] [PubMed] [Google Scholar]
- 61.Fernández-Esparrach G, Ginès A, García P, et al. Incidence and clinical significance of hyperamylasemia after endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of pancreatic lesions: A prospective and controlled study. Endoscopy. 2007;39:720–4. doi: 10.1055/s-2007-966719. [DOI] [PubMed] [Google Scholar]
- 62.Eloubeidi MA, Gress FG, Savides TJ, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: A pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004;60:385–9. doi: 10.1016/s0016-5107(04)01714-6. [DOI] [PubMed] [Google Scholar]
- 63.Eloubeidi MA, Tamhane A, Varadarajulu S, et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: A prospective evaluation. Gastrointest Endosc. 2006;63:622–9. doi: 10.1016/j.gie.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 64.Gress F, Michael H, Gelrud D, et al. EUS-guided fine-needle aspiration of the pancreas: Evaluation of pancreatitis as a complication. Gastrointest Endosc. 2002;56:864–7. doi: 10.1067/mge.2002.129602. [DOI] [PubMed] [Google Scholar]
- 65.Mezzi G, Arcidiacono PG, Carrara S, et al. Complication after endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of rectal lesion. Endoscopy. 2007;39(Suppl 1):E137. doi: 10.1055/s-2007-966318. [DOI] [PubMed] [Google Scholar]
- 66.Voss M, Hammel P, Molas G, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–9. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut. 1999;44:720–6. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee LS, Saltzman JR, Bounds BC, et al. EUS-guided fine needle aspiration of pancreatic cysts: A retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005;3:231–6. doi: 10.1016/s1542-3565(04)00618-4. [DOI] [PubMed] [Google Scholar]
- 69.Ngamruengphong S, Swanson KM, Shah ND, et al. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015;64:1105–10. doi: 10.1136/gutjnl-2014-307475. [DOI] [PubMed] [Google Scholar]
- 70.Plougmann JI, Klausen P, Toxvaerd A, et al. DNA sequencing of cytopathologically inconclusive EUS-FNA from solid pancreatic lesions suspicious for malignancy confirms EUS diagnosis. Endosc Ultrasound. 2020;9:37–44. doi: 10.4103/eus.eus_36_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trisolini E, Armellini E, Paganotti A, et al. KRAS mutation testing on all non-malignant diagnosis of pancreatic endoscopic ultrasound-guided fine-needle aspiration biopsies improves diagnostic accuracy. Pathology. 2017;49:379–86. doi: 10.1016/j.pathol.2016.12.348. [DOI] [PubMed] [Google Scholar]
- 72.Park JK, Lee YJ, Lee JK, et al. KRAS mutation analysis of washing fluid from endoscopic ultrasound-guided fine needle aspiration improves cytologic diagnosis of pancreatic ductal adenocarcinoma. Oncotarget. 2017;8:3519–27. doi: 10.18632/oncotarget.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Itoi T, Sofuni A, Fukushima N, et al. Ribonucleotide reductase subunit M2 mRNA expression in pretreatment biopsies obtained from unresectable pancreatic carcinomas. J Gastroenterol. 2007;42:389–94. doi: 10.1007/s00535-007-2017-0. [DOI] [PubMed] [Google Scholar]
- 74.Ma G, Sun Y, Fu S. Evaluation of S100A4 mRNA in EUS-FNA specimens for the assessment of chemosensitivity to gemcitabine from patients with unresectable pancreatic cancer. Int J Clin Exp Patho. 2015;8:13284–8. [PMC free article] [PubMed] [Google Scholar]
- 75.Yamada R, Mizuno S, Uchida K, et al. Human equilibrative nucleoside transporter 1 expression in endoscopic ultrasonography-guided fine-needle aspiration biopsy samples is a strong predictor of clinical response and survival in the patients with pancreatic ductal adenocarcinoma undergoing gemcitabine-based chemoradiotherapy. Pancreas. 2016;45:761–71. doi: 10.1097/MPA.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawano M, Kaino S, Amano S, et al. Heat shock protein 27 expression in EUS-FNA samples can predict gemcitabine sensitivity in pancreatic cancer. In Vivo. 2018;32:637–42. doi: 10.21873/invivo.112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larson BK, Tuli R, Jamil LH, et al. Utility of endoscopic ultrasound-guided biopsy for next-generation sequencing of pancreatic exocrine malignancies. Pancreas. 2018;47:990–5. doi: 10.1097/MPA.0000000000001117. [DOI] [PubMed] [Google Scholar]
- 78.Elhanafi S, Mahmud N, Vergara N, et al. Comparison of endoscopic ultrasound tissue acquisition methods for genomic analysis of pancreatic cancer. J Gastroenterol Hepatol. 2019;34:907–13. doi: 10.1111/jgh.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tiriac H, Bucobo JC, Tzimas D, et al. Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment. Gastrointest Endosc. 2018;87:1474–80. doi: 10.1016/j.gie.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tiriac H, Belleau P, Engle DD, et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8:1112–29. doi: 10.1158/2159-8290.CD-18-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]