Abstract
Background and Objectives:
EUS-FNA is applied widely in clinical practice, but there remains a lack of authentic training models. The present study aimed to develop a novel swine training model and to perform a preliminary assessment of its feasibility and efficacy.
Materials and Methods:
To create an internal lesion-like target, empty shells of iodine-125 seeds were implanted into the caudate lobe of the liver in Bama minipigs. A training program involving 10 trainees was subsequently carried out, in which a total of 60 needlings were performed, composed of 6 for each trainee obtained during two training steps. Comparisons of procedure-related variables were conducted between the two. Trainees completed a questionnaire to assess their basic endoscopic experiences and reasonability of the model.
Results:
A target region of 2.0 cm × 2.0 cm in diameter was successfully established on the caudate lobe in all implanted pigs. In the training program, the average procedure time decreased from the first to the second step and the average time for the total 30 needlings' obtainment was significantly shorter for the second training step (23.8 ± 4.5 min vs. 40.9 ± 9.0 min, P < 0.001). For the second step, there was also a significant improvement in total success rate (86.7% vs. 56.7%, P = 0.020) and accuracy rate (76.7% vs. 43.3%, P = 0.017). All trainees scored the effectiveness of the model highly and all reported improved confidence after the training.
Conclusion:
This novel swine training model could authentically mimic clinical EUS-FNA, providing an effective in vivo practice tool for novices before clinical practice.
Keywords: animal model, EUS, FNA, swine model, training model
INTRODUCTION
EUS has been used widely in clinical practice, emerging as a well-established and critical tool in the diagnosis and evaluation of esophagogastrointestinal and pancreaticobiliary diseases. Over the past few years, however, the role of EUS has evolved drastically within the field of therapeutic interventions. A wide range of EUS-guided techniques, including the EUS-FNA, injection, drainage, and anastomosis, were developed and have promoted an expansive prospect for clinical application of this technology.[1] As the application of EUS continues to expand, a prominent contradiction has arisen between the ever-growing clinical demands and the limited number of competent endosonographers.[2] In China, the number of credentialed endosonographers is far from sufficient to cover the large population, and there is a significant spatial disequilibrium of EUS development among different geographical regions in the nation. The result is a need for efficient and authentic EUS training greater than ever before.
According to current guidelines, achieving competency in all aspects of EUS requires a minimum of 150 supervised cases, 50 of which should involve EUS-FNA.[3,4] However, the training in EUS-FNA itself faces remarkable challenges because of its long and steep learning curve.[5] Chinese centers with adequate case volume for training remain low in number. Moreover, the practice of training novices in EUS-FNA on actual patients is related to a series of safety and ethics issues. Effective training models for EUS-FNA will help to overcome these shortcomings, improving preclinical training while decreasing the number of supervised training cases involving actual patients. Of the several models that have been developed to date for EUS training, they are mostly ersatz, inadaptable to EUS-FNA, and not amenable to repeated use.[6,7,8,9,10,11]
Here, we describe our development of a novel swine model to facilitate “hands-on” training of EUS-FNA by creating a target region on the caudate lobe of the liver via implantation of empty shells of iodine-125 seeds. This study encompassed a preliminary assessment of the feasibility and efficacy of this model for EUS-FNA training.
MATERIALS AND METHODS
Study design
This was a prospective pilot study involving the creation of an in vivo animal model for EUS-FNA training and subsequent evaluation of the model among trainees. All procedures were conducted at a tertiary digestive center with training qualifications for advanced endoscopic interventions in China. The study was approved by the Institutional Animal Care and Use Committee before initiation.
Animals
A total of five 6-month-old male Bama minipigs (Sus scrofa), weighing 23.5–27.0 kg, were used. The general health condition of each pig was observed for 1 week before the study to ensure optimum health. All pigs were fasted for 24 h before the EUS-FNA procedures and 24 h after the procedures with intramuscular injection of prophylactic antibiotics using benzathine benzylpenicillin (16.5 mg/kg/day) and proton-pump inhibitor (omeprazole, 10 mg/day). On the 2nd day after EUS-FNA, a liquid diet was initiated, with gradual advancement to normal diet. All EUS-FNA procedures were performed under general anesthesia with intubation and under carbon dioxide insufflations. All animals were closely monitored by veterinarians throughout the entire study period.
Creation of the in vivo animal model
All animal models were developed by a single skilled endoscopist (KX-W) with experiences of more than 1000 EUS-FNAs. A linear array echoendoscope (EG-530UT, Fujifilm, Tokyo, Japan) and echoprocessor system (SU-7000, Fujifilm, Tokyo, Japan) were used for both model creation and the subsequent training program. In practice, after intubation of the echoendoscope into the stomach, the ultrasound anatomic structures were sequentially revealed as aorta, celiac trunk, superior mesenteric artery, splenic artery/vein, pancreas, portal vein, and liver. The echoendoscope was then positioned and maintained with the imaging of the caudate lobe of the liver in the center of the ultrasound chart display. A 19-gauge needle (Boston Scientific, Natick, MA, USA) was then passed through the accessory channel of the echoendoscope and advanced into the caudate lobe of the liver under EUS guidance.
In order to create a target region, empty shells of iodine-125 seeds (Xinke Pharmaceutical, Shanghai, China) were implanted into the caudate lobe [Video 1]. We have previously reported the implantation of iodine-125 seeds in the treatment of patients with pancreatic cancer.[12,13,14] The seeds used in the present study were similar as previously reported (4.5-mm length and 0.8-mm diameter) but did not contain any radioactive nuclide [Figure 1]. Four seeds were implanted into each animal, and the formation of a lesion-like target region on the caudate lobe of the liver was observed on the next day [Video 2 and Figure 1].
Figure 1.
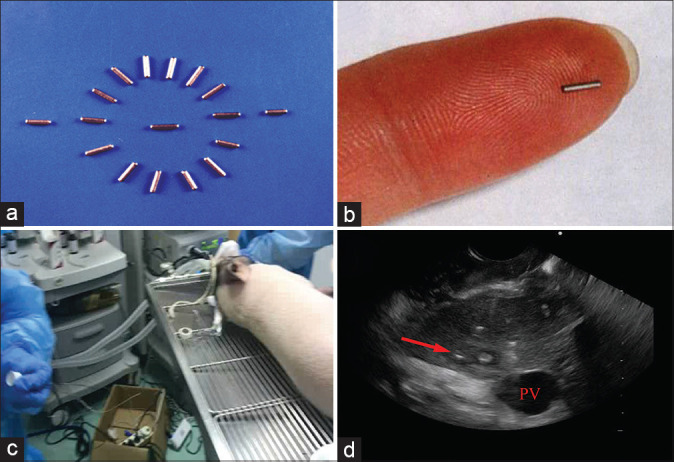
Establishment of the novel swine model. (a and b) The feature of the empty shells of iodine-125 seeds; (c) the scene when implanting the seeds in Bama minipigs; (d) the created target region in the caudate lobe (red arrow)
Training program
A total of 10 trainees with experience in performing gastroscopy and colonoscopy were enrolled in the study's training program. Each completed a 1-month course to obtain theoretical knowledge of anatomy and EUS and to observe numerous expert operator procedures performed on clinical patients. Subsequently, the trainees were progressed to two hands-on training steps using the novel animal model, with the second step being conducted at 1 week after the first. The 10 trainees were randomly assigned to the five animal models, resulting in a two-trainee-per-pig pattern. Every trainee was allowed to perform three separate EUS-FNA procedures each step. A 22-gauge needle (Cook Medical Inc., Bloomington, IN, USA) was used for all EUS-FNA procedures.
The procedures of EUS-FNA were performed the same as in clinical practice. The needle was advanced into the target region, with visualization of the needle in real time. After guidance into the target region, the needle was pushed back and forth 10 times with the stylet slow-pull method. Then, the needle was withdrawn and detached from the echoendoscope. The aspirated materials were expulsed onto slides using a syringe or a stylet. The yields of specimens were assessed by an experienced supervisor (KX-W). The echoendoscope was withdrawn after each procedure, and the trainee took turns to prevent meaningless consecutive procedures.
Training assessment
During every EUS-FNA procedure, the trainees were asked to display the main anatomic structures for a standard abdominal EUS examination (listed above), to locate the target region on the caudate lobe by tracing vessels, and to perform EUS-FNA in avoidance of intervening vessels using color Doppler. The trainees were instructed to pay careful attention to tactile feelings in their fingers and hands when the needle was passing through the liver. The average procedure time, accuracy, safety, and success rate were recorded and compared between the two training steps. The accuracy was assessed by appropriate puncturing of the needle tip into the target region formed by the implanted seeds. When the needle tip was seen to be forwarded right into the target region on the ultrasound chart display, the procedure was deemed to be accurate; otherwise, the procedure was deemed inaccurate, regardless of the specimen acquisition. The procedure was evaluated as safe when the following criteria were met: no obvious puncture of the vessels, as monitored by EUS view of the needle into the vessels; the needle tip being within the target region while pushing and pulling; and not losing EUS view of the needle. The procedure was considered successful upon the accurate interpretation of the main structures plus revelation of the created target region and adequate obtainment of specimens. The aspirated material was evaluated by the supervisor via visual inspection according to the criteria for macroscopic on-site evaluation previously reported.[15] In general, the samples were macroscopically assessed according to the presence of either a definite tissue core with scanty blood clots, a visible tissue core or tissue fragments mixed with blood clots, or only blood without any tissue. The yields of the first two types were considered adequate. After the two animal training steps, the trainees were asked to complete a questionnaire,[16] which gathered basic information about their endoscopic experiences and reasonability of the model; the latter covered the complexity of operative skills, the operative similarity between the model and the real patient, the effectiveness of the model in EUS training, and the confidence of trainees after undergoing the training on a 0- to 10-point scale.
Statistical analysis
SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Data were expressed as mean ± standard deviation or as percentage. Statistical significance was calculated by Student's t-test. A comparison of categorical data was performed using Fisher's exact test. A two-sided P ≤ 0.05 was considered to indicate statistical significance.
RESULTS
Successful establishment of the novel model
All five pigs were successfully implanted with the empty shells of iodine-125 seeds. A lesion-like target region was observed on the caudate lobe of the liver by the seeds in all pigs the day after implantation. The region was similarly round-shaped with a diameter of about 2.0 cm × 2.0 cm [Figure 1]. The target region persisted and all animals survived throughout the entire course of the study without any adverse event or death, suggesting that the animals could be used repeatedly for future trainings.
EUS-FNA training using the novel model
The 10 trainees performed a total of 60 EUS-FNA training procedures using the novel swine model. The average time of 3 needlings for a single trainee shortened from the first training step to the second [Figure 2a]. Furthermore, the average time for the total 30 needlings was significantly shorter for the second training step (23.8 ± 4.5 min vs. first: 40.9 ± 9.0 min, P < 0.001) [Figure 2b]. In the total 30 needlings performed in the first training step, 17 were considered successful, resulting in a success rate of 56.7%; the success rate was significantly better for the second training step (86.7% [26/30]; P = 0.020). The accuracy rate also showed a significant improvement from the first to the second training step (43.3% (13/30) to 76.7% (23/30); P = 0.017). Although we also observed an elevation of the safety rate in the second training step, the difference failed to reach the threshold for statistical significance (P = 0.067). The results of the outcomes are summarized in Table 1.
Figure 2.
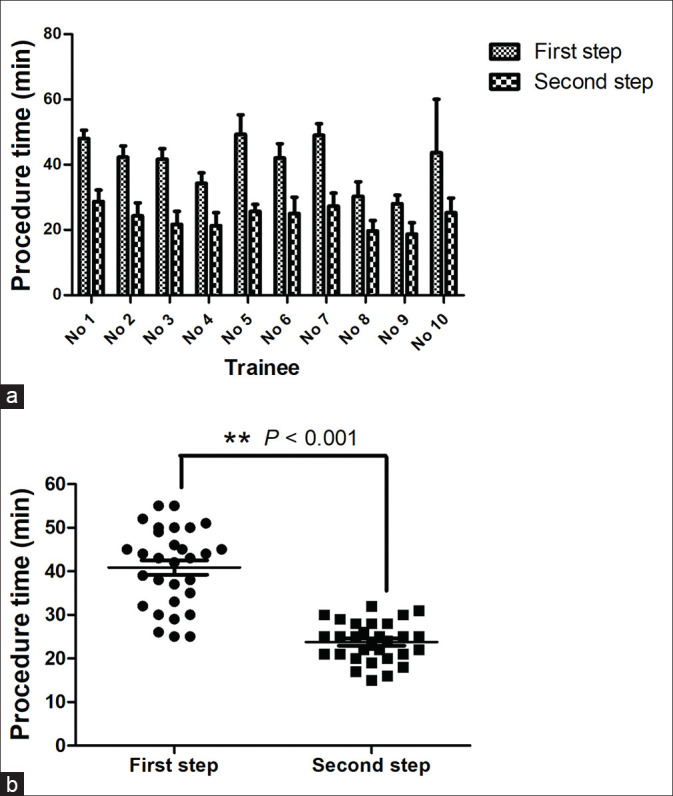
Comparison of the procedure time between two training steps. (a) The average time was decreased for every individual trainee; (b) the average time of the total 30 needlings was significantly shorter in the second training step than that in the first
Table 1.
Comparisons between the first and second EUS-FNA training steps using the novel swine model
| Variable | First step | Second step | P |
|---|---|---|---|
| Procedure time* | 40.9±9.0 | 23.8±4.5 | <0.001 |
| Success rate | 56.7% (17/30) | 86.7% (26/30) | 0.020 |
| Accuracy rate | 43.3% (13/30) | 76.7% (23/30) | 0.017 |
| Safety rate | 30.0% (9/30) | 56.7% (17/30) | 0.067 |
*Data are presented as mean±SD. Bold text indicates a statistically significant difference. SD: Standard deviation
Cost-effectiveness
The cost of the model mainly includes a startup cost of purchasing the animals (1500 CNY per pig). Other expenses are the fees for the training arena plus animal anesthesia (500 CNY for 4 h). The endoscopes and all accessories were all recycled and sterilized after clinical use. The empty seeds were provided by the company for free.
Questionnaire analysis
All trainees scored high, indicating good effectiveness of the model for EUS-FNA training, and all evaluated the operative similarity to be adequate to that in real clinical patients. All trainees also reported having felt more at ease and comfortable in determining the anatomy, positioning the echoendoscope, keeping the target region in place, and proceeding with the puncture. All trainees reported an improved confidence after undergoing the training.
DISCUSSION
In the present study, we established a novel swine model for “hands-on” EUS-FNA training. To the best of our knowledge, this is hitherto the first live model that could best mimic clinical EUS-FNA with good reproducibility and durability. The caudate lobe – a small distinct portion of the liver situated on the posterior surface and lying within the confines between the main portal vein and left portal vein anteriorly and the inferior vena cava posteriorly – was utilized to create a target region by implantation of empty shells of iodine-125 seeds. The surrounding vessels could help the new learners to better understand the anatomic structures and obtain skills of tracing a vessel to find the target lesion. Moreover, they are helpful in fostering the learners' awareness of avoiding vessels while puncturing the needle into the target lesion. In our preliminary evaluation of the feasibility and effectiveness of the novel model in EUS-FNA training, the trainees were asked to locate the target region on the caudate lobe by recognition and interpretation of relevant anatomic structures. EUS-FNA was then carried out with emphasis on the trainee's tactile feelings in handling the echoendoscope and needling within the liver. All trainees appraised the effectiveness of the model in their EUS training as high, which was confirmed by a significantly reduced procedure time and elevated rates of overall success and accuracy from the first to the second training step. Although the increase in safety rate failed to show a statistical significance, this may be explained by the relatively strict criteria used in our study to define a “safe” operation (i.e., avoiding the vessels, controlling the needle tip within the target region, and not losing EUS view of the needle while pushing and pulling). Honing of these advanced skills may require more sessions of training or practice on more clinical cases. Nonetheless, all trainees reported improvement in their skill confidence after undergoing the training. We observed persistence of the seeds in position for at least 6 months, suggesting that the pigs could be reused for repeated training procedures in a tertiary teaching hospital. However, it is currently difficult to determine exactly how long the pigs be used for the training, since whether the tissue damages caused by repeated EUS-FNA procedures and the consequent processes such as bleeding, inflammation, and tissue reconstruction would exert influence on the reusability of the model and tactile feeling while needling still needs further investigation.
EUS-FNA was initially applied as an accurate and minimally invasive method for the sampling of pancreatic masses. The procedure of EUS-FNA involves comprehensive abilities that are foundational for all interventional EUS procedures, including recognition and interpretation of anatomic structures, tracing vessels and locating the target lesion, positioning and stable maintenance of the scope, puncturing of the needle into a target, and avoiding the vessels.[17] Therefore, the learning and mastering of EUS-FNA should be achieved before the practice of other advanced therapeutic EUS procedures. Training for EUS-FNA needs to address different stages, and as such, the following sequence is generally recommended: (1) acquisition of cognitive and theoretical knowledge; (2) use of computer-based simulator or phantoms; (3) use of ex vivo and/or live animal models; and (4) clinical training supervised by experts. Models play a critical role in the training process before introducing the skill into their clinical practice.[18] Any training model should be as authentic as possible, and for EUS-FNA, it should provide visualization and scope positioning that are consistent to that in real clinical cases. The model should provide reproducibility and accessibility. Durability is also important, meaning that the model must hold up through many procedures performed under novice hands in a series of training sessions.
Until today, many training models for EUS-FNA have been reported, including phantoms, computer-based simulators, ex vivo models using isolated organs from animals, and live animal models;[6,7,8,9,10,11] yet, each features substantial limitations. Phantoms are easy to use and require minimal preparation but lack realism. Ex vivo animal models generally require more extensive preparation and disposal after use, being more or less rapidly damaged or destroyed. Computer-based simulators have the advantage of prolonged use at minimal additional expense after a one-time startup cost, and they are valuable tools for initial training in EUS; however, these simulators do not offer verisimilitude to the tactile feel of puncturing a human lesion and the experience of adverse events, such as bleeding and perforation. Therefore, achieving realistic properties as well as reproducibility and durability of models for EUS-FNA training remains challenging.
Animal models have long been used for teaching therapeutic endoscopy. A live swine is the best model from an anatomical perspective, in comparison with human beings. As such, swine may provide the direct tactile feedback of penetration through the gastrointestinal wall into the target lesion while handling the needle. In fact, among the models available for hands-on training, live swine are the most realistic and can support the improvement of EUS-FNA skills. However, a healthy swine does not normally present with masses or enlarged lymph nodes. In the first report of a swine model for EUS-FNA training, the normal pancreas was utilized for practicing needle puncture without a target lesion.[7] This model was later improved by creating a submucosal lesion in the stomach via injection of lipid emulsion and a focal pseudo-mediastinal lymph node via injection of saline solution.[8] Another model, reported by Fritscher-Ravens et al.,[9] also induced lymphadenopathy in the mediastinum by injection of graphite. Since the created submucosal lesions or mediastinal lymph nodes were located very proximal to the endoscope, they did not optimally mimic the complexities that may be encountered in hepatopancreatobilliary EUS-FNA. Moreover, the reproducibility and durability of these models were also unclear due to the probable degrading of the submucosal lesions and lymph nodes.
There are several limitations of the present study. First, this was a single-center study conducted at a tertiary hospital, and the sample size of trainees was relatively small. Second, the quality of the obtained specimens was not assessed histologically but was judged by the supervisor. Since whether the specimens were obtained from within the target region or not (mainly the liver) could not be distinguished histologically, we consider it not very necessary to perform histological assessment. Instead, the supervisor monitored the whole course of every EUS-FNA procedure. The trainees were asked to display the main anatomic structures, to locate the target region and to perform EUS-FNA with the needle tip being seen to be forwarded right into the target region on the ultrasound chart display. The aspirated material was also macroscopically evaluated by the supervisor as we introduced above. These measures have covered the fundamental knowledge and skills that were required for the training of EUS-FNA. Finally, we did not carry out tests or verifications of the trainees' performances on clinical patients after undergoing the training in comparison with those who did not undergo the training. Therefore, large-scale, randomized, and placebo-controlled clinical study is needed to more definitely know whether this novel swine model could serve as a robust tool in reducing the number of supervised examinations in actual patients required for the attainment of competency and improving the learning curve.
CONCLUSION
We have created a novel in vivo model for EUS-FNA training. This authentic and reusable model may play an important role in enabling novices to practice and develop safe and effective EUS-FNA procedures before clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos Available on: www.eusjournal.com
REFERENCES
- 1.Braden B, Gupta V, Dietrich CF. Therapeutic EUS: New tools, new devices, new applications. Endosc Ultrasound. 2019;8:370–81. doi: 10.4103/eus.eus_39_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong JYY, Kongkam P, Ho KY. Training in endoscopic ultrasonography: An Asian perspective. Dig Endosc. 2017;29:512–6. doi: 10.1111/den.12802. [DOI] [PubMed] [Google Scholar]
- 3.Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811–4. doi: 10.1016/s0016-5107(01)70082-x. [DOI] [PubMed] [Google Scholar]
- 4.Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 5.Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33–7. doi: 10.1016/s0016-5107(03)02028-5. [DOI] [PubMed] [Google Scholar]
- 6.Sorbi D, Vazquez-Sequeiros E, Wiersema MJ. A simple phantom for learning EUS-guided FNA. Gastrointest Endosc. 2003;57:580–3. doi: 10.1067/mge.2003.141. [DOI] [PubMed] [Google Scholar]
- 7.Bhutani MS, Hoffman BJ, Hawes RH. A swine model for teaching endoscopic ultrasound (EUS) imaging and intervention under EUS guidance. Endoscopy. 1998;30:605–9. doi: 10.1055/s-2007-1001364. [DOI] [PubMed] [Google Scholar]
- 8.Bhutani MS, Aveyard M, Stills HF., Jr Improved model for teaching interventional EUS. Gastrointest Endosc. 2000;52:400–3. doi: 10.1067/mge.2000.108408. [DOI] [PubMed] [Google Scholar]
- 9.Fritscher-Ravens A, Cuming T, Dhar S, et al. Endoscopic ultrasound-guided fine needle aspiration training: Evaluation of a new porcine lymphadenopathy model forin vivo hands-on teaching and training, and review of the literature. Endoscopy. 2013;45:114–20. doi: 10.1055/s-0032-1325931. [DOI] [PubMed] [Google Scholar]
- 10.Hoshi K, Irisawa A, Shibukawa G, et al. Validation of a realistic, simple, and inexpensive EUS-FNA training model using isolated porcine stomach. Endosc Int Open. 2016;4:E1004–8. doi: 10.1055/s-0042-110094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim GH, Bang SJ, Hwang JH. Learning models for endoscopic ultrasonography in gastrointestinal endoscopy. World J Gastroenterol. 2015;21:5176–82. doi: 10.3748/wjg.v21.i17.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Z, Du Y, Li Z, et al. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: A prospective pilot study. Endoscopy. 2008;40:314–20. doi: 10.1055/s-2007-995476. [DOI] [PubMed] [Google Scholar]
- 13.Wang K, Jin Z, Du Y, et al. Evaluation of endoscopic-ultrasound-guided celiac ganglion irradiation with iodine-125 seeds: A pilot study in a porcine model. Endoscopy. 2009;41:346–51. doi: 10.1055/s-0028-1119588. [DOI] [PubMed] [Google Scholar]
- 14.Wang KX, Jin ZD, Du YQ, et al. EUS-guided celiac ganglion irradiation with iodine-125 seeds for pain control in pancreatic carcinoma: A prospective pilot study. Gastrointest Endosc. 2012;76:945–52. doi: 10.1016/j.gie.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Oh D, Seo DW, Hong SM, et al. The impact of macroscopic on-site evaluation using filter paper in EUS-guided fine-needle biopsy. Endosc Ultrasound. 2019;8:342–7. doi: 10.4103/eus.eus_34_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Fang J, Jin Z, et al. Use of simulator for EUS training in the diagnosis of pancreatobiliary diseases. Endosc Ultrasound. 2019;8:25–30. doi: 10.4103/2303-9027.252232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryozawa S, Fujita N, Irisawa A, et al. Current status of interventional endoscopic ultrasound. Dig Endosc. 2017;29:559–66. doi: 10.1111/den.12872. [DOI] [PubMed] [Google Scholar]
- 18.Parra-Blanco A, González N, González R, et al. Animal models for endoscopic training: Do we really need them? Endoscopy. 2013;45:478–84. doi: 10.1055/s-0033-1344153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


