Abstract
Background
Ebola virus (EBOV) disease has killed thousands of West and Central Africans over the past several decades. Many who survive the acute disease later experience post-Ebola syndrome, a constellation of symptoms whose causative pathogenesis is unclear.
Methods
We investigated EBOV-specific CD8+ and CD4+ T-cell responses in 37 Sierra Leonean EBOV disease survivors with (n = 19) or without (n = 18) sequelae of arthralgia and ocular symptoms. Peripheral blood mononuclear cells were infected with recombinant vesicular stomatitis virus encoding EBOV antigens. We also studied the presence of EBOV-specific immunoglobulin G, antinuclear antibodies, anti–cyclic citrullinated peptide antibodies, rheumatoid factor, complement levels, and cytokine levels in these 2 groups.
Results
Survivors with sequelae had a significantly higher EBOV-specific CD8+ and CD4+ T-cell response. No differences in EBOV-specific immunoglobulin G, antinuclear antibody, or anti–cyclic citrullinated peptide antibody levels were found. Survivors with sequelae showed significantly higher rheumatoid factor levels.
Conclusion
EBOV-specific CD8+ and CD4+ T-cell responses were significantly higher in Ebola survivors with post-Ebola syndrome. These findings suggest that pathogenesis may occur as an immune-mediated disease via virus-specific T-cell immune response or that persistent antigen exposure leads to increased and sustained T-cell responses.
Keywords: Ebola virus, post-Ebola sequelae, T-cell response
In this study, we evaluated Ebola-specific T-cell responses in Ebola virus disease survivors with or without post-Ebola syndrome, and we found that survivors with postinfectious sequelae had significantly higher CD8+ T-cell responses than survivors without sequelae.
Ebola virus (EBOV) disease (EVD) is a devastating and fatal disease that has plagued central Africa for decades [1]. EBOV, the causative pathogen of EVD, swept through West Africa in 2013–2016, killing thousands, leaving increasing poverty and significant social disruption [2]. More than 28 000 West Africans contracted the disease, and 11 310 died, giving the outbreak a staggering 40% mortality rate [3]. Of the 17 000 survivors, many continue to experience debilitating symptoms for months, or even years after viremia has cleared [4, 5]. These symptoms have been collectively defined as post-Ebola syndrome (PES) [4, 5].
PES, a constellation of physical and psychiatric symptoms reported in EBOV survivors, was initially associated with Congolese and Ugandan outbreaks, and has been further characterized since the West African epidemic [4, 6–9]. Hallmark symptoms of PES are similar to those present after other viral infections, including arthralgia and ocular symptoms, as well as more general symptoms, including fatigue, myalgia, and headache. These symptoms occur with much higher frequency in EVD survivors than in their household contact controls [4–7]. After the 1995 EBOV outbreak in Kikwit, Democratic Republic of Congo, some of the first descriptions of PES were published [6, 7]. One group reported that 4 of 21 survivors evaluated reported ophthalmalgia, photophobia, hyperlacrimation, and visual acuity loss. Examination revealed uveitis in all 4 patients [6]. About 60% of survivors from the same outbreak continued to report myalgia and arthralgia after 21 months [7].
The 2013–2016 Ebola epidemic in West Africa has left survivors with similar sequelae [4, 8, 9]. Survivors developed arthralgia and 33% of survivors experienced ocular symptoms and signs of uveitis, along with myriad other general symptoms that persisted for >504 days [4]. Another study in Sierra Leone found rates of arthralgia and ocular manifestations of 76% and 50%, respectively, in postinfectious individuals [5]. More than 30% of participants reporting new ophthalmalgia had uveitis diagnosed by means of slit-lamp examination, and uveitis was associated with higher EBOV polymerase chain reaction during acute illness [5].
The pathogenesis of PES is unclear. Autoimmune disease due to molecular mimicry and immune complex deposition into joint tissue has been observed in hepatitis arthritis and could be a possible mechanism of pathogenesis of PES [10]. Alternatively, survivors with severe EVD could have experienced direct viral injury at the time of infection or a delayed hypersensitivity reaction to viral antigens [11], resulting in continued symptoms. Given that persistent viral antigen exposure and high viral titers during the acute infection are associated with PES, we investigated whether differences in cell-mediated and humoral immunity to EBOV were associated with PES. We also questioned whether an increase in peripheral circulating antibodies, such as antinuclear antibody (ANA), anti–cyclic citrullinated peptide antibody (anti-CCP), and rheumatoid factor (RF), might be associated with PES. We designed a cross-sectional study to evaluate EBOV-specific adaptive responses and indicators of autoimmunity in Ebola survivors with or without PES.
METHODS
Study Site and Participants
Kenema Government Hospital in Kenema, Sierra Leone, was the site of our study and is the Ministry of Health and Sanitation, Government of Sierra Leone referral hospital for Kenema District. Participants were registered and recruited by the Ebola Survivors Association of Kenema, Kono, and Kailahun districts, had a documented clinical history of EVD and were treated at an Ebola treatment unit.
Participants were surveyed for PES every 6 months. We defined individuals as having PES if they reported arthralgia and ≥2 of the following ocular symptoms: dryness, burning, loss of vision, blurry vision, tearing, pain, photophobia, foreign body sensation, eye redness, which we thought was suggestive of uveitis [12]. Participants were classified as not having sequelae if they reported no symptoms on their first survey. One participant reported only ocular pruritus and was included in the cohort without sequelae, given the mildness of his reported symptom and lack of correlation of pruritus alone with uveitis.
This study was approved by Scripps Research, Tulane University’s Human Research Protection Program, and the Sierra Leone Ethics and Scientific Review Committee. All subjects provided written consent. The study participant identifiers are 1740090, 2034870, 2827730, 6809170, 5656880, 1195170, 5164310, 6394110, 0937800, 5792800, 1233210, 9478070, 8197950, 8939540, 8496920, 6444640, 7308250, 4279150, 0030080, 0021700, 6827870, 5840770, 5311660, 9126840, 5987500, 8158490, 5709400, 4961230, 2090830, 6107720, 5201180, 8666680, 3015790, and 2370690.
EBOV-Specific Antibody Analysis
Participants’ immunoglobulin G (IgG) levels were tested with the ReEBOV IgG/immunoglobulin M enzyme-linked immunosorbent assay (ELISA) Kit (Zalgen Labs). The kits used were nonstandard Zalgen kits and were coated with antibodies against viral protein (VP) 40. Positive cutoffs were defined as 3 standard deviations above the average IgG optical density value of negative controls.
Peripheral Blood Mononuclear Cell Isolation
Blood was collected in BD Vacutainer EDTA tubes (BD Biosciences). Peripheral blood mononuclear cell (PBMC) isolation was performed at Kenema Government Hospital. Vacutainer tubes were centrifuged at 430g, and undiluted plasma was removed for additional analysis. Blood was diluted with phosphate-buffered saline (PBS) and layered onto Lymphoprep (Stemcell Technologies) and then centrifuged for 30 minutes at 450g without brake. The buffy coat was removed, diluted with PBS, and then centrifuged at 230g. The cell pellet was resuspended in PBS and then centrifuged at 430g. PBMCs were resuspended in 3 mL of CryoStor Freezing Media (Biolife Solutions) and slow frozen for 4 hours at ‒80°C before storage in a liquid nitrogen dry shipper and shipment to the United States.
Recombinant Single-Cycle Infectious Vesicular Stomatitis Virus Preparation and T-Cell Assay
Recombinant single-cycle infectious vesicular stomatitis virus (rscVSV) encoding EBOV genes (Makona strain G3845) for nucleoprotein (NP), glycoprotein (GP), soluble glycoprotein (sGP), VP24, VP30, VP35, and VP40 proteins were prepared and used for T-cell stimulation and quantification of EBOV-specific T-cell responses, as described elsewhere [13–16]. PBMCs from EBOV survivors were plated in a 96-well plate in Roswell Park Memorial Institute 1640 medium with 10% fetal bovine serum, and infected with rscVSVs encoding full-length EBOV proteins NP, GP, sGP, VP24, VP30, VP35, and VP40, with a multiplicity of infection of 15. As a negative control, PBMCs were infected with rscVSV encoding enhanced green fluorescent protein, to evaluate CD8+ T-cell activation to the VSV vector. For a positive control, PBMCs were incubated with antibodies against human CD3 and CD28, at concentrations of 60 and 20 µg/µL, respectively. PBMCs were incubated at 37°C, brefeldin A was added at a concentration of 4 ng/mL after 4 hours, and then cells continued overnight incubation.
Cells were subsequently stained with fluorescein isothiocyanate–conjugated antihuman CD3 clone HIT3α (Biolegend), peridinin-chlorophyll protein–cyanine 5.5–conjugated antihuman CD4 clone OKT4 (BioLegend), and BV421-conjugated antihuman CD8 clone RPA-T8 (BD Biosciences) antibodies at a dilution of 1:200 for 1 hour at 4°C. The cells were washed and then fixed with BD Cytofix (BD Biosciences) for 20 minutes at 4°C. The cells were washed with BD Perm/Wash buffer and then stained with phycoerythrin–cyanine 7–conjugated antihuman interferon (IFN) γ clone B27 (BD Biosciences), allophycocyanin-conjugated antihuman tumor necrosis factor (TNF) α clone MAb11 (BD Biosciences), and PE-conjugated antihuman interleukin 2 (IL-2) clone 5344.11 (BD Biosciences) antibodies diluted in BD Perm/Wash buffer for 1 hour, at 4°C. Cells were suspended in fluorescence-activated cell sorter buffer in preparation for flow cytometry. LSR II (Becton Dickinson) was used for flow cytometry data collection, and FlowJo software, version 10 (TreeStar) for analysis. Cells were gated on live lymphocytes, single cells, and CD3+, CD8+, or CD4+ cells and were considered activated if positive for both IFN-γ and TNF-α at 1.2 log10 greater than the median base population of T cells. The methods of this assay have been detailed elsewhere [14–16].
Human Epithelial Cell Immunofluorescence Staining for ANA Detection
NOVA-Lite HEp-2 ANA substrate slides (Inova Diagnostics) were used to detect ANA in the plasma of EBOV survivors with or without sequelae. Staining of slides was performed consistently with the manufacturer’s instructions. Slides were mounted using Vectashield Mounting Medium with DAPI (Vector Laboratories) and observed using an Olympus BH2 microscope. Digital images of ANA patterns were captured using a Leica DFC 365 FX camera and analyzed using Leica Application Suite AF software (Leica Microsystems). Positive samples were scaled from 1 (lowest) to 4 (highest) based on fluorescence intensity. Negative samples were scored as 0. Controls were used on each assay. The scoring was done by a partially blinded assessor and verified by a second blinded assessor.
ANA, RF, and Anti-CCP ELISA Protocol
ANA, RF, and anti-CCP ELISAs (Inova) were performed according to manufacturer’s instructions. The plates were read on an ELISA plate reader with a 450-nm filter, with the reference filter set at 600–650 nm.
Neutralization Protocol
Pseudovirus was generated using EBOV GP (MakonaΔmuc) in an HIVΔENV (SG3) backbone. The virus was titered in TZM cells, with a target luminescence of 10 000 relative luminescence units. Serum samples were was diluted and incubated with pseudotype virus for 1 hour. Adherent TZM cells were trypzinized, prepared in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum, and plated. The mixture was incubated for 48 hours and then incubated with Bright-Glo substrate (Promega) diluted in lysis buffer. Luminescence was measured, and neutralization percentages were calculated.
Multiplex Assay
The human high-sensitivity T-cell multiplex and complement multiplex assays (Millipore) were performed according to the manufacturer’s instructions. Data were collected using a Luminex 200 instrument (Luminex).
RESULTS
CD8+ and CD4+ T-Cell Responses in EBOV Survivors With or Without Sequelae
Nineteen survivors had sequelae of joint pain plus at least 2 ocular symptoms. Eighteen survivors reported no sequelae at all, except for 1 survivor’s report of ocular pruritus (Table 1). The mean ages of survivors with or without sequelae were 31 and 29 years, respectively. Participant survivors were mostly women, 63% of those with (n = 12) and 67% of those without (n = 12) sequelae.
Table 1.
Demographics and Clinical Findings in 37 Survivors of Ebola Virus Diseasea
group
| Characteristic | Survivors With Sequelae (n = 19) | Survivors Without Sequelae (n = 18) |
|---|---|---|
| Demographics | ||
| Age, mean, y | 31 | 29 |
| Female sex, no. (%) | 12 (63) | 12 (67) |
| Clinical findings, no. | ||
| Arthralgia | 18 | 0 |
| Ocular symptoms | 19 | 1 |
| Blurry vision | 6 | 0 |
| Complete loss of vision | 0 | 0 |
| Eye redness | 5 | 0 |
| Pain | 10 | 0 |
| Burning | 7 | 0 |
| Dry eyes | 0 | 0 |
| Tearing eyes | 9 | 0 |
| Sensation of foreign body | 5 | 0 |
| Light sensitivity | 6 | 0 |
| Flashes of light | 6 | 0 |
| Pruritus | 10 | 1 |
| Eye floaters | 4 | 0 |
aOne survivor was without arthralgia but had several ocular symptoms concerning for uveitis so was included in the sequelae group.
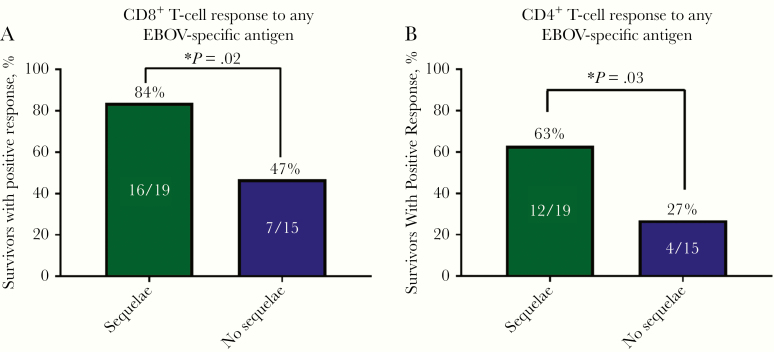
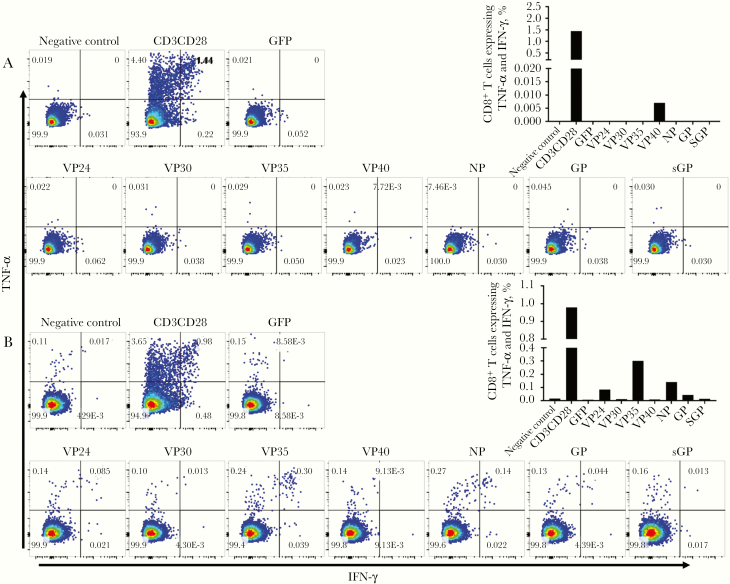
To assess EBOV-specific T-cell responses in EVD survivors, we used a library of rscVSVs encoding EBOV antigens (NP, GP, sGP, VP24, VP30, VP35, and VP40). These reagents are able to stimulate virus-specific responses in both CD8+ and CD4+ T cells and have been used to identify CD8+ T-cell epitopes from EVD survivors. We considered CD3+ T cells expressing both IFN-γ and TNF-α at least 1.2 log10 higher than the median response to negative controls (rscVSV expressing enhanced green fluorescent protein and no infection controls) as EBOV antigen-specific responses. CD8+ T-cell expression of IL-2 was low; therefore, IL-2 was not included in the analysis and results but can be seen in Supplementary Figure 1. All EBOV survivors with sequelae were included in the T-cell analysis, but only 15 of the 18 survivors without sequelae had PBMCs available for analysis. Of the EBOV survivors with sequelae, 16 of 19 participants (84%) had positive CD8+ T-cell responses to ≥1 of the 7 EBOV antigens, compared with 7 of 15 (47%) without sequelae (P = .02) (Figure 1A). Similarly, EBOV survivors with sequelae had higher CD4+ T-cell responses than those without sequelae (63% vs 27%; P = .03) (Figure 1B). Examples of negative and positive CD8+ T-cell responses are shown in Figure 2.
Figure 1.
CD8+ (A) and CD4+ (B) T-cell responses to any Ebola virus (EBOV)–specific antigen in survivors of EBOV disease with (n = 19) or without (n = 15) sequelae, defined as joint pain along with ≥2 reported ocular symptoms. (P values calculated with Student t tests.)
Figure 2.
Examples of Ebola virus (EBOV)–specific CD8 T-cell responses in survivors of EBOV disease without (A) or with (B) sequelae. Abbreviations: GFP, green fluorescent protein; GP, glycoprotein; IFN, interferon; NP, nucleoprotein; sGP soluble glycoprotein; TNF, tumor necrosis factor; VP, viral protein. (For scientific notation, 7.72E‒3, for example represents 7.72 X 103; the unit measured is events.)
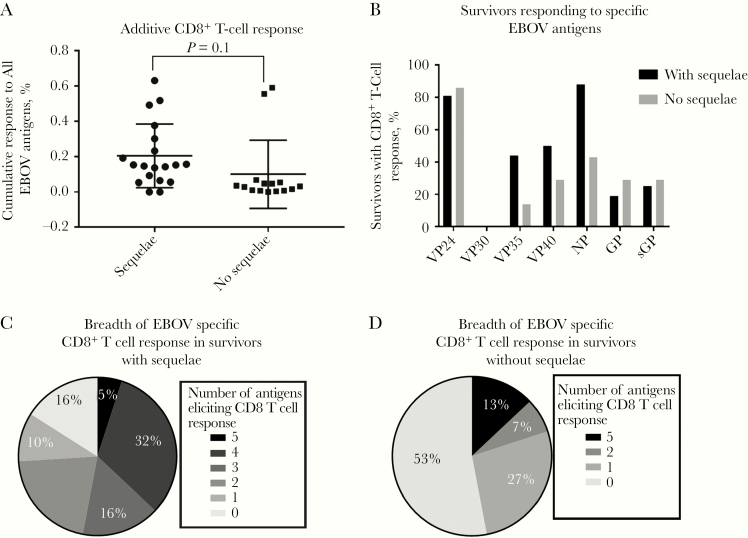
We did not observe significant differences between the combined percentage responses of EBOV-specific CD8+ T cells to all EBOV antigens (NP, GP, sGP, VP24, VP30, VP35, and VP40) between the 2 groups whether we included (Figure 3A) or excluded nonresponders (P = .1 and P = .6, respectively). We found no significant difference in the breadth of response to EBOV antigens between the 2 groups; however, most survivors with sequelae responded to a total of 4 EBOV proteins (6 of 19 [32%]), whereas most of the survivors without sequelae responded to none (7 of 15 [47%]) or to just 1 EBOV antigen (4 of 15 [27%]) (Figure 3C). Of the survivors who responded to EBOV antigens (excluding nonresponders), those with sequelae frequently responded to VP24 (81%), NP (88%), and VP40 (50%), and those without sequelae most frequently responded to VP24 (86%), NP (43%), GP (29%), and sGP (29%) (Figure 3D).
Figure 3.
A, Combined percent of Ebola virus (EBOV)–specific CD8+ T-cell responses to 7 EBOV antigens (nucleoprotein [NP], glycoprotein [GP], soluble glycoprotein [sGP], viral protein [VP] 24, VP30, VP35, and VP40) for each survivor with or without sequelae (P = .1, calculated with Student t test). B, Frequency of survivor response to each EBOV antigen (excluding survivors with no CD8+ T-cell response in the 24-hour overnight assay) (n = 16 with sequelae; n = 7 without sequelae). C, Frequency of survivors with or without sequelae by number of EBOV antigens showing positive CD8+ T-cell response. D, Frequency of survivor response to each EBOV antigen. Survivors with no CD8+ T cell response were excluded from this figure. Survivor with sequelae n = 16; Without sequelae n = 7.
Levels of EBOV-Specific IgG Antibody in EVD Survivors With or Without PES
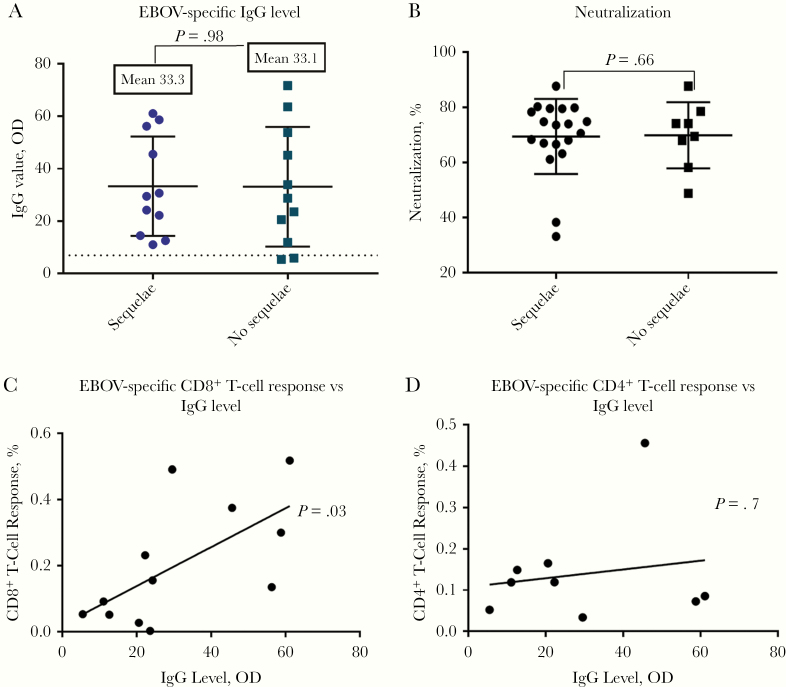
Because we observed that survivors with sequelae were more likely to have memory CD4+ T-cell responses, we asked whether levels of EBOV-specific antibodies differed between the 2 groups. We found no significant differences in EBOV-specific IgG levels to EBOV VP40 between the EBOV survivor groups with (n = 11) or without (n = 11) PES (P = .98) (Figure 4A). We also assessed differences in neutralizing antibodies between those with (n = 19) or without sequelae (n = 8). Serum samples from survivors were assessed for their ability to inhibit infection of EBOV GP pseudotyped virus. No differences were observed in the neutralizing capacity of the 2 groups (Figure 4B). When we assessed whether both the humoral and cellular arms of the immune system were active in the survivors, we found a significant correlation between the sum of EBOV-specific CD8 T-cell responses and EBOV-specific IgG antibody titers among the EVD survivors with a CD8 T-cell response to any EBOV antigen (n = 12) (r = 0.64; P = .03) (Figure 4C). However, there was no correlation between the sum of EBOV-specific CD4 T-cell responses and antibody titers (P = .7) (Figure 4D).
Figure 4.
A, Ebola virus (EBOV)–specific immunoglobulin G (IgG) levels determined using ReEBOV IgG enzyme-linked immunosorbent assay, testing antibodies against viral protein 40 (n = 11 with sequelae; n = 11 without sequelae; P = .98, calculated with Student t test). B, Percentage of neutralization of EBOV antibodies by EBOV glycoprotein pseudotype virus in survivors with (n = 19) or without (n = 8) post-Ebola sequelae (P = .66; Student t test). C, Summed percentage of CD8+ T-cell response to EBOV antigens is significantly parallel to EBOV-specific IgG levels, excluding nonresponders (P = .03; 2-tailed Student t test). D, Summed percentage CD4+ T-cell response to EBOV antigens is not correlated with EBOV-specific IgG values, excluding nonresponders (P = .7; 2-tailed Student t test). Abbreviation: OD, optical density.
ANA, RF, and Anti-CCP Antibody Assays in EBOV Survivors With or Without Sequelae
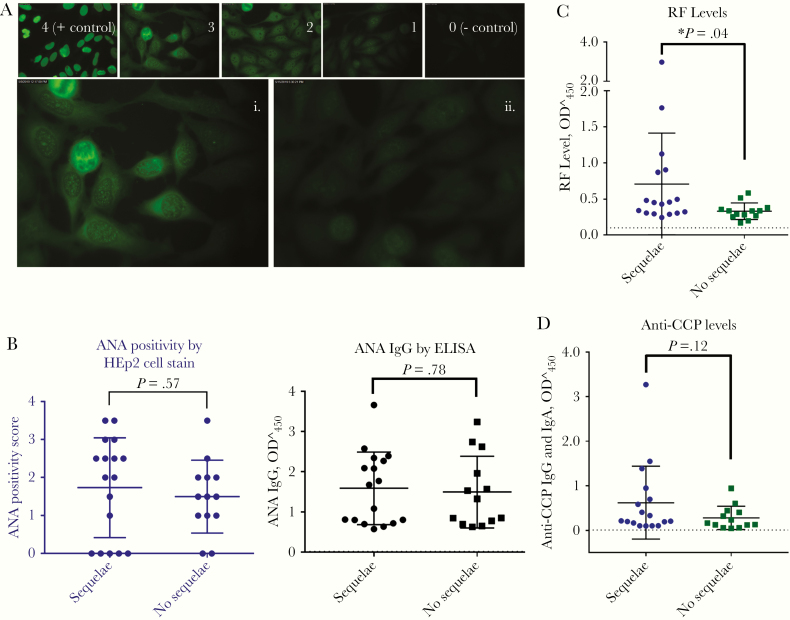
We next assessed peripheral circulating autoantibody levels of ANA, RF, and anti-CCP, which are associated with arthritis and autoimmune disease. EVD survivors with or without sequelae had no difference in circulating ANA levels, by either human epithelial type 2 immunofluorescence staining or ELISA (P = .57 and .69, respectively) (Figure 5A and 5B). RF levels were significantly higher in survivors with sequelae than in those without sequelae (0.71 vs 0.33; P = .04) (Figure 5C), a finding consistent with the reported arthralgia. There was 1 outlier in the survivor group with sequelae, however when this value was removed from the data set, the P value remained .04. There was no difference in anti-CCP antibody levels between the 2 groups (P = .12) (Figure 5D).
Figure 5.
A, Antinuclear antibody (ANA) immunoglobulin G (IgG) levels by human epithelial type 2 (HEp2) immunofluorescence assay, in survivors of Ebola virus (EBOV) disease with or without sequelae. Panels 0–4 represent examples of brightness scoring scale. Also shown are examples of survivors with (i) and without (ii) sequelae. B, ANA in EBOV survivors with (n = 17) or without (n = 13) sequelae, measured with both HEp2 cell immunofluorescence assay and enzyme-linked immunosorbent assay (ELISA) (P = .57 and .78, respectively, calculated with Student t test). C, Rheumatoid factor (RF) levels are significantly higher in EBOV survivors with sequelae than in those without sequelae (P = .04; Student t test). D, Anti–cyclic citrullinated peptide antibody (anti-CCP) antibody levels in EBOV survivors with or without sequelae, measured with ELISA (P = .12; Student t test). Dotted lines in B–D indicate negative control levels, as provided by the test kits. Abbreviation: OD450, optical density at 450 nm.
Cytokine and Complement Levels in EBOV survivors With or Without PES
Finally, we determined whether survivors with PES had a signature of immune activation by measuring a panel of cytokines associated with T-cell responses and complement levels (Supplementary Figure 2). We found no differences in IFN-inducible T-cell α-chemoattractant, granulocyte-macrophage colony-stimulating factor, fractalkine, IFN-γ, IL-2, interleukin 13 (IL-13), interleukin 10, 12, 21, 4, 23, 5, 6, 7, and 8, macrophage inflammatory protein (MIP) 3α, MIP-1α, IL-22, or TNF-α values between EBOV with (n = 17) or without PES (n = 12). IL-13 levels were significantly higher in EBOV survivors with PES (4.5 vs 6.8 pg/mL; P = .03). Conversely, MIP-1β levels were significantly higher in EBOV survivors without PES (10.7 vs 16.4 pg/mL; P = .03), as were interleukin 1β (IL-1β) levels (23.7 vs 31.6 pg/mL; P = .03). There were no differences in complement levels for C1q, C3, or C4 between the 2 groups.
DISCUSSION
EVD survivors with PES had significantly higher EBOV-specific CD8+ and CD4+ T-cell responses than survivors without PES. We suggest 3 possible explanations for the pathogenesis of observed PES. First, PES could be caused by virally mediated injury. PES is associated with higher levels of viremia during infection [5] and the presence of virus in immune privileged sites during convalescence [17, 18]. Cellular injury during acute infection may be more severe in those with higher viral titers. In addition, the presence of virus during convalescence could mediate cellular injury either through direct viral cytopathic effects or by immune responses to infected cells. Others have observed that survivors with more severe disease and higher levels of viremia during acute infection have higher levels of activated CD8+ T cells during acute disease and in convalescence [19]. Moreover, survivors from the West African Ebola outbreak had a 3.3 increased odds ratio for uveitis for every 5-point decrease in EBOV polymerase chain reaction cycle threshold at the time of acute infection, as described by Mattia et al [5]. Together, these data indicate that severe EVD characterized by high viral titers both induces a robust T-cell response and increases the likelihood of PES during convalescence.
Although higher T-cell responses in individuals with PES are likely a result of robust and continued antigen exposure, whether T cells play a direct role in disease is unclear. EBOV has been isolated months after infection from semen, vaginal fluid, aqueous humor, breast milk, and cerebrospinal fluid, indicating that EBOV can persist after viremic clearance [7, 17, 18]. Persistent antigen exposure at sites with decreased immune surveillance, such as joint spaces and the anterior chamber of the eye, may lead to intermittent viremia, with recurrent CD8+ T-cell activation. Continued antigen exposure may explain the higher T-cell responses we found in our study participants with sequelae. We did not evaluate synovial fluid or aqueous humor for EBOV RNA owing to regulation limitations imposed on our study. Such studies to evaluate suspected sites of persistent antigen exposure for EBOV RNA are under consideration.
Alternatively, the pathogenesis of PES may reflect an EBOV-triggered autoimmune-mediated disease, a possibility that we investigated in this study. Although we observed elevated CD4+ responses in EBOV survivors with sequelae, there were no differences in anti-VP40 IgG levels or neutralizing antibodies between survivors with and those without sequelae. We also quantified markers of autoimmune disease to determine whether virus-induced autoreactivity might play a role in PES. The presence of ANA has been observed after infections with several RNA and DNA viruses [20].
Although we observed high ANA levels in some EVD survivors, levels did not differ significantly between those with and those without sequelae. Levels of peripheral anti-CCP antibodies, which are autoantibodies seen in other rheumatologic disorders, were similar in EVD survivors with or without sequelae. Similar levels of ANA and anti-CCP in both groups suggests that immune complex deposition into articular tissue may not explain the post-EBOV symptom of arthralgia; however, this finding does not exclude the possibility that autoantibodies are present at the site of tissue damage (eg, synovium), or that other autoantibodies exist. Pathologic evaluation of articular tissue for antibody or T-cell deposits would be needed to confirm a role for autoimmunity. The small number of participants in our study may have prevented the detection of significant differences in circulating autoantibodies between EBOV survivors with and those without sequelae. RF levels were significantly higher in survivors with sequelae. RF elevation is associated with autoimmune rheumatoid arthritis, viral illnesses [21] and hepatitis C virus–induced mixed cryoglobulinemia [22], for which arthralgia is a hallmark symptom [22].
Although we found no differences in antibody responses, measuring both the neutralizing capacity of survivor serum samples and the presence of anti-VP40 IgGs, we cannot exclude the possibility that antibodies or antibody-antigen complexes contribute to PES. Although antibodies are made, neutralizing antibodies do not usually appear until several months after clinical survival [23], suggesting that EBOV-specific T cells are likely to play an essential role in control of acute infection and viral clearance. Nevertheless, arthralgia in PES cases would be consistent with symptoms associated with articular immune complex deposition. Clearance of these complexes may cause or exacerbate disease. Additional studies should be performed to measure the presence of articular immune complexes in survivors with or without PES.
The third possible mechanism of PES involves the role that HLA plays in shaping T-cell responses. Elevated T-cell responses in those with PES implicate HLAs as a genetic contributor to disease. Evaluating these genetic associations would require hundreds of participants, owing to the diversity of HLA alleles among our cohort. However, because T-cell responses are critical for viral clearance and, as we have shown, are elevated in survivors with PES, HLA association studies may identify those with an underlying genetic predisposition to PES. The HLA alleles for our study participants are publicly available on the Center for Viral Systems Biology online database [24].
We found that IL-13 levels were significantly higher in EBOV survivors with sequelae. IL-13 is secreted by CD4+ T cells, and these differences are likely related to higher CD4+ T-cell activation in survivors with PES. Contrary to our expected observation, MIP-1β and IL-1β levels were significantly lower in survivors without PES. MIP-1β forms reversible polymers that buries the receptor binding site, rendering aggregates inactive [25]. Thus, while levels of MIP-1β are lower in those without PES, it is unclear whether there are differences in MIP-1β activity between groups. We detected lower levels of IL-1β in survivors without PES, but it is difficult to interpret the significance of this finding given that background IL-1β levels in the general Sierra Leonean population are unknown.
In the current study, we used EBOV antigen-encoding rscVSVs to measure T-cell responses in EVD survivors [14]. The advantage of this system is the ability to determine T-cell responses while retaining antigen processing and presentation. This biological process is critical for selection of antigenic peptides and may limit false-positive responses, which is especially important when evaluating T-cell responses in developing countries where antigenic burden is high. The limited sample quantity from EVD survivors did not allow for analysis of T-cell responses specific to RNA-dependent RNA polymerase. However, studies examining T-cell analysis of other negative-strand RNA viruses have consistently shown that responses to the polymerase are nearly always subdominant, likely owing to low expression of this antigen during infection [26, 27]. We measured T-cell responses by a short, overnight stimulation, which likely captures only the most dominant and abundant memory T-cell responses. Longer antigen stimulation would promote the proliferation of low precursor, subdominant EBOV-specific T cells.
PES is a major burden on West African society, leading to loss of productivity, work capacity, and income. Further investigation of the causes of PES may aid in identifying interventions in affected individuals. To our knowledge, ours is the first study to compare EBOV-specific T cells to PES and clearly document a direct correlation between PES and the presence of EBOV-specific T cells and RF, suggesting EBOV-induced immunologic pathogenesis and a possible autoimmune role.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge and thank the participants of the study who generously shared their time for the purpose of research, and the government of Sierra Leone for allowing us to conduct this research. We thank the staff of Kenema Government Hospital and the Lassa Ward, especially Simbirie Jalloh for her management of the Lassa fever program and Francis Baimba for his assistance with phlebotomy.
Author contributions. S. M. L., J. M. M., K. M. P., J. C. d. l. T., R. F. G., J. S. S., M. B. A. O., and B. M. S. designed the studies and experiments. M. B. and L. M. B. designed and provided resources for experiments. S. M. L., S. S., M. M., A. G., J. D. S., B. C., A. S., K. S., and I. B. conducted experiments. S. M. L., M. B. A. O., and B. M. S. analyzed the data and wrote the manuscript. S. M. L., L. K., M. M., F. A. H., M. Y., M. G., D. S. G., and J. S. S. chose and enrolled participants into the study, collected blood samples, and maintained databases for clinical information. S. M. L., R. F. G., J. S. S., M. B. A. O., and B. M. S. provided project direction and oversight.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (contract grant HHSN272201400048C under BAA-NIAID-DAIT-NIHAI2013167) and the National Institutes of Health (grant 1R01AI123535).
Potential conflicts of interest. J. C. d. l. T. reports grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), during the conduct of the study. L. M. B. is a cofounder of Zalgen Labs. L. M. B. is also a board member for the Viral Hemorrhagic Fever Consortium (vhfc.org), a partnership of academic and industry scientists developing diagnostics, therapeutics, and vaccines for Lassa Fever and other severe diseases. (Tulane University and various industry partners have filed United States and foreign patent applications on behalf of the consortium for several of these technologies; if commercial products are developed, consortium members may receive royalties or profits.) R. F. G. reports grants from the NIH, during the conduct of the study, and other support from Zalgen Labs, outside the submitted work. J. S. S. reports grants from the NIH, during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Khan AS, Tshioko FK, Heymann DL, et al. Commission de Lutte Contre les Epidémies à Kikwit. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. J Infect Dis 1999; 179(suppl 1):S76–86. [DOI] [PubMed] [Google Scholar]
- 2. Nuriddin A, Jalloh MF, Meyer E, et al. Trust, fear, stigma and disruptions: community perceptions and experiences during periods of low but ongoing transmission of Ebola virus disease in Sierra Leone, 2015. BMJ Glob Health 2018; 3:e000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Center for Disease Control and Prevention. 2014–2016 Ebola outbreak in West Africa https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/index.html. Accessed 9 September 2019.
- 4. Mohammed H, Vandy AO, Stretch R, et al. Sequelae and other conditions in Ebola virus disease survivors, Sierra Leone, 2015. Emerg Infect Dis 2017; 23:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattia JG, Vandy MJ, Chang JC, et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross sectional study. Lancet Infect Dis 2016; 16:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kibadi K, Mupapa K, Kuvula K, et al. Late ophthalmologic manifestations in survivors of the 1995 Ebola virus epidemic in Kikwit, Democratic Republic of the Congo. J Infect Dis 1999; 179(suppl 1):S13–4. [DOI] [PubMed] [Google Scholar]
- 7. Rowe AK, Bertolli J, Khan AS, et al. Commission de Lutte Contre les Epidémies à Kikwit Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. J Infect Dis 1999; 179(suppl 1):S28–35. [DOI] [PubMed] [Google Scholar]
- 8. Wilson H, Amo-Addae M, Kenu E. Post Ebola syndrome among Ebola virus disease survivors in Montserrado County, Liberia 2016. Biomed Res Int 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The PREVAIL III Study Group. A longitudinal study of Ebola sequelae in Liberia. New Eng J Med 2019; 380:924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pawlotsky JM, Roudot-Thoraval F, Simmonds P, et al. Extrahepatic immunologic manifestations in chronic hepatitis C and hepatitis C virus serotypes. Ann Intern Med 1995; 122:169–73. [DOI] [PubMed] [Google Scholar]
- 11. Roy F. Ocular differential diagnosis. 3rd ed. Philadelphia, PA: Lea & Febiger, 1984. [Google Scholar]
- 12. Amissah-Arthur MB, Poller B, Tunbridge A, Adebajo A. Musculoskeletal manifestations of Ebola virus. Rheumatology 2018; 57:28–31. [DOI] [PubMed] [Google Scholar]
- 13. Whitt M. Generation of VSV pseudotypes and using recombinant ΔG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J Virol Methods 2010; 169:365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakabe S, Sullivan BM, Hartnett JN, et al. Analysis of CD8+ T cell response during the 2013–2016 Ebola epidemic in West Africa. Proc Natl Acad Sci U S A 2018; 115:E7578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakabe S, Hartnett JN, Ngo N, et al. Identification of common CD8+ T cell epitopes from Lassa fever survivors in Nigeria and Sierra Leone. J Virol 2020; 94(12):e00153-20. doi: 10.1128/JVI.00153-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sullivan BM, Sakabe S, Hartnett JN, et al. High crossreactivity of human T cell responses between Lassa virus lineages. PLoS Pathog 2020; 16:e1008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uyeki TM, Erickson BR, Brown S, et al. Ebola virus persistence in semen of male survivors. Clin Infect Dis 2016; 62:1552–5. [DOI] [PubMed] [Google Scholar]
- 18. MacIntyre C, Chughtai A. Recurrence and reinfection- a new paradigm for the management of Ebola virus disease. Int J Infect Dis 2016; 43:58–61. [DOI] [PubMed] [Google Scholar]
- 19. McElroy AK, Akondy RS, Davis CW, et al. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci U S A 2015; 112:4719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oldstone M. Virus induced autoimmune disease: viruses in the production and prevention of autoimmune disease. In: Membranes and viruses in immunopathology. New York, NY: Academic Press, 1972:469. [Google Scholar]
- 21. Shimerling R, Delbanco T. The rheumatoid factor: an analysis of clinical utility. Am J Med 1991; 91:528–34. [DOI] [PubMed] [Google Scholar]
- 22. Ferri C, Sebastiani M, Giuggioli D, et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum 2004; 33:355–74. [DOI] [PubMed] [Google Scholar]
- 23. Davis CW, Jackson KJL, McElroy AK, et al. Longitudinal analysis of the human B cell response to Ebola virus infection. Cell 2019; 177:1566–1582.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Center for Viral Systems Biology. HLA genotype sequencing of hemorrhagic fever survivors data https://data.cvisb.org/dataset/hla. Accessed 2 April 2020.
- 25. Graham GJ, MacKenzie J, Lowe S, et al. Aggregation of the chemokine MIP-1α is a dynamic and reversible phenomenon: biochemical and biological analyses. J Biol Chem 1994; 269:4974–8. [PubMed] [Google Scholar]
- 26. Bui HH, Peters B, Assarsson E, Mbawuike I, Sette A. Ab and T cell epitopes of influenza A virus, knowledge and opportunities. Proc Natl Acad Sci U S A 2007; 104:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terajima M, Babon JA, Co MD, Ennis FA. Cross-reactive human B cell and T cell epitopes between influenza A and B viruses. Virol J 2013; 10:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.