Abstract
Deletion of the pfhrp2 gene in Plasmodium falciparum can lead to false-negative rapid diagnostic test (RDT) results, constituting a major challenge for evidence-based malaria treatment. Here we analyzed the whole genome sequences of 138 P. falciparum clinical samples collected from the China-Myanmar boarder for pfhrp2 and pfhrp3 gene deletions. We found pfhrp2 and pfhrp3 deletions in 9.4% and 3.6% of samples, respectively, with no samples harboring deletions of both genes. The pfhrp2 deletions showed 2 distinct breakpoints, representing 2 different chromosomal deletion events. A phylogenetic analysis performed using genome-wide single-nucleotide polymorphisms revealed that the 2 pfhrp2 breakpoint groups as well as all the pfhrp3-negative parasites formed separate clades, suggesting they might have resulted from clonal expansion of pfhrp2- and pfhrp3-negative parasites. These findings highlight the need for urgent surveys to determine the prevalence of pfhrp2-negative parasites causing false-negative RDT results and a plan for switching of RDTs pending the survey results.
Keywords: Plasmodium falciparum, pfhrp2 deletion, Southeast Asia, genome, clonal expansion
Whole genome analysis identified almost 10% Plasmodium falciparum clinical isolates from the China-Myanmar border area harboring the pfhrp2 gene deletion, which was likely the result of clonal expansion.
The extensive deployment of vector control strategies and the adoption of evidence-based management of malaria cases have resulted in a remarkable decline of global malaria incidence [1]. The ability to accurately and rapidly diagnose malaria infections is essential for the control of malaria transmission [2]. Starting in 2010, the World Health Organization (WHO) guidelines for the treatment of malaria recommend only using antimalarials to treat confirmed cases of malaria [3], a departure from the previous practice of symptom-based treatment of suspected malaria cases. This cornerstone policy change was made possible by the widespread use of rapid diagnostic tests (RDTs) as the point-of-care diagnostic of malaria [4, 5]. In 2018, 412 million RDTs were sold by manufacturers worldwide [1].
The most commonly used RDTs detect the presence in blood circulation of the histidine-rich protein (HRP) 2, an abundant and stable antigen exclusively expressed in Plasmodium falciparum [5]. While false-negative RDT results are known to occur for different reasons such as operator error, poor storage conditions, and low-parasite density infections [6], there is an increasing concern of false-negative RDTs attributed to the genetic variation and deletion of the pfhrp2 gene [7]. Since HRP3 shares epitopes with HRP2, HRP2-based RDTs, in the absence of pfhrp2, can sometimes detect P. falciparum infection, but this requires a higher parasite density [8]. Both the pfhrp2 and pfhrp3 genes are located in the subtelomeric regions, which are prone to deletion [9]. Studies from genetic crosses suggest little to no fitness cost associated with the loss of pfhrp2 [9, 10], whereas genetic crosses showing a lower frequency of progeny carrying the pfhrp3 deletion suggest fitness cost of pfhrp3-negative parasites under laboratory or experimental conditions [11]. As deletions of both genes render the parasites undetectable by the HRP2-based RDTs, reliance on HRP2-based RDTs to guide antimalarial treatment has presumably exerted strong selective pressure, driving the spread of parasites lacking the pfhrp2 gene [12, 13]. Genetic studies using microsatellite markers indicated the emergence of pfhrp2 deletion in multiple parasite lineages as well as relatedness of certain strains suggestive of expansion of lineages [14–16].
The geographical distribution of pfhrp2/3 deletions is heterogeneous, probably reflecting the spontaneous nature in the emergence of these deletions [14, 17]. The deletion of the pfhrp2 gene was first identified in Peru in 2010, and retrospective samples missing both genes reached approximately 22% [18]. Since then, deletion of pfhrp2 and/or pfhrp3 has been reported in other countries of the Americas [15, 16, 19, 20], Africa [17, 21–29], and Asia [30–33]. The WHO currently recommends switching to pan-malaria RDTs in regions where parasites with pfhrp2 deletion cause > 5% false-negative RDT results [34]. In some areas such as Peru and Eritrea, the prevalence of pfhrp2 deletion has already risen to very high levels, necessitating the switch to non-HRP2 RDTs [17, 35]. Therefore, it is imperative to monitor P. falciparum–endemic areas where the use of HRP2 RDTs is common to ensure that the tests are still effective.
A modeling study suggests that the use of HRP2 RDTs will lead to the spread of pfhrp2 deletions and that this is more likely to occur in areas with lower transmission [12]. The Greater Mekong Subregion (GMS) of Southeast Asia has a low-endemicity setting of malaria, where the emergence of artemisinin resistance in P. falciparum a decade ago is a major global concern. False-negative RDTs due to pfhrp2 deletion would delay drug treatment and sustain malaria transmission, threatening the artemisinin resistance containment plan and the regional malaria elimination goal. Studies aimed at characterizing the situation of pfhrp2 deletion and false-negative RDTs in the GMS are rare. Our previous study comparing polymerase chain reaction (PCR) with HRP2 RDTs showed the pfhrp2 deletion in 4 of 97 clinical P. falciparum isolates from the China-Myanmar border [32]. Yet, mining of the genome sequencing data from 1099 P. falciparum clinical parasites from the GMS did not produce clear evidence of pfhrp2 deletion, albeit pfhrp3 deletion has been confirmed in 19 isolates [36]. To more accurately determine the situation of pfhrp2 deletions in the GMS, we procured 138 P. falciparum clinical isolates from the China-Myanmar border for whole genome sequencing (WGS). Bioinformatic and phylogenetic analyses were conducted to document pfhrp2/3 deletions in this parasite population and to understand the genetic origins of these deletion strains.
MATERIALS AND METHODS
Parasite Samples
Plasmodium falciparum clinical isolates used in this study were archived samples collected in 2007 (n = 21), 2008 (n = 29), 2009 (n = 64), 2010 (n = 6), and 2011 (n = 16) from patients presenting with uncomplicated malaria in village clinics and township hospitals near the Laiza area, Kachin State, Myanmar at the China-Myanmar border. In addition, the samples also included 2 isolates collected in 2004 in Yunnan Province, China. Parasites were confirmed to be monoclonal after genotyping at the merozoite surface protein (msp) 1, msp2, and glutamate-rich protein. Parasites were typically cultured for about 2 weeks in type O+ human red blood cells in complete medium supplemented with 6% human AB serum under an atmosphere of 90% N2/5% O2/5% CO2 before the isolation of parasite genomic DNA.
Genome Sequencing and PCR Confirmation
Parasite genomic DNA was extracted from cultured isolates using the Wizard Genomic DNA Purification Kit (Promega). WGS libraries were prepared using the Illumina TruSeq DNA PCR-Free kit and sequenced on the HiSeq 2500 system. To validate the sequencing results, amplification of pfhrp2 and pfhrp3 exon 2 was performed using primers and conditions as described elsewhere [8]. Genes immediately flanking pfhrp2 (Pf3D7_0831600 and Pf3D7_0832000) and pfhrp3 (PF3D7_1371300, PF3D7_1371900, and PF3D7_1372400) were amplified using primers and PCR conditions described in Supplementary Table 1 and illustrated in Supplementary Figure 1. In addition, for the isolates with pfhrp2 deletion, PCR was designed to confirm the breakpoints predicted from WGS (Supplementary Table 1).
Sequence Alignment and Single-Nucleotide Polymorphism Calling
The quality of the reads was accessed using factqc version 0.11.5. Trimmomatic was used to remove adapter sequences and reads where the sequencing quality dropped below a Phred score of 20 [37]. The reads were aligned to P. falciparum 3D7 reference genome 41 using BWA mem version 0.7.12. The bam files were sorted using samtools version 1.3.1. The alignments were sorted and indexed using picard version 2.18.3. The base quality scores were recalibrated using GATK version 3.7 using a training set of high-quality single-nucleotide polymorphisms (SNPs) and indels from a genetic cross-study [38]. Haplotype caller from GATK was used to call SNPs and the variant call format files combined and genotyped using GATK with the ploidy number set to 1. Prior to filtering out low-quality variants, SNPs and indels were separated using the SelectVariants tool from GATK. Variant recalibration was performed on the SNPs using GATK with the tranche score set to 90% (90% true positive rate) using SNPs from an earlier study [38].
Detection of pfhrp2/3 Deletion
Samtools version 1.3.1 depth was used to calculate the sequencing coverage for the areas around pfhrp2 (chromosome 8: 1 355 000–1 455 000 bp) and pfhrp3 (chromosome 13: 2 792 191–2 892 191 bp). When calculating the coverage, the minimum base quality was set to 30. A custom R script (R version 3.4.1) was used to average the read coverage into 100 bp–sized bins and the bins were plotted using ggplot version 3.0.0. The coverage plots were manually inspected for decreases in coverage around pfhrp2 and pfhrp3.
Phylogenetic Analysis
SNPs were converted to a major and minor allele matrix using bcftools version 1.3.1. A custom R script was then used to remove SNPs with all the same values and SNPs with missing calls in any of the samples. The filtered data was converted into a pseudochromosome using a custom python script. This pseudochromosome was used as input to RAxML version 8.2.12 to perform 1000 × bootstrapping (model GTRACT) with ML search. The extended haplotype homozygosity (EHHS), a probability score for all SNPs at a site to be homozygous, was calculated using the R package (R version 3.4.1) rehh version 3.0.1 [39]. The starts of pfhrp2 (Pf3D7_08_v3 position 1 374 236) and pfhrp3 (Pf3D7_13_v3 position 2 840 727) were used, respectively, as the focal points for calculating EHHS.
Evaluation of pfhrp2/3 Deletion on the Performance of an HRP2-Based RDT
The BinaxNOW malaria test (Alere) was used to evaluate the impact of pfhrp2/3 deletion on the potential performance of HRP2-based RDTs. This RDT, coated with anti-HRP2 antibodies on the P. falciparum test line (T1) and anti-aldolase antibodies on the pan-Plasmodium test line (T2), qualitatively detects both HRP2 and the pan aldolase, respectively. For validation purpose, parasite isolates were cultured to 0.33%–0.8% parasitemia at 2% hematocrit, and 15 µL of culture was used for each RDT strip following the manufacturer’s instructions with the results being read in 15 minutes.
RESULTS
Identification of Isolates With pfhrp2/3 Deletions
To accurately determine the prevalence of pfhrp2/3 deletion in parasite populations from the China-Myanmar border area of the GMS, we performed WGS of 138 archived P. falciparum isolates. The sequencing reads were aligned to the P. falciparum 3D7 reference genome to detect SNPs and copy number variations. On average, 6 281 654 of 150 bp reads were obtained for each isolate, giving approximately 37 times coverage of the genome. Sequencing coverage in the regions surrounding pfhrp2 and pfhrp3 was graphed and visually inspected for decreased coverage indicative of deletion events. From the 138 samples, 13 (9.4%) and 5 (3.6%) showed evidence of pfhrp2 and pfhrp3 deletions, respectively. No samples harbored both pfhrp2 and pfhrp3 deletions.
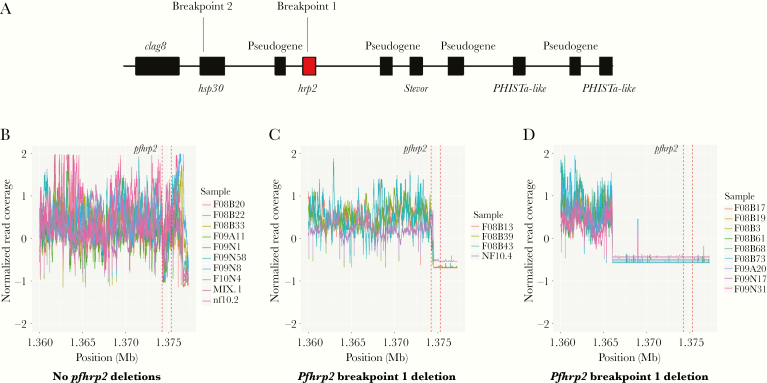
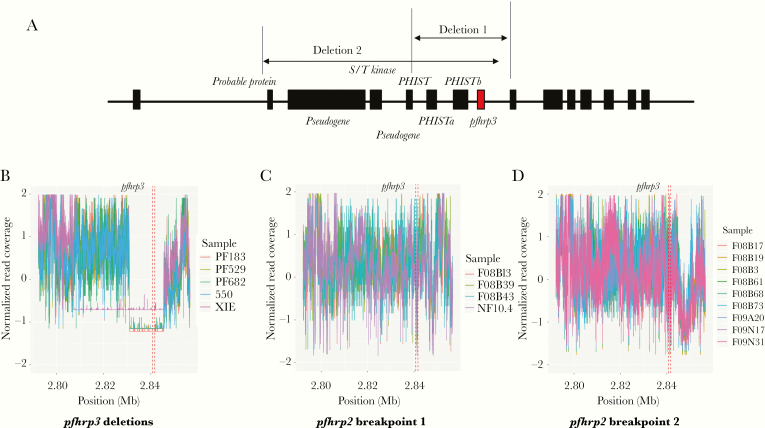
Close examination of the parasites with pfhrp2 deletion revealed 2 distinctive breakpoints in the pfhrp2-negative parasites (Figure 1). Four (2.9%) isolates (3 from 2008 and 1 from 2010) had a breakpoint at approximately 1 374 558 bp of chromosome 8, leading to the loss of most of exon 2 and all of exon 1 of pfhrp2 (Figure 1B), whereas 9 (6.5%) isolates (6 from 2008 and 3 from 2009) had a breakpoint at approximately 1 366 000 bp, resulting in the complete loss of pfhrp2 (Figure 1C). Six genes were lost in the breakpoint-1 group and 8 genes were lost in the breakpoint-2 group (Figure 1A). Due to the highly repetitive nature of the subtelomeric regions, it was not possible to accurately map the chromosomal ends (Supplementary Figure 2), but it was likely that entire chromosomal ends were lost from the breakpoints in these isolates with pfhrp2 deletion. Similarly, 2 different breakpoints were identified in the 5 isolates carrying the pfhrp3 deletion (Figure 2). Four isolates had a deletion between approximately 2 831 000 and 2 845 439 bp and 1 isolate that was collected in 2004 had a deletion between approximately 2 807 324 and 2 845 439 bp. There were up to 8 genes lost in the isolates with pfhrp3 deletion (Figure 2).
Figure 1.
Deletions of pfhrp2 from 138 clinical samples collected from the China-Myanmar border area. A, Schematic of the chromosome 8 end to illustrate the positions of the 2 breakpoints at and near pfhrp2. Breakpoints 1 and 2 start at approximately 1 374 558 bp and 1 366 000 bp, respectively. B, Normalized coverage in the pfhrp2 region from 10 random samples without pfhrp2 deletions. C, Normalized coverage in the pfhrp2 region for the breakpoint 1 samples. D, Normalized coverage in the pfhrp2 region for the breakpoint 2 samples. Vertical dotted lines indicate the location of the pfhrp2 gene. The online version of this figure is in color.
Figure 2.
Deletions of pfhrp3 from 138 clinical samples collected from the China-Myanmar border area. A, Schematic of the chromosome 13 end to illustrate the positions of the breakpoints near pfhrp3. Deletions 1 and 2 start at approximately 2 831 000 bp and 2 807 324 bp, respectively. Both deletions extend to approximately 2 845 439 bp. B–D, Normalized coverage near the pfhrp3 locus for samples containing pfhrp3 deletions (B), pfhrp2 breakpoint 1 deletions (C), and pfhrp2 breakpoint 2 deletions (D). These samples do not have both pfhrp2 and pfhrp3 deletions. Vertical dotted lines indicate the location of the pfhrp3 gene. The online version of this figure is in color.
For confirmation of the genome sequencing results, primers were designed to amplify exon 2 of pfhrp2 and its flanking genes (Pf3D7_0831600 and PF3D7_0832000) as well as exon 2 of pfhrp3 and its flanking genes (PF3D7_1371300, PF3D7_1371900, and PF3D7_1372400). The PCR results were consistent with the WGS data (Supplementary Table 2).
Clonal Expansion of Parasites Harboring pfhrp2/3 Deletions
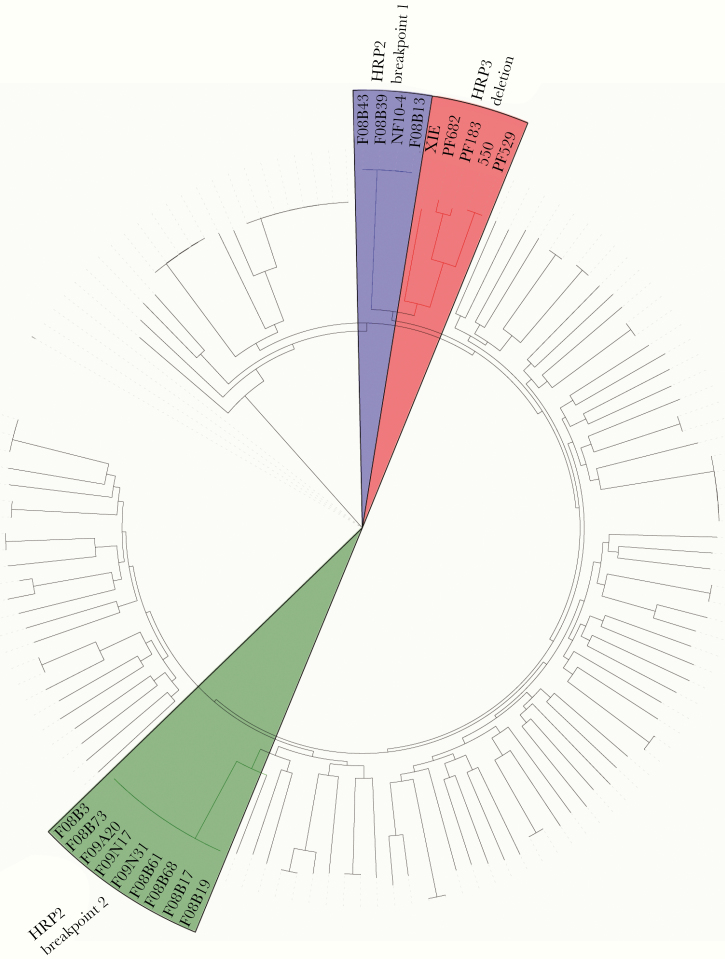
Using 62 845 SNPs from the genome sequencing data, we performed phylogenetic analysis of the parasite isolates. The 2 pfhrp2 breakpoint groups formed 2 distinct clusters with strong bootstrap supports, suggesting that the pfhrp2 deletion has evolved independently in parasites with different genetic backgrounds (Figure 3). Moreover, parasite isolates within each pfhrp2 breakpoint group were genetically closely related, indicating they might have arisen from clonal expansion of the pfhrp2-deletion lineages. Interestingly, the pfhrp2 breakpoint-1 group and parasites with the pfhrp3 deletion formed a single clade despite the fact that no strains contained both deletions.
Figure 3.
Phylogenetic tree of the parasite samples suggesting clonal expansion of parasites carrying pfhrp2 or pfhrp3 deletions. Phylogenetic analysis was performed using single-nucleotide polymorphisms called from whole genome sequencing in RAxML version 8.2.12. The 2 pfhrp2 breakpoint clusters and the pfhrp3 deletion cluster are highlighted in green, blue, and pink, respectively in the online colored version. These clusters appear as shaded in the grayscale version.
pfhrp2 Deletion and False-Negative RDT Results
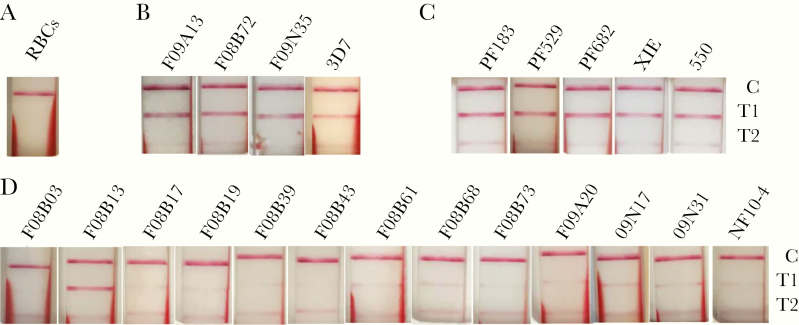
Since the primary epitopes targeted by the HRP2 antibodies are located in exon 2, pfhrp2 deletion in both breakpoint groups would result in the lack of HRP2 expression in these isolates, which may lead to false-negative HRP2 RDTs. Yet, pfhrp2 deletion may not result in RDT failure given the potential cross-reactivity with the HRP3 antigen. To evaluate whether the parasite lines with pfhrp2 deletion would result in RDT negativity, we used in vitro cultures of 21 clinical parasite isolates (13 with pfhrp2 deletion, 5 with pfhrp3 deletion, and 3 randomly chosen without pfhrp2/3 deletion) for tests using the BinaxNOW malaria RDT. As expected, the 3 isolates without pfhrp2/3 deletion and the reference strain 3D7 all produced a solid band at the HRP2 test line in the RDT (Figure 4). Similarly, all 5 isolates with pfhrp3 deletion showed a clear band at the HRP2 test line, consistent with the presence of pfhrp2 gene in these isolates. In contrast, all but 1 parasite isolate with pfhrp2 deletion produced no visible band or only a faint band at the HRP2 test line, although the parasitemias of the pfhrp2-deletion isolates tested, ranging from 0.33% (16 500 parasites/µL) to 0.8% (40 000 parasites/µL), were much higher than the detection threshold of the BinaxNOW malaria RDT.
Figure 4.
Evaluation of field parasite isolates by using the BinaxNow rapid diagnostic test (RDT). Parasites cultured in vitro to 0.33%–0.8% parasitemia at 2% hematocrit were used for the RDT assays. A–D, RDT results of red blood cells (RBCs) only as negative control (A), 3D7 reference and random parasite samples without pfhrp2/3 deletions (B), samples with pfhrp3 deletions (C), and samples with pfhrp2 deletions (D). The 3 bands C, T1, and T2 indicate control, HRP2, and aldolase, respectively. Note the prominent T1 band for all control samples and those with pfhrp3 deletions. For pfhrp2 deletion samples, only 1 parasite (F08B13) showed a prominent T1 band, whereas the remaining 12 isolates showed no or a faint T1 band. The online version of this figure is in color.
DISCUSSION
We utilized archived P. falciparum clinical samples from the China-Myanmar border to provide solid evidence on the emergence of the pfhrp2 deletion problem in this region. Parasites with pfhrp2 deletion show significant geographical heterogeneity, and their prevalence has reached such high levels in South America [16, 18–20] and some African areas [17, 24, 26] that HRP2-based RDTs need to be replaced. In Asia, this problem is understudied and sporadic reports indicate much lower prevalence of pfhrp2-negative parasites [31, 32, 36, 40]. In India, the overall prevalence of pfhrp2-deleted parasites was low (< 3%) [31, 40], but the distribution was highly heterogeneous. In a small area of Odisha, India, pfhrp2-negative parasites reached almost 10% (38/384) [33]. Similarly, although the analysis of available P. falciparum genomes from the GMS did not show clear evidence of pfhrp2 deletion [36], our analysis of the newly sequenced P. falciparum genomes from the China-Myanmar border identified almost 10% of parasites with pfhrp2 deletion. Using a PCR method, we reported pfhrp2 deletion in 4.3% of P. falciparum clinical isolates in this region [32]. While this difference could be attributed to the methods used to detect pfhrp2, the relatively high prevalence of pfhrp2 deletion approaching 10% warrants further scrutiny of the situation. Especially with the goal of eliminating P. falciparum parasites from the GMS by 2025, intensified malaria interventions led to sharp declines of malaria incidence, a condition predicted to favor the evolution of pfhrp2-negative parasites [12]. One strategy being advocated and evaluated to reduce transmission is to detect and eliminate asymptomatic P. falciparum reservoirs, thus disrupting the transmission cycle. Although HRP2-based ultrasensitive RDTs were shown to improve the detection sensitivity for asymptomatic infections [41], they are also likely to be compromised by pfhrp2 deletion.
Using a genomic approach, we provided clear evidence to show that the pfhrp2-deleted parasites formed 2 distinctive lineages, indicating that pfhrp2 deletion in this parasite population involved independent evolution in different genetic backgrounds, similar to what has been observed in Peru [14]. Furthermore, the genetic relatedness of parasites within each pfhrp2-negative lineage strongly suggests clonal expansion of the pfhrp2-deleted lines. One could speculate that the pfhrp2-negative parasites could be from a single place and single point in time, suggesting that these were a clustered set of infections being transmitted locally. However, this was not the case as the samples were collected over 5 years and from different locations and hence would be representative of the parasites circulating in this region. Clonal expansion of pfhrp2-negative parasites has also been shown to be mainly responsible for the drastically increased HRP2 RDT-negative cases in Eritrea in recent years [17]. In Eritrea, the clonal expansion of pfhrp2-deleted parasites was concurrent with the wide use of and compliance with HRP2-based RDTs before treatment, pointing to the possibility that the exclusive use of HRP2-based RDTs is a strong selection force driving the spread of pfhrp2-negative parasites. In the GMS, evidence-based treatment of malaria cases has also been promoted as an important strategy for regional malaria elimination. HRP2-based RDTs have been the major malaria diagnostics in addition to microscopy at the China-Myanmar border [42]. While we cannot exclude other biological processes that may favor the selection and spread of pfhrp2-deleted parasites, the increased use of HRP2-based RDTs in our study region could be one of the driving forces leading to clonal expansion of pfhrp2 deletion. In this border region, the P. falciparum population has experienced a genetic bottleneck in recent years [43], which may also be correlated with the increased use of HRP2 RDTs.
Evidence from other studies suggests that deletions of pfhrp2 and pfhrp3 genes occur frequently, and often co-occur in parasites [18]. In many parts of the world such as Peru and Central America, pfhrp3 deletions are more common than pfhrp2 deletions [18, 20]. The pfhrp3 deletion parasites from the China-Myanmar border were from a lineage closely related to 1 of the pfhrp2-negative lineage, but no parasites harbored deletions of both genes. Why there would be a clonal expansion of pfhrp3 deletions in the absence of a pfhrp2 deletion is not clear, since the loss of pfhrp3 while retaining an intact pfhrp2 would not confer an ability to evade detection by a pfhrp2 RDT. It is also interesting to note that the 2 breakpoint groups of pfhrp3 formed a single clade, suggesting that the genetic backgrounds of these parasites may make them vulnerable to pfhrp3 deletion.
Reports to date indicate that chromosomal breakpoints leading to pfhrp2 and pfhrp3 deletions are highly variable. Most studies have not performed the sequencing necessary to accurately map the breakpoints [44]. It has been shown that chromosomal breaks often occurred in the exon 2 of pfhrp2 with genes from 1 or both flanking sides deleted, whereas others showed that deletions occurred only in pfhrp2 or pfhrp3, with the flanking genes intact [15–20, 22, 24, 25, 31, 32, 44, 45]. Analysis of the existing P. falciparum WGS data identified a pfhrp2 deletion that spanned 14 kb and a pfhrp3 deletion that spanned 47 kb [36]. In this study we identified pfhrp2 deletions that spanned 25 kb and 34 kb and identified pfhrp3 deletions that spanned 14 kb and 38 kb. Taken together, the pfhrp2 and pfhrp3 loci are prone to deletions, but the breakpoints appear to be variable.
From a practical perspective, the identification of a substantial proportion of parasites from the GMS with pfhrp2 deletion suggests that HRP2-based RDTs would miss these patients. With our in vitro culture, although the pfhrp2-deleted P. falciparum parasites were tested at significantly higher parasitemias than the detection limit of the BinaxNow RDT, only 1 of the 13 pfhrp2-deleted parasites showed a clear band in the test line. This could have resulted from the presence of pfhrp3 since the 2 histidine-rich proteins share cross-reactive epitopes [46]. Testing of the samples with other HRP2-based RDTs is needed to confirm such a result, as different HRP2-based RDTs may target different epitopes [47]. Under field conditions, the pfhrp2 deletion would greatly increase the probability of false-negative diagnosis [26]. Our previous comparison for the performance of RDTs in the China-Myanmar border area has already identified clinical febrile P. falciparum cases that were microscopy positive, but negative by 2 different HRP2-based RDTs [32, 42], suggesting that this problem has been persisting. In the pursuance of eliminating P. falciparum parasites by 2025 in the GMS, there is an urgent need to survey this situation to determine whether it is time to consider switching to non-HRP2 alternatives of diagnosis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This study was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant number U19AI089672). L. C. was also supported by a Cohen Foundation scholarship.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World malaria report 2019.https://www.who.int/publications-detail/world-malaria-report-2019. Accessed 19 May 2020.
- 2. Perkins MD, Bell DR. Working without a blindfold: the critical role of diagnostics in malaria control. Malar J 2008; 7(Suppl 1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Guidelines for the treatment of malaria. 2nd ed. Geneva, Switzerland: WHO Press, 2010. [Google Scholar]
- 4. Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg 2007; 77:119–27. [PubMed] [Google Scholar]
- 5. Mouatcho JC, Goldring JPD. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol 2013; 62:1491–505. [DOI] [PubMed] [Google Scholar]
- 6. Abba K, Deeks JJ, Olliaro P, et al. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev 2011; 7:CD008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poti KE, Sullivan DJ, Dondorp AM, Woodrow CJ. HRP2: transforming malaria diagnosis, but with caveats. Trends Parasitol 2020; 36:112–26. [DOI] [PubMed] [Google Scholar]
- 8. Baker J, McCarthy J, Gatton M, et al. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J Infect Dis 2005; 192:870–7. [DOI] [PubMed] [Google Scholar]
- 9. Scherf A, Mattei D. Cloning and characterization of chromosome breakpoints of Plasmodium falciparum: breakage and new telomere formation occurs frequently and randomly in subtelomeric genes. Nucleic Acids Res 1992; 20:1491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker-Jonah A, Dolan SA, Gwadz RW, Panton LJ, Wellems TE. An RFLP map of the Plasmodium falciparum genome, recombination rates and favored linkage groups in a genetic cross. Mol Biochem Parasitol 1992; 51:313–20. [DOI] [PubMed] [Google Scholar]
- 11. Wellems TE, Walliker D, Smith CL, et al. A histidine-rich protein gene marks a linkage group favored strongly in a genetic cross of Plasmodium falciparum. Cell 1987; 49:633–42. [DOI] [PubMed] [Google Scholar]
- 12. Gatton ML, Dunn J, Chaudhry A, Ciketic S, Cunningham J, Cheng Q. Implications of parasites lacking Plasmodium falciparum histidine-rich protein 2 on malaria morbidity and control when rapid diagnostic tests are used for diagnosis. J Infect Dis 2017; 215:1156–66. [DOI] [PubMed] [Google Scholar]
- 13. Watson OJ, Slater HC, Verity R, et al. Modelling the drivers of the spread of Plasmodium falciparum hrp2 gene deletions in sub-Saharan Africa. Elife 2017; 6. doi:10.7554/eLife.25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akinyi S, Hayden T, Gamboa D, et al. Multiple genetic origins of histidine-rich protein 2 gene deletion in Plasmodium falciparum parasites from Peru. Sci Rep 2013; 3:2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdallah JF, Okoth SA, Fontecha GA, et al. Prevalence of pfhrp2 and pfhrp3 gene deletions in Puerto Lempira, Honduras. Malar J 2015; 14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dorado EJ, Okoth SA, Montenegro LM, et al. Genetic characterisation of Plasmodium falciparum isolates with deletion of the pfhrp2 and/or pfhrp3 genes in Colombia: the Amazon region, a challenge for malaria diagnosis and control. PLoS One 2016; 11:e0163137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berhane A, Anderson K, Mihreteab S, et al. Major threat to malaria control programs by Plasmodium falciparum lacking histidine-rich protein 2, Eritrea. Emerg Infect Dis 2018; 24:462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gamboa D, Ho MF, Bendezu J, et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One 2010; 5:e8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akinyi Okoth S, Abdallah JF, Ceron N, et al. Variation in Plasmodium falciparum histidine-rich protein 2 (Pfhrp2) and Plasmodium falciparum histidine-rich protein 3 (Pfhrp3) gene deletions in Guyana and Suriname. PLoS One 2015; 10:e0126805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fontecha G, Mejía RE, Banegas E, et al. Deletions of pfhrp2 and pfhrp3 genes of Plasmodium falciparum from Honduras, Guatemala and Nicaragua. Malar J 2018; 17:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koita OA, Doumbo OK, Ouattara A, et al. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg 2012; 86:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wurtz N, Fall B, Bui K, et al. Pfhrp2 and pfhrp3 polymorphisms in Plasmodium falciparum isolates from Dakar, Senegal: impact on rapid malaria diagnostic tests. Malar J 2013; 12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amoah LE, Abankwa J, Oppong A. Plasmodium falciparum histidine rich protein-2 diversity and the implications for PfHRP 2: based malaria rapid diagnostic tests in Ghana. Malar J 2016; 15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parr JB, Verity R, Doctor SM, et al. Pfhrp2-deleted Plasmodium falciparum parasites in the Democratic Republic of the Congo: a national cross-sectional survey. J Infect Dis 2017; 216:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menegon M, L’Episcopia M, Nurahmed AM, Talha AA, Nour BYM, Severini C. Identification of Plasmodium falciparum isolates lacking histidine-rich protein 2 and 3 in Eritrea. Infect Genet Evol 2017; 55:131–4. [DOI] [PubMed] [Google Scholar]
- 26. Kozycki CT, Umulisa N, Rulisa S, et al. False-negative malaria rapid diagnostic tests in Rwanda: impact of Plasmodium falciparum isolates lacking hrp2 and declining malaria transmission. Malar J 2017; 16:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Funwei R, Nderu D, Nguetse CN, et al. Molecular surveillance of pfhrp2 and pfhrp3 genes deletion in Plasmodium falciparum isolates and the implications for rapid diagnostic tests in Nigeria. Acta Trop 2019; 196:121–5. [DOI] [PubMed] [Google Scholar]
- 28. Plucinski MM, Herman C, Jones S, et al. Screening for pfhrp2/3-deleted Plasmodium falciparum, non-falciparum, and low-density malaria infections by a multiplex antigen assay. J Infect Dis 2019; 219:437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomson R, Beshir KB, Cunningham J, et al. Pfhrp2 and pfhrp3 gene deletions that affect malaria rapid diagnostic tests for Plasmodium falciparum: analysis of archived blood samples from 3 African countries. J Infect Dis 2019; 220:1444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kumar N, Pande V, Bhatt RM, et al. Genetic deletion of HRP2 and HRP3 in Indian Plasmodium falciparum population and false negative malaria rapid diagnostic test. Acta Trop 2013; 125:119–21. [DOI] [PubMed] [Google Scholar]
- 31. Bharti PK, Chandel HS, Ahmad A, Krishna S, Udhayakumar V, Singh N. Prevalence of pfhrp2 and/or pfhrp3 gene deletion in Plasmodium falciparum population in eight highly endemic states in India. PLoS One 2016; 11:e0157949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li P, Xing H, Zhao Z, et al. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 in the China-Myanmar border area. Acta Trop 2015; 152:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pati P, Dhangadamajhi G, Bal M, Ranjit M. High proportions of pfhrp2 gene deletion and performance of HRP2-based rapid diagnostic test in Plasmodium falciparum field isolates of Odisha. Malar J 2018; 17:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. World Health Organization. False-negative RDT results and implications of new reports of P. falciparum histidine-rich protein 2/3 gene deletions.https://www.who.int/malaria/publications/atoz/information-note-hrp2-based-rdt/en/. Accessed 19 May 2020.
- 35. Maltha J, Gamboa D, Bendezu J, et al. Rapid diagnostic tests for malaria diagnosis in the Peruvian Amazon: impact of pfhrp2 gene deletions and cross-reactions. PLoS One 2012; 7:e43094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sepúlveda N, Phelan J, Diez-Benavente E, et al. Global analysis of Plasmodium falciparum histidine-rich protein-2 (pfhrp2) and pfhrp3 gene deletions using whole-genome sequencing data and meta-analysis. Infect Genet Evol 2018; 62:211–9. [DOI] [PubMed] [Google Scholar]
- 37. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014; 30:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miles A, Iqbal Z, Vauterin P, et al. Indels, structural variation, and recombination drive genomic diversity in Plasmodium falciparum. Genome Res 2016; 26:1288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gautier M, Klassmann A, Vitalis R. rehh 2.0: a reimplementation of the R package rehh to detect positive selection from haplotype structure. Mol Ecol Resour 2017; 17:78–90. [DOI] [PubMed] [Google Scholar]
- 40. Kumar Bharti P, Singh Chandel H, Krishna S, et al. Sequence variation in Plasmodium falciparum histidine rich proteins 2 and 3 in Indian isolates: implications for malaria rapid diagnostic test performance. Sci Rep 2017; 7:1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Z, Soe TN, Zhao Y, et al. Geographical heterogeneity in prevalence of subclinical malaria infections at sentinel endemic sites of Myanmar. Parasit Vectors 2019; 12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yan J, Li N, Wei X, et al. Performance of two rapid diagnostic tests for malaria diagnosis at the China-Myanmar border area. Malar J 2013; 12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lo E, Zhou G, Oo W, et al. Molecular inference of sources and spreading patterns of Plasmodium falciparum malaria parasites in internally displaced persons settlements in Myanmar-China border area. Infect Genet Evol 2015; 33:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng Q, Gatton ML, Barnwell J, et al. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J 2014; 13:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murillo Solano C, Akinyi Okoth S, Abdallah JF, et al. Deletion of Plasmodium falciparum histidine-rich protein 2 (pfhrp2) and histidine-rich protein 3 (pfhrp3) genes in Colombian parasites. PLoS One 2015; 10:e0131576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee N, Baker J, Andrews KT, et al. Effect of sequence variation in Plasmodium falciparum histidine-rich protein 2 on binding of specific monoclonal antibodies: implications for rapid diagnostic tests for malaria. J Clin Microbiol 2006; 44:2773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee N, Gatton ML, Pelecanos A, et al. Identification of optimal epitopes for Plasmodium falciparum rapid diagnostic tests that target histidine-rich proteins 2 and 3. J Clin Microbiol 2012; 50:1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.