Abstract
Objectives
One-fifth of Covid-19 patients suffer a severe course of Covid-19 infection; however, the specific causes remain unclear. Mast cells (MCs) are activated by SARS-CoV-2. Although only recently recognized, MC activation syndrome (MCAS), usually due to acquired MC clonality, is a chronic multisystem disorder with inflammatory and allergic themes, and an estimated prevalence of 17%. This paper describes a novel conjecture explaining how MCAS might cause a propensity for severe acute Covid-19 infection and chronic post-Covid-19 illnesses.
Methods
Observations of Covid-19 illness in patients with/without MCAS were compared with extensive clinical experience with MCAS.
Results
The prevalence of MCAS is similar to that of severe cases within the Covid-19-infected population. Much of Covid-19’s hyperinflammation is concordant with manners of inflammation which MC activation can drive. Drugs with activity against MCs or their mediators have preliminarily been observed to be helpful in Covid-19 patients. None of the authors’ treated MCAS patients with Covid-19 suffered severe infection, let alone mortality.
Conclusions
Hyperinflammatory cytokine storms in many severely symptomatic Covid-19 patients may be rooted in an atypical response to SARS-CoV-2 by the dysfunctional MCs of MCAS rather than a normal response by normal MCs. If proven, this theory has significant therapeutic and prognostic implications.
Keywords: Covid-19, SARS-CoV-2, Mast cell activation syndrome, Mast cell activation disease, Medical hypothesis
Introduction
Since December 2019, the Covid-19 pandemic, due to the SARS-CoV-2 coronavirus, has been rapidly spreading throughout many parts of the world. It has been calamitous to the personal health and finances of millions and also–largely due to the infection’s high mortality rate–to global healthcare systems and societal economic welfare. Approximately 15–20% of Covid-19-infected patients suffer a severe form of the acute infection (Bulut and Kato, 2020, Rabec et al., 2020, Grasselli et al., 2020) hallmarked by hyperinflammatory cytokine storms causing far more morbidity and mortality than from any direct viral cytotoxicity. This has a high mortality risk (Zhou et al., 2020a)– ≥50% in some subpopulations (e.g. patients with cardiac injury or requiring continuous renal replacement therapy)–(Fominskiy et al., 2020, Shi et al., 2020, Bhatraju et al., 2020, Chen et al., 2020) and requires hospitalisation and, often, mechanical ventilation. The Covid-19 cytokine storm is characterised by rapid proliferation and hyperactivation of T cells, macrophages, and natural killer cells, and the overproduction of >150 inflammatory cytokines and chemical mediators released by immune or nonimmune cells (Sun et al., 2020, Mangalmurti and Hunter, 2020). Among these inflammatory cells, mast cells (MCs) may play an important role because when they recognise viral products, they are activated and synthesise many chemokines and cytokines. In addition, some cytokines secreted by other cells such as T cells, damaged epithelial and endothelial cells (Mukai et al., 2018), or even by themselves (Hermans et al., 2019), stimulate MC activation. MCs regulate the functions of immune cells such as dendritic cells, monocytes/macrophages, granulocytes, T cells, B cells and NK cells. They also recruit immune cells to inflamed tissue by secreting chemokines and other mediators which locally increase vascular permeability (Abraham and St John, 2010, Krystel-Whittemore et al., 2016, St John et al., 2011).
The roles of MCs in coronavirus-induced inflammation (Kritas et al., 2020, Kılınc and Kılınc, 2020, Theoharides, 2020, Zhou et al., 2020b) and cytokine storms (Theoharides, 2020) have been recently discussed. Although MCs can recognise viruses by diverse mechanisms (e.g. Toll-like receptor 3 detection of viral double-stranded ribonucleic acid (RNA), viral sphingosine-1-phosphate (S1P) binding to S1P receptors, and retinoic acid-induced gene I (RIG-I) recognition of uncapped viral RNA) (Criado et al., 2020), they also express angiotensin converting enzyme 2 (ACE2), now known as the principal receptor for SARS-CoV-2, thus defining a route by which MCs could also become hosts for this virus (Theoharides, 2020). MCs also express many serine proteases (including tryptase), which are necessary for SARS-CoV-2 infection (Theoharides, 2020). Some risk factors for a severe form of Covid-19 infection have been identified (e.g. older age, obesity and/or other chronic pre-existing illness); however, specific mechanisms by which such factors would permit more severe infection remain unclear. After an acute infection with Covid-19, many people soon manifest a variety of chronic and often inflammatory multisystem illnesses (Wang et al., 2020, Bulut and Kato, 2020, Scala and Pacelli, 2020, Troyer et al., 2020, Hays, 2020).
Another mystery about the Covid-19 pandemic is why the infection is mildly symptomatic or even asymptomatic in the majority of those who are infected but is severely symptomatic, often life-threatening, in a sizeable minority. In other words, what causes the immune system to so catastrophically suddenly overreact in certain Covid-19 patients while remaining properly regulated in the majority? Another important question regards the aetiology of chronic post-Covid-19 illnesses. Although solid data on which these questions can be answered are not yet available, this paper summarises the evidence suggesting that mast cell activation disease–the majority of which comprises the prevalent, but only recently recognised, mast cell activation syndrome (MCAS)–fits very well with these puzzling observations.
This study offers a potentially important conjecture spurred by (1) familiarity (across several thousand cases over the last dozen years) with MCAS (mainly presenting as a chronic multisystem polymorbidity of general MC-mediator-driven themes of inflammation ± allergic-type issues) (Afrin et al., 2016a, Afrin et al., 2020) and (2) the theory that Covid-19 inflammatory illnesses may be due to abnormal hyperactivation by SARS-CoV-2 of the dysfunctional, likely mutated portion of the MC population underlying primary MCAS, as opposed to normal activation of normal MCs by the virus.
Primary MCAS has been thought by some to underlie, to at least some extent, many of the risk factors identified thus far for severe Covid-19 infection (Afrin, 2016b). MCAS is known to permanently escalate its baseline level of dysfunction of the affected MCs shortly after a major stressor (likely due to acquisition–due to complex interactions between epigenetic abnormalities and the stressor’s induced cytokine storm–of additional mutations by the mutated stem cells from which the mutated/dysfunctional MCs are derived) (Molderings, 2015, Haenisch et al., 2014, Molderings, 2016, Altmüller et al., 2017, Haenisch et al., 2012, Molderings et al., 2010, Molderings et al., 2007). As such, the assortment of (generally inflammatory) post-Covid-19 illnesses seen in many Covid-19 patients would be a natural course for MCAS. In fact, Covid-19 would be far from the first infection for which post-infectious chronic multisystem inflammatory illness is increasingly being suspected to be rooted in (initiation of, or more likely escalation of pre-existing) MCAS (e.g. Epstein-Barr virus infection, tick-borne infections) rather than chronic active infection (Afrin, 2016b, Kempuraj et al., 2020). Again, since MCAS is a chronic multisystem inflammatory disease (with intermittent acute flares) if it is nothing else, it is possible that at least some of the patients previously thought to have suffered repeat bouts of Covid-19 infection might have only suffered an initial bout of infection followed some time later by symptomatic flaring of escalated MCAS (e.g. fatigue, myalgias).
Of further interest, estimates of MCAS prevalence (17%, at least in the developed world) (Molderings et al., 2013) closely correspond with estimates of prevalence of severe Covid-19 infection. MCs–present in all vascularised tissues but dominant at the environmental interfaces and in vessel walls (Akin and Metcalfe, 2004)–are activated by the SARS-Cov-2 coronavirus which causes Covid-19 infection (Kritas et al., 2020, Theoharides, 2020, Zhou et al., 2020b), leading to MC activation and resulting release of various subsets of the MC’s >1000 potent multi-action mediators (Ibelgaufts, 2020), including: biogenic amines (e.g. histamine), proteases (e.g. tryptase and chymase), cytokines (e.g. interleukins and TNF-α), eicosanoids (e.g. prostaglandins and leukotrienes), heparin, and growth factors, at least some of which are increasingly thought to play key roles in driving the hyperinflammation of severe Covid-19 illness (Kempuraj et al., 2020, Valent et al., 2020).
A significant number of fatal cases of Covid-19 infection are due to cardiovascular complications such as pulmonary embolism, thromboembolism, sepsis and multiorgan failure. It has been shown that MCs play a significant role in promoting thrombotic diseases and complications; it has also been shown that stabilising MCs helps to prevent fatal sepsis (Ramos et al., 2010). As another example, neuropsychiatric disease appears common in both MCAS (Afrin et al., 2015) and Covid-19 illness (Romero-Sánchez et al., 2020), and although the acute and subacute neurological disease in Covid-19 illness is thought to be principally due to inflammation-induced coagulation, the authors conjecture that chronic neuropsychiatric symptoms may be due more to escalated (and likely pre-existing) MCAS. Additionally, some of the drugs or drug classes at least preliminarily shown to be helpful in modulating the severity of Covid-19 infection (e.g. famotidine (Freedberg et al., 2020) and aspirin) (Viecca et al., 2020), and for which anti-viral actions seem extremely unlikely, have actions which include inhibiting MC activation or antagonising released MC mediators. Other drugs or drug classes with activity against MCs or their released mediators have been proposed for, or are actively involved in, trials against Covid-19 infection, including: cromolyn (Sestili and Stocchi, 2020, Sepay et al., 2020, Gigante et al., 2020), flavonoids (Theoharides, 2020), leukotriene inhibitors (Almerie and Kerrigan, 2020), Janus kinase (JAK) inhibitors (Goker Bagca and Biray Avci, 2020, Seif et al., 2020, Luo et al., 2020, Spinelli et al., 2020, Meyer et al., 2020), dexamethasone (Meyer et al., 2020, RECOVERY Collaborative Group et al., 2020), low-dose naltrexone (Sims, 2020), quercetin (Onal, 2020, Colunga Biancatelli et al., 2020), and ascorbic acid (Colunga Biancatelli et al., 2020).
MCAS remains a relatively unrecognised entity, despite its great prevalence, which has likely been ‘camouflaged’ by its extreme heterogeneity of clinical presentation (Afrin et al., 2016a, Afrin et al., 2017), as driven by its underlying extreme mutational heterogeneity. Although MCAS in some patients may be purely secondary to another process (e.g. autoimmunity or cancer), it clearly is a primary disease for those in whom it is currently possible to demonstrate MC-relevant clonality in the clinical laboratory. This is performed either by KIT mutation analysis (currently largely limited to probing by polymerase chain reaction for codon 816 mutations, almost always present in mastocytosis but rarely found in MCAS) or by flow cytometry for cell surface co-expression of CD117 together with either CD25 and/or CD2. In the majority of MCAS cases, the disease is currently ‘idiopathic’, solely because clonality cannot be demonstrated through the available clinical testing. Studies to date have consistently shown, via sequencing of MC isolates obtained from MCAS patients, that the MCs in almost all MCAS patients bear a wide variety of mutations across KIT (just not in codon 816) and also dozens of other MC regulatory genes (Molderings et al., 2007, Molderings et al., 2010, Afrin et al., 2016c, Altmüller et al., 2017).
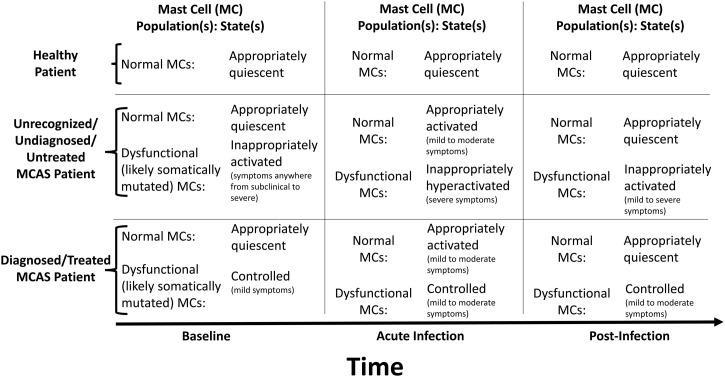
Therefore, mainly due to this extreme clinical heterogeneity and recent recognition of the existence of the disease (implying that most physicians remain unaware of it), most MCAS patients remain undiagnosed and untreated, and therefore their dysfunctional MCs, whether causing mild or severe illness, are uncontrolled and may react inappropriately to SARS-CoV-2 (Table 1 ). Another confounding issue is that many MCAS patients who have been undiagnosed for decades tend to minimise their problems, sometimes deceivingly declaring themselves as ‘healthy’. This perhaps accounts for some of the many severe Covid-19 patients described as ‘healthy’ prior to infection. In the authors' own MCAS patients (i.e., patients already diagnosed and treated, and thus already with at least partial control over their MCAS; note many of these patients had long suffered severe courses of MCAS prior to diagnosis and having it brought under at least partial control with treatment) who have come to suffer Covid-19 infection, none of them have suffered a severe course of the infection (i.e., none have required mechanical ventilation, let alone died), and we conjecture it is precisely because their dysfunctional MCs were already under at least partial control throughout the acute infection that they have not suffered severe courses, though their MCAS still places them at increased risk for developing post-infectious illness (Figure 1 ).
Table 1.
Organ and system involvement in mast cell activation syndrome. Conditions highlighted in bold are also seen in Covid-19 acute infection and/or post-infectious syndrome.
| Organ/system | Symptom/finding |
|---|---|
| Constitutional | Fatigue, fevers, chills, weight loss, weight gain |
| Ears, nose and throat | Conjunctivitis, rhinitis, sinusitis, dysosmia/anosmia, tinnitus, hearing loss, dysgeusia/ageusia, sore throat |
| Neurologic | Headaches, migraines, brain fog, anxiety, depression, insomnia, seizures |
| Cardiovascular | Chest pain, palpitations, hypotension |
| Pulmonary | Cough, dyspnoea, wheezing |
| Urogenital | Frequency, urgency, dysuria, pelvic pain |
| Oesophageal | Heartburn, dysphagia, globus, chest pain |
| Stomach | Dyspepsia, nausea, vomiting |
| Small intestine/colon | Bloating, food intolerance, abdominal pain, diarrhoea, constipation |
| Hepatic | Elevated transaminases, hepatomegaly |
| Salivary Glands | Swelling |
| Lymphatics | Lymphadenopathy |
| Dermatologic | Flushing, pruritis, urticaria, haemangiomas, nodules, rashes, alopecia |
| Musculoskeletal | Myalgias, arthralgias, oedema |
Figure 1.
Illustration of proposed model. Normal mast cells (MCs) react normally to SARS-CoV-2, participating in driving mild to moderate symptoms through the network of inflammatory cells, and returning to a quiescent state once the virus has been eradicated. Some of the MCs will be abnormal/dysfunctional and prone to constitutive and reactive hyperactivation if mast cell activation syndrome (MCAS) is present. If MCAS is undiagnosed and thus untreated, the abnormal MCs may react inappropriately and excessively to SARS-CoV-2, driving a hyperinflammatory state via excessive release of their mediators and excessive recruitment (also via their released mediators) of other inflammatory cells. If MCAS is diagnosed and treated, the abnormal MCs will be relatively controlled, diminishing their aberrant hyperreactivity to SARS-CoV-2. As major stressors (such as infections and hyperinflammation) can induce major escalations in baseline MC dysfunction in MCAS (likely via induction of additional mutations in the stem cells and multipotent progenitors at the root of the patient’s population of dysfunctional MCs), the abnormal MCs in MCAS will have potential to drive post-Covid inflammatory syndrome (with clinical specifics dependent on the mutational profiles in the individual patient’s MCs), but the severity of that syndrome may be mitigated by recognition/diagnosis of the patient’s MCAS and pharmacologic control of the patient’s dysfunctional MCs.
Based on current knowledge, Covid-19 infection causes mild to moderate symptoms in the majority of patients. However, these early data also suggest that even if symptoms are just ‘mild to moderate’ during the acute infection, fibrotic lung damage develops in some, potentially leading to long-term complications for a subset of patients (Spagnolo et al., 2020, Leask, 2020, Lechowicz et al., 2020, George et al., 2020). It is well known that over-activated MCs play a crucial role in the development of fibrotic conditions. Given that up to 17% of the population is generally pre-disposed to developing syndromes and diseases related to MC activation (Molderings et al., 2013), it is conceivable that people with this predisposition might have increased risk of developing the chronic respiratory, neurologic or other illnesses increasingly being seen following acute Covid-19 illness. Furthermore, the MC activation induced by Covid-19 infection could increase the risk of poor outcomes in undiagnosed or uncontrolled MCAS patients. Lung biopsies from Covid-19 patients clearly show a significantly increased number of activated MCs compared to healthy controls, demonstrating an important role of MCs during Covid-19 infections (Zhou et al., 2020b).
MCAS-targeted therapy (e.g. inexpensive, safe histamine H1 and H2 receptor antagonists) immediately upon recognition or suspicion of onset of Covid-19 illness might mitigate the severity of the illness. The impact on reducing hospitalisations, morbidity and mortality warrants further investigation. Evaluation of MCAS in patients who develop chronic post-Covid-19 illnesses is also recommended.
The fact that MCs normally activate in response to infection precludes diagnostic testing for MCAS (i.e. testing for elevated levels in blood and urine of mediators relatively specific to MCs such as tryptase, heparin, histamine and derivatives, prostaglandin D2 and derivatives) (Afrin et al., 2020, Afrin and Molderings, 2014) during acute Covid-19 infection. However, the potential personal and societal implications of the conjecture described here are huge and rapid formal investigation is recommended. Such investigation should include, at a bare minimum, a pilot clinical trial empirically initiating MCAS-targeted therapy in patients newly presenting with suspected Covid-19 illness and in whom careful history-taking (regardless of the initially asserted state of prior health) reveals chronic inflammatory and/or allergic issues suspicious for MCAS. Initial empiric MCAS-targeted therapy could include at least histamine H1 and H2 receptor antagonists. Note that most MCAS-targeted therapies are sufficiently safe to make their empiric initiation reasonable.
The signalling networks in all inflammatory diseases are extremely complex, and other inflammatory cells besides MCs are inescapably involved in generating the hyperinflammation of Covid-19 infection (e.g. the extreme hyperferritinaemia seen in some cases might easily be a macrophage activation syndrome or secondary haemophagocytic lymphohistiocytosis sparked by a Covid-19-driven escalation of MCAS more so than direct virus-driven macrophage activation, given that hyperferritinaemia is certainly not seen in all patients with severe Covid-19 infection) (Gómez-Pastora et al., 2020, Ruscitti et al., 2020, Ruan et al., 2020, Mehta et al., 2020). However, it is felt that the clinical patterns seen thus far in the Covid-19 population suggest that MCAS (likely pre-existing) is the root issue in many, perhaps even most, of those suffering ‘severe’ infection.
The role that cytokine storms play in severe cases of Covid-19 is what would be expected if the conjecture described here is correct. Hyperactive MCs can get into a continuous activation loop, leading to cytokine storms which can result in the fluid build-up and pulmonary and other damage often seen in severe Covid-19 patients. In sum, although most MCAS patients do not present with Covid-19-like hyperinflammation, MCAS is an extraordinarily heterogeneous disease and it is felt that MCAS (likely pre-existing) ‘fits’ well with most of the behaviours of severe Covid-19 infection observed thus far. Blocking MC mediators in Covid-19 patients may help calm MCs and cytokine storms, which may result in better outcomes, including lower mortality rates. Furthermore, using MC stabilisers such as antihistamines and cromolyn may help to prevent a significant increase in post-Covid-19 chronic illnesses, which in a significant proportion of such patients may be driven by chronic persistent MC activation. These MC-targeted treatment suggestions may be relevant for all Covid-19 patients, not just those with pre-existing MC diseases.
Conflict of interest
Drs. Afrin and Weinstock are uncompensated volunteer medical advisors to the start-up company MC Sciences, Ltd. Dr. Molderings is the chief medical officer of the start-up company MC Sciences, Ltd. All authors disclaim any financial conflicts of interest. All authors report that they had full access to all of the text in this submission and take responsibility for the integrity of any factual statements and analysis. This work has not been previously presented in any other form or venue.
Sources of support
No funding or other support was received for this work from any source.
Author contributions
Dr. Afrin initiated the project and was the principal writer. All other authors contributed equally to the editing of the paper.
Ethical approval
No human subjects were involved in this work and, as such, ethical approval was not required for the development of this article.
References
- Abraham S.N., St John A.L. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrin L.B., Molderings G.J. A concise, practical guide to diagnostic assessment for mast cell activation disease. World J Hematol. 2014;3(1):1–17. doi: 10.5315/wjh.v3.i1.1. https://www.wjgnet.com/2218-6204/journal/v3/i1/index.htm available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrin L.B., Pöhlau D., Raithel M. Mast cell activation disease: an underappreciated cause of neurologic and psychiatric symptoms and diseases. Brain Behav Immun. 2015;50:314–321. doi: 10.1016/j.bbi.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Afrin L.B., Butterfield J.H., Raithel M., Molderings G.J. Often seen, rarely recognized: mast cell activation disease–a guide to diagnosis and therapeutic options. Ann Med. 2016;48(3):190–201. doi: 10.3109/07853890.2016.1161231. [DOI] [PubMed] [Google Scholar]
- Afrin L.B. Mast cell activation disease and the modern epidemic of chronic inflammatory disease. Transl Res. 2016;174:33–59. doi: 10.1016/j.trsl.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Afrin L.B., Cichocki F., Hoeschen A. Mast cell regulatory gene variants are common in mast cell activation syndrome. Blood. 2016;128(22):4878. doi: 10.1182/blood.V128.22.4878.4878. [DOI] [Google Scholar]
- Afrin L.B., Self S., Menk J., Lazarchick J. Characterization of mast cell activation syndrome. Am J Med Sci. 2017;353(3):207–215. doi: 10.1016/j.amjms.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrin L.B., Ackerley M.B., Bluestein L.S. Diagnosis of mast cell activation syndrome: a global “consensus-2”. Diagnosis (Berl) 2020 doi: 10.1515/dx-2020-0005. /j/dx.ahead-of-print/dx-2020-0005/dx-2020-0005.xml [published online ahead of print, 22 April 2020] [DOI] [PubMed] [Google Scholar]
- Akin C., Metcalfe D.D. The biology of Kit in disease and the application of pharmacogenetics. J Allergy Clin Immunol. 2004;114(1):13–20. doi: 10.1016/j.jaci.2004.04.046. [DOI] [PubMed] [Google Scholar]
- Almerie M.Q., Kerrigan D.D. The association between obesity and poor outcome after COVID-19 indicates a potential therapeutic role for Montelukast. Med Hypotheses. 2020;143:109883. doi: 10.1016/j.mehy.2020.109883. [published online ahead of print, 27 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmüller J., Haenisch B., Kawalia A. Mutational profiling in the peripheral blood leukocytes of patients with systemic mast cell activation syndrome using next-generation sequencing. Immunogenetics. 2017;69(6):359–369. doi: 10.1007/s00251-017-0981-y. [DOI] [PubMed] [Google Scholar]
- Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically Ill patients in the Seattle region–case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut C., Kato Y. Epidemiology of COVID-19. Turk J Med Sci. 2020;50(SI-1):563–570. doi: 10.3906/sag-2004-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [published correction appears in BMJ. 2020;368:m1295] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colunga Biancatelli R.M.L., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado P.R., Pagliari C., Criado R.F.J., Marques G.F., Belda W., Jr. What the physicians should know about mast cells, dendritic cells, urticaria, and omalizumab during COVID-19 or asymptomatic infections due to SARS-CoV-2? Dermatol Ther. 2020 doi: 10.1111/dth.14068. [published online ahead of print, 25 July 2020] [DOI] [PubMed] [Google Scholar]
- Fominskiy E.V., Scandroglio A.M., Monti G. Prevalence, characteristics, risk factors, and outcomes of invasively ventilated COVID-19 patients with acute kidney injury and renal replacement therapy. Blood Purif. 2020:1–8. doi: 10.1159/000508657. [published online ahead of print, 13 July 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [published online ahead of print, 06 April 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg D.E., Conigliaro J., Wang T.C. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.053. S0016-5085(20)34706-5 [published online ahead of print, 22 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30225-3. [published online ahead of print, 15 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigante A., Aquili A., Farinelli L. Sodium chromo-glycate and palmitoylethanolamide: a possible strategy to treat mast cell-induced lung inflammation in COVID-19. Med Hypotheses. 2020;143:109856. doi: 10.1016/j.mehy.2020.109856. [published online ahead of print, 19 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker Bagca B., Biray Avci C. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.06.013. S1359-6101(20)30158-1 [published online ahead of print, 20 June 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pastora J., Weigand M., Kim J. Hyperferritinemia in critically ill COVID-19 patients–is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. 2020;509:249–251. doi: 10.1016/j.cca.2020.06.033. [published online ahead of print, 21 June 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B., Nöthen M.M., Molderings G.J. Systemic mast cell activation disease: the role of molecular genetic alterations in pathogenesis, heritability and diagnostics. Immunology. 2012;137(3):197–205. doi: 10.1111/j.1365-2567.2012.03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B., Fröhlich H., Herms S., Molderings G.J. Evidence for contribution of epigenetic mechanisms in the pathogenesis of systemic mast cell activation disease. Immunogenetics. 2014;66(5):287–297. doi: 10.1007/s00251-014-0768-3. [DOI] [PubMed] [Google Scholar]
- Hays P. Clinical sequelae of the novel coronavirus: does COVID-19 infection predispose patients to cancer? Future Oncol. 2020;16(20):1463–1474. doi: 10.2217/fon-2020-0300. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hermans M., van Lennep J.R., van Daele P. Mast cells in cardiovascular disease: from bench to bedside. Int J Mol Sci. 2019;20:3395. doi: 10.3390/ijms20143395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibelgaufts H. 2020. “Mast Cells” in COPE: Cytokines and Cells Online Pathfinder Encyclopaedia. Available at: http://www.cells-talk.com/index.php/page/copelibrary?key=mast%20cells [Accessed 2 August 2020] [Google Scholar]
- Kempuraj D., Selvakumar G.P., Ahmed M.E. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist. 2020 doi: 10.1177/1073858420941476. [published online ahead of print, 18 July 2020] [DOI] [PubMed] [Google Scholar]
- Kılınc E., Kılınc Y.B. Mast cell stabilizers as a supportive therapy can contribute to alleviate fatal inflammatory responses and severity of pulmonary complications in COVID-19 infection. Anadolu Kliniği Tıp Bilimleri Dergisi. 2020:111–119. [Google Scholar]
- Kritas S.K., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents. 2020;34(1) doi: 10.23812/20-Editorial-Kritas. [published online ahead of print, 04 February 2020] [DOI] [PubMed] [Google Scholar]
- Krystel-Whittemore M., Dileepan K.N., Wood J.G. The mast cell: a multi-functional mast cell. Front Immunol. 2016;6:620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. COVID-19: is fibrosis the killer? J Cell Commun Signal. 2020;14(2):255. doi: 10.1007/s12079-020-00569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechowicz K., Drożdżal S., Machaj F. COVID-19: the potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J Clin Med. 2020;9(6):1917. doi: 10.3390/jcm9061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Li Y.X., Jiang L.J., Chen Q., Wang T., Ye D.W. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol Sci. 2020;41(8):531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020 doi: 10.1016/j.immunol.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer L., Verbist K.C., Albeituni S. JAK/STAT pathway inhibition sensitizes CD8 T cells to dexamethasone-induced apoptosis in hyperinflammation. Blood. 2020 doi: 10.1182/blood.2020006075. [published online ahead of print, 12 June 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molderings G.J., Kolck U.W., Scheurlen C., Brüss M., Homann J., Von Kügelgen I. Multiple novel alterations in Kit tyrosine kinase in patients with gastrointestinally pronounced systemic mast cell activation disorder. Scand J Gastroenterol. 2007;42(9):1045–1053. doi: 10.1080/00365520701245744. [DOI] [PubMed] [Google Scholar]
- Molderings G.J., Meis K., Kolck U.W., Homann J., Frieling T. Comparative analysis of mutation of tyrosine kinase kit in mast cells from patients with systemic mast cell activation syndrome and healthy subjects. Immunogenetics. 2010;62(11-12):721–727. doi: 10.1007/s00251-010-0474-8. [DOI] [PubMed] [Google Scholar]
- Molderings G.J., Haenisch B., Bogdanow M., Fimmers R., Nöthen M.M. Familial occurrence of systemic mast cell activation disease. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0076241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molderings G.J. The genetic basis of mast cell activation disease–looking through a glass darkly. Crit Rev Oncol Hematol. 2015;93(2):75–89. doi: 10.1016/j.critrevonc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Molderings G.J. Transgenerational transmission of systemic mast cell activation disease-genetic and epigenetic features. Transl Res. 2016;174:86–97. doi: 10.1016/j.trsl.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Mukai K., Tsai M., Saito H. Mast cells as sources of cytokines, chemokines and growth factors. Immunol Rev. 2018;282:121–150. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://clinicaltrials.gov/ct2/show/NCT04377789Onal H. Effect of Quercetin on Prophylaxis and Treatment of COVID-19. 2020. Available at: [Accessed 2 August 2020].
- Rabec C., Gonzalez-Bermejo J., Respiratory Support, Chronic Care Group GAVO 2 of the French Society of Respiratory Diseases SPLF, GAVO 2 collaborators Respiratory support in patients with COVID-19 (outside intensive care unit). A position paper of the Respiratory Support and Chronic Care Group of the French Society of Respiratory Diseases [published online ahead of print, 2020 May 27] Respir Med Res. 2020;78:100768. doi: 10.1016/j.resmer.2020.100768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos L., Peña G., Cai B., Deitch E.A., Ulloa L. Mast cell stabilization improves survival by preventing apoptosis in sepsis. J Immunol. 2010;185(1):709–716. doi: 10.4049/jimmunol.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group, Horby P., Lim W.S. Dexamethasone in hospitalized patients with Covid-19-preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [published online ahead of print, 17 July 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 doi: 10.1212/WNL.0000000000009937. [published online ahead of print, 01 June 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [Supplemented by: Ruan Q., Yang K., Wang W., Jiang L., Song J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(6):1294–1297. DOI: 10.1007/s00134-020-06028-z.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscitti P., Berardicurti O., Di Benedetto P. Severe COVID-19, another piece in the Puzzle of the hyperferritinemic syndrome. An immunomodulatory perspective to alleviate the storm. Front Immunol. 2020;11:1130. doi: 10.3389/fimmu.2020.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala S., Pacelli R. Fighting the host reaction SARS-CoV-2 in critically ill patients: the possible contribution of off-label drugs. Front Immunol. 2020;11:1201. doi: 10.3389/fimmu.2020.01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif F., Aazami H., Khoshmirsafa M. JAK inhibition as a new treatment strategy for patients with COVID-19. Int Arch Allergy Immunol. 2020;181(6):467–475. doi: 10.1159/000508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepay N., Sepay N., Al Hoque A., Mondal R., Halder U.C., Muddassir M. In silico fight against novel coronavirus by finding chromone derivatives as inhibitor of coronavirus main proteases enzyme. Struct Chem. 2020:1–10. doi: 10.1007/s11224-020-01537-5. [published online ahead of print, 13 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestili P., Stocchi V. Repositioning chromones for early anti-inflammatory treatment of COVID-19. Front Pharmacol. 2020;11:854. doi: 10.3389/fphar.2020.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [published online ahead of print, 25 Mar 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://clinicaltrials.gov/ct2/show/NCT04365985Sims M. Study of Immunomodulation Using Naltrexone and Ketamine for COVID-19 (SINK COVID-19). 2020. Available at [Accessed 2 August 2020].
- Spagnolo P., Balestro E., Aliberti S. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30222-8. [published online ahead of print, 15 May 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli F.R., Conti F., Gadina M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci Immunol. 2020;5(47):eabc5367. doi: 10.1126/sciimmunol.abc5367. [DOI] [PubMed] [Google Scholar]
- St John A.L., Rathore A.P., Yap H. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc Natl Acad Sci U S A. 2011;108:9190–9195. doi: 10.1073/pnas.1105079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Wang T., Cai D. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020:S1359–S6101. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides T.C. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors. 2020;46(3):306–308. doi: 10.1002/biof.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent P., Akin C., Bonadonna P. Risk and management of patients with mastocytosis and MCAS in the SARS-CoV-2 (COVID-19) pandemic: expert opinions. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.06.009. S0091-6749(20)30839-3 [published online ahead of print, 2020 Jun 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viecca M., Radovanovic D., Forleo G.B., Santus P. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res. 2020;158:104950. doi: 10.1016/j.phrs.2020.104950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [published correction appears in Lancet. 2020;395(10229):1038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Ren L., Zhang L. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6) doi: 10.1016/j.chom.2020.04.017. 883–890.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]