Ibrutinib is a novel Bruton’s tyrosine kinase inhibitor associated with dramatic improvements in the outcomes of various B-cell hematologic malignancies and is now standard lifelong therapy in those without intolerance or disease-progression.(1) However, there is a >4- fold increased risk of incident atrial arrhythmias.(2) Recently, unexplained cases of incident ventricular arrhythmias (VAs) and sudden cardiac death (SCD) have been reported among ibrutinib-users.(3) Yet, whether ibrutinib is associated with VAs in long-term follow-up is unknown.
Leveraging data from a large and contemporary U.S.-based Comprehensive Cancer Center registry cohort of consecutive patients treated with ibrutinib from 2009–2016 following Institutional Review Board approval, we explored the rate of incident (first-ever) symptomatic VAs.(2) Study patients included adults ≥18 years old, treated with ibrutinib for a hematologic malignancy. Patients with incomplete medical records for the variables of interest were excluded. We manually searched all subject charts for incident VAs. Symptomatic premature ventricular tachycardia, sustained ventricular tachycardia (VT), and ventricular fibrillation (VF) were considered incident VAs.(4) Arrhythmia episodes were graded using the Common Terminology Criteria for Adverse Events v4.03, followed by adjudication by two independent cardiologists.(4) VA-associated symptoms included chest pain, palpitations, dizziness, syncope, heart failure (HF) symptoms, and SCD.
Our primary outcome was the development of incident symptomatic VAs following ibrutinib initiation. The secondary outcome was the development of any symptomatic arrhythmia [supraventricular tachycardia (SVT) or VA]. Follow-up began from ibrutinib initiation.
Univariable and multivariable Fine and Gray regression analyses accounting for competing risks were preformed to determine the association between baseline covariates and outcomes. Survival analysis techniques were used to estimate the cumulative incidence of VAs. Person-year incidence rates for VA development on ibrutinib were calculated. Additionally, subgroup analysis among patients without baseline coronary artery disease (CAD) and/or left ventricular ejection fraction of <50% (HFrEF) was performed. Observed idiopathic VA rates were compared to expected incidence rates derived from published contemporary data from the Rochester Epidemiology Project using calculated relative risks (RRs).(4) Absolute excess risk (AER) was calculated, by subtracting the expected number of cases from the number of observed, multiplying by 100,000, and finally dividing by person-years at-risk. A Naranjo Probability Score was calculated for each event to determine the likelihood of ibrutinib-association, with a score of ≥6, suggestive of at least probable association.
Overall, 582 patients (63.8±10.9 years, 69.8% male, 45.0% hypertensive, 13.1% diabetic, 11.3% CAD, 2.4% HF) were treated with ibrutinib for hematologic malignancies. Most had an ECOG performance status of 0–1 (92.5%); concurrent anticancer therapy was used in 175 (30.1%), with 9 (1.5%) receiving anthracyclines; complete cohort characteristics were previously described.(2) Over a median follow-up of 32 months (range 0.7–73) 11 patients developed symptomatic VAs, of which 7 (one SCD/VF, and two recurrent sustained-VT) had at least probable association with ibrutinib. Ninety-four developed any symptomatic arrhythmia (18 with non-AF SVTs). Among those with VAs of at least probable ibrutinib-association, the median time-to-event was 16 months (range 0.7–57.6), while the median time-to-any arrhythmic event was 8.6 months (range 0.2–61.7). Over the 1,134 person-years of ibrutinib exposure, the corresponding estimated 100,000 person-year VA incidence rate was 617.
No cardiovascular or electrocardiographic variables, including QTc, were found to be associated with VAs. But, male gender, prior AF, HF, CAD, diabetes, widened QRS, and valvular disease, respectively, were associated with development of any arrhythmia (VA+SVT). In a multivariable model, only prior AF was associated with arrhythmic events.
Moreover, among those without baseline CAD and HFrEF, 6 patients had an incident VA event, corresponding to an estimated 100,000 person-year incidence rate of 596 (Figure). When compared to a reported idiopathic VA incidence of 48.1 among similar non-ibrutinib subjects (4), this translated into an observed versus expected RR of 12.4 (p<0.001) and AER of 548. Enhanced automaticity through late-sodium currents is a proposed mechanism.(3) Limitations include the potential some intermittent baseline or post-initiation events were unrecognized, and that VAs range in severity.
Figure:
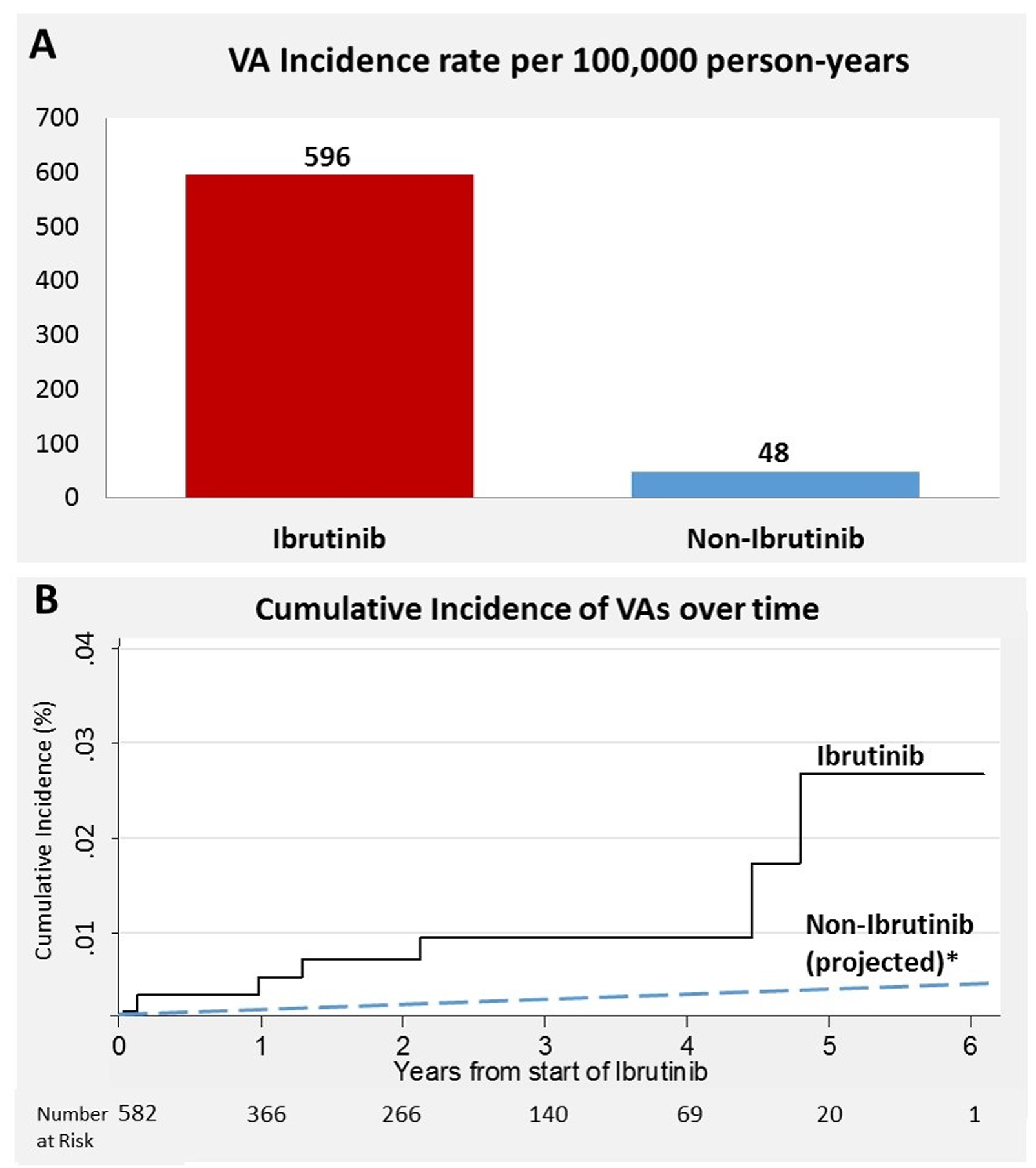
Ventricular arrhythmia (VA) crude (A) and cumulative (B) incidence rates in ibrutinib-users versus an ibrutinib-free population (4). *Assumes a linear event-rate over time.
In summary, ibrutinib is associated with a markedly increased risk of incident VA events in long-term follow-up, even after accounting for baseline cardiovascular disease. Given the expanding use of this therapy, close monitoring is warranted. Further research targeting the mechanism(s) of arrhythmias following ibrutinib initiation is needed.
Acknowledgements:
The authors acknowledge and thank the patients and their families treated at the Ohio State University Comprehensive Cancer Center. The manuscript’s content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: This work was supported in part by an NIH P50-CA140158 award, as well as by K23 CA178183, R01 CA197870, R35 CA197734, and K12-CA133250 grants, and the D. Warren Brown, and Four Winds Foundations.
Disclosures: Dr. Byrd has received research funding from Acerta Pharma, Pharmacyclics, Inc, and Genentech. Dr. Awan has received research funding from Innate Pharma and provided consulting services to Gilead Sciences, Pharmacyclics, Inc, Janssen, Abbvie, and Novartis Oncology. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiczer TE, Levine LB, Brumbaugh J, et al. Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Advances. 2017;1:1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lampson BL, Yu L, Glynn RJ, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129(18):2581–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirichand S, Killu AM, Padmanabhan D, et al. Incidence of Idiopathic Ventricular Arrhythmias: A Population-Based Study. Circulation: Arrhythmia and Electrophysiology. 2017;10:e004662. [DOI] [PMC free article] [PubMed] [Google Scholar]


