Abstract
Background:
Accurate estimates of the risk of transfusion-transmitted infectious diseases are essential for monitoring the safety of blood supply and evaluating the potential effects of new screening tests.
Objective:
The aim was to determine changes over time in blood donor population infection rates of hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV-1, 2) and syphilis.
Materials and Methods:
Changes in rates of HBV, HCV, HIV-1, 2, and syphilis infections were evaluated by comparing yearly prevalence rates for blood donors over 3 years, that is, between January 1, 2012 and December 31, 2014. Serological tests were done according to the standard operating procedures and manufacturer's instructions and included the following: tests for hepatitis B surface antigen; antibodies to HCV and HIV-1, 2 and rapid plasma reagin test for syphilis.
Results:
Nearly 2.54 of the total screened blood donors were reactive for one of the four transfusion transmitted infections (TTIs) with higher prevalence in replacement (3%) than voluntary donors (2.3%) and in male (2.54%) than female (2.3%) donors. TTI tend to be more (54.7%) in younger population of 18–30 years. HCV infection is the most common of all TTI (50%).
Conclusion:
The rising prevalence rates of HIV; HBV; HCV and syphilis among different groups suggests that blood transfusion is still very unsafe in this community and emphasis should be laid on donor education and donor self-exclusion, implementation of strict donor screening criteria, pre-donation counseling, and more sensitive screening methods. Furthermore, donors with a history of sexually transmitted infections should be totally excluded from all donations.
Keywords: Hepatitis B virus; hepatitis C virus; human immunodeficiency virus-1, 2, transfusion transmitted infections
INTRODUCTION
Blood safety remains an issue of major concern in transfusion medicine in developing countries where national blood transfusion services and policies, appropriate infrastructure, trained personnel and financial resources are lacking. This is aggravated by the predominance of family and replacement donors (RD), rather than regular voluntary blood donors.[1] Transfusion transmitted infections (TTIs), that is, hepatitis B virus (HBV), hepatitis C virus (HCV), and Human immunodeficiency virus (HIV) causes serious mortality, morbidity, and financial burden and are, thus, a major global health problem.[2] Monitoring time trends and prevalence of infectious diseases in the blood donor population provides a mechanism to assess the safety of the blood supply, effectiveness of donor deferral criteria and other screening measures; gives an idea of epidemiology of these diseases in the community.[3] Blood centers have implemented education programs, continuing medical educations and screening procedures aimed at reducing the risk of TTI and efforts are under way to improve screening of donors. Infectious units donated in the window period, the time between infection and detectability by screening tests are more liable for infections not to be detected by routine screening tests. Therefore, more sensitive and specific screening tests such as chemiluminescence immunoassays (CLIA) and nucleic acid amplification testing (NAT) with maximum reduction in window period have been implemented worldwide. Direct per cutaneous exposures, transfusion of blood or blood products, transplantation of organs or tissues from infectious donors, and sharing of contaminated needles among injection drug users, have been associated with the most efficient transmission of TTI.[4] With the advent of NAT Western countries have decreased the risk of TTI to a major extent. Despite this dramatic progress, India is far from achieving a zero risk blood supply as it depends heavily on RD and the escalating cost of the medical care make the desired results even more difficult to obtain.[5]
Study aim
Evaluating and monitoring the prevalence of TTI in blood donors is a valuable index of donor selection and blood safety. This study analyzed the trends of TTI among blood donors of Patiala and adjoining areas of Punjab during a 3-year period.
MATERIALS AND METHODS
All the eligible blood donors were requested to fill up the blood donor questionnaire cum consent form formulated as per the rules laid down in Drugs and Cosmetics Act, Ministry of Health and Family Welfare, Government of India.[6] Each donor was selected by a Medical officer after a detailed medical history and brief physical examination. Informed consent was obtained from all the donors that their blood will be tested for the five mandatory TTI, that is, hepatitis B, hepatitis C, HIV-1 and 2, syphilis and malaria. Donors were asked not to donate if they had AIDS-related symptoms or HIV-related risk behavior including injecting intravenous drugs, being a male who had sex with another male, promiscuous behavior or having a sexual relationship with a prostitute.
All the donor samples were examined using commercial third generation enzyme linked immunosorbent assays (ELISA) kits, that is, erbalisa hepatitis B; Transasia – Biomedicals Ltd., or Hepalisa; J Mitra for hepatitis B surface antigen (HBsAg), erbalisa hepatitis C Transasia - Biomedicals Ltd., or HCV Microlisa, J Mitra for Anti HCV and Erbalisa HIV 1 and 2; Transasia - Biomedicals Ltd., or HIV Microlisa; J. Mitra for anti HIV-1, 2. Rapid screening kits, that is, Hepacard; J. Mitra for HBsAg, HCV Tridot; J. Mitra for Anti HCV and HIV Tridot; J Mitra for Anti HIV-1, 2 were used for screening in case of dire emergency. Carbogen; Tulip Diagnostics (P) Ltd: Rapid plasma reagin (RPR) Card Test was used for screening for syphilis. RPR reactive donor samples were retested using specific test treponema pallidum haemagglutination. All tests were performed according to the manufacturer's instructions. External quality controls (provided along with the testing kit) and internal quality controls (prepared in-house from confirmed reactive donors) both for positive control and negative control were run in parallel along with each new run. Moreover, the reactive samples with one test kit were repeated in duplicate with another test kit or same test kit with different lot number. Once positive was considered as positive and that blood and its components were discarded properly as per departmental protocol to add to the patient safety.
Statistical analysis
Data retrieved was tabulated and statistical evaluation was performed using SPSS software. Annual prevalence of HBsAg, anti HCV, anti HIV, and syphilis among the blood donors was evaluated.
RESULTS
The prevalence of hepatitis infections was higher than that of retroviral infections. Total 15,056 donors were selected and screened for TTI over these 3 years with 10,765 (71.5%) voluntary donors (VD) and 4291 (28.5%) RD; 14,579 (96.8%) male and 477 (3.2%) female donors. 382, that is, 2.54% of the screened blood donors were reactive for one of the four TTI.
Out of total blood donors screened, TTI were present in 1.68% VD and 0.85% RD and their incidence was more among male (2.46%) than female (0.073%) donors. When incidence was calculated among same category such as gender wise; 2.30% of females and 2.54% of males were infected while according to type of donation, 3% of RD and 2.3% of VD were infected. Among total infected donors 11 (2.9%) were females and 371 (97.1%) were males; 129 (33.8%) were RD and 253 (66.2%) were VD [Table 1].
Table 1.
Distribution of transfusion transmitted infections among blood donors according to gender and type of donation
| Category | Category types | Total donors screened | Percentage of TTIs among total blood donors screened | Percentage of TTIs among same category | Frequency and percentage of TTIs among gender and donation type |
|---|---|---|---|---|---|
| Gende | Female | 477 | 0.073 | 2.30 | 11 (2.9) |
| Male | 14,579 | 2.46 | 2.54 | 371 (97.1) | |
| Type of donors | Replacement | 4291 | 0.85 | 3 | 129 (33.8) |
| Voluntary | 10,765 | 1.68 | 2.3 | 253 (66.2) |
TTIs=Transfusion transmitted infections
Over these 3 years 20122014, 191 (50%) donors were reactive for Anti HCV; 151 (39.5%) were reactive for HBsAg, 32 (8.4%) were reactive for anti HIV; 6 (1.6%) were reactive for syphilis and 2 (0.5%) were reactive for both Anti HIV and Anti HCV [Figure 1]. There were 136 (35.6%) donors reactive for TTI in the year 2012, 115 (30.1%) in 2013 and 131 (34.3%) in the year 2014 [Figure 2].
Figure 1.
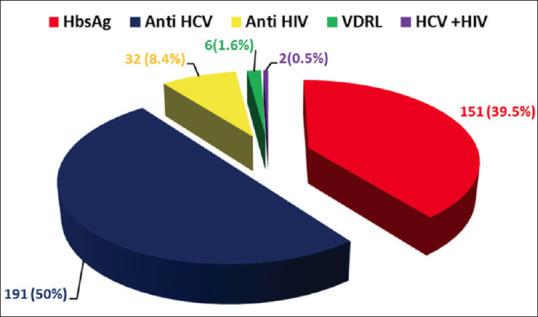
Pie - chart depicting the frequency of hepatitis B virus, hepatitis C virus, human immunodeficiency virus and syphilis infections among blood donors
Figure 2.
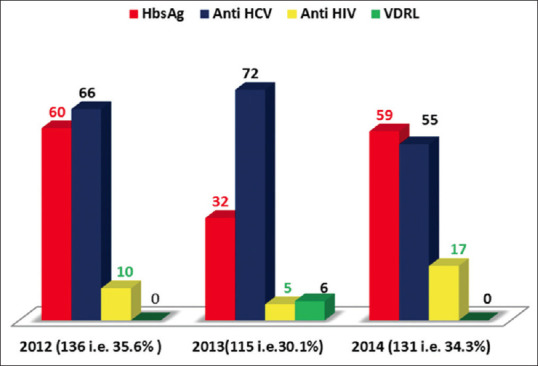
Frequency of transfusion transmitted infections among blood donors in the years 2012, 2013 and 2014
Out of 5488 donors screened in the year 2012, 136 (2.47%) were reactive for TTI; out of 5207 donors, 115 (2.21%) were reactive in 2013 and out of 4361 donors screened in the year 2014, 131 (3.00%) were reactive for TTI. According to the total donors screened yearly; the prevalence in ascending order was 2013, 2012 and 2014.
Prevalence of HBV was higher and similar in 2012 and 2014 i.e., 60 (39.7%) and 59 (39.1%). Prevalence of HCV was highest in 2013, that is, 71 (37.2%). HIV prevalence sharply increased in 2014 from 10 (31.3%) in 2012; 5 (15.6%) in 2013 to 17 (53.1%) in 2014. 6 donors reactive for syphilis were detected only in 2013. 2 (0.5%) of the infected persons had multiple infections (HIV + HCV). In year 2012, prevalence of TTI in ascending order was HIV (7.4%), HBV (44.1%) and HCV (47.8%); in 2013 it was HIV (4.3%), syphilis (5.2%), HBV (27.8%) and HCV (61.7%,) whereas in 2014, it was HIV (13.0%), HCV (42.0%) and HBV (45.0%) [Table 2].
Table 2.
Distribution of hepatitis B virus, hepatitis C v irus, human immunodeficiency v irus and syphilis infections in year 2012, 2013 and 2014
| Type of infection | 2012 | 2013 | 2014 | Total |
|---|---|---|---|---|
| HBV | ||||
| Count | 60 | 32 | 59 | 151 |
| Percentage within type of infection | 39.7 | 39.7 | 39.7 | 100.0 |
| Percentage within year | 44.1 | 27.8 | 45.0 | 45.0 |
| HCV | ||||
| Count | 65 | 71 | 55 | 191 |
| Percentage within type of infection | 34.0 | 37.2 | 28.8 | 100.0 |
| Percentage within year | 47.8 | 61.7 | 42.0 | 50.0 |
| HCV + HIV | ||||
| Count | 1 | 1 | 0 | 2 |
| Percentage within type of infection | 50.0 | 50.0 | 0 | 100.0 |
| Percentage within year | 0.7 | 0.9 | 0 | 0.5 |
| HIV | ||||
| Count | 10 | 5 | 17 | 32 |
| Percentage within type of infection | 31.3 | 15.6 | 53.1 | 100.0 |
| Percentage within year | 7.4 | 4.3 | 13.0 | 8.4 |
| Syphilis | ||||
| Count | 0 | 6 | 0 | 6 |
| Percentage within type of infection | 0 | 100.0 | 0 | 100.0 |
| Percentage within year | 0 | 5.2 | 0 | 1.6 |
| Total | ||||
| Count | 136 | 115 | 131 | 382 |
| Total Percentage within type of infection | 35.6 | 30.1 | 34.3 | 100.0 |
| Total Percentage within year | 100.0 | 100.0 | 100.0 | 100.0 |
HBV=Hepatitis B virus; HCV=Hepatitis C virus; HIV=Human immunodeficiency virus
In the year 2012, HBV (44.1%) and HCV (47.8%) reactive donors formed the major chunk of reactive donors while HIV reactivity was 7.4%. In 2013; HCV reactive donors showed a sudden rise, that is, 61.7% while HIV reactivity declined further to 4.3% but 6 (5.2%) reactive donors for syphilis were also noticed in 2013 which itself is a risk factor for increasing TTI. Year 2014, showed the trends of TTI similar to 2012 with HBV and HCV reactivity rate of 45% and 42% with a rise in HIV prevalence 13% [Table 2].
All the reactive blood donors were categorized into 3 age groups and highest number of reactive donors were found in 18–30 years age group, that is, 209 (55%) [Figure 3].
Figure 3.
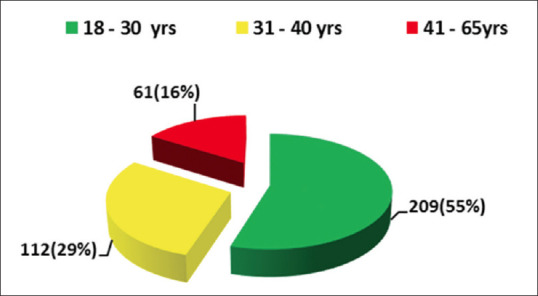
Frequency and percentage of transfusion transmitted infections among three different age categories
All the TTI were found to be more among VD except for two donors who were reactive for both HIV and HCV and both of them were RD. Incidence of HCV was also higher among RD 58.1%. TTI were found to be more in RD 3% compared to 2.3% in VD which was significant. Incidence of HCV infection was higher among both VD (45.8%) and RD (58.1%) and in both genders, that is, male (50.1%) and female (45.5%) donors. Incidence of TTI was highest among 18-30 years age group with HBV (61.6%), HCV (50.3%) and HIV (56.3%) while incidence of syphilis (45.5%) was highest among 41–65 years age group. Higher frequency of HCV was noticed with increasing age group [Table 3].
Table 3.
Distribution of hepatitis B virus, hepatitis C virus, human immunodeficiency virus and syphilis infections among replacement and voluntary donors; male and female donors and among the donors of three different age groups
| Type of infection | Type of donor | Gender | Age groups (years) | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Replacement | Voluntary | Male | Female | 18-30 | 31-40 | 41-65 | ||
| HBV | ||||||||
| Count | 42 | 109 | 146 | 5 | 93 | 39 | 19 | 151 |
| Percentage within type of infection | 27.8 | 72.2 | 96.7 | 3.3 | 61.6 | 25.8 | 12.6 | 100.0 |
| Percentage within donor type | 32.6 | 43.1 | 39.4 | 45.5 | 44.5 | 34.8 | 31.1 | 39.5 |
| HCV | ||||||||
| Count | 75 | 116 | 186 | 5 | 96 | 62 | 33 | 191 |
| Percentage within type of infection | 39.3 | 60.7 | 97.4 | 2.6 | 50.3 | 32.5 | 17.3 | 100.0 |
| Percentage within donor type | 58.1 | 45.8 | 50.1 | 45.5 | 45.9 | 55.4 | 54.1 | 50.0 |
| HCV + HIV | ||||||||
| Count | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 2 |
| Percentage within type of infection | 100.0 | 0 | 100.0 | 0 | 50.0 | 0 | 50.0 | 100.0 |
| Percentage within donor type | 1.6 | 0 | 0.5 | 0 | 0.5 | 0 | 1.6 | 0.5 |
| HIV | ||||||||
| Count | 9 | 23 | 31 | 1 | 18 | 10 | 4 | 32 |
| Percentage within type of infection | 28.1 | 71.9 | 96.9 | 3.1 | 56.3 | 31.3 | 12.5 | 100.0 |
| Percentage within donor type | 7.0 | 9.1 | 8.4 | 9.1 | 8.6 | 8.9 | 6.6 | 8.4 |
| Syphilis | ||||||||
| Count | 1 | 5 | 6 | 0 | 1 | 1 | 4 | 6 |
| Percentage within type of infection | 16.7 | 83.3 | 100.0 | 0 | 16.7 | 16.7 | 45.5 | 100.0 |
| Percentage within donor type | 0.8 | 2.0 | 1.6 | 0 | 0.5 | 0.9 | 6.6 | 1.6 |
| Total | ||||||||
| Count | 129 | 253 | 371 | 11 | 209 | 112 | 61 | 382 |
| Percentage within type of infection | 33.8 | 66.2 | 97.1 | 2.9 | 54.7 | 29.3 | 16.0 | 100.0 |
| Percentage within donor type | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
HBV=Hepatitis B virus; HCV=Hepatitis C virus; HIV=Human immunodeficiency virus
Reactive donors were also divided into 10 categories depending on occupations prevalent in this region and occupations with high risk of contracting TTI. Highest frequency of TTI were found in donors doing private job 117 (30.6%) followed by agriculture 92 (24.1%), students 66 (17.3%) and businessmen 43 (11.3%) which constituted 83.3% of the total reactive donors. Of all the TTI, incidence of HCV was highest among all the occupations [Table 4].
Table 4.
Distribution of hepatitis B virus, hepatitis C virus, human immunodeficiency virus and venereal disease research laboratory infections among ten common occupations in Punjab
| Type of infection | Occupation | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agriculture Business Driver Government job House wife Laborer Medico NRI Private job Student | |||||||||||
| HBV | |||||||||||
| Count | 32 | 20 | 5 | 9 | 2 | 7 | 0 | 1 | 35 | 40 | 151 |
| Percentage within type of infection | 21.2 | 13.2 | 3.3 | 6.0 | 1.3 | 4.6 | 0 | 0.7 | 23.2 | 26.5 | 100.0 |
| Percentage within occupation | 34.8 | 46.5 | 27.8 | 52.9 | 33.3 | 36.8 | 0 | 100.0 | 29.9 | 60.6 | 39.5 |
| HCV | |||||||||||
| Count | 53 | 17 | 12 | 6 | 4 | 11 | 3 | 0 | 69 | 16 | 191 |
| Percentage within type of infection | 27.7 | 8.9 | 6.3 | 3.1 | 2.1 | 5.8 | 1.6 | 0 | 36.1 | 8.4 | 100.0 |
| Percentage within occupation | 57.6 | 39.5 | 66.7 | 35.3 | 66.7 | 57.9 | 100.0 | 0 | 59.0 | 24.2 | 50.0 |
| HCV + HIV | |||||||||||
| Count | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| Percentage within type of infection | 0 | 50.0 | 0 | 0 | 0 | 50.0 | 0 | 0 | 0 | 0 | 100.0 |
| Percentage within occupation | 0 | 2.3 | 0 | 0 | 0 | 5.3 | 0 | 0 | 0 | 0 | 0.5 |
| HIV | |||||||||||
| Count | 6 | 4 | 1 | 1 | 0 | 0 | 0 | 0 | 10 | 10 | 32 |
| Percentage within type of infection | 18.8 | 12.5 | 3.1 | 3.1 | 0 | 0 | 0 | 0 | 31.3 | 31.3 | 100.0 |
| Percentage within occupation | 6.5 | 9.3 | 5.6 | 5.9 | 0 | 0 | 0 | 0 | 8.5 | 15.2 | 8.4 |
| VDRL | |||||||||||
| Count | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 6 |
| Percentage within type of infection | 16.7 | 16.7 | 0 | 16.7 | 0 | 0 | 0 | 0 | 50.0 | 0 | 100.0 |
| Percentage within occupation | 1.1 | 2.3 | 0 | 5.9 | 0 | 0 | 0 | 0 | 2.6 | 0 | 1.6 |
| Total | |||||||||||
| Count | 92 | 43 | 18 | 17 | 6 | 19 | 3 | 1 | 117 | 66 | 382 |
| Percentage within type of infection | 24.1 | 11.3 | 4.7 | 4.5 | 1.6 | 5.0 | 0.8 | 0.3 | 30.6 | 17.3 | 100.0 |
| Percentage within occupation | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
HBV=Hepatitis B virus; HCV=Hepatitis C virus; HIV=Human immunodeficiency virus; VDRL=Venereal disease research laboratory; NRI=Nonresident of India
DISCUSSION
The prevalence of these TTI varies by nationality and geography,[7] 71.5% of the blood donors had donated voluntarily in the blood donation camps organized by different clubs, religious organizations, offices, political parties, etc., or voluntarily at the blood donation center. Therefore, the prevalence of viral carrier rates in blood donors is similar to that of the general population. Thus, the data highlighting the increase of TTI among the blood donors is of concern indicating that the occurrence of these infections among the VD should be monitored carefully and the possible causes evaluated.
Male donors formed the bulk of donor population (96.8%) similar to the Pahuja et al. study, while prevalence of TTI was similar in both the genders 2.30% in females and 2.54% in males.[5]
Occurrence of TTI among blood donors was compared in the 3 years, to assess the trends in 3 consecutive years. The prevalence in ascending order was 2013 (2.21%), 2012 (2.47%) and 2014 (3.0%) indicating an increasing trend which might be due to the increasing TTI among general population or more probably due to the implementation of 100% ELISA testing of blood donors in year 2014. Due to the epidemic of dengue in Northern India in the later months of the year 2013, the emergency requirement of blood and its components increased, moreover replacement and family donations increased and some of the blood and its components were screened by rapid testing techniques and issued in emergency. This resulted in lower detection of TTI for collections in window period. It indicates the necessity for implementation of more sensitive screening techniques such as CLIA or NAT which are not feasible in all blood donation centers but screening by ELISA (3rd or 4th generation) should be implemented, which is comparatively cost effective. When 100% ELISA testing was implemented in our center in 2014, increasing trends of TTI were noticed even more than year 2012.
RD constitutes the largest group of blood donors in India[8] while in present study it constituted 28.5% of the total donors. Incidence of TTI was significantly higher among RD (3%) than VD (2.3%). Therefore, a large scale multidisciplinary approach towards enhancement of voluntary blood donation needs to be undertaken by the Government of India.
Transmission of HBV by blood components negative for HBsAg can occur in acute phase of infection during the seronegative window period, or during chronic stages (occult infection). These HBsAg negative individuals but positive for HBV DNA form a continuous source of infection.[9] A widespread hepatitis B immunization program should be initiated which may lead to decrease in hepatitis B prevalence in the future (by preventing early childhood infection and providing immunity for adolescents and adults before they engage in high-risk behaviors). In the present study, seroprevalence of HBsAg was highest among 18–30 years age group (61.6%). Younger age at acquisition of infection continues to be the most important predictor of chronic carriage. HBV vaccination programs will decrease the future global burden of HBV infection.[10,11] In India transfusion associated HBV is estimated to be approximately 50% or more in multiply transfused patients and approximately 1.5% in postsurgical recipients.[12] Blood containing anti-HBc with or without detectable presence of HBsAg might be infectious, therefore routine blood donor screening for anti-HBc is recommended to decrease the risk of post transfusion HBV infection.[13]
The present study showed increasing trends of TTI similar to study by Bhattacharya et al.[13] Rising trends could be curtailed by focusing efforts on primary prevention strategies to reduce or eliminate the risk for transmission from nosocomial exposures (e.g., blood transfusion, unsafe injection practices) and high-risk practices.[14] Due to limitations in current blood screening practices in developing countries, donation by such individuals is a potential source of TTI to the recipients.[15,16,17,18] The risk of transmitting TTI by the transfusion of screened blood is very small in Western world, and newer screening tests have reduced the risk even further.[19,20,21,22,23,24] However, this is not applicable to developing countries, like India where cost constraint is still a big obstacle, making the availability of newer screening techniques limited to few centers.
Similar to Ameen et al. and Sharma et al., prevalence of anti-HCV was significantly higher among RD (58.1%) versus VD (45.8%).[25,26] Hepatitis C acquisition is a serious risk for multi-transfused patients.[27] Furthermore, anti HCV reactivity was highest of all the TTI in all the age groups, indicating its increasing trend with age. Because there is no vaccine and no post exposure prophylaxis for HCV, the focus of primary prevention efforts should be safer blood supply, safe injection practices in health care and other settings, and decreasing the number of people who initiate injection drug use.[28] Socioeconomic development, health education programs, and adult hepatitis B immunization programs targeting high-risk groups should be initiated.[29]
Current donor screening procedures include pre - donation counseling and confidential direct questioning by doctors and trained staff about health and specific high-risk behaviors. Because of strict donor screening, the number of TTI in high risk groups, like long route drivers (4.7%), nonresident of India (0.3%) and medicos (0.8%), were comparatively less although frequency of these occupations in general population were also less. HIV and HBV infection were found to be more common among younger age groups (students, private employees). In contrast to study by El Beltagy et al., in present study incidence of TTI among people of lower socioeconomic status, that is, laborers was lower (5%) and higher among students (17.3%).[29] Hence, further improvement in behavioral screening is desirable. Although some strategies may be difficult to implement in light of chronic blood shortages, approaches to improve deferral procedures include the following: (1) Increasing education and awareness of risk factors and the importance of safe blood donations, (2) ensuring that donor recruiters and sponsors do not inappropriately pressurize persons who may be ineligible for donation, (3) decreasing test-seeking behavior by encouraging testing at alternative test sites, (4) further identification or characterization of important risk behaviors, and (5) strategies to reach donors who are reluctant to admit that they engaged in high-risk behaviors.
Hepatitis B vaccination and programs designed to encourage intravenous drug users to stop using, seek treatment, and utilize harm reduction techniques, should be implemented.[30] Among blood donors, prior blood or blood product transfusion, intranasal cocaine use, intravenous drug use, sexual promiscuity, tattooing, and ear piercing are risk factors for TTI.[31] The high frequency of intravenous drug use was unexpected, because donors had denied such use when questioned directly at the time of their blood donations. Finally, implementation of more sensitive tests (such as NAT) that detect infection earlier will further decrease risks of TTI.[32]
The present study showed an increasing trend of hepatitis B and C and a dramatic rise in HIV over 3 years. Moreover, prevalence of TTI was quite higher among younger age group (18–30 years) and students which was an alarming situation. From these findings, it can be concluded that awareness regarding TTI was poor among the donor population. To curb down the prevalence of TTI, it is necessary to implement pre donation counseling sessions and self-exclusion options for eligible blood donors. Counseled healthy donors get aware of the need of enhanced blood safety and therefore would take care to remain non infected by these infections. Therefore, providing adequate pre donation information with emphasis on healthy life style and behavior, face to face donor counseling, strict maintenance of privacy and confidentiality during donor screening, especially during outdoor blood donation camps to elicit hidden risk factors are recommended which will prevent blood donation by people with high risk factors. Switching over to more sensitive TTI screening tests and then repeat evaluation, monitoring and comparison of trends of TTI after their implementation are future perspectives of present study.
CONCLUSION
Stringent measures need to be taken on urgent basis including dissemination of information, strict blood donor screening criteria and implementation of sensitive screening tests like NAT, inclusion of antibody to hepatitis B core antigen or other sensitive markers to the screening protocol. In addition, referral of people engaged in high risk activities to counselors and rehabilitation centers is recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mbanya DN, Takam D, Ndumbe PM. Serological findings amongst first-time blood donors in Yaoundé, Cameroon: Is safe donation a reality or a myth? Transfus Med. 2003;13:267–73. doi: 10.1046/j.1365-3148.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman SH, Kuhns MC, Todd DS, Glynn SA, McNamara A, DiMarco A, et al. Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: Implications for transfusion transmission and donor screening. Transfusion. 2003;43:696–704. doi: 10.1046/j.1537-2995.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 3.Glynn SA, Kleinman SH, Schreiber GB, Busch MP, Wright DJ, Smith JW, et al. Trends in incidence and prevalence of major transfusion-transmissible viral infections in US blood donors, 1991 to 1996. Retrovirus Epidemiology Donor Study (REDS) JAMA. 2000;284:229–35. doi: 10.1001/jama.284.2.229. [DOI] [PubMed] [Google Scholar]
- 4.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26(3 Suppl 1):62S–5S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 5.Pahuja S, Sharma M, Baitha B, Jain M. Prevalence and trends of markers of hepatitis C virus, hepatitis B virus and human immunodeficiency virus in Delhi blood donors: A hospital based study. Jpn J Infect Dis. 2007;60:389–91. [PubMed] [Google Scholar]
- 6.Malik V. Drugs and Cosmetics Act 1940. 16th ed. Lucknow: Eastern Book Company; 2003. pp. 279–303. [Google Scholar]
- 7.El-Hazmi MM. Prevalence of HBV, HCV, HIV-1, 2 and HTLV-I/II infections among blood donors in a teaching hospital in the Central region of Saudi Arabia. Saudi Med J. 2004;25:26–33. [PubMed] [Google Scholar]
- 8.Makroo RN, Salil P, Vashist RP. Trends of HIV infection in the blood donors of Delhi. Indian J Pathol Microbiol. 1996;39:139–42. [PubMed] [Google Scholar]
- 9.Liu CJ, Chen DS, Chen PJ. Epidemiology of HBV infection in Asian blood donors: Emphasis on occult HBV infection and the role of NAT. J Clin Virol. 2006;36(Suppl 1):S33–44. doi: 10.1016/s1386-6532(06)80007-7. [DOI] [PubMed] [Google Scholar]
- 10.Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. 2004;38(10 Suppl 3):S158–68. doi: 10.1097/00004836-200411003-00008. [DOI] [PubMed] [Google Scholar]
- 11.Tandon BN, Acharya SK, Tandon A. Epidemiology of hepatitis B virus infection in India. Gut. 1996;38(Suppl 2):S56–9. doi: 10.1136/gut.38.suppl_2.s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saraswat S, Banerjee K, Chaudhury N, Mahant T, Khandekar P, Gupta RK, et al. Post-transfusion hepatitis type B following multiple transfusions of HBsAg-negative blood. J Hepatol. 1996;25:639–43. doi: 10.1016/s0168-8278(96)80232-7. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya P, Chandra PK, Datta S, Banerjee A, Chakraborty S, Rajendran K, et al. Significant increase in HBV, HCV, HIV and syphilis infections among blood donors in West Bengal, Eastern India 2004-2005: Exploratory screening reveals high frequency of occult HBV infection. World J Gastroenterol. 2007;13:3730–3. doi: 10.3748/wjg.v13.i27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mast EE, Alter MJ, Margolis HS. Strategies to prevent and control hepatitis B and C virus infections: A global perspective. Vaccine. 1999;17:1730–3. doi: 10.1016/s0264-410x(98)00415-0. [DOI] [PubMed] [Google Scholar]
- 15.Weber B, Melchior W, Gehrke R, Doerr HW, Berger A, Rabenau H. Hepatitis B virus markers in anti-HBc only positive individuals. J Med Virol. 2001;64:312–9. doi: 10.1002/jmv.1052. [DOI] [PubMed] [Google Scholar]
- 16.Wang JT, Wang TH, Sheu JC, Shih LN, Lin JT, Chen DS. Detection of hepatitis B virus DNA by polymerase chain reaction in plasma of volunteer blood donors negative for hepatitis B surface antigen. J Infect Dis. 1991;163:397–9. doi: 10.1093/infdis/163.2.397. [DOI] [PubMed] [Google Scholar]
- 17.Weinberger KM, Bauer T, Böhm S, Jilg W. High genetic variability of the group-specific a-determinant of hepatitis B virus surface antigen (HBsAg) and the corresponding fragment of the viral polymerase in chronic virus carriers lacking detectable HBsAg in serum. J Gen Virol. 2000;81(Pt 5):1165–74. doi: 10.1099/0022-1317-81-5-1165. [DOI] [PubMed] [Google Scholar]
- 18.Allain JP. Occult hepatitis B virus infection: Implications in transfusion. Vox Sang. 2004;86:83–91. doi: 10.1111/j.0042-9007.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685–90. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 20.Burnouf T, Radosevich M. Reducing the risk of infection from plasma products: Specific preventative strategies. Blood Rev. 2000;14:94–110. doi: 10.1054/blre.2000.0129. [DOI] [PubMed] [Google Scholar]
- 21.Dodd RY, Notari EP, 4th, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. 2002;42:975–9. doi: 10.1046/j.1537-2995.2002.00174.x. [DOI] [PubMed] [Google Scholar]
- 22.Busch MP, Glynn SA, Stramer SL, Strong DM, Caglioti S, Wright DJ, et al. Anew strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion. 2005;45:254–64. doi: 10.1111/j.1537-2995.2004.04215.x. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien SF, Yi QL, Fan W, Scalia V, Kleinman SH, Vamvakas EC. Current incidence and estimated residual risk of transfusion-transmitted infections in donations made to Canadian Blood Services. Transfusion. 2007;47:316–25. doi: 10.1111/j.1537-2995.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 24.Nascimento MC, Mayaud P, Sabino EC, Torres KL, Franceschi S. Prevalence of hepatitis B and C serological markers among first-time blood donors in Brazil: A multi-center serosurvey. J Med Virol. 2008;80:53–7. doi: 10.1002/jmv.21046. [DOI] [PubMed] [Google Scholar]
- 25.Ameen R, Sanad N, Al-Shemmari S, Siddique I, Chowdhury RI, Al-Hamdan S, et al. Prevalence of viral markers among first-time Arab blood donors in Kuwait. Transfusion. 2005;45:1973–80. doi: 10.1111/j.1537-2995.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 26.Sharma RR, Cheema R, Vajpayee M, Rao U, Kumar S, Marwaha N, et al. Prevalence of markers of transfusion transmissible diseases in voluntary and replacement blood donors. Natl Med J India. 2004;17:19–21. [PubMed] [Google Scholar]
- 27.Al-Sheyyab M, Batieha A, El-Khateeb M. The prevalence of hepatitis B, hepatitis C and human immune deficiency virus markers in multi-transfused patients. J Trop Pediatr. 2001;47:239–42. doi: 10.1093/tropej/47.4.239. [DOI] [PubMed] [Google Scholar]
- 28.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 29.El Beltagy KE, Al Balawi IA, Almuneef M, Memish ZA. Prevalence of hepatitis B virus markers among blood donors in a tertiary hospital in Tabuk, Northwestern Saudi Arabia. Int J Infect Dis. 2008;12:495–9. doi: 10.1016/j.ijid.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Gostin LO, Lazzarini Z, Jones TS, Flaherty K. Prevention of HIV/AIDS and other blood-borne diseases among injection drug users. A national survey on the regulation of syringes and needles. JAMA. 1997;277:53–62. [PubMed] [Google Scholar]
- 31.Conry-Cantilena C, VanRaden M, Gibble J, Melpolder J, Shakil AO, Viladomiu L, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med. 1996;334:1691–6. doi: 10.1056/NEJM199606273342602. [DOI] [PubMed] [Google Scholar]
- 32.Meena M, Jindal T, Hazarika A. Prevalence of hepatitis B virus and hepatitis C virus among blood donors at a tertiary care hospital in India: A five-year study. Transfusion. 2011;51:198–202. doi: 10.1111/j.1537-2995.2010.02801.x. [DOI] [PubMed] [Google Scholar]


