Abstract
Background:
Semantic variant primary progressive aphasia (svPPA), a clinical syndrome characterized by loss of semantic knowledge, is associated with neurodegeneration that starts in the anterior temporal lobe (ATL) and gradually spreads towards posterior temporal and medial frontal areas. At the earliest stages, atrophy may be predominantly lateralized to either the left or right ATL, leading to different clinical profiles with greatest impairment of word comprehension or visual/social semantics, respectively.
Methods & Procedures:
We report the in-depth longitudinal investigation of cognitive and neuroanatomical features of JB, an unusual case of ATL neurodegeneration with relative sparing of left lateral ATL regions.
Outcomes & Results:
Over the course of nine years, neurodegeneration progressed to involve bilateral temporo-lateral and frontal regions, resulting in a relatively symmetric and diffuse frontotemporal atrophy pattern. In parallel, JB developed greater behavioral, cognitive, and language impairments, as well as signs of motor neuron disease at her last evaluation. Episodic memory and socio-emotional processing deficits arose, likely secondary to semantic verbal deficits, while visuospatial processing, executive function, and non-semantic language abilities remained largely unaffected throughout the course of the disease.
Conclusions:
The details of this rare case of early medial more than lateral ATL degeneration are consistent with a bilateral organization of the semantic system and, crucially, with a functional dissociation between medial paralimbic and lateral neocortical temporal regions. Cases of frontotemporal dementia (FTD) such as JB, who initially do not meet current clinical criteria for svPPA and instead present with some features of behavioral variant FTD, highlight the need for specific criteria for the right temporal variant of FTD that we propose could be called semantic variant FTD.
Keywords: primary progressive aphasia, semantic dementia, right temporal variant, frontotemporal dementia, motor neuron disease
Introduction
Semantic variant of primary progressive aphasia (svPPA) is a clinical syndrome marked by progressive selective impairment of semantic memory (Hodges, Patterson, Oxbury, & Funnell, 1992; Snowden, Goulding, & Neary, 1989; Warrington, 1975). Current clinical criteria require presence of naming and single-word comprehension deficits, as well as at least three additional features among impaired object knowledge, surface dyslexia or dysgraphia, spared repetition, or spared speech production (Gorno-Tempini et al., 2011). SvPPA has been associated with bilateral neurodegeneration of the anterior temporal lobe (ATL), yet early cases often present with atrophy lateralized to either the left or right hemisphere (e.g., Chan et al., 2009).
Clinical differences have been described between cases with predominantly left-sided ATL or right-sided atrophy: while the former show more impairment in naming, word comprehension and reading of irregular words, the latter presents with behavioral and emotional changes as well as isolated loss of semantic knowledge for famous people (Binney et al., 2016; Brambati et al., 2009; Chan et al., 2009; Evans, Heggs, Antoun, & Hodges, 1995; Gainotti, Barbier, & Marra, 2003; Galton et al., 2001; Gorno-Tempini, Dronkers, et al., 2004; R. J. Perry et al., 2001; Snowden, Thompson, & Neary, 2004; Thompson et al., 2004). However, as the disease progresses towards bilateral atrophy of the temporal and frontal lobes, left-sided and right-sided variants overlap in their clinical profiles (Chan et al., 2009; Seeley et al., 2005). Thus, despite the initial differences, the two variants are better conceived as extremes along a continuum within the same disorder rather than two distinct syndromes (Binney et al., 2016; Kumfor et al., 2016). When cases of right-temporal atrophy are diagnosed early, they might mainly show emotional/behavioral changes without impairment in verbal semantics, word-comprehension, and naming. In this instance, they might not meet criteria for svPPA and instead meet the broad criteria for behavioral variant frontotemporal dementia (bvFTD). Diagnostic criteria for bvFTD include progressive deterioration of behaviour and/or cognition, together with at least three of the following symptoms: 1) early behavioural disinhibition, 2) early apathy or inertia, 3) early loss of sympathy or empathy, 4) early perseverative, stereotyped or compulsive/ritualistic behaviour, 5) hyperorality and dietary changes, or 6) executive/generation deficits with relative sparing of memory and visuospatial functions (Rascovsky et al., 2011).
In relation to the ATL, structural and functional differences have been identified not only between left and right temporal lobes, but also between medial and lateral portion of each ATL. Converging evidence from cyto-architectonic, chemo-architectonic and pathological markers allow parcellation of the temporal pole cortex in at least 6 different regions (Ding, Van Hoesen, Cassell, & Poremba, 2009). The heterogeneity of the ATL is corroborated by the observation of distinct functional (Pascual et al., 2015) and structural (Papinutto et al., 2016) connectivity profiles. While ventromedial regions appear to be preferentially connected with limbic and visual systems, leading to a functional specialization for socio-emotional and visual stimuli, dorsolateral regions show preferential connectivity with language and auditory systems, leading to a functional specialization for verbal stimuli. Coherently, current theoretical models consider the ATL as a semantic hub processing multimodal information in a graded, rather than unitary, fashion (e.g., Rice, Hoffman, & Lambon Ralph, 2015).
Clinically, the degree to which neurodegeneration affects dorsolateral (neocortical) or ventromedial (limbic) regions likely determines how prominent the observed changes in language and behavior are, respectively. This could explain why the behavioral symptoms observed in individuals with the right variant of ATL degeneration can overlap with those detected in the bvFTD (Rascovsky et al., 2011), even though the underlying pathology greatly differs (D. C. Perry et al., 2017; Spinelli et al., 2017). Single case studies that accurately describe the symptoms associated with a specific pattern of ATL atrophy can thus be instrumental in developing new hypotheses on the organization of the semantic system.
In this case report, we outline nine years of cognitive, language, and anatomical data in an individual suffering from bilateral ATL neurodegeneration who presents with an unusual clinical profile. A striking dissociation between virtually spared linguistic abilities and deteriorated non-verbal semantic processing and emotional/behavioral changes was coupled with atrophy affecting bilateral medial portions of the ATL, with relative sparing of left dorsolateral ATL. Hard to fit into current clinical criteria, this case called for an in-depth neuro-cognitive investigation by means of experimental tasks focusing specifically on verbal vs. non-verbal semantic knowledge and socio-emotional functions. Following the parallel evolution of cognitive symptoms and anatomical changes, our longitudinal data suggest that relative sparing of the lateral portion of the left ATL supported JB’s residual lexical-semantic processing abilities. Thus, this case provides information on the organization of the semantic system both within and across the right and left ATL, in particular highlighting the functional difference between medial paralimbic and lateral neocortical regions. Finally, it underlines the need for clinical criteria embracing all presentations of semantic variant of PPA and FTD.
Materials and Methods
Case presentation
JB is a 67-year-old right-handed woman, who presented to the UCSF Memory and Aging Center in December of 2009 after four years of progressive socially inappropriate behavior, compulsive and repetitive manners, increased mental rigidity, poor emotional recognition, and people and place semantic problems. Before symptom onset, she was a high-functioning woman with a Master’s degree in Liberal Arts (18 years of education), who formerly worked as a teacher and writer. She has been followed by us annually, with a total of seven visits, until August 2018. The subject of this case report gave written consent to participate in accordance with the Institutional Review Board of the University of California San Francisco.
Her first symptoms, around 2005, included compulsive behavioral changes and semantic deficits for known people. Her husband reported changes in her eating schedule and diet. After implantation of dental braces, she started blending all her food and concomitantly adopted a regimented eating schedule and healthy diet, with a detailed menu of specific items eaten at specific times of the day. At the time of her first visit in 2009, she had been eating the same menu every day for at least two years. She also developed an enhanced interest in puzzles and games, which she played for a set amount of time each day, aiming at winning a specific number of times every single day. She planned and listed all her daily activities on a full-page log, and check-marked them when accomplished. Her detailed calendar included scheduling of when to be intimate with her spouse.
Premorbidly, her husband described her as a happy person who liked socializing with people, was even-tempered, and well-organized. With the onset of the disease, these personality traits became exaggerated, and she developed an even more positive perspective and started hugging people she had only recently met. Her spouse noticed that she started to lose interest in news, politics and other global issues, previously topics she was eager to discuss, and avoided negative and violent movies and other media altogether. She had also become more extroverted, talkative, and disinhibited, and had trouble recognizing others’ emotions and social cues. She was very verbose, making it difficult to interrupt or change topics. She started sharing intimate personal details with family and friends, and later also with strangers. She did not recognize that this made her spouse uncomfortable, or understand why when she was told so. Overall, JB had poor insight into her deficits. She did acknowledge certain behavioral changes such as hugging more and being more talkative. However, she attributed these to her family’s cultural roots and being a social person, and stated that these are not a problem for her.
About two years prior to the first visit, semantic deficits were noticed; she had difficulty recognizing familiar faces and retrieving proper names. For example, at a high school reunion, JB was unable to recognize her classmates’ faces or remember their names. Similarly, she had trouble remembering the names for her Christmas card list. These difficulties progressed and, by her second visit, she could not recognize the names and faces of the neuropsychologist and physician who had tested her one-on-one for more than an hour the week before. Moreover, JB developed a problem recognizing places; she would fail to recognize places she had been before, and at the same time have a wrongful feeling of familiarity with places she had never been before.
At no time during her disease did JB have delusions or hallucinations, depression, anxiety, aggression, trouble sleeping, or sensory symptoms. Her past medical history was significant for hearing impairment, hyperlipidemia, and impaired glucose tolerance. With regards to family history, there are no known relatives with dementia or neurodegenerative disease.
During her first visit in 2009, neuropsychological testing revealed impaired retrieval of semantic information for people as well as mild naming difficulties but spared single word comprehension (see below for details). Based on her clinical presentation (i.e., semantic difficulties for people, compulsions rigidity and disinhibition, with sparing of single word comprehension and reading), we expected asymmetric, right greater than left, ATL atrophy. Instead, structural MRI showed fairly bilateral (yet slightly more right-sided) ATL atrophy, with a surprisingly medial greater than lateral involvement. Not meeting criteria for PPA nor for bvFTD, she received a generic diagnosis of frontotemporal dementia. The peculiar relative sparing of verbal semantics despite clearly notable left (medial) ATL atrophy was the observation that motivated our in-depth study. JB was followed annually, allowing observation of how semantic and behavioral symptoms slowly progressed over time. In April 2011, her clinical findings met the diagnostic criteria for predominantly right svPPA.
Throughout the years, she maintained an active social life including daily hikes, up to an hour, with friends or her spouse. Moreover, she continued to cook, drive, shop, pay bills, use the computer, and do household chores. However, as of her fourth visit, her symptoms had clearly worsened. She had developed difficulty with spatial navigation even in familiar places, seemingly because she could not recognize known houses and landmarks. Moreover, her behavior gradually changed with increasing disinhibition and child-like manners (e.g., dancing in public places). Occasionally, she would stick out her tongue, wink her eye, or giggle during examinations. These symptoms further developed over time and by her sixth visit she would, for instance, approach strangers, touching and kissing without realizing this could make people uncomfortable. She also started making playful sounds and exaggerated movements throughout the examinations. JB’s mental rigidity gradually spread to all daily activities: by her third visit, she used to inflexibly schedule not only when to exercise and when to take medications, but also when to playing games and the number of times she had to win. Devoted to prayer even premorbidly, she slowly increased her praying to over an hour daily and developed a strict script for it.
Concurrently, the semantic problems continued to worsen over time. Since her second visit, she had clear difficulty with confrontation naming: she could not name beaver (“it is eating part of the tree trunk,” with no knowledge of where it lives, despite cues), tongs (“ice cube holder”), or tripod (also not with phonemic cues, and with no conceptual knowledge about it). At her fourth visit in 2013, the physician reported few errors in her spontaneous speech (e.g., “urinologist” for “urologist”). During her fourth visit she was able to name “knuckles,” during her fifth visit she called them “hand bumps,” and during her sixth visit she called them “finger joints.” In 2016, at her sixth visit, her semantic impairment had become even more prominent and now included not only semantic impairment and anomia for people and places, but also mild surface dyslexia and dysgraphia (e.g., “yaucht” for yacht), and single-word comprehension deficits, such as difficulty understanding the words insomnia, octopus, and ostrich. She could name a neurological hammer a “hammer” but could not say what it was used for. During the same visit, her spouse reported that her difficulty remembering familiar faces and places had notably worsened. Her repetition and speech comprehension remained relatively spared throughout all annual visits, as did reading, writing, and spelling of regular words.
During all of her visits, her neurological examination was mostly unremarkable until the last visit in August 2018 (14 years from first symptom) when she presented with an eight-month history of progressive worsening muscle weakness and multiple falls. Her cranial nerves exam revealed spastic, slow, and strained speech, tongue fasciculations, and increased jaw jerk. Her upper extremity motor exam demonstrated bilateral thenar and hypothenar atrophy and intrinsic hand muscle weakness. Her lower extremity exam showed bilateral lower extremity weakness, right foot drop, diffuse hyperreflexia including crossed adduction, positive Hoffman sign, and upgoing toes. Therefore, during her last visit, electromyography was performed to aid diagnosis of neuromuscular disorder.
Cognitive, language, and social evaluation
Between her initial diagnostic visit in December 2009 and her last follow-up in August 2018, JB was evaluated on a variety of neuropsychological tests. Besides the comprehensive battery of cognitive and language tasks routinely administered in our center (see Table 1), she underwent in-depth assessment of semantic (both verbal and non-verbal) and socio-emotional processing (see Table 2). Each of the described tests was administered during at least three and up to a total of seven visits; no visit occurred in 2012 or 2017. The majority of these tests are described in detail in Gorno-Tempini, Rankin, et al. (2004); for those that are not, additional information follows below.
Table 1.
JB’s demographic and neuropsychological profile
| Visit number (Year) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Scoring | Controls m (SD) | 1 (2009) | 2 (2011) | 3 (2013) | 4 (2014) | 5 (2015) | 6 (2016) | 7 (2018) | |
| Age at testing | 67 | 69 | 71 | 72 | 73 | 74 | 76 | ||
| CDR | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | ||
| MMSE | Max. 30 | 29.5 (0.7) | 28 | 29 | 27 | 27 | 27 | 26 | 27 |
| Visuospatial processing | |||||||||
| Benson complex figure - copy | Max. 17 | 15.1 (1.7) | 15 | 16 | NC | 11* | 14 | 16 | 16 |
| VOSP Number Location | Max. 10 | 9.1 (1.0) | 10 | 10 | NC | 10 | 10 | 10 | 10 |
| Episodic memory | |||||||||
| CVLT short delay free recall | Max. 9 | 7.9 (1.6) | 6 | 6 | 5 | 5 | 5 | 4* | 3* |
| CVLT long delay free recall | Max. 9 | 7.3 (1.6) | NC | 7 | 5 | 1* | 0* | 2* | 2* |
| CVLT recognition | Max. 9 | 8.7 (0.9) | 7 | 9 | 5* | 4* | 5* | 7 | 7 |
| Benson complex figure - delay | Max. 17 | 10.9 (3.9) | NC | 8 | NC | 7 | 5 | 7 | 0* |
| Executive functioning | |||||||||
| Digit span - forward | Max. 9 | 6.9 (1.2) | 9 | 9 | 9 | 8 | 7 | 8 | 9 |
| Digit span - backward | Max. 8 | 4.9 (1.1) | 5 | 7 | 6 | 7 | 6 | 5 | 5 |
| Stroop | # correct | 88.3 (18.5) | 84 | 84 | NC | 73 | 68 | 80 | 69 |
| Trails | Max. 14 | 13.9 (0.9) | NC | 14 | 14 | 14 | 14 | 14 | 14 |
| Language | |||||||||
| Syntax comprehension | % correct | 98.5 (1.8) | 100 | 100 | 100 | NC | 96 | 100 | 100 |
| WAB Sequential Commands | Max. 80 | 80 (0) | 80 | 62 | 80 | 80 | 80 | 80 | NC |
| WAB Auditory Word Recognition | Max. 60 | 60 (0) | 60 | 60 | 60 | 60 | 60 | 60 | NC |
| WAB Spontaneous Speech | Max. 20 | 20 (0) | 20 | 20 | 20 | 20 | 20 | 20 | NC |
| WAB Repetition | Max. 100 | 99.5 (0.9) | 100 | 100 | NC | 100 | 100 | 100 | NC |
| PPVT | Max. 16 | 15.7 (0.6) | 16 | 14* | NC | 14* | 13* | 13* | 11* |
| Verbal fluency - semantic | # correct | 21.2 (3.6) | 14 | 12* | 9* | 11* | 14 | 9* | 6* |
| Verbal fluency - phonemic | # correct | 16.6 (6.8) | 6 | 13 | 9 | 12 | 11 | 11 | 8 |
| BNT | Max. 15 | 14.4 (0.7) | 12* | 13 | 13 | 11* | 11* | 6* | 8* |
Note.
= more than 2 standard deviations below the mean of controls; NC = not collected, CDR = Clinical Dementia Rating Scale; MMSE = Mini-Mental State Examination; VOSP = Visual Object and Space Perception Battery; CVLT = California Verbal Learning Test; WAB = Western Aphasia Battery; PPVT = Peabody Picture Vocabulary Test; BNT = Boston Naming Test
Table 2.
In-depth assessment of JB’s semantic and socio-emotional processing
| Visit number (Year) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Scoring | Controls m (SD) | 1 (2009) | 2 (2011) | 3 (2013) | 4 (2014) | 5 (2015) | 6 (2016) | 7 (2018) | |
| Semantic knowledge - verbal | |||||||||
| OOT - words | Max. 20 | 17.8 (1.9) | NC | 18 | 20 | 19 | NC | NC | NC |
| Camels & Cactus Test - words | Max. 64 | 60.7 (2.1) | NC | 55* | 52* | 50* | NC | NC | NC |
| Cambridge Naming Test | Max. 64 | 62.1 (1.9) | 57* | 61 | 59 | 58* | NC | NC | NC |
| Verbal fluency - semantic | # correct | 21.2 (3.6) | 14 | 12* | 9* | 11* | 14 | 9* | 6* |
| Semantic knowledge - non-verbal | |||||||||
| OOT - pictures | Max. 20 | 16.8 (2.2) | NC | 15 | 12* | 11* | 14 | 13 | NC |
| Camels & Cactus Test - pictures | Max. 64 | 58.9 (3.1) | NC | 48* | 52* | 50* | NC | NC | |
| Pyramids & Palm Trees - pictures | Max. 52 | 51.8 (0.4) | 50* | 49* | 49* | 51 | NC | NC | NC |
| Category Comprehension Test | Max. 64 | 63.1 (0.5) | NC | 63 | 60* | NC | NC | 61* | NC |
| Socio-emotional processing | |||||||||
| CATS Name Emotional Prosody | Max. 12 | 11.4 (0.9) | 12 | 12 | NC | 12 | 12 | 12 | 3* |
| CATS Affect Matching | Max. 16 | 13.5 (1.5) | 14 | 15 | NC | 11 | 13 | 12 | 12 |
| TASIT Emotion Evaluation Test | Max. 14 | 11.7 (1.6) | 11 | 8* | NC | 10 | 7* | 7* | NC |
| TASIT Social Inference | Max. 60 | 54.2 (4.7) | 34* | 24* | NC | 34* | 29* | 30* | NC |
| Faces processing | |||||||||
| Benton facial recognition | Max. 7 | 6.8 (0.8) | NC | NC | 6 | 3* | 1* | 1* | 1* |
| FF Naming | Max. 16 | 13.0 (2.6) | 9 | 7* | 4* | 4* | 2* | 4* | 3* |
| FF Semantic Association - names | Max. 10 | 9.8 (0.3) | 7* | 9* | 9* | 8* | 8* | 8* | NC |
| FF Recognition | Max. 20 | 19.9 (0.3) | 15* | 17* | 16* | 14* | 15* | 16* | NC |
| FF Semantic Association - pictures | Max. 10 | 9.2 (0.6) | NC | 9 | 9 | 7* | 8 | 7* | NC |
Note.
= more than 2 standard deviations below the mean of controls; NC = not collected; OOT = Over-regular Object Test; CATS = Comprehensive Affect Testing System; TASIT = The Awareness of Social Inference Test; FF = UCSF Famous Faces Test
A functional assessment of the severity of her dementia symptoms was made using the Clinical Dementia Rating Scale (CDR; Morris, 1993) through a semi-structured interview with her spouse. General cognition was assessed with the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975). Visuospatial processing was tested with the Benson complex figure, which is a modified version of the Rey-Osterrieth complex figure, and the Number Location test from the Visual Object Space Perception (VOSP) battery (Warrington & James, 1991). Episodic memory was evaluated with the California Verbal Learning Test (CVLT; Delis, Kramer, Kaplan, & Ober, 2000) and a 10-minute delayed free recall of the Benson complex figure for verbal and visual memory, respectively. Executive functioning was examined with forward and backward Digit Span tasks from the Wechsler Memory Scale-third edition (WMS-III; Wechsler, 1997), the incongruent version of the Stroop test in 60 seconds, and a modified version of the Trails sequencing task (Reitan, 1958), in which one had to alternately connect numbers and days of the week within 120 seconds. Semantic knowledge was evaluated using various tests, both verbally and non-verbally. Verbal semantic knowledge was assessed with the Peabody Picture Vocabulary Test (PPVT; Dunn, Dunn, Bulheller, & Häcker, 1965), the abbreviated 15-item Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983; Mack, Freed, Williams, & Henderson, 1992), and semantic fluency (animals). We tested verbal and non-verbal semantic associations with the picture and word versions, respectively, of the Pyramids and Palm Trees test (Howard & Patterson, 1992). Various domains of non-semantic language abilities were evaluated as well. Lexical retrieval and access were assessed with letter fluency (words beginning with ‘d’) and the Western Aphasia Battery (WAB; Kertesz, 1982) Auditory Word Recognition subtest. Sentence comprehension was assessed with the Western Aphasia Battery (WAB) Sequential Commands subtest, and a syntactic comprehension task, in which one hears a sentence and has to select the matching picture from two options (Wilson et al., 2010). We assessed speech and language production with the WAB subtests for Spontaneous Speech and Repetition.
JB also completed the word and picture versions of the 20-item four-alternative forced-choice Over-regular Object Test (OOT; Rice, Caswell, Moore, Hoffman, & Lambon Ralph, 2018; Rogers, Lambon Ralph, Hodges, & Patterson, 2004). In this test, one sees four pictures of the same object or animal; one of the pictures is a correct representation of the object or animal while three pictures have been visually manipulated such that they have missing, added, or changed characteristics. Additionally, during her second, fourth, and fifth visit, JB was tested on a selection of tests from the Cambridge Semantic Memory Test Battery (Adlam, Patterson, Bozeat, & Hodges, 2010) for an in-depth assessment of her verbal and non-verbal semantic knowledge, including the 64-item Naming Test, Category Comprehension Test, and the word and picture versions of the Camels and Cactus Test. In the Cambridge Naming Test, one names a line-drawing. In the Category Comprehension Test, one hears the name of an object and has to identify its line drawing, which is depicted together with nine distractors from the same semantic category. In the Camels and Cactus Test, one has to decide which of two visual items is best associated with the target visual item; items are either all pictures or words, depending on the version. Each of the Cambridge tests consists of half natural versus half man-made items, evenly distributed among eight categories: domestic animals, foreign animals, birds, fruits, vehicles, large household items, small household items, and tools. Person-specific semantic information was assessed with an experimental battery including confrontation naming and recognition of famous faces (FF), and their semantic association with pictures and names (another famous face/name with the same career, e.g., a fellow actor or politician) in a triplet matching task (Borghesani et al., 2019; Gorno-Tempini, Rankin, et al., 2004).
Socio-emotional processing was tested with the Name Emotional Prosody and Affect Matching subtests of the Comprehensive Affect Testing System (CATS; Froming, Levy, Schaffer, & Ekman, 2006). In the Name Emotional Prosody test, one hears a content-neutral sentence and has to indicate which emotion-prosody the speaker used (i.e., happy, sad, angry, frightened, or neutral). In the Affect Matching test, one has to match the pictured emotion of a target character with the same emotion displayed by one of five other pictured characters. We also administered the short version of the Emotion Evaluation Test and the Social Inference–Minimal Test of The Awareness of Social Inference Test (TASIT; McDonald, Flanagan, Rollins, & Kinch, 2003). In the abbreviated Emotion Evaluation Test, one chooses which of seven basic emotions (happiness, surprise, anger, sadness, fear, disgust or neutral) an actor depicts in each of 14 videos. In the Social Inference–Minimal Test, one sees an everyday conversation and has to answer four questions per script indicating the speakers’ beliefs, meaning, intentions, and feelings for each of ten neutral and five paradoxical (e.g., sarcastic) scripts. Unfamiliar face processing to assess prosopagnosia was tested with a short version of the Benton Facial Recognition Test (Benton & Van Allen, 1968).
Scores from controls were derived from Gorno-Tempini, Rankin, et al. (2004) for the MMSE, Benson complex figure tasks, CVLT, Digit Span tasks, BNT, verbal fluency tasks (semantic and letter), Western Aphasia Battery tests, and Pyramids & Palm Trees test, from Watson et al. (2018) for the Stroop and PPVT, from Corbett, Jefferies, Burns, and Lambon Ralph (2015) for the Cambridge battery, from Binney et al. (2016) for the CATS, from Rice et al. (2018) for the OOT, and from 10 age-matched controls for the Number Location subtest of the Visual Object and Space Perception Battery (VOSP), Trails test, Syntax Comprehension test, Benton Facial Recognition test, Famous Faces tests, and the TASIT. Comparing JB scores with published elderly healthy controls data allowed us to compute z-scores describing her deviation from normal performance. In Table 1 and 2 we report JB’s raw scores while marking those falling more than two standard deviations below controls’ performance. Moreover, to quantify (and compare) the overall impairment in verbal and non-verbal semantic, for those time point where at least 3 tasks for each domain were administered, we computed an average z-score for verbal and one for non-verbal semantic. It should be acknowledged that relying on control data from published studies grants comparison with previous studies, yet carries an important limitation in a longitudinal single-case such as ours: the same reference values are used across different time points thus demographic variables such as age are not perfectly matched with that of our participant.
Neuroanatomical analysis
JB underwent high-resolution structural brain MRI at baseline and in each follow-up visit—images were acquired within 90 days of neuropsychological testing. During her first five visits, three-dimensional images were obtained on a 3 Tesla (3T) Siemens TrioTim MRI scanner equipped with a body transmit coil and an 8-channel receive head coil. Structural MRI sequences included a magnetization prepared rapid gradient echo (MPRAGE) to obtain whole-brain T1-weighted images (160 sagittal slices; slice thickness = 1 mm; field of view = 256 x 256 mm; matrix = 256 x 240; voxel size 1.0 x 1.0 x 1.0 mm; repetition time = 2300 ms; echo time = 2.98 ms; inversion time = 900 ms; flip angle = 9 degrees). During her sixth visit, whole-brain three-dimensional T1-weighted images were acquired on a 3T Siemens Prisma MRI scanner. This scanner was equipped with a 64-channel transmit and receiver head coil using a MPRAGE sequence (160 sagittal slices; slice thickness = 1 mm; field of view = 256 mm2; matrix = 256 x 240; voxel size 1.0 x 1.0 x 1.0 mm3; repetition time = 2300 ms; echo time = 2.9 ms; inversion time = 900 ms; flip angle = 9 degrees).
Structural T1-weighted images were preprocessed using SPM12. Preprocessing involved bias-correction, segmentation into tissue compartments, and spatial normalization using a single generative model with the standard SPM12 parameters. To optimize intersubject registration, the participant’s image was warped to a template derived from 300 confirmed neurologically healthy older adults (ages 44-86, M±SD: 67.2±7.3; 113 males, 186 females) scanned with one of three magnet strengths (1.5T, 3T, 4T), using affine and nonlinear transformations with the help of the Diffeomorphic Anatomical Registration through Exponentiated Lie algebra (DARTEL) toolbox (Ashburner, 2007). Spatially normalized, segmented, and modulated gray matter images were smoothed using an 8-mm FWHM isotropic Gaussian kernel. Finally, JB’s maps were compared to 534 confirmed neurologically healthy controls from the UCSF MAC Hilblom Cohort (age range 44-99 years, M±SD: 68.7±9.1; 220 male/302 female), adjusted for age, sex, total intracranial volume, and magnet strength. Obtained W-scores are interpreted like z-scores, with M = 0/SD = 1. Negative W-scores represent below-average volume and scores < −1.50 fall below the 7th percentile and can thus be considered clinically abnormal. To evaluate whole brain spread of atrophy, we computed peak and average deviation from controls in volumetric region of interest (ROIs) derived from the Neuromorphometrics ROI probabilistic atlas.
The ATL is known to be a heterogeneous region in terms not only of its cyto-architectonic and chemo-architectonic characteristics (Ding et al., 2009), but also with respect to its functional and structural connectivity profiles (Papinutto et al., 2016; Pascual et al., 2015). To better highlight atrophy distribution and progression within the ATL, we thus developed a novel parcellation based on previous findings identifying portions of the ATL preferentially connected with specific cortical regions (Papinutto et al., 2016). Four masks were built in each hemisphere isolating the anterior dorsolateral ATL (yellow in Figure 2b, preferentially connected with the superior occipital pole and the superior temporal gyrus), the posterior dorsolateral ATL (purple, preferentially connected with the medial temporal gyrus), the anterior ventromedial ATL (green, preferentially connected with the orbitofrontal cortex), and the posterior ventromedial ATL (blue, preferentially connected with the inferior occipital pole and the inferior temporal gyrus).
Figure 2. Atrophy distribution and progression.
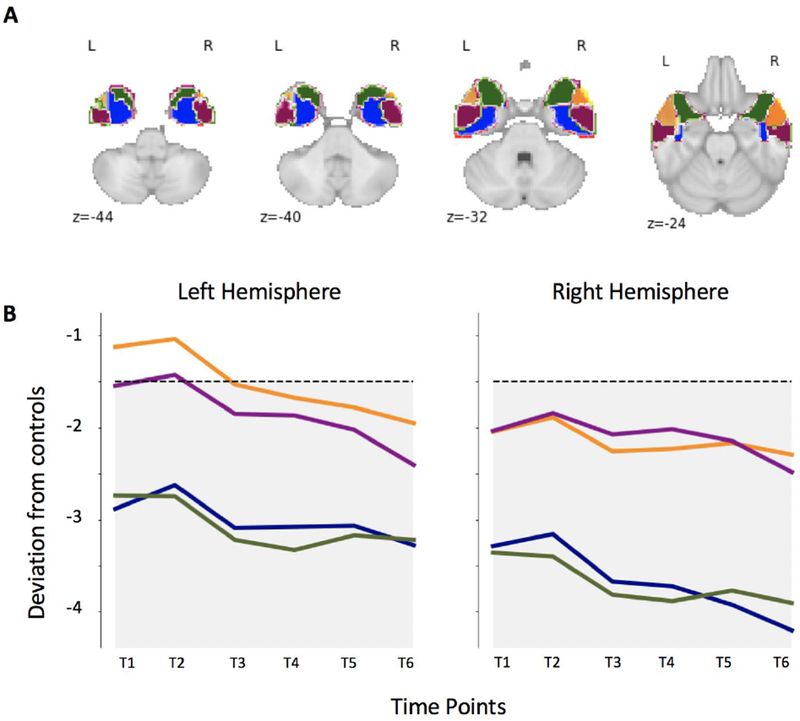
A) Ad-hoc ROIs parcellating the ATL in portions known to be preferentially connected to specific cortical areas, respectively: the anterior dorsolateral ROI (yellow) to superior occipital pole and superior temporal gyrus; the posterior dorsolateral ROI (purple) to medial temporal gyrus; the anterior ventromedial ROI (green) to orbitofrontal cortex; the posterior ventromedial ROI to (blue) to inferior occipital pole and inferior temporal gyrus.
B) At time 1, the atrophy affects the right more than the left hemisphere, and the medial more than lateral regions within each ATL. The dotted line indicates the threshold below which scores are considered clinically abnormal. The only region above such threshold of significant impairment is the left anterior dorsolateral region at time point 1 and 2. As the disease progresses, a decline is observed across all ROIs in both hemispheres.
To compute an overall index of atrophy lateralization, we merged the 4 ipsilateral ROIs obtaining two masks of left (Av_Left) vs. right (Av_Right) ATL. The laterality index was calculated as the ratio between the difference in volume loss between the two hemispheres and their sum, i.e.: (Av_Right - Av_Left) / (Av_Right + Av_Left). Negative values would indicate a predominantly left atrophy, while positive ones a predominantly right atrophy.
Results
Neuropsychological evaluation
Results of JB’s full neuropsychological examination are reported in Table 1, while the findings of the additional assessment of verbal and non-verbal semantic, as well as socio-emotional processing, are reported in Table 2.
JB’s general cognition mildly declined throughout the disease (MMSE ranged from 29 to 26, CDR from 0.5 to 1). Executive functioning was normal to high compared to controls, with above average scores on the forward and backward digit span tasks and normal-ranged scores on the Stroop task without signs of decline. She obtained the maximum score on the Trails task during every visit, within a time range of 32-60 seconds, which is well within the maximum allowed time of 120 seconds. Her visuospatial processing abilities remained relatively spared, for instance, she scored at ceiling on the VOSP Number Location test during all visits. On episodic memory, JB performed slightly below normal during her first visit, with scores worsening over the course of the disease. She failed to perform the delayed recall of the Benson figure during her first visit and performed variably over the years, yet performing only one standard deviation (SD) below controls’ mean up until visit six.
Overall, JB’s language abilities were within normal range, with various aspects remaining unaffected during all years. For instance, she obtained the maximum score on the WAB Auditory Word Recognition test at every visit and scored at ceiling on nearly every occasion in both the syntax comprehension and WAB Sequential Commands tests, including during her last visits. Her speech and language production were unimpaired as shown by maximum scores in all years on the Spontaneous Speech and Repetition subtests of the WAB. Her phonemic fluency remained relatively stable as well, fluctuating around one SD below normal controls. While fluent in her spontaneous speech throughout the course of the disease, her speech pattern had changed with the last visit. She has abnormally high pitch breaks in the middle or towards the end of sentences, including declarative ones. This pattern was exhibited in both spontaneous speech and single word naming tasks. Prosody was grossly normal, but she showed signs of dysphonia with moderately rough quality of speech and pitch breaks, slight dysarthria, and slightly reduced variation in speaking speed.
During the first visits, JB’s semantic knowledge showed a clear dissociation: her verbal semantic processing was less impaired than her non-verbal abilities. For example, comparing the word and picture versions of the Camels and Cactus Test, both tests elicited below-normal performance; nonetheless, she obtained a score of 55 out of 64 on the verbal part, but only 48 out of 64 on the non-verbal part. Similarly, on the word version of the OOT she scored 18 and 20 out of 20 in her second and third visit, respectively, but on the picture version only 15 and 12 out of 20. Throughout the years, her scores on verbal semantic tasks declined to approximately the same impairment level as her non-verbal semantic knowledge. Her semantic fluency, below normal during all visits, was initially better than her phonemic one yet rapidly declined. This pattern, associated with left-lateralized lateral temporal lobe atrophy, is fairly typical (Baldo, Schwartz, Wilkins, & Dronkers, 2006; Vonk et al., 2019). On the Cambridge tests, which dissociate between natural and man-made objects, JB showed worse performance on natural items irrespective of the task (i.e., production and comprehension) and input material (i.e., words and pictures) throughout the visits. Comparing the average z-score for verbal and non-verbal semantic allows appreciation of how, even though performance in both domains progressively decreased, non-verbal semantic processing is more impaired that the verbal counterpart: in 2011, verbal = −1.45, non-verbal = −2.87 (difference = 1.42); in 2013, verbal = −2.03, non-verbal = −4.38 (difference = 2.35); in 2014, verbal = −2.39, non-verbal = −2.50 (difference = 0.11). Average z-scores for other years could not calculated as only one or two in-depth tasks were administered for verbal and non-verbal semantics.
JB’s person-specific semantic knowledge was very impaired from early on. Her scores on the Famous Faces tests were extremely low compared to controls since the first visit, and fluctuated around the same score throughout the years—except for naming famous faces, on which her score of 9 out of 16 at visit one declined to a score of 1 at visit seven. Her facial recognition of unfamiliar faces was initially slightly less impaired compared to processing famous faces, with a score of 6 out of 7 at visit three, but this ability also declined over the years with a score of 1 at visit seven.
Finally, socio-emotional functioning was significantly impaired as of JB’s first visit and slightly declined over the course of the years as observed by her performance on the TASIT Social Inference and Emotion Evaluation tasks. Her social inference score on the TASIT was only 34 out of 60 at visit one, and further declined to a score of 30 at visit six. Remarkably, her basic emotion processing, as tested by matching an emotion to a face or prosody on the CATS, remained relatively preserved. This pattern suggests that the deficit originates from a faulty semantic interpretation of emotions, i.e., a difficulty in placing emotions into context.
Neuromuscular analysis
Throughout the course of the disease, JB’s neurological examination was mostly unremarkable until the last visit when she was wheelchair-bound due to an eight-month history of progressive worsening muscle weakness and multiple falls. Right median and ulnar motor and sensory nerve conduction studies were performed with surface electrodes. Electromyography studies of the bilateral lower and right upper limb were performed with concentric electrodes. The nerve conduction studies revealed absent right median compound muscle action potential (CMAP) and reduced amplitude for the right ulnar compound muscle action potential. The distal motor latency for the right ulnar CMAP was prolonged. The electromyography studies showed fibrillation potentials in every muscle sampled except one, increased incidence of long duration motor unit action potentials in the left tibialis anterior and gastrocnemius muscle, and reduced and/or absent recruitment of motor unit action potentials in every muscle tested. These abnormal electrodiagnostic studies provide evidence for subacute chronic denervation of every muscle tested in the right upper and bilateral lower extremities and support the diagnosis of amyotrophic lateral sclerosis (ALS).
Neuroanatomical analysis
At every visit, there was a slight but noticeable progression of atrophy. At her first visit, JB’s MRI scan showed bilateral but asymmetrical atrophy in the medial part of the ATL, more prominent on the right than the left (Figure 1A–B). Across the visits, her atrophy in this region progressed bilaterally, but more quickly on the left than right, reducing the asymmetry. To quantify the temporal evolution of the atrophy lateralization, we computed a lateralization index comparing volume loss in a left vs. right masks of the ATL. Such atrophy lateralization index was positive across all six time points, denoting a predominantly right atrophy, yet progressively closer to 0: time point 1 = 0.128, time point 2 = 0.135, time point 3 = 0.099, time point 4 = 0.087, time point 5 = 0.089, time point 6 = 0.085. Moreover, the atrophy started as most severe in the medial parts of the right and left ATL anterior temporal lobes and gradually spread to include more lateral regions as well (Figure. 2B).
Figure 1. JB’s atrophy pattern at the first visit.
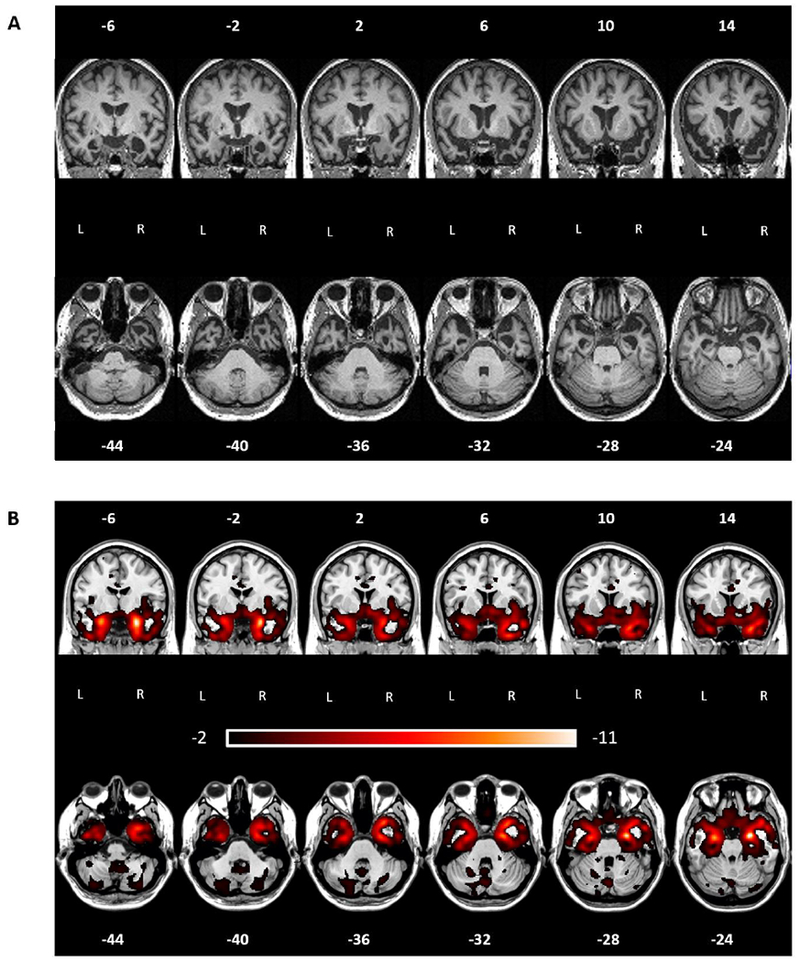
A) coronal and axial cuts of a normalized MRI showing clear ATL neurodegeneration with right greater than left, and medial greater than lateral atrophy. B) coronal and axial cuts of w-maps. W-values indicated deviations from controls, with values below −1.5 indicating clinically abnormal regions (below the 7th percentile compared to healthy controls).
Few regions outside of the ATL were involved, but notably the bilateral orbitofrontal cortex, insulae, amygdalae, hippocampi, and parahippocampal regions. Both amygdalae and hippocampi presented marked neurodegeneration early on in the course of the disease, while orbitofrontal, insular and parahippocampal regions showed predominantly right atrophy at the first visit, progressing to bilateral atrophy during follow-up scans. In Supplementary Figure 1 we show whole brain cuts at each time point, and in Supplementary Table 1 we report all regions showing clinically abnormal GM volume (w-maps values < −1.50) across the six visits. For each ROI, we report the minimum w-score (i.e., maximum deviation from the controls) and its coordinates, along with the average w-score for that region. This data corroborates the radiological read of JB’s anatomical images by quantifying the progressive spread of atrophy from anterior temporal pole to posterior temporal and frontal cortices. It should be noted how, while an involvement of frontal cortices, in particular orbitofrontal and medial ones, can be appreciated, atrophy impact in these regions is, since the first visit and through the development of the disease, less prominent than the impact on the temporal lobes, in particular in the ATL.
Discussion
This report describes the clinical, neuropsychological, neuroimaging, and neuromuscular progression over the course of nine years of a woman with bilateral, right greater than left, and medial greater than lateral, ATL atrophy. Our observations have important implications for the clinical assessment of individuals with temporal degeneration as well as for neuro-cognitive theories on the organization of the semantic system.
At her first visit, JB did not meet criteria for svPPA (Gorno-Tempini et al., 2011), showing fairly modest naming and single-word comprehension deficits limited to the category of famous people, with spared repetition and speech production. Her first chief symptoms involved compulsive behavioral symptoms and non-verbal semantic knowledge deficits, with relative sparing of other cognitive functions. Throughout the years, her clinical profile worsened to include impairments in emotional processing, social functioning, and verbal semantic knowledge. Other aspects of language, as well as low-level visual processing and executive functions, remained relatively spared until late in the disease course. JB’s initial presentation thus included symptoms usually associated with right-sided svPPA (e.g., deficit in person-specific knowledge and recognition of familiar faces), as well as characteristic features of bvFTD (e.g., dietary changes).
Individuals with right-sided ATL degeneration often reach clinical attention late in the course of the disease, sometimes after psychiatric consultation, and are inconsistently classified as having svPPA or bvFTD. Since these syndromes are associated with different probability of underlying pathologies, a differential diagnosis between the two conditions is clinically relevant (Rohrer et al., 2010; Spinelli et al., 2017). Therefore, this case highlights the need for more detailed clinical criteria enabling early differential diagnosis between different clinical presentations of ATL atrophy. JB’s profile suggests that, while sparing of lexical-semantic processing might be a common feature of right-sided svPPA and bvFTD, a specific pattern of non-verbal and social semantic impairment, with changes in emotion processing, rigidity, and person-knowledge deficits might help drawing the distinction. Moreover, JB’s clinical presentation highlights how critical functional dissociations can be associated, not only with differential involvement of left and right ATL, but also between medial and lateral aspects of these regions. JB showed marked involvement of right more than left ventromedial limbic portions of the ATL and relatively sparing of the left dorsolateral neocortical ones, providing evidence in support of the ATL parcellation suggested by cyto-architectonic markers as well as structural and functional imaging findings (Ding et al., 2009; Papinutto et al., 2016; Pascual et al., 2015).
As JB’s clinical profile changed dramatically throughout the disease progression, her atrophy progression paralleled her cognitive and behavioral symptoms. At her first visit, neurodegeneration of the ATL, amygdala, and hippocampus was characterized by right greater than left asymmetry. At this time, her general verbal semantic knowledge (i.e., tools, animals, fruits) was relatively spared, with the exception of famous people’s proper names. Instead, she showed clear deficits in semantic processing for famous people and non-verbal semantics. Her chief complaint was remembering familiar names and faces, and her deficit in this domain was confirmed by tests of face and proper name processing and person-specific semantic information. Various comparisons between individuals with svPPA and those bvFTD have reported that a deficit in person-specific knowledge and recognition of familiar faces is only present, and therefore characteristic, of predominantly right semantic variants (e.g., Kamminga et al., 2015; Thompson et al., 2004). Coherently, abilities of facial recognition are associated with the right temporal pole, particularly the right inferior temporal regions that extent to a network including right inferior and superior frontal regions and right parietal regions (Andreasen et al., 1996; Andrews & Ewbank, 2004; Borghesani et al., 2019; Evans et al., 1995; Gorno-Tempini et al., 1998; Gorno-Tempini, Rankin, et al., 2004; Leveroni et al., 2000). Crucially, our detailed parcellation of the ATL showed a striking medial more than lateral pattern within both hemispheres. For the first two years, the dorsolateral portion of the left ATL was virtually spared, likely supporting her residual verbal semantic and linguistic functions.
JB’s first and most marked symptom were compulsive dietary changes, as she started to blend all her food, became overly concerned about maintaining a healthy diet, and became compulsive and ritualistic about eating habits. Changes in food preferences and eating habits are an often-present symptom of predominantly right svPPA (e.g., Chan et al., 2009; Gorno-Tempini, Rankin, et al., 2004; Ikeda, Brown, Holland, Fukuhara, & Hodges, 2002). These observed dietary changes are not consistent with the typical eating and food preference changes observed in bvFTD, where there is a marked preference for carbohydrates and sweets often accompanied by a profound gain in weight (Ahmed et al., 2016; Aiello, Silani, & Rumiati, 2016; Piguet, 2011). The representation of taste and its pleasantness has been linked to areas in the orbitofrontal cortex, amygdala, and insula (e.g., O’Doherty, Rolls, Francis, Bowtell, & McGlone, 2001). Gradually, as her disease progressed, her strict dietary restrictions and rigidity disappeared, and instead she developed a pathological sweet tooth: she gained 10 pounds between her fourth and fifth visit and was subsequently diagnosed as pre-diabetic. This unhealthy change would be a typical symptom of bvFTD (Rascovsky et al., 2011; Shinagawa et al., 2009). The increased preference for carbohydrates and sugary foods has been associated with atrophy in the bilateral posterolateral orbitofrontal cortex and right anterior insula (Whitwell et al., 2007), which indeed parallels with the emergence JB’s pathological sweet tooth.
JB’s early behavioral symptoms included becoming more extroverted, talkative, and disinhibited, all consistently linked to the right ventrolateral prefrontal cortex (e.g., Dillon & Pizzagalli, 2007; Horn, Dolan, Elliott, Deakin, & Woodruff, 2003). The frontal lobe—right more than left (e.g., Tranel, Bechara, & Denburg, 2002)—has been associated with social functioning and emotion processing. More specifically, social functioning has been linked to the anterior rostral medial frontal cortex (e.g., Amodio & Frith, 2006), and emotion processing to the ventromedial prefrontal and medial orbitofrontal cortices, as well as the amygdala (e.g., Britton et al., 2006; Northoff et al., 2000). Thus, the behavioral changes observed in our case strongly support the involvement of these anatomies in social functioning and contextual emotion processing. Critically, appropriate semantically-driven personal evaluations have been linked with the integrity of the so-called semantic appraisal network (SAN) including temporal poles, orbitofrontal cortex, and ventral striatum (Ranasinghe et al., 2016; Seeley, Zhou, & Kim, 2012; Yeo et al., 2011). Further studies should aim at defining (and comparing) the degree of SAN damage in bvFTD and (right-sided) svPPA.
While the disease affected bilateral temporal lobe structures at first, primarily the right medial ones, it gradually spread to include more ventromedial frontal areas as well as more posterior temporal ones. As expected, this evolution was accompanied by worsening of verbal semantic knowledge (Brambati et al., 2009; Kumfor et al., 2016). The discrepancy between verbal and non-verbal semantic abilities could be best appreciated in the experimental tasks we administered; both semantic associations tests used (Over-regular Object Test and Camel and Cactus Test) allow direct comparison of semantic processing of words and pictures. Initially, JB was clearly more impaired in the non-verbal conditions than their verbal counterparts. Consistently with the progressive decline of verbal semantic processing, evening out the dissociation initially observed, cognitive tasks relying on lexical-semantic processing worsened, as exemplified by the sharp decline of her verbal episodic memory (compared to a relatively stable visual episodic memory). Another observation was that JB performed worse on natural items than man-made objects. Noppeney et al. (2007) showed that participants with herpes simplex virus encephalitis, who had primarily medial ATL damage, exhibited a category-specific deficit for living things, while their participants with semantic dementia, who had primarily bilateral posterior and lateral ATL damage, were equally impaired on both categories. While JB’s atrophy pattern progressed to posterior and lateral regions, the atrophy pattern in the medial regions remained more advanced than in the dorsolateral regions (Figure 2)—in parallel, JB’s performance on man-made objects remained better than on natural items throughout her visits.
As is true for most individuals with svPPA, many cognitive abilities and functions remained remarkably unaffected throughout disease evolution. For example, her executive functions and low-level visuospatial processing remained spared, in line with preservation of dorsolateral frontal and occipital cortices, respectively. While lexical-semantic abilities specifically linked to the left ATL declined, other aspects of language classically associated with left perisylvian damage remained spared and she kept performing at ceiling on tests of speech/language production, syntax comprehension and repetition. Finally, it should be acknowledged that JB was a highly educated writer, a feature that might have granted her an enhanced cognitive reserve for language.
By her last visit, nine years after initial diagnosis, 13 years after the first symptoms, JB presented wheelchair-bound, with wasting and weakness in lower limbs, as well as impaired bulbar function (e.g., dysarthria and reduced speaking speed). The results of an electromyogram confirmed she met criteria for motor neuron disease, namely the presence of upper and lower motor neuron dysfunction (Geevasinga et al., 2016), suggesting that the disease had spread to motor neurons in frontal brain regions as well as to motor nuclei of the brain and the spinal cord. The comorbidity of frontotemporal dementia and motor neuron disease has been well-described in the literature, but the clinical diagnosis may be under-recognized (Olney, Spina, & Miller, 2017). Overlap between the two syndromes occurs in up to 15% of individuals who first present with frontotemporal dementia and up to 30% of individuals who first present with motor neuron disease (Lomen-Hoerth, 2011).
In sum, this case suggests that initial medial right-sided temporal lobe atrophy, with relative sparing of the left dorsolateral temporal cortex, can lead to behavioral and emotional changes in the context of only mildly impaired verbal semantics relative to the non-verbal semantic impairment. It also exemplifies how, over the course of nine years, the spread of the neurodegeneration parallels cognitive and behavioral changes: from predominantly right-sided ventromedial ATL atrophy and mostly non-verbal semantic and emotional symptoms, to diffuse bilateral frontotemporal atrophy and global semantic, behavioral and neuromuscular deficits. Our findings provide valuable insights not only on the lateralization of semantic representations within and across the right and left ATL, but also into the clinical assessment of individuals with asymmetric ATL degeneration. In particular, cases of frontotemporal lobar degeneration such as JB, who initially do not meet current clinical criteria for svPPA and instead present with some features of bvFTD, highlight the need for specific criteria for the right temporal variant of frontotemporal dementia (FTD) with medial ATL atrophy, as its early disease course so markedly deviates from both left-sided svPPA and classic bvFTD. We propose that this syndrome could be called semantic variant FTD.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health (M.GT., NINDS R01 NS050915), (M.GT., NIDCD K24 DC015544), (B.M., NIA P50 AG023501), and by Alzheimer Nederland (J.V.). We thank JB and her family for their time and effort dedicated to our research. We kindly thank Grace Rice and Matthew Lambon Ralph for providing control data of the Over-regular Object Test.
Footnotes
Declaration of interests
The authors declare that they have no competing interests.
References
- Adlam ALR, Patterson K, Bozeat S, & Hodges JR (2010). The Cambridge Semantic Memory Test Battery: Detection of semantic deficits in semantic dementia and Alzheimer’s disease. Neurocase, 16(3), 193–207. [DOI] [PubMed] [Google Scholar]
- Ahmed RM, Irish M, Henning E, Dermody N, Bartley L, Kiernan MC, … Hodges JR (2016). Assessment of eating behavior disturbance and associated neural networks in frontotemporal dementia. JAMA Neurology, 73(3), 282–290. [DOI] [PubMed] [Google Scholar]
- Aiello M, Silani V, & Rumiati RI (2016). You stole my food! Eating alterations in frontotemporal dementia. Neurocase, 22(4), 400–409. [DOI] [PubMed] [Google Scholar]
- Amodio DM, & Frith CD (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–277. doi: 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Andreasen N, O’Leary D, Arndt S, Cizadlo T, Hurtig R, Rezai K, … Hichwa R. (1996). Neural substrates of facial recognition. Neurosciences, 8, 139–146. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, & Ewbank MP (2004). Distinct representations for facial identity and changeable aspects of faces in the human temporal lobe. Neuroimage, 23(3), 905–913. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, & Dronkers NF (2006). Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. Journal of the International Neuropsychological Society, 12(6), 896–900. [DOI] [PubMed] [Google Scholar]
- Benton AL, & Van Allen MW (1968). Impairment in facial recognition in patients with cerebral disease. Trans Am Neurol Assoc, 93, 38–42. [PubMed] [Google Scholar]
- Binney RJ, Henry ML, Babiak M, Pressman PS, Santos-Santos MA, Narvid J, … Rankin KP. (2016). Reading words and other people: A comparison of exception word, familiar face and affect processing in the left and right temporal variants of primary progressive aphasia. Cortex, 82, 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani V, Narvid J, Battistella G, Shwe W, Watson C, Binney RJ, … Gorno-Tempini ML. (2019). “Looks familiar, but I do not know who she is”: The role of the anterior right temporal lobe in famous face recognition. Cortex, 115, 72–85. doi: 10.1016/j.cortex.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, … Gorno-Tempini ML (2009). Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiology of Aging, 30(1), 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, & Liberzon I (2006). Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage, 31(1), 397–409. doi: 10.1016/j.neuroimage.2005.11.027 [DOI] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, … Fox NC. (2009). The clinical profile of right temporal lobe atrophy. Brain, 132(5), 1287–1298. [DOI] [PubMed] [Google Scholar]
- Corbett F, Jefferies E, Burns A, & Lambon Ralph MA (2015). Deregulated semantic cognition contributes to object-use deficits in Alzheimer’s disease: A comparison with semantic aphasia and semantic dementia. Journal of Neuropsychology, 9(2), 219–241. doi: 10.1111/jnp.12047 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). California Verbal Learning Test - Second Edition. The Psychological Corporation: San Antonio, TX [Google Scholar]
- Dillon DG, & Pizzagalli DA (2007). Inhibition of action, thought, and emotion: a selective neurobiological review. Applied and Preventive Psychology, 12(3), 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW, Cassell MD, & Poremba A (2009). Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. Journal of Comparative Neurology, 514(6), 595–623. doi: 10.1002/cne.22053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM, Bulheller S, & Häcker H (1965). Peabody Picture Vocabulary Test Manual. Minneapolis, MN: American Guidance Service Circle Pines. [Google Scholar]
- Evans JJ, Heggs AJ, Antoun N, & Hodges JR (1995). Progressive prosopagnosia associated with selective right temporal lobe atrophy. A new syndrome? Brain, 118 ( Pt 1), 1–13. doi: 10.1093/brain/118.1.1 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Froming K, Levy M, Schaffer S, & Ekman P (2006). The comprehensive affect testing system. Psychology Software, Inc; Available online at: http://www.psychologysoftware.com/CATS.htm. [Google Scholar]
- Gainotti G, Barbier A, & Marra C (2003). Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain, 126(Pt 4), 792–803. doi: 10.1093/brain/awg092 [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, … Hodges N (2001). Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology, 57(2), 216–225. [DOI] [PubMed] [Google Scholar]
- Geevasinga N, Loy CT, Menon P, de Carvalho M, Swash M, Schrooten M, … Higashihara M. (2016). Awaji criteria improves the diagnostic sensitivity in amyotrophic lateral sclerosis: A systematic review using individual patient data. Clinical Neurophysiology, 127(7), 2684–2691. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, … Miller BL. (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, … Grossman M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, & Frackowiak RS (1998). The neural systems sustaining face and proper-name processing. Brain, 121 (Pt 11), 2103–2118. doi: 10.1093/brain/121.11.2103 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Rankin KP, Woolley JD, Rosen HJ, Phengrasamy L, & Miller BL (2004). Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex, 40(4), 631–644. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, & Funnell E (1992). Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain, 115(6), 1783–1806. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, & Woodruff PW (2003). Response inhibition and impulsivity: an fMRI study. Neuropsychologia, 41(14), 1959–1966. [DOI] [PubMed] [Google Scholar]
- Howard D, & Patterson K (1992). Pyramids and palm trees: A test of semantic access from pictures and words. Bury St. Edmonds, UK: Thames Valley Test Company. [Google Scholar]
- Ikeda M, Brown J, Holland AJ, Fukuhara R, & Hodges JR (2002). Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 73(4), 371–376. doi: 10.1136/jnnp.73.4.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamminga J, Kumfor F, Burrell JR, Piguet O, Hodges JR, & Irish M (2015). Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. Journal of Neurology, Neurosurgery, and Psychiatry, 86(10), 1082–1088. doi: 10.1136/jnnp-2014-309120 [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (1983). Boston Naming Test. Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Kertesz A (1982). Western aphasia battery test manual. New York, NY: Grune & Stratton. [Google Scholar]
- Kumfor F, Landin-Romero R, Devenney E, Hutchings R, Grasso R, Hodges JR, & Piguet O (2016). On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain, 139(3), 986–998. [DOI] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, & Rao SM (2000). Neural systems underlying the recognition of familiar and newly learned faces. Journal of Neuroscience, 20(2), 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomen-Hoerth C (2011). Clinical phenomenology and neuroimaging correlates in ALS-FTD. Journal of Molecular Neuroscience, 45(3), 656–662. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, & Henderson VW (1992). Boston Naming Test: shortened versions for use in Alzheimer’s disease. Journal of Gerontology, 47(3), 154–158. [DOI] [PubMed] [Google Scholar]
- McDonald S, Flanagan S, Rollins J, & Kinch J (2003). TASIT: A new clinical tool for assessing social perception after traumatic brain injury. Journal of Head Trauma Rehabilitation, 18(3), 219–238. [DOI] [PubMed] [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 43(11), 2412–2414. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Patterson K, Tyler LK, Moss H, Stamatakis EA, Bright P, … Price CJ. (2007). Temporal lobe lesions and semantic impairment: a comparison of herpes simplex virus encephalitis and semantic dementia. Brain, 130(4), 1138–1147. [DOI] [PubMed] [Google Scholar]
- Northoff G, Richter A, Gessner M, Schlagenhauf F, Fell J, Baumgart F, … Heinze HJ. (2000). Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: a combined fMRI/MEG study. Cerebral Cortex, 10(1), 93–107. doi: 10.1093/cercor/10.1.93 [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, & McGlone F (2001). Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology, 85(3), 1315–1321. doi: 10.1152/jn.2001.85.3.1315 [DOI] [PubMed] [Google Scholar]
- Olney NT, Spina S, & Miller BL (2017). Frontotemporal dementia. Neurologic Clinics, 35(2), 339–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papinutto N, Galantucci S, Mandelli ML, Gesierich B, Jovicich J, Caverzasi E, … Gorno-Tempini ML. (2016). Structural connectivity of the human anterior temporal lobe: A diffusion magnetic resonance imaging study. Human Brain Mapping, 37(6), 2210–2222. doi: 10.1002/hbm.23167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, & Dickerson BC (2015). Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cerebral Cortex, 25(3), 680–702. doi: 10.1093/cercor/bht260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Brown JA, Possin KL, Datta S, Trujillo A, Radke A, … Seeley WW. (2017). Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain, 140(12), 3329–3345. doi: 10.1093/brain/awx254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Rosen HR, Kramer JH, Beer JS, Levenson RL, & Miller BL (2001). Hemispheric dominance for emotions, empathy and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase, 7(2), 145–160. [DOI] [PubMed] [Google Scholar]
- Piguet O (2011). Eating disturbance in behavioural-variant frontotemporal dementia. J Mol Neurosci, 45(3), 589–593. doi: 10.1007/s12031-011-9547-x [DOI] [PubMed] [Google Scholar]
- Ranasinghe KG, Rankin KP, Pressman PS, Perry DC, Lobach IV, Seeley WW, … Miller BL. (2016). Distinct Subtypes of Behavioral Variant Frontotemporal Dementia Based on Patterns of Network Degeneration. JAMA Neurology, 73(9), 1078–1088. doi: 10.1001/jamaneurol.2016.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, … Miller BL. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills, 8(3), 271–276. [Google Scholar]
- Rice GE, Caswell H, Moore P, Hoffman P, & Lambon Ralph MA (2018). The Roles of Left Versus Right Anterior Temporal Lobes in Semantic Memory: A Neuropsychological Comparison of Postsurgical Temporal Lobe Epilepsy Patients. Cerebral Cortex, 1–15. doi: 10.1093/cercor/bhx362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GE, Hoffman P, & Lambon Ralph MA (2015). Graded specialization within and between the anterior temporal lobes. Annals of the New York Academy of Sciences, 1359, 84–97. doi: 10.1111/nyas.12951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Hodges JR, & Patterson K (2004). Natural selection: The impact of semantic impairment on lexical and object decision. Cognitive Neuropsychology, 21(2-4), 331–352. [DOI] [PubMed] [Google Scholar]
- Rohrer J, Geser F, Zhou J, Gennatas E, Sidhu M, Trojanowski J, … Seeley W. (2010). TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology, 75(24), 2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, & Rosen HJ (2005). The natural history of temporal variant frontotemporal dementia. Neurology, 64(8), 1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Zhou J, & Kim EJ (2012). Frontotemporal dementia: what can the behavioral variant teach us about human brain organization? Neuroscientist, 18(4), 373–385. doi: 10.1177/1073858411410354 [DOI] [PubMed] [Google Scholar]
- Shinagawa S, Ikeda M, Nestor P, Shigenobu K, Fukuhara R, Nomura M, & Hodges J (2009). Characteristics of abnormal eating behaviours in frontotemporal lobar degeneration: a cross-cultural survey. Journal of Neurology, Neurosurgery & Psychiatry, 80(12), 1413–1414. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, & Neary D (1989). Semantic dementia: A form of circumscribed cerebral atrophy. Behavioural Neurology, 2(3), 167–182. [Google Scholar]
- Snowden JS, Thompson JC, & Neary D (2004). Knowledge of famous faces and names in semantic dementia. Brain, 127(Pt 4), 860–872. doi: 10.1093/brain/awh099 [DOI] [PubMed] [Google Scholar]
- Spinelli EG, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F, … Meyer M. (2017). Typical and atypical pathology in primary progressive aphasia variants. Annals of Neurology, 81(3), 430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Graham KS, Williams G, Patterson K, Kapur N, & Hodges JR (2004). Dissociating person-specific from general semantic knowledge: roles of the left and right temporal lobes. Neuropsychologia, 42(3), 359–370. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, & Denburg NL (2002). Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex, 38(4), 589–612. [DOI] [PubMed] [Google Scholar]
- Vonk JMJ, Rizvi B, Lao PJ, Budge M, Manly JJ, Mayeux R, & Brickman AM (2019). Letter and Category Fluency Performance Correlates with Distinct Patterns of Cortical Thickness in Older Adults. Cerebral Cortex, 29(6), 2694–2700. doi: 10.1093/cercor/bhy138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK (1975). The selective impairment of semantic memory. The Quarterly journal of experimental psychology, 27(4), 635–657. [DOI] [PubMed] [Google Scholar]
- Warrington EK, & James M (1991). The visual object and space perception battery.
- Watson CL, Possin K, Allen IE, Hubbard HI, Meyer M, Welch AE, … Gorno-Tempini ML. (2018). Visuospatial Functioning in the Primary Progressive Aphasias. Journal of the International Neuropsychological Society, 24(3), 259–268. doi: 10.1017/s1355617717000984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997). Wechsler Memory Scale - III. San Antonio, TX: Psychological Coorporation. [Google Scholar]
- Whitwell JL, Sampson EL, Loy CT, Warren JE, Rossor MN, Fox NC, & Warren JD (2007). VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage, 35(1), 207–213. doi: 10.1016/j.neuroimage.2006.12.006 [DOI] [PubMed] [Google Scholar]
- Wilson SM, Dronkers NF, Ogar JM, Jang J, Growdon ME, Agosta F, … Gorno-Tempini ML. (2010). Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. The Journal of Neuroscience, 30(50), 16845–16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


