The left atrium (LA) is integral in maintaining hemodynamic function, serving as a reservoir, conduit and pump for left ventricular (LV) filling, and regulator of volume through natriuretic peptide secretion. Consequently, LA dysfunction is associated with adverse clinical implications, highlighting the importance of its identification. This is particularly true in the setting of atrial fibrillation (AF) and heart failure with preserved ejection fraction (HFpEF), as both syndromes are highlighted by LA dysfunction. Herein, we argue for the systematic identification of LA myopathy to guide tailored therapy in AF and HFpEF.
AF, HFpEF, and LA Myopathy
Atrial fibrillation–predominant HFpEF is a unique clinical phenotype, characterized by LA mechanical dysfunction, congestive symptoms, and poor prognosis. Indeed, LA myopathy as defined by reduced LA reservoir strain is common among patients with HFpEF and comorbid AF and is associated with worse pulmonary vascular resistance, right ventricular function, and peak oxygen consumption.1 Furthermore, these patients may have abnormalities in right atrial function that lead to increased venous congestion. Such patients with AF-predominant HFpEF may have marked LA remodeling without substantial alterations in LV performance. While the loss of LA contractile function because of AF undoubtedly contributes to the clinical manifestations of LA myopathy, reduced LA compliance also adversely affects hemodynamics.2 Additionally, mitral annular dilation results in atrial functional mitral regurgitation, further contributing to impaired LA function.3 These findings suggest that LA myopathy may occur disproportionately to LV dysfunction in AF-predominant HFpEF.
LA Myopathy in AF: An Underappreciated Phenotype of HFpEF
Atrial fibrillation and HFpEF share common pathophysiologic features, including a relative deficiency in nitric oxide.4 Additionally, both syndromes share overlapping symptoms, as dyspnea and exercise intolerance are hallmarks of each disease in isolation. Such nonspecific symptoms in isolated AF are commonly deemed secondary to arrhythmia alone. However, such patients likely have substantial LA myopathy and restoring sinus rhythm may not completely alleviate their symptoms. Such patients should be considered to have AF-predominant HFpEF. In fact, among a cohort of patients with AF and unexplained dyspnea, the probability of undiagnosed HFpEF was more than 50%.5 Indeed, a recently developed risk score for HFpEF (the H2FpEF score) identified AF as the most important clinical variable that predicts HFpEF among patients with dyspnea.5 Thus, LA myopathy secondary to AF and HFpEF is a major contributor to disease pathogenesis.
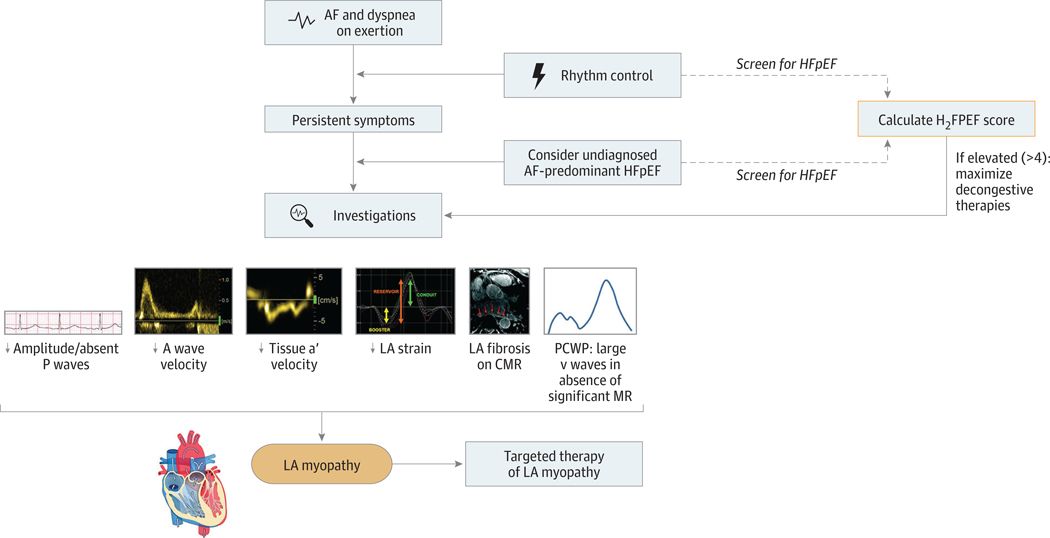
While identifying LA myopathy in patients with AF maybe challenging, there are potential signs to heighten clinical suspicion (Figure). Among patients with AF who have sinus rhythm restored, low-amplitude P waves signal LA myopathy. In addition, subtle echocardiographic abnormalities may increase the likelihood for LA myopathy. Doppler imaging results may reveal low A wave or tissue a’ velocities, corresponding to poor LA contraction despite restored sinus rhythm. Finally, poor LA reservoir or contractile strain, which can be measured by speckle-tracking echocardiography, is a hallmark of LA myopathy.1 A blunted increase in LA reservoir strain after volume challenge may provide further evidence for LAmyopathy.2 The presence of macroscopic LA scar on cardiac magnetic resonance imaging is an additional sign of LA remodeling, but images are technically challenging.6 Perhaps most important is longitudinal follow-up after restoring sinus rhythm, as those with LA myopathy will have persistent dyspnea or exercise intolerance.
Figure. Identification of the Left Atrial (LA) Myopathy Phenotype in Atrial Fibrillation (AF) and Heart Failure With Preserved Ejection Fraction (HFpEF).
Atrial fibrillation is commonly associated with mechanical LA dysfunction and, in the presence of dyspnea on exertion, represents an underappreciated form of HFpEF. Patients with AF and dyspnea should be systematically screened for HFpEF using the simple and validated H2FPEF risk score (eg, at the time of cardioversion). Among patients with persistent dyspnea after restoration of sinus rhythm, the presence of LA myopathy as noted through various investigations identifies a cohort of patients who could benefit from therapies targeting LAmyopathy. Portions of this figure were reproduced with permission from Quail et al6 (https://creativecommons.org/licenses/by/4.0/). CMR indicates cardiac magnetic resonance; MR, mitral regurgitation; PCWP, pulmonary capillary wedge pressure.
Treating LAMyopathy
Once identified, there are limited treatment options for LA myopathy in AF-predominant HFpEF. Given that symptoms of dyspnea may be secondary to an exercise-induced rise in LA pressure, up-titration of loop diuretic therapy may be considered but is unlikely to yield symptom improvement. In LA myopathy, LA pressure is typically elevated even when overall volume status is well controlled. Thus, further diuresis is typically not possible because of symptoms (lightheadedness) or worsening renal function. While mineralocorticoid receptor antagonists should be considered among those with AF-predominant HFpEF given their beneficial effects in the overall HFpEF cohort, it remains unclear if mineralocorticoid receptor antagonists have targeted LA effects. Indeed, spironolactone initiation has been associated with maintenance of sinus rhythm among patients with AF and HF,7 suggesting LA-specific benefits. However, spironolactone did not (1) reduce new-onset AF in the TOPCAT trial (NCT0094302) or (2) improve exercise capacity among patients with permanent AF (NCT02673463). Further investigation is required to understand the LA-specific effects of other pharmacotherapies. Given the role of the LA in natriuretic peptide secretion and a possible signal of improved outcomes among patients with AF treated with angiotensin receptor neprilysin inhibitors in HFpEF (NCT01920711), the influence of angiotensin receptor neprilysin inhibitors on LA function may be of interest. Indeed, sex-specific effects of sacubitril-valsartan on LA function should also be investigated given the drug’s beneficial effect in women. Finally, hemodynamic assessment using wireless pulmonary artery pressure monitoring may be useful in this HFpEF cohort to optimize decongestive therapies.
Efforts to offload the myopathic LA may improve symptom burden among those with persistent symptoms after restoring sinus rhythm. An interatrial shunt device placed to unload the LA and transfer surplus plasma volume to the venous system has demonstrated promise. In the Reduce Elevated Left Atrial Pressure in Patients with Heart Failure I trial, an interatrial shunt device reduced pulmonary capillary wedge pressure during exercise compared with a sham procedure.8 A phase 3 sham-controlled trial (REDUCE LAP-HF II;NCT03088033) is underway. Other devices under investigation that unload the LA hold promise, including an LA to coronary sinus shunt device9 and an LA decompression device.10 It remains unclear whether unloading the LA actively reverses underlying LA myopathy.
Atrial fibrillation–predominant HFpEF is a distinct clinical entity characterized by LA dysfunction leading to congestive symptoms and poor clinical outcomes. Patients with AF who have persistent symptoms despite restoration of sinus rhythm may have LA myopathy and undiagnosed HFpEF. There are several diagnostic clues to identify LA myopathy. We advocate for systematically identifying patients with AF who may have undiagnosed HFpEF using these signs, as this could result in increased recognition of AF-predominant HFpEF and allow for the treatment of persistent symptoms. While current therapies targeting LA myopathy are lacking, novel treatments to unload the LA offer promise in enhancing therapeutic options for patients with AF to alleviate symptoms moving forward.
Acknowledgments
Conflict of Interest Disclosures: Dr Shah has received research grants from the National Institutes of Health (R01 HL107577, R01 HL127028, R01 HL140731, and R01 HL149423), the American Heart Association (16SFRN28780016 and 15CVGPSD27260148), Actelion, AstraZeneca, Corvia, and Novartis and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Ironwood, Merck, MyoKardia, Novartis, Sanofi, and United Therapeutics. He is also the principal investigator of the Corvia REDUCE LAP-HF I and II trials. Dr Patel is supported by National Heart, Lung, and Blood Institute T32 postdoctoral training grant T32HL069771.
Contributor Information
Ravi B. Patel, Feinberg School of Medicine, Division of Cardiology, Northwestern University Chicago, Illinois..
Sanjiv J. Shah, Feinberg School of Medicine, Division of Cardiology, Northwestern University Chicago, Illinois..
REFERENCES
- 1.Freed BH, Daruwalla V, Cheng JY, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9(3):e003754. doi: 10.1161/CIRCIMAGING.115.003754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obokata M, Negishi K, Kurosawa K, et al. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6(7):749–758. doi: 10.1016/j.jcmg.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 3.Deferm S, Bertrand PB, Verbrugge FH, et al. Atrial functional mitral regurgitation: JACC review topic of the week. J Am Coll Cardiol. 2019;73(19): 2465–2476. doi: 10.1016/j.jacc.2019.02.061 [DOI] [PubMed] [Google Scholar]
- 4.Cai H, Li Z, Goette A, et al. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106(22):2854–2858. doi: 10.1161/01.CIR.0000039327.11661.16 [DOI] [PubMed] [Google Scholar]
- 5.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BAA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138 (9):861–870. doi: 10.1161/CIRCULATIONAHA.118.034646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quail M, Grunseich K, Baldassarre LA, et al. Prognostic and functional implications of left atrial late gadolinium enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2019;21(1):2. doi: 10.1186/s12968-018-0514-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RienstraM Hobbelt AH, Alings M, et al. ; RACE 3 Investigators. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. 2018;39(32):2987–2996. doi: 10.1093/eurheartj/ehx739 [DOI] [PubMed] [Google Scholar]
- 8.Feldman T, Mauri L, Kahwash R, et al. ; REDUCE LAP-HF I Investigators and Study Coordinators. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): a phase 2, randomized, sham-controlled trial. Circulation. 2018;137(4):364–375. doi: 10.1161/CIRCULATIONAHA.117.032094 [DOI] [PubMed] [Google Scholar]
- 9.Simard T, Labinaz M, Zahr F, et al. TCT-87 levoatrial to coronary sinus shunting as a novel strategy for symptomatic heart failure: first-in-human experience. J AmColl Cardiol. 2019; 74:B87. doi: 10.1016/j.jacc.2019.08.128 [DOI] [Google Scholar]
- 10.Burkhoff D, Maurer MS, Joseph SM, et al. Left atrial decompression pump for severe heart failure with preserved ejection fraction: theoretical and clinical considerations. JACC Heart Fail. 2015;3(4): 275–282. doi: 10.1016/j.jchf.2014.10.011 [DOI] [PubMed] [Google Scholar]