Abstract
Objective
To identify risk factors for venous thromboembolism (VTE) and to examine the association of VTE and survival in women with uterine carcinosarcoma.
Methods
This multicenter retrospective study examined 906 women who underwent primary surgical treatment for stage I-IV uterine carcinosarcoma. Time-dependent analyses were performed for cumulative incidence of VTE after surgery on multivariate models.
Results
There were 72 (7.9%) women who developed VTE after surgery with 1-, 2-, and 5-year cumulative incidences being 5.1%, 7.3%, and 10.2%, respectively. On multivariate analysis, older age (hazard ratio [HR] per year 1.03, P = 0.012), non-Asian race (HR 6.28, P< 0.001), large body habitus (HR per kg/m2 1.04, P = 0.014), residual disease at surgery (HR 3.04, P = 0.003), tumor size ≥5 cm (HR2.73, P = 0.003), and stage IV disease (HR2.12, P = 0.025) were independently associated with increased risk of developing VTE. A risk pattern analysis identified that obese Non-Asian women with large tumors (13.7% of population) had the highest incidence of VTE (2-year cumulative rate, 26.1%) whereas Asian women with no residual disease (47.1% of population) had the lowest (2-year cumulative rate, 1.6%) (P< 0.001). Presence of carcinoma/sarcoma in metastatic sites was significantly associated with increased risk of VTE compared to carcinoma alone (2-year rates, 31.2% versus 8.4%, P = 0.049). VTE was independently associated with decreased progression-free survival on multivariate models (5-year rates, 24.9% versus 47.2%, HR 1.46,95%CI 1.05–2.04, P = 0.026).
Conclusion
Our study suggests that VTE represents a surrogate marker of aggressive tumor behavior and diminished patient condition in uterine carcinosarcoma; obese Non-Asian women with large tumors carry a disproportionally high risk of VTE, suggesting that long-term prophylaxis may benefit this population.
Keywords: Uterine carcinosarcoma, Venous thromboembolism, Deep vein thrombosis, Pulmonary embolism, Risk factors, Survival
1. Introduction
Uterine carcinosarcoma is a rare high-grade endometrial cancer and represents the phenomena of epithelial-to-mesenchymal transition (EMT) with the sarcoma component being dedifferentiated from the carcinoma component [1]. Uterine carcinosarcoma is typically a disease of the elderly, a population that often has multiple medical comorbidities and physical deconditioning [1]. Moreover, women with uterine carcinosarcoma frequently present with advanced-stage disease [2]; all of these are known to be risk factors for developing venous thromboembolism (VTE) [3–5].
VTE is a common clinical problem in women with gynecologic malignancies, including ovarian (VTE incidence, 6.4–23.2%), cervical (12.3%), and endometrial (8.1%) cancers [6–9]. Across these three cancer types, VTE is recognized as a manifestation of aggressive tumor characteristics such as advanced or metastatic disease (VTE incidence, 23.5–44.8%), thrombocytosis (22.3–25.0%), high-risk histology (ovarian clear cell carcinoma 11.9–43.1%; and uterine serous/clear cell carcinomas 28.6–29.2%), as well as poor patient condition including hypoalbuminemia (19.3–53.2%) [6–9]. Additionally, women who develop VTE have worse survival outcomes as demonstrated in published studies [6–9].
Because uterine carcinosarcoma has similar clinical and molecular characteristics to other high-risk endometrial cancer types [10,11], and is associated with risk factors for developing VTE, it is likely that women with uterine carcinosarcoma carry a high risk of developing VTE. To date there has been no prior study examining the significance of VTE solely in the uterine carcinosarcoma population. Therefore, identifying a subgroup of women with an increased risk of VTE may alter the management of uterine carcinosarcoma.
The objective of this study was (i) to examine incidence and risk factors for VTE and (ii) to examine the association of VTE and survival in women with uterine carcinosarcoma.
2. Patients and methods
2.1. Study eligibility
This is a secondary analysis of a formerly organized surgical database for uterine carcinosarcoma [2,12–15]. Previously, we conducted a large- scale multicenter retrospective review of women with stage I–IV uterine carcinosarcoma who underwent primary hysterectomy-based surgical treatment between 1993 and 2013. We obtained Institutional Review Board approval at each participating institution. There were 26 institutions from the United States and Japan that participated in this study (906 cases). By utilizing this surgical database, we queried the cases of women who developed VTE after the initial surgery for uterine carcinosarcoma.
2.2. Clinical information
From the database, we obtained the following information for the analysis: patient baseline characteristics, pathology results, initial treatment information, and survival outcomes. For patient characteristics, patient age (continuous), country (USA versus Japan), race (White, Black, Hispanic, and Asian), body mass index (BMI, continuous), gravidity (null versus multi), and preoperative CA-125 level (continuous) were evaluated.
Histopathologic results included carcinoma component (low-grade versus high-grade), sarcoma component (homologous versus heterologous), tumor size from the uterine specimen (≥5 versus <5 cm) [16], presence of sarcoma dominance (yes versus no), depth of myometrial tumor invasion (inner-half versus outer-half), lympho-vascular space invasion (LVSI, present versus absent), pelvic and para-aortic lymph node status (metastasis, non-metastasis, and not examined), and cancer stage (I, II, III, and IV). In addition, histology subtypes of the carcinoma component were examined as classified previously [2].
Treatment information abstracted included: residual disease at the end of primary hysterectomy-based surgery (yes versus no), use of postoperative chemotherapy (yes versus no), and use of postoperative radiotherapy (yes versus no). For survival outcomes, progression-free survival (PFS) and cause-specific survival (CSS) were recorded.
2.3. Evaluation of VTE
Information regarding VTE, itemized in the universal data record form at the time of data collection, was examined for the time interval between the hysterectomy-based surgery and the last follow-up date. Type of VTE was grouped into deep venous thrombosis (DVT) or pulmonary embolism (PE). Across the study sites, the diagnosis of VTE was made by radiographic imaging modalities including computed tomography, pulmonary angiogram, ventilation perfusion lung scan, or Doppler study. This database does not contain information regarding prophylactic anti-coagulation.
2.4. Histopathologic evaluation
For this multicenter retrospective study, gynecologic pathologists at each institution reviewed the archived histopathology slides to determine the type of carcinoma and sarcoma component, sarcoma dominance, myometrial tumor invasion, and LVSI. Among metastatic sites, histology patterns were also examined for carcinoma alone, sarcoma alone, and both carcinoma and sarcoma. The details of the methodology are described in our previous study [2].
2.5. Study definition
Cancer stage was reclassified based on the 2009 International Federation of Gynecology and Obstetrics (FIGO) system [17]. Sarcoma dominance was defined as a sarcoma component comprising >50% of the primary tumor in the hysterectomy specimen. Lymph node ratio was defined as the percent proportion of lymph node containing tumor cells among the sampled nodes. PFS was defined as the time interval between the date of hysterectomy and the date of the first recurrence/progression of disease or last follow-up. CSS was defined as the time interval between the date of surgical staging and the date of death due to uterine carcinosarcoma or last follow-up. Women who were alive at the last follow-up or died of other disease were censored.
2.6. Statistical consideration
The primary interest of analysis was to estimate incidence of VTE and to identify independent risk factors for VTE in women with uterine carcinosarcoma. The secondary interest of analysis was to examine the association of VTE and survival in uterine carcinosarcoma. Continuous variables were assessed for the normality with the Kolmogorov-Smirnov test, expressed with mean (±SD) or median (interquartile range) as appropriate. Statistical significance of continuous variables was examined by Student t-test or Mann-Whitney U test as appropriate. Categorical variables were evaluated with chi-square test or Fisher exact test as appropriate.
Because development of VTE after the diagnosis of uterine carcinosarcoma is a time-dependent event, we used a log-rank test for univariate analysis and a Cox proportional hazard regression model for multivariate analysis to identify the significant risk factors for VTE that developed during the follow-up time after the surgical treatment for uterine carcinosarcoma. In the multivariate model, all the covariates with P < 0.05 on univariate analysis were entered in the initial model, and the least significant covariate was removed from the model until the model included only significant covariates with P < 0.05 in the final model (conditional backward method). Magnitude of the statistical significance was expressed with hazard ratio (HR) and 95% confidence interval (CI). Cumulative incidence curves were constructed by the Kaplan-Meier method.
In an attempt to predict a subgroup of women with increased risk of VTE, a recursive partitioning analysis was performed to construct a regression-tree model for VTE risk pattern [18]. All independent risk factors of VTE were entered in the analysis, and chi-square automatic interaction detector method was used for the model. Among the determined nodes in this analysis, cumulative incidence curves of VTE were constructed with a log-rank test to characterize a subgroup of women with increased risk of VTE.
On survival analysis, we used various models to examine the durability of an independent association of VTE and survival outcome. In each, the association was adjusted for (i) age alone, (ii) age and stage, (iii) age, stage, residual disease, postoperative chemotherapy, and postoperative radiotherapy, and (iv) age, stage, residual disease, postoperative chemotherapy, postoperative radiotherapy, carcinoma type, tumor size, sarcoma dominance, and myometrial invasion. The rationale for the adjustment models were that these covariates are known to be a priori survival factors and that interaction between these covariates can be assessable in each layer of the adjustment model [2].
The variance inflation factor was determined among covariates in multivariate analysis, and a value of ≥2 was defined as multicollinearity in this study. In multivariate analyses, over-adjustment was assessed with the ratio of events-of-interest per the entered covariates, and a cutoff level of < 10 was interpreted as over-adjustment in this study. A P < 0.05 was considered statistically significant with two-tailed hypothesis, and Statistical Package for Social Science software (IBM SPSS, version 24.0, Armonk, NY, USA) was used for all the analyses. The STROBE guidelines were consulted to outline the results of retrospective cohort studies [19].
3. Results
Among 906 women in the study cohort, there were 72 (7.9%, 95%C1 6.2–9.7) women who developed VTE. Among the 72 VTE cases, the majority was DVT alone (n = 38, 52.9%) followed by PE alone (n = 23, 31.9%) and both DVT and PE (n = 11,15.3%). Nearly one sixth (n = 11,15.3%) of VTE were diagnosed at the time of uterine carcinosarcoma diagnosis. Peri-operative diagnosis of VTE within 30 days from surgery was seen in < 10% of all VTE cases (n = 7, 9.7%). The most common timing of VTE was after recurrence (n = 23, 31.9%) followed by the time period between one to six months after surgery (n = 17, 23.6%).
Baseline patient characteristics are shown in Table 1. Women who developed VTE were more likely to be older compared to those who did not (median, 65 versus 63 years, P = 0.028). Non-Asian women had higher risks of VTE compared to Asian women (P < 0.001), and Black women had the highest incidence of VTE (21.4%) followed by Hispanic (13.6%) and White (12.3%) women. Residents in Japan had a significantly lower incidence of VTE compared to those in the United States (3.2% versus 13.2%, P < 0.001). Women who developed VTE had a larger body habitus compared to those who did not (median BM1, 29.7 versus 23.1 kg/m2, P < 0.001).
Table 1.
Patient demographics based on VTE (N = 906).
| Characteristics | VTE (−) | VTE (+) | P-value |
|---|---|---|---|
| Number | 834 (92.1%) | 72 (7.9%) | |
| Age (year) | 63 (IQR 14) | 65 (IQR 15) | 0.028 |
| Race | <0.001 | ||
| White | 249 (87.7%) | 35 (12.3%) | |
| Black | 66 (78.6%) | 18 (21.4%) | |
| Hispanic | 19 (86.4%) | 3 (13.6%) | |
| Asian | 466 (96.9%) | 15 (3.1%) | |
| Others | 18 (94.7%) | 1 (5.3%) | |
| Country | <0.001 | ||
| USA | 376 (86.8%) | 57 (13.2%) | |
| Japan | 458 (96.8%) | 15 (3.2%) | |
| Gravidity | 0.17 | ||
| Null | 124 (89.2%) | 15 (10.8%) | |
| Multi | 684 (92.7%) | 54 (7.3%) | |
| BMI (kg/m2) | 23.1 (IQR 6.0) | 29.7 (IQR 11.4) | <0.001 |
| CA-125 (IU/L) | 22.3 (IQR 47) | 32.2 (IQR 80) | 0.21 |
| Residual disease at surgery | 0.008 | ||
| No | 721 (93.2%) | 53 (6.8%) | |
| Yes | 84 (84.8%) | 15 (15.2%) | |
| Postope chemotherapy | 0.24 | ||
| No | 276 (93.6%) | 19 (6.4%) | |
| Yes | 549 (91.2%) | 53 (8.8%) | |
| Postope radiotherapy | 0.33 | ||
| No | 616 (92.5%) | 50 (7.5%) | |
| Yes | 208 (90.4%) | 22 (9.6%) |
Median (IQR) or number (%) per row is shown. Mann-Whitney U test, Fisher exact test, or chi-square test for P-values. Significant P-values are emboldened. Abbreviations: IQR, interquartile range; BMI, body mass index; CA-125, cancer antigen 125; and VTE, venous thromboembolism.
Treatment types were compared between the two groups (Table 1). Women who had residual tumor at the end of hysterectomy-based surgery were more likely to develop VTE compared to those who did not (15.2% versus 6.8%, P = 0.008). Postoperative chemotherapy use (8.8% versus 6.4%) as well as radiotherapy use (9.6% versus 7.5%) was not associated with development of VTE (both, P > 0.05).
Association of tumor characteristics and VTE was examined (Table 2). Women whose tumors were high-grade carcinoma were more likely to develop VTE compared to those who had low-grade carcinoma (9.2% versus 4.7%, P = 0.028). Among carcinoma types, serous histology had the highest 2-year cumulative incidence of VTE (10.7%) followed by mixed (10.3%), grade 3 endometrioid (7.6%), and clear cell (6.9%) histology types (Table S1). Serous histology had a significantly increased risk of VTE compared to grade 1 endometrioid type (10.7% versus 6.3%, P = 0.031).
Table 2.
Tumor characteristics based on VTE (N = 906).
| Characteristics | VTE (−) | VTE (+) | P-value |
|---|---|---|---|
| Carcinoma | 0.028 | ||
| Low-grade | 244 (95.3%) | 12 (4.7%) | |
| High-grade | 590 (90.8%) | 60 (9.2%) | |
| Sarcoma | 0.32 | ||
| Homologous | 495 (92.9%) | 38 (7.1%) | |
| Heterologous | 339 (90.9%) | 34 (9.1%) | |
| Tumor size | 0.037 | ||
| <5 cm | 301 (94.7%) | 17 (5.3%) | |
| ≥5 cm | 510 (90.6%) | 53 (9.4%) | |
| Sarcoma dominance | 0.61 | ||
| No | 486 (92.6%) | 39 (7.4%) | |
| Yes | 333 (91.5%) | 31 (8.5%) | |
| Myometrial invasion | 0.39 | ||
| Inner half | 435 (92.8%) | 34 (7.2%) | |
| Outer half | 392 (91.2%) | 38 (8.8%) | |
| LVSI | 0.61 | ||
| Absent | 332 (92.7%) | 26 (7.3%) | |
| Present | 500 (91.7%) | 45 (8.3%) | |
| PLN metastasis | 0.047 | ||
| No | 464 (94.7%) | 26 (5.3%) | |
| Yes | 155 (90.1%) | 17 (9.9%) | |
| PAN metastasis | 0.37 | ||
| No | 308 (96.0%) | 13 (4.0%) | |
| Yes | 74 (93.7%) | 5 (6.3%) | |
| LNR (%)a | |||
| Pelvic | 16.7 (IQR 32.9) | 15.5 (IQR 23.4) | 0.71 |
| Para-aortic | 40.0 (IQR 85.9) | 33.4 (IQR 77.6) | 0.89 |
| Stage | <0.001 | ||
| I | 422 (94.8%) | 23 (5.2%) | |
| II | 61 (93.8%) | 4 (6.2%) | |
| III | 253 (91.7%) | 23 (8.3%) | |
| IV | 98 (81.7%) | 22 (18.3%) |
Median (IQR) or number (%) per row is shown. Mann-Whitney U test, Fisher exact test, or chi-square test for P-values. Significant P-values are emboldened. Abbreviations: IQR, interquartile range; LVSI, lympho-vascular space invasion; PLN, pelvic lymph node; PAN, para-aortic lymph node; LNR, lymph node ratio; and VTE, venous thromboembolism.
Among node metastatic cases.
Large tumor size was significantly associated with development of VTE (9.4% versus 5.3%, P = 0.037). Women who had pelvic lymph node metastasis had a higher incidence of VTE compared to those who did not (9.9% versus 5.3%, P = 0.047). The extent of pelvic or para-aortic lymph node metastasis was not associated with development of VTE (both, P> 0.05). Women who had advanced disease had a significantly higher incidence of VTE compared to those who did not (stage IV versus I-III, 18.3% versus 5.2–8.3%, P < 0.001).
A multivariate analysis was performed to identify the independent clinico-pathological factors associated with development of VTE (Table 3). We found that older age (HR per year 1.03, 95%CI 1.01–1.06, P = 0.012), non-Asian ethnicity (HR 6.28, 95%CI 2.74–14.4, P < 0.001), large body habitus (HR per kg/m2 1.04,95%CI 1.01–1.07, P = 0.014), residual tumor at hysterectomy-based surgery (HR3.04,95%CI1.38–6.72, P = 0.003), uterine tumor size ≥5 cm (HR 2.73, 95%CI 1.39–5.33, P = 0.003), and stage IV disease (HR 2.12, 95%CI 1.10–4.07, P = 0.025) were independently associated with increased risk of developing VTE. Carcinoma component did not remain an independent risk factor for VTE in this two-tier model as well as in the histology type-specific model (Table S2). In a post-hoc analysis median BMI was examined across race. The results showed that Asians had the lowest BMI among the races (White 29.4, Black 32.4, Hispanic 29.1, and Asian 22.3 kg/m2, P < 0.001).
Table 3.
Independent clinico-pathological factors for VTE (N = 906).
| Characteristics | No. | 2-yr (%) | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| Age (year) | 906 | n.a. | 1.04 (1.02–1.07) | 0.002 | 1.03 (1.01–1.06) | 0.012 |
| Race | ||||||
| Asian | 481 | 2.5% | 1 | 1 | ||
| Non-Asian | 409 | 13.7% | 6.16 (3.27–11.6) | <0.001 | 6.28 (2.74–14.4) | <0.001 |
| BMI (kg/m2) | 863 | n.a. | 1.06 (1.04–1.08) | <0.001 | 1.04 (1.01–1.07) | 0.014 |
| Residual disease at surgery | ||||||
| No | 774 | 6.0% | 1 | 1 | ||
| Yes | 99 | 19.0% | 2.85 (1.50–5.40) | 0.001 | 3.04 (1.38–6.72) | 0.006 |
| Carcinoma | ||||||
| Low-grade | 256 | 3.1% | 1 | |||
| High-grade | 650 | 8.8% | 2.32 (1.18–4.56) | 0.012 | ||
| Tumor size | ||||||
| <5 cm | 318 | 3.9% | 1 | 1 | ||
| ≥5 cm | 563 | 9.2% | 2.41 (1.30–4.46) | 0.004 | 2.73 (1.39–5.33) | 0.003 |
| Stage | ||||||
| I-III | 786 | 5.6% | 1 | 1 | ||
| IV | 120 | 20.0% | 4.35 (2.54–7.46) | <0.001 | 2.12 (1.10–4.07) | 0.025 |
Log-rank test for univariate analysis and Cox proportional hazard regression model for multivariate analysis. Variables with P < 0.05 in univariate analysis were entered in the initial model, and only variable with P < 0.05 are listed in the final model for conditional backward method. Event-to-variable ratio ≥ 10 indicates absence of over-adjustment. Multicollinearity was seen between race and country as well as nodal status and stage: race and stage were therefore entered in the model and country and lymph node status were not entered in the model. Significant P-values are emboldened. Abbreviations: VTE, venous thromboembolism; 5-yr (%), 5-year cumulative incidence of VTE; HR, hazard ratio; and CI, confidence interval; BMI, body mass index; PLN, pelvic lymph node; and PAN, para-aortic lymph node.
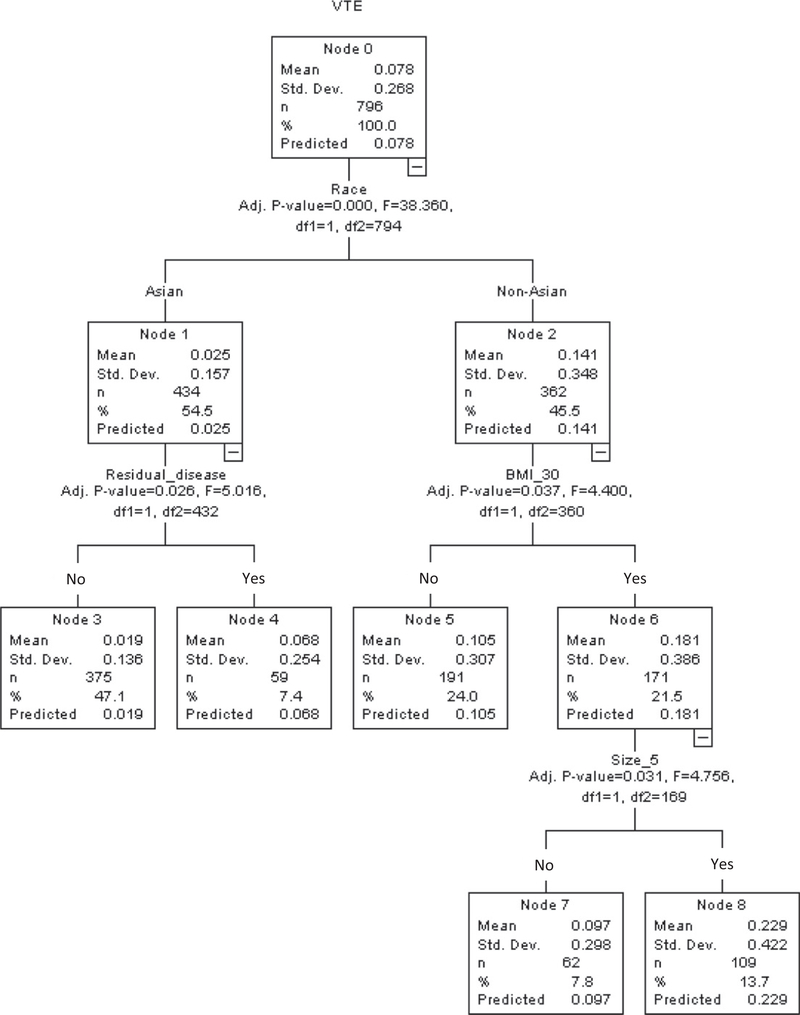
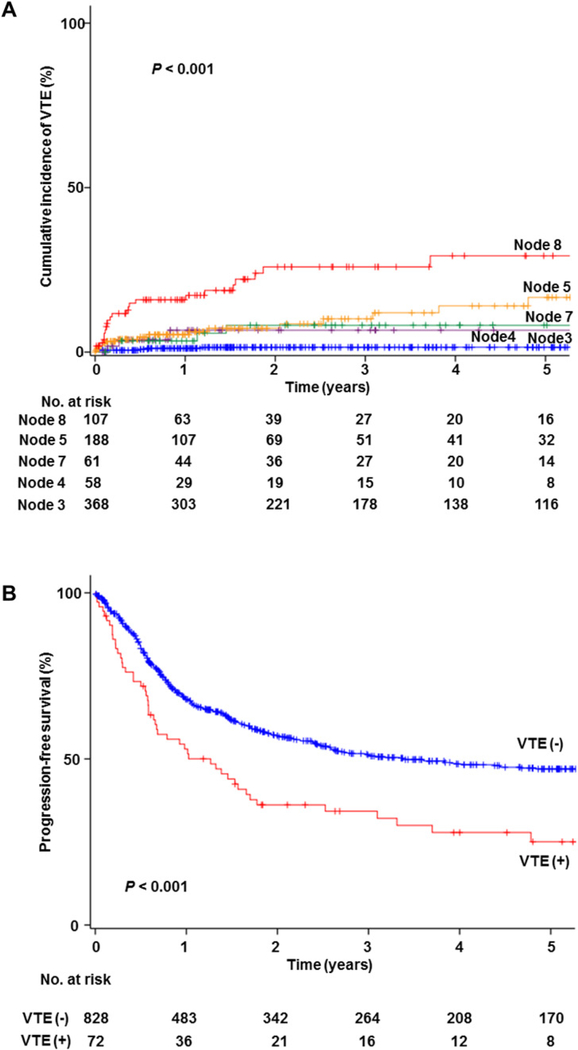
By utilizing these six independent risk factors for VTE, a recursive partitioning analysis was performed to construct a regression-tree model (Fig. 1). A total of 796 women with available data on these risk factors were clustered into the five groups (node 3, 4, 5, 7, and 8). Cumulative incidences of VTE were significantly associated with the patterns of these independent risk factors for VTE (P < 0.001; Fig. 2A). Obese Non-Asian women with large tumors, representing 13.7% of population, had the highest incidence of VTE (2-year cumulative rate, 26.1%; node 8). Asian women with no residual disease, representing 47.1% of population, had the lowest incidence of VTE (2-year cumulative rate, 1.6%; node 3).
Fig. 1.
Regression tree model for venous thromboembolism. A recursive partitioning analysis was performed to construct a regression-tree model for VTE risk. All independent risk factors of VTE were entered in the analysis (Table 3), and chi-square automatic interaction detector method was used for the model. Abbreviations: VTE, venous thromboembolism; and BMI, body mass index.
Fig. 2.
Kaplan-Meier curves for venous thromboembolism. Log-rank test for P-values. Follow-up time was truncated at 5 years from surgical treatment. A) Cumulative incidence of VTE based on the risk factor patterns in decision-tree model, and B) progression-free survival based on VTE. Abbreviation: VTE, venous thromboembolism.
Histology patterns of lymph node and omental metastases were examined for VTE risk. Among 193 women with available histology patterns, carcinoma alone (n = 147, 76.2%) was the most common histology pattern followed by sarcoma alone (n = 24,12.4%) and both carcinoma and sarcoma (n = 22,11.4%). Presence of carcinoma/sarcoma in metastatic sites was significantly associated with increased risk of VTE compared to carcinoma alone (2-year rates, 31.2% versus 8.4%, P = 0.049). Women with carcinoma alone and sarcoma alone at these metastatic sites had similar incidences of VTE (8.4% versus 5.6%, P = 0.56).
Survival analysis was performed (Table 4). Median follow-up time for women without events was 32.2 months for the VTE group and 36.5 months for the non-VTE group. There were 49 (68.1%) women who developed recurrence/progression or death from uterine carcinosarcoma in the VTE group; whereas 380 (45.7%) women developed these events in the non-VTE group. On univariate analysis, VTE was significantly associated with decreased PFS (24.9% versus 47.2%, P < 0.001; Fig. 2B) and CSS (43.6% versus 60.2%, P = 0.001). On multivariate models, association of VTE and decreased PFS remained independent after controlling for patient demographics, tumor factors, and treatment types (HR 1.46, 95%CI 1.05–2.04, P = 0.024; Model 4). Association of VTE and CSS did not reach statistical significance in the same model (HR 1.34,95%CI 0.91–1.97, P = 0.14; Model 4). Type of VTE was not associated with survival (all, P> 0.05).
Table 4.
Multivariate models for association of VTE and survival outcome (N = 906).
| Progression-free survival |
Cause-specific survival |
|||
|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Unadjusted | 1.78 (1.32–2.40) | <0.001 | 1.79 (1.27–2.53) | 0.001 |
| Model 1 | 1.67 (1.24–2.26) | 0.001 | 1.65 (1.17–2.33) | 0.004 |
| Model 2 | 1.39 (1.03–1.88) | 0.03 | 1.33 (0.94–1.88) | 0.11 |
| Model 3 | 1.42 (1.03–1.96) | 0.03 | 1.40 (0.97–2.01) | 0.07 |
| Model 4 | 1.46 (1.05–2.04) | 0.026 | 1.34 (0.91–1.97) | 0.14 |
Cox proportional hazard regression models for multivariate analysis. Model 1: adjusted for age (continuous). Model 2: adjusted for age and stage (I-II versus III-IV). Model 3: adjusted for age, stage, residual disease, postope chemotherapy (yes versus no), and postope radiotherapy (yes versus no). Model 4: adjusted for age, stage, residual disease, postope chemotherapy, postope radiotherapy, carcinoma type (low- versus high-grade), tumor size (≥5 versus < 5 cm), sarcoma dominance (yes versus no), and myometrial invasion (inner-half versus outer-half). Event-to-variable ratio ≥ 10 indicates absence of over-adjustment. Significant P-values are emboldened. Abbreviations: VTE, venous thromboembolism; HR, hazard ratio; and CI, confidence interval.
4. Discussion
Our study found that women with uterine carcinosarcoma carry a high-risk of VTE that is associated with aggressive tumor characteristics and poor patient condition. Moreover, women who have multiple risk factors have a considerably increased risk of VTE. Both tumor and patient factors related to VTE warrant further discussion.
Based on our study, patient-related factors for developing VTE include old age, large body habitus, and race. Generally, increasing age is known to be a risk factor for developing VTE [3,4]. It is likely that women with a geriatric age have multiple medical comorbidities and decreased daily activity, thus increasing VTE risk. Obesity is a state of chronic inflammation and increased unopposed estrogen [20]. Increased inflammatory cytokines related to excess visceral adiposity, including IL-6, result in an increased risk of VTE [6,20,21]. Excess adiposity not only increases aromatase activity resulting in estrogen production but also decreases sex hormone-binding globulin resulting in increased bioactive estrogen [20]. Such increased levels of circulating estrogen may result in an increased risk of VTE in obese women particularly in the elderly [22].
In our study we found that race was not only a risk factor for developing VTE, but was the factor with the largest magnitude of statistical significance. Asian women had the smallest body habitus among the examined patients. This may indirectly result in a lower VTE incidence as large body habitus was found to be an independent risk factor for VTE in our study. Asian women may carry less risk of obesity-related medical comorbidities compared to others; which could further directly or indirectly impact VTE risk. Generally, race is an important factor for VTE and needs to be considered for risk-stratification [23].
Based on the results of this investigation, tumor-related factors for developing VTE were residual tumor at surgery, large uterine tumor size, and stage IV disease. Moreover, presence of both carcinoma/sarcoma at the metastatic site, a possible histopathologic evidence of EMT, was a significant factor for VTE. These findings imply a role of the EMT in VTE development. EMT is a unique tumor characteristic in uterine carcinosarcoma and is associated with aggressive tumor behavior [1, 11]. EMT is known to be a mechanism by which cancer metastasizes via induction of inflammatory cytokines [24,25]. Various inflammatory cytokines induce EMT via alteration in expression of adhesion molecules and transcription factors [26,27], and similar to the induction of EMT, inflammatory cytokines can also cause VTE [28,29]. In the setting of metastatic disease, circulating tumor cells activate coagulation factors resulting in VTE formation [30]. Taken together, it is speculated that the triad of inflammation, EMT, and VTE interact closely in metastatic uterine carcinosarcoma.
Another possible tumor-related factor unique to uterine carcinosarcoma may include P1K3CA, which is known to be commonly mutated and amplified in uterine carcinosarcoma [10,11]. PIK3CA mutation upregulates NF-κB, leading to induction of EMT and IL-6 dependent STAT3 activation, and elevation of IL-6 transcription occurs due to the mutated PIK3CA gene [31,32]. Because increased IL-6 is associated with increased risk of VTE via induction of pro-coagulant factors [6, 21], it may be speculated that PIK3CA mutation in uterine carcinosarcoma induces the IL-6 elevation that causes thromboembolic events.
In our study, serous histology in the carcinoma component had the highest incidence of VTE. Uterine carcinosarcoma shares many clinical and molecular similarities to another type of high-grade endometrial cancer, uterine serous carcinoma [10,11,33]. Uterine serous carcinoma molecularly and clinically mimics high-grade serous ovarian carcinoma where IL-6 mediated paraneoplastic thrombocytosis is known to be a mechanism of tumor progression [9,34,35]. Because thrombocytosis is associated with increased risk of VTE and serous histology is one of the common carcinoma components in uterine carcinosarcoma [2], it is speculated that IL-6 dependent thrombocytosis may result in increased risk of VTE in uterine carcinosarcoma.
A strength of this study is that it is likely the first to report on the significance of VTE solely in patients with uterine carcinosarcoma. Given the rarity of uterine carcinosarcoma, the findings in this study have important implications for daily practice and management regarding this disease. Moreover, sample size and event number for VTE in this study were large enough to conduct multivariate analysis. There are also a few limitations to this study. First, this study was conducted retrospectively, making it possible to have missed confounding factors in the analysis. For example, we only collected symptom-based VTE; exact incidences of sub-clinical VTE were not captured as assessment for biological markers such as plasma d-dimer was not routinely performed [36]. Moreover, this database does not have information on past history of VTE, which is a major risk factor for subsequent VTE.
Our surgical database lacks information on treatment type and compliance related to VTE. Because heparin is considered as the standard treatment for VTE in cancer patients and is suggested to demonstrate anti-tumor effects via inhibition of tumor proliferation, metastasis, and angiogenesis as shown in pre-clinical studies [37], one may argue that lack of information on heparin use could have interfered with the results of survival analysis. However, a recent meta-analysis has shown that heparin use has a small effect on mortality among cancer patients [38]. We also did not have information for postoperative VTE prophylaxis in our database. However, we found that the majority of VTE in women with uterine carcinosarcoma were diagnosed outside the perioperative period. In addition, our study did not contain biological data such as oncogene mutation, albumin level, platelet counts, and IL-6 levels. Thus, while the proposed mechanism for tumor-related VTE for uterine carcinosarcoma sounds plausible, it remains unknown if these genetic signatures are associated with aggressive tumor characteristics resulting in metastatic disease and increased risk of VTE. Lastly, patient medical comorbidities and performance status, which are important risk factors for VTE, were not available in our dataset.
Given the decreased quality of life associated with VTE and heparin injection as well as cost related to therapeutic heparin treatment, an effort to reduce VTE would be clinically meaningful. In this study, we found that women with uterine carcinosarcoma who had certain risk factors carry a disproportionally high risk of VTE (obese non-Asian with large tumor), and this subgroup may be a candidate population for VTE prophylaxis. Aspirin and statins are shown to reduce the risk of VTE [39,40]. These pharmacologic agents are relatively inexpensive compared to other anti-thromboembolic agents and may possibly decrease cancer mortality [41–44], and thus, further study for risks and benefits as well as cost-effectiveness would be warranted.
Supplementary Material
HIGHLIGHTS.
Venous thromboembolism (VTE) was examined in uterine carcinosarcoma (UCS).
Approximately 8% of women with UCS developed VTE.
Patient factors for VTE: older age, large body habitus, and non-Asian
Tumor factors for VTE: residual disease, large tumor, and stage IV disease
VTE was associated with decreased survival in UCS.
Acknowledgments
Financial support
Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
Disclosure statement
There is no conflict of interest in all authors for this study.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2017.11.036.
References
- [1].Cantrell LA, Blank SV, Duska LR, Uterine carcinosarcoma: a review of the literature, Gynecol. Oncol 137 (2015) 581–588. [DOI] [PubMed] [Google Scholar]
- [2].Matsuo K, Takazawa Y, Ross MS, Elishaev E, Podzielinski I, Yunokawa M, Sheridan TB, Bush SH, Klobocista MM, Blake EA, Takano T, Matsuzaki S, Baba T, Satoh S, Shida M, Nishikawa T, Ikeda Y, Adachi S, Yokoyama T, Takekuma M, Fujiwara K, Hazama Y, Kadogami D, Moffitt MN, Takeuchi S, Nishimura M, Iwasaki K, Ushioda N, Johnson MS, Yoshida M, Hakam A, Li SW, Richmond AM, Machida H, Mhawech-Fauceglia P, Ueda Y, Yoshino K, Yamaguchi K, Oishi T, Kajiwara H, Hasegawa K, Yasuda M, Kawana K, Suda K, Miyake TM, Moriya T, Yuba Y, Morgan T, Fukagawa T, Wakatsuki A, Sugiyama T, Pejovic T, Nagano T, Shimoya K, Andoh M, Shiki Y, Enomoto T, Sasaki T, Mikami M, Shimada M, Konishi I, Kimura T, Post MD, Shahzad MM, Im DD, Yoshida H, Omatsu K, Ueland FR, Kelley JL, Karabakhtsian RG, Roman LD, Significance of histologic pattern of carcinoma and sarcoma components on survival outcomes of uterine carcinosarcoma, Ann. Oncol 27 (2016) 1257–1266. [DOI] [PubMed] [Google Scholar]
- [3].Tritschler T, Aujesky D, Venous thromboembolism in the elderly: a narrative review, Thromb. Res 155 (2017) 140–147. [DOI] [PubMed] [Google Scholar]
- [4].Geldhof V, Vandenbriele C, Verhamme P, Vanassche T, Venous thromboembolism in the elderly: efficacy and safety of non-VKA oral anticoagulants, Thromb. J 12 (2015) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wun T, White RH, Epidemiology of cancer-related venous thromboembolism, Best Pract. Res. Clin. Haematol 22 (2009) 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Matsuo K, Hasegawa K, Yoshino K, Murakami R, Hisamatsu T, Stone RL, A Prévis R, Hansen JM, Ikeda Y, Miyara A, Hiramatsu K, Enomoto T, Fujiwara K, Matsumura N, Konishi I, Roman LD, Gabra H, Fotopoulou C, Sood AK, Venous thromboembolism, interleukin-6 and survival outcomes in patients with advanced ovarian clear cell carcinoma, Eur.J. Cancer 51 (2015) 1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Matsuo K, Moeini A, Machida H, Fullerton ME, Shabalova A, Brunette LL, Roman LD, Significance of venous thromboembolism in women with cervical cancer, Gynecol. Oncol 142 (2016) 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Matsuo K, Yessaian AA, Lin YG, Pham HQ, Muderspach LI, Liebman HA, Morrow CP, Roman LD, Predictive model of venous thromboembolism in endometrial cancer, Gynecol. Oncol 128 (2012) 544–551. [DOI] [PubMed] [Google Scholar]
- [9].Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, Rupairmoole R, Armaiz-Pena GN, Pecot CV, Coward J, Deavers MT, Vasquez HG, Urbauer D, Landen CN, Hu W, Gershenson H, Matsuo K, Shahzad MM, King ER, Tekedereli I, Ozpolat B, Ahn EH, Bond VK, Wang R, Drew AF, Gushiken F, Lamkin D, Collins K, DeGeest K, Lutgendorf SK, Chiu W, Lopez-Berestein G, Afshar-Kharghan V, Sood AK, Paraneoplastic thrombocytosis in ovarian cancer, N. Engl.J. Med 366 (2012) 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhao S, Bellone S, Lopez S, Thakral D, Schwab C, English DP,Black J, Cocco E, Choi J, Zammataro L, Predolini F, Bonazzoli E, Bi M, Buza N, Hui P, Wong S, Abu-Khalaf M, Ravaggi A, Bignotti E, Bandiera E, Romani C, Todeschini P, Tassi R, Zanotti L, Odicino F, Pecorelli S, Donzelli C, Ardighieri L, Facchetti F, Falchetti M, Silasi DA, Ratner E, Azodi M, Schwartz PE, Mane S, Angioli R, Terranova C, Quick CM, Edraki B, Bilguvar K, Lee M, Choi M, Stiegler AL, Boggon TJ, Schlessinger J, Lifton RP, Santin AD, Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition, Proc. Natl. Acad. Sci. U. S. A 113 (2016) 12238–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cherniack AD, Shen H, Walter V, Stewart C, A Murray B, Bowlby R, Hu X, Ling S, Soslow RA, Broaddus RR, Zuna RE, Robertson G, Laird PW, Kucherlapati R, Mills GB, Weinstein JN, Zhang J, Akbani R, Levine DA, Integrated molecular characterization of uterine carcinosarcoma, Cancer Cell 31 (2017) 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Matsuo K, Ross MS, Bush SH, Yunokawa M, Blake EA, Takano T, Ueda Y, Baba T, Satoh S, Shida M, Ikeda Y, Adachi S, Yokoyama T, Takekuma M, Takeuchi S, Nishimura M, Iwasaki K, Yanai S, Klobocista MM, Johnson MS, Machida H, Hasegawa K, Miyake TM, Nagano T, Pejovic T, Shahzad MM, Im DD, Omatsu K, Ueland FR, Kelley JL, Roman LD, Tumor characteristics and survival outcomes of women with tamoxifen-related uterine carcinosarcoma, Gynecol. Oncol 144 (2016) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Matsuo K, Omatsu K, Ross MS, Johnson MS, Yunokawa M, Klobocista MM, Im DD, Bush SH, Ueda Y, Takano T, Blake EA, Hasegawa K, Baba T, Shida M, Satoh S, Yokoyama T, Machida H, Adachi S, Ikeda Y, Iwasaki K, Miyake TM, Yanai S, Nishimura M, Nagano T, Takekuma M, Takeuchi S, Pejovic T, Shahzad MM, Ueland FR, Kelley JL, Roman LD, Impact of adjuvant therapy on recurrence patterns in stage I uterine carcinosarcoma, Gynecol. Oncol 145 (2017) 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Matsuo K, Johnson MS, Im DD, Ross MS, Bush SH, Yunokawa M, Blake EA, Takano T, Klobocista MM, Hasegawa K, Ueda Y, Shida M, Baba T, Satoh S, Yokoyama T, Machida H, Ikeda Y, Adachi S, Miyake TM, Iwasaki K, Yanai S, Takeuchi S, Nishimura M, Nagano T, Takekuma M, Shahzad MMK, Pejovic T, Omatsu K, Kelley JL, Ueland FR, Roman LD, Survival outcome of women with stage IV uterine carcinosarcoma who received neoadjuvant chemotherapy followed by surgery,J. Surg. Oncol (2017) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Matsuo K, Ross MS, Yunokawa M, Johnson MS, Machida H, Omatsu K, Klobocista MM, Im DD, Satoh S, Baba T, Ikeda Y, Bush SH, Hasegawa K, Blake EA, Takekuma M, Shida M, Nishimura M, Adachi S, Pejovic T, Takeuchi S, Yokoyama T, Ueda Y, Iwasaki K, Miyake TM, Yanai S, Nagano T, Takano T, Shahzad MMK, Ueland FR, Kelley JL, Roman LD, Salvage chemotherapy with taxane and platinum for women with recurrent uterine carcinosarcoma, Gynecol. Oncol 147 (2017) 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ferguson SE, Tornos C, Hummer A, R Barakat R, Soslow RA, Prognostic features of surgical stage I uterine carcinosarcoma, Am. J. Surg. Pathol 31 (2007) 1653–1661. [DOI] [PubMed] [Google Scholar]
- [17].Pecorelli S, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium, Int. J. Gynaecol. Obstet 105 (2009) 103–104. [DOI] [PubMed] [Google Scholar]
- [18].Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R, Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials, Int. J. Radiat. Oncol. Biol. Phys 37 (1997) 745–751. [DOI] [PubMed] [Google Scholar]
- [19].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines forreporting observational studies, BMJ 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Onstad MA, Schmandt RE, Lu KH, Addressing the role of obesity in endometrial cancer risk, prevention, and treatment, J. Clin. Oncol 34 (2016) 4225–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kerr R, Stirling D, Ludlam CA, Interleukin 6 and haemostasis, Br.J. Haematol 115 (2001) 3–12. [DOI] [PubMed] [Google Scholar]
- [22].Archer DF, Oger E, Estrogen and progestogen effect on venous thromboembolism in menopausal women, Climacteric 15 (2012) 235–240. [DOI] [PubMed] [Google Scholar]
- [23].White RH, Keenan CR, Effects of race and ethnicity on the incidence of venous thromboembolism, Thromb. Res 123 (2009) S11–7. [DOI] [PubMed] [Google Scholar]
- [24].Yeung KT, Yang J, Epithelial-mesenchymal transition in tumor metastasis, Mol. Oncol 11 (2017) 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Karlsson MC, Gonzalez SF, Welin J,Fuxe J, Epithelial-mesenchymal transition in cancer metastasis through the lymphatic system, Mol. Oncol 11 (2017) 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jung HY, Fattet L, Yang J, Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis, Clin. Cancer Res 21 (2014) 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bharti R, Dey G, Mandal M, Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: a snapshot ofIL-6 mediated involvement, Cancer Lett 375 (2016) 51–61. [DOI] [PubMed] [Google Scholar]
- [28].Saghazadeh A, Rezaei N, Inflammation as a cause of venous thromboembolism, Crit. Rev. Oncol. Hematol 99 (2016) 272–285. [DOI] [PubMed] [Google Scholar]
- [29].Rodriguez AL, Wojcik BM, Wrobleski SK, Myers DD Jr., Wakefield TW, Diaz JA, Statins, inflammation and deep vein thrombosis: a systematic review, J. Thromb. Thrombolysis 33 (2012) 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mego M, Karaba M, Minarik G, Benca J, Sedlackova T, Tothova L, Vlkova B, Cierna Z, Janega P, Luha J, Gronesova P, Pindak D, Fridrichova I, Celec P, Reuben JM, Cristofanilli M, Mardiak J, Relationship between circulating tumor cells, blood coagulation, and urokinase-plasminogen-activator system in early breast cancer patients,BreastJ 21 (2015) 155–160. [DOI] [PubMed] [Google Scholar]
- [31].Hutti JE, Pfefferle AD, Russell SC, Sircar M, Perou CM, Baldwin AS, Oncogenic PI3K mutations lead to NF-kappaB-dependent cytokine expression following growth factor deprivation, Cancer Res 72 (2012) 3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chandler RL, Damrauer JS, Raab JR, Schisler JC, Wilkerson MD, Didion JP, Starmer J, Serber D, Yee D, Xiong J, Darr DB, Pardo-Manuel de Villena F, Kim WY, Magnuson T, Coexistent ARID1A-PIK3CA mutations promote ovarian clearcell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling, Nat. Commun 6 (2015) 6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sagae S, Susumu N, Viswanathan AN, Aoki D, Backes FJ, Provencher DM, Vaughan M, Creutzberg CL, Kurzeder C, Kristensen G, Lee C, Kurtz JE, Glasspool RM, Small W Jr., Gynecologic Cancer InterGroup (GCIG) consensus review for uterine serous carcinoma, Int. J. Gynecol. Cancer 24 (2014) S83–9. [DOI] [PubMed] [Google Scholar]
- [34].Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA, Integrated genomic characterization of endometrial carcinoma, Nature 497 (2013) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moore KN, Fader AN, Uterine papillary serous carcinoma, Clin. Obstet. Gynecol 54 (2011)278–291. [DOI] [PubMed] [Google Scholar]
- [36].Satoh T, Matsumoto K, Uno K, Sakurai M, Okada S, Onuki M, Minaguchi T, Tanaka YO, Homma S, Oki A, Yoshikawa H, Silent venous thromboembolism before treatment in endometrial cancer and the risk factors, Br. J. Cancer 99 (2008) 1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kuderer NM, Ortel TL, Francis CW, Impact of venous thromboembolism and anticoagulation on cancer and cancer survival, J. Clin. Oncol 27 (2009) 4902–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Akl EA, Kahale LA, Ballout RA, Barba M, Yosuico VE, van Doormaal FF, Middeldorp S, Bryant A, Schunemann H, Parenteral anticoagulation in ambulatory patients with cancer, Cochrane Database Syst. Rev (2014), CD006652. [DOI] [PubMed] [Google Scholar]
- [39].Simes J, Becattini C, Agnelli G, Eikelboom JW, Kirby AC, Mister R, Prandoni P, Brighton TA, Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration, Circulation 130 (2014) 1062–1071. [DOI] [PubMed] [Google Scholar]
- [40].Li L, Zhang P, Tian JH, Yang K, Statins for primary prevention of venous thromboembolism, Cochrane Database Syst. Rev (2014), CD008203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nielsen SF, Nordestgaard BG, Bojesen SE, Statin use and reduced cancer-related mortality, N. Engl.J. Med 367 (2012) 1792–1802. [DOI] [PubMed] [Google Scholar]
- [42].Matsuo K, Cahoon SS, Yoshihara K, Shida M, Kakuda M, Adachi S, Moeini A, Machida H, Garcia-Sayre J, Ueda Y, Enomoto T, Mikami M, Roman LD, Sood AK, Association of low-dose aspirin and survival of women with endometrial cancer, Obstet. Gynecol 128 (2016) 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nevadunsky NS, Van Arsdale A, Strickler HD, A Spoozak L, Moadel A, Kaur G, Girda E, Goldberg GL, Einstein MH, Association between statin use and endometrial cancer survival, Obstet. Gynecol 126 (2015) 144–150. [DOI] [PubMed] [Google Scholar]
- [44].Takiuchi T, Blake EA, Matsuo K, Sood AK, Brasky TM, Aspirin use and endometrial cancer risk and survival, Gynecol. Oncol (2017) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.