Abstract
Background
To examine recurrence patterns in women with stage I uterine carcinosarcoma (UCS) stratified by adjuvant therapy pattern.
Methods
We examined 443 cases of stage I UCS derived from a retrospective cohort of 1192 UCS cases from 26 institutions. Adjuvant therapy patterns after primary hysterectomy-based surgery were correlated to recurrence patterns.
Results
The most common adjuvant therapy was chemotherapy alone (41.5%) followed by chemotherapy/radiotherapy (15.8%) and radiotherapy alone (8.4%). Distant-recurrence was the most common recurrence pattern (5-year cumulative rate, 28.1%) followed by local-recurrence (13.3%). On multivariate analysis, chemotherapy but not radiotherapy remained an independent prognostic factor for decreased risk of local-recurrence (5-year cumulative rates 8.7% versus 19.8%, adjusted-hazard ratio [HR] 0.46, 95% confidence interval [CI] 0.25–0.83, P = 0.01) and distant-recurrence (21.2% versus 38.0%, adjusted-HR 0.41, 95%CI 0.27–0.62, P < 0.001). The chemotherapy/radiotherapy group had a lower 5-year cumulative local-recurrence rate compared to the chemotherapy alone group but it did not reach statistical significance (5.1% versus 10.1%, adjusted-HR 0.46, 95%CI 0.13–1.58, P = 0.22). Radiotherapy significantly decreased local-recurrence when tumors had high-grade carcinoma, sarcoma component dominance, and deep myometrial tumor invasion (all, P < 0.05); and combining radiotherapy with chemotherapy was significantly associated with decreased local-recurrence compared to chemotherapy alone in the presence of multiple risk factors (5-year cumulative rates, 2.5% versus 21.8%, HR 0.12, 95%CI 0.02–0.90; P = 0.013) but not in none/single factor (P = 0.36).
Conclusion
Adjuvant chemotherapy appears to be effective to control both local- and distant-recurrences in stage I UCS; adding radiotherapy to chemotherapy may be effective to control local-recurrence when the tumor exhibits multiple risk factors.
Keywords: Uterine carcinosarcoma, Stage I, Chemotherapy, Radiotherapy, Recurrence, Survival outcome
1. Introduction
Uterine carcinosarcoma is a rare but aggressive high-grade endometrial cancer, representing a biphasic tumor with the sarcoma element being dedifferentiated from the carcinoma component [1–6]. The majority of uterine carcinosarcomas are diagnosed as stage I disease, and surgery with total hysterectomy, salpingo-oophorectomy, and lymphadenectomy remains the standard primary treatment approach [7,8]. Due to poor survival outcome even in stage I disease [9,10], adjuvant therapy after primary surgical treatment is an important consideration in the management of uterine carcinosarcoma [7,8].
Various studies have demonstrated the effectiveness of postoperative systemic chemotherapy for early-stage uterine carcinosarcoma [11,12]. This approach is based on the rationale that stage I disease can develop substantially high incidence of distant-recurrence in the absence of adjuvant chemotherapy [12]. A large-scale nation-wide study has shown a recent increase in the use of chemotherapy and chemo-radiotherapy for early-stage uterine carcinosarcoma [13]. This study also demonstrated that chemotherapy and chemo-radiotherapy were associated with improved survival compared to no treatment for early-stage uterine carcinosarcoma; however, no direct comparison was performed between chemotherapy alone and chemo-radiotherapy, making it difficult to evaluate the role of adding radiotherapy to chemotherapy in the management of stage I uterine carcinosarcoma [13].
Because the role of adjuvant radiotherapy is questionable for early-stage uterine carcinosarcoma in controlling local recurrence in women who also receive chemotherapy [11,13–18], identifying the predictors of radiotherapy response will be useful to maximize the benefit of radiotherapy and minimize the adverse effects related to this treatment modality. The objective of the study was to examine recurrence patterns and survival outcome of women with stage I uterine carcinosarcoma who received adjuvant therapy with chemotherapy and radiotherapy.
2. Patients and methods
2.1. Eligibility
We utilized the previously organized dataset for uterine carcinosarcoma from a multi-center international study that was conducted in 26 academic and/or regional cancer centers in the United States and Japan [19,20]. In this large-scale multicenter collaboration, consecutive cases of stages I—IV uterine carcinosarcoma were retrospectively reviewed for histopathology findings. We obtained Institutional Review Board approval at each participating institution. Inclusion criteria were consecutive cases of stage I uterine carcinosarcoma that underwent primary hysterectomy-based surgical treatment with available adjuvant therapy information between 1993 and 2013. Exclusion criteria included stages II—IV disease, neoadjuvant radiotherapy or chemotherapy, no hysterectomy status, incorrect diagnosis, and absence of archived histopathology slides for evaluation. The STROBE guidelines were consulted to outline the results of retrospective cohort studies [21].
2.2. Clinical information
We abstracted the following information from archived medical records for the eligible cases: patient demographics, histopathology results, treatment type, and survival outcomes. For patient demographics, patient age at surgery, country, ethnicity, body mass index (BMI), parity, and preoperative CA-125 level were collected. Histopathologic findings included carcinoma type, sarcoma element, dominant histology component, cancer stage, tumor size, lymphovascular space invasion (LVSI), and depth of myometrial tumor invasion. Treatment information abstracted included: use of neoadjuvant therapy, and surgical details regarding hysterectomy and pelvic/para-aortic lymphadenectomy, and type of postoperative adjuvant therapy. Adjuvant radiotherapy type included whole pelvic radiotherapy (WPRT) and intracavitary brachytherapy (ICBT). Adjuvant chemotherapy information included type and number of administered cycles. For survival outcomes, disease-free survival (DFS) and overall survival (OS) were recorded. Among recurrent cases, anatomical locations of the first recurrent site were abstracted.
2.3. Histologic evaluation
Gynecologic pathologists reviewed the archived histopathology hematoxylin-eosin and where available immunohistochemically stained slides at each participating institution to evaluate the histologic subtypes of carcinoma and sarcoma components [19]. We grouped the carcinoma components into low-grade (grades 1–2 endometrioid) and high-grade (grade 3 endometrioid, serous, clear cell, undifferentiated, and mixed histology) subtypes, and grouped the sarcoma components into homologous (endometrial stromal sarcoma, leiomyosarcoma, fibrosarcoma, and undifferentiated sarcoma) and heterologous (rhabdomyosarcoma, osteosarcoma, chondrosarcoma, and liposarcoma) subtypes. We examined the proportions of carcinoma and sarcoma components in a semi-quantitative fashion within the primary tumor site in the hysterectomy specimen.
2.4. Study definition
Cutoff values for patient age, CA-125 level, depth of myometrial tumor invasion, and tumor size were based on prior studies [22–24]. We re-classified the cancer stage based on the 2009 International Federation of Gynecology and Obstetrics (FIGO) system [24]. A sarcoma component comprising > 50% of the primary tumor in the hysterectomy specimen was defined as sarcoma dominance. Myometrial tumor invasion > 50% was defined as deep invasion. Adjuvant chemotherapy regimen was categorized into taxane and platinum combination regimen (taxane/platinum-based), regimens containing ifosfamide (ifosfamide-based), and others. In the combination of chemotherapy and radiotherapy group, chemotherapy refers systemic chemotherapy but not concurrent chemotherapy during radiotherapy as a radiosensitizer. WPRT refers to external beam pelvic radiation and ICBT refers vaginal cuff radiation. DFS was defined as the time interval between the date of hysterectomy and the date of the first recurrence of disease or last follow-up. OS was defined as the time interval between the date of hysterectomy and the date of death due to uterine carcinosarcoma or last follow-up. Local-recurrence refers vaginal cuff and/or pelvic recurrence. Distant-recurrence refers recurrence other than local-recurrence.
2.5. Statistical analysis
The primary analysis of interest was to examine survival outcome and recurrence patterns across the adjuvant therapy patterns. The secondary analysis of interest was to examine the association of tumor factors and adjuvant radiotherapy response. Continuous variables, expressed with mean (± SD) or median (range), were examined by one-way ANOVA test or Kruskal-Wallis H test as appropriate. Categorical variables were evaluated with chi-square test.
Survival curves were constructed by Kaplan-Meier method [25], and the statistical significance between the curves were assessed by log-rank test for univariate analysis. We used a Cox proportional hazard regression model for multivariate analysis to determine the independent prognostic factors for survival and recurrence [26]. Covariates with P < 0.20 in univariate analysis were entered in the initial model. Least significant covariates were removed from the model until the final model retains only covariates with P < 0.05 (conditional backward methods) [27]. The relatively liberal P-value cutoffs for covariate selection were used due to small sample size in our study. Magnitude of the statistical significance was expressed with hazard ratio (HR) and 95% confidence interval (CI).
The variance inflation factor was determined among covariates in multivariate analysis, and a value of ≥ 2 was defined as multicollinearity in this study [28]. In multivariate analysis, over-adjustment was assessed with the ratio of events-of-interest per the entered covariates, and a cutoff level of < 10 was interpreted as over-adjustment in this study [29,30]. A P < 0.05 was considered statistically significant (all, 2-tailed). Statistical Package for Social Science software (SPSS, version 12.0, Chicago, IL) was used for all the analyses.
3. Results
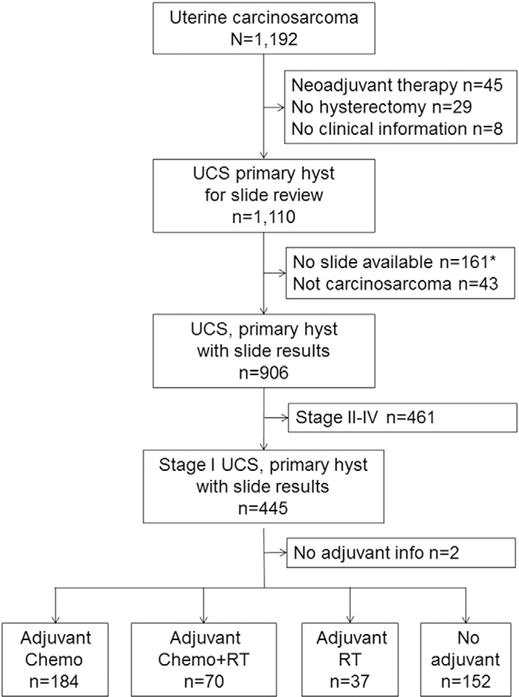
We identified 443 women with stage I uterine carcinosarcoma who had histology slide review and adjuvant therapy information available for analysis (Fig. 1). Patient demographics of the entire cohort are shown in Table 1. Mean age of patients was 64.6 with the majority being Asian (n = 261, 59.6%). The majority of the tumors had a high-grade carcinoma component (n = 291, 65.7%), homologous sarcoma element (n = 270, 60.9%), and stage IA disease (n = 293, 66.1%). Sarcoma dominance was seen in 177 (40.7%) cases. Nearly a half of tumors expressed LVSI (n = 194, 43.9%). Women with stage I uterine carcinosarcoma commonly underwent pelvic lymphadenectomy (n = 327, 73.8%) but not para-aortic lymphadenectomy (n = 191, 43.1%). The most common adjuvant radiotherapy was WPRT-based (n = 89, 83.2%) among those who received adjuvant radiotherapy. A taxane-platinum doublet was the most common adjuvant chemotherapy choice (n = 168, 66.1%) among those who received adjuvant chemotherapy.
Fig. 1.
Study selection schema (N = 1192). *including 2 cases that sarcoma component was not determined. Abbreviations: UCS, uterine carcinosarcoma; hyst, hysterectomy; chemo, chemotherapy alone; and RT, radiotherapy alone.
Table 1.
Patient demographics for stage I uterine carcinosarcoma (n = 443).
| Age | 64.6 (±10.4) |
| <60 years | 145 (32.7%) |
| ≥60 years | 298 (67.3%) |
| Race | |
| Caucasian | 126 (28.8%) |
| African | 34 (7.8%) |
| Hispanic | 11 (2.5%) |
| Asian | 261 (59.6%) |
| Unknown | 6 (1.4%) |
| Area | |
| United States | 189 (42.7%) |
| Japan | 254 (57.3%) |
| BMI | 26.5 (±8.0) |
| <30 kg/m2 | 333 (78.9%) |
| ≥30 kg/m2 | 89 (21.1%) |
| Parity | |
| Nulliparous | 70 (16.2%) |
| Multiparous | 363 (83.8%) |
| CA-125 | 16(2–735) |
| <30 IU/L | 232 (52.4%) |
| ≥30 IU/L | 79 (17.8%) |
| Not measured | 132 (29.8%) |
| Carcinoma component | |
| Low-gradea | 152 (34.3%) |
| High-gradeb | 291 (65.7%) |
| Sarcoma component | |
| Homologous | 270 (60.9%) |
| Heterologous | 173 (39.1%) |
| Sarcoma dominance | |
| No | 258 (59.3%) |
| Yes | 177 (40.7%) |
| Tumor size | |
| <5 cm | 195 (45.8%) |
| ≥5 cm | 231 (54.2%) |
| Myometrial invasion | |
| ≤50% | 293 (66.1%) |
| >50% | 150 (33.9%) |
| LVSI | |
| No | 248 (56.1%) |
| Yes | 194 (43.9%) |
| Pelvic lymphadenectomy | |
| Performed | 327 (73.8%) |
| Not performed | 116 (26.2%) |
| Sampled pelvic nodes | 20 (1–81) |
| Aortic lymphadenectomy | |
| Performed | 191 (43.1%) |
| Not performed | 252 (56.9%) |
| Sampled para-aortic nodes | 9 (1 –72) |
| Adjuvant radiotherapy | |
| None | 336 (75.8%) |
| WPRT ± ICBTc | 89 (20.1%) |
| ICBT alone | 18 (4.1%) |
| Adjuvant chemotherapy | |
| None | 189 (42.7%) |
| Taxane/platinum-based | 168 (37.9%) |
| Ifosfamide-based | 61 (13.8%)e |
| Others | 25 (5.6%) |
| Chemotherapy cycle | 6 (1–9)d |
| Adjuvant therapy pattern | |
| None | 152 (34.3%) |
| RT alone | 37 (8.4%) |
| Chemotherapy alone | 184 (41.5%) |
| Chemotherapy + RT | 70 (15.8%) |
| Recurrence sites (any) | |
| Local | 51 (11.5%) |
| Vaginal cuff | 24 (5.4%) |
| Pelvis | 33 (7.5%) |
| Distant | 106 (24.0%) |
Notes to Table 1
Number (%), mean (±SD), or median (range) is shown. Missing information included race (n = 5), BMI (n = 21), parity (n = 10), sarcoma dominance (n = 8), tumor size (n = 17), LVSI (n = 1), and anatomical recurrent site (n = 9). Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; LVSI, lymphovascular space invasion; ICBT, intracavitary brachytherapy; WPRT, whole pelvic radiotherapy; and RT, radiotherapy.
Grade 1 endometrioid (n = 68) and grade 2 endometrioid (n = 84).
Grade 3 endometrioid (n = 115), serous (n = 62), clear cell (n = 10), undifferentiated (n = 18), mixed (n = 83), and others (n = 3).
Including 2 cases with extended field radiotherapy to para-aortic lymph nodes.
Median cycles were 6 (1–7) for chemotherapy/radiotherapy group and 6(1–9) for chemotherapy alone group.
Including 12 cases of ifosfamide and paclitaxel.
3.1. Adjuvant therapy pattern
Adjuvant therapy patterns were examined (Table 2). The most common pattern was chemotherapy alone (n = 184, 41.5%) followed by systemic chemotherapy and radiotherapy (n = 70, 15.8%), and radiotherapy alone (n = 37, 8.4%). The most common sequence pattern in the chemotherapy/radiotherapy group was systemic chemotherapy followed by radiotherapy (n = 39, 54.2%), sandwich therapy (n = 31, 43.1%), and radiotherapy followed by systemic chemotherapy (n = 2, 2.8%). There were152(34.3%) women who did not receive any adjuvant therapy after hysterectomy-based surgery, and these women were more likely to be older (age ≥ 60 years, 79.6%) than those who received adjuvant therapy and less likely to undergo pelvic and para-aortic lymphadenectomy (59.9% and 25.0%, respectively; all, P < 0.001). Women who received chemotherapy/radiotherapy were more likely be obese and to receive care in the United States (both, P < 0.001). While women with a low-grade carcinoma component were more likely to receive chemotherapy alone for adjuvant therapy (P < 0.001), the type of sarcoma element was not associated with adjuvant therapy pattern (P = 0.48). Women whose tumors had LVSI or sarcoma dominance were more likely to receive chemotherapy/radiotherapy (both, P < 0.05). Women who did not have pelvic lymphadenectomy were more likely to receive radiotherapy (P < 0.001). Median number of chemotherapy cycles were six for both the chemotherapy alone and the chemotherapy/radiotherapy groups (range 1–9), and 88.6% of chemotherapy/radiotherapy group received ≥ 4 cycles (62 out of 70 cases).
Table 2.
Patient demographics based on adjuvant therapy for stage I uterine carcinosarcoma (n = 443).
| Characteristic | Chemotherapy alone n = 184 (41.5%) | Chemotherapy/radiotherapy n = 70 (15.8%) | Radiotherapy alone n = 37 (8.4%) | None n = 152 (34.3%) | P-value |
|---|---|---|---|---|---|
| Age | 61.8 (±8.9) | 63.1 (±9.0) | 62.5 (±11.5) | 69.3 (±10.7) | <0.001 |
| <60 years | 73 (39.7%) | 24 (34.3%) | 17 (45.9%) | 31 (20.4%) | |
| ≥60 years | 111 (60.3%) | 46 (65.7%) | 20 (54.1%) | 121 (79.6%) | |
| Race | <0.001 | ||||
| Caucasian | 23 (12.7%) | 42 (60.0%) | 23 (62.2%) | 38 (25.3%) | |
| African | 5 (2.8%) | 16 (22.9%) | 2 (5.4%) | 11 (7.3%) | |
| Hispanic | 3 (1.7%) | 6 (8.6%) | 1 (2.7%) | 1 (0.7%) | |
| Asian | 148 (81.8%) | 6 (8.6%) | 9 (24.3%) | 98 (65.3%) | |
| Unknown | 2 (1.1%) | 0 | 2 (5.4%) | 2 (1.3%) | |
| Area | <0.001 | ||||
| United States | 36 (19.6%) | 68 (97.1%) | 29 (78.4%) | 56 (36.8%) | |
| Japan | 148 (80.4%) | 2 (2.9%) | 8 (21.6%) | 96 (63.2%) | |
| BMI | 24.4 (±5.7) | 32.9 (±11.0) | 27.0 (±5.3) | 26.2 (±8.0) | <0.001 |
| <30 kg/m2 | 158 (87.3%) | 30 (46.9%) | 22 (73.3%) | 123 (83.7%) | |
| ≥30 kg/m2 | 23 (12.7%) | 34 (53.1%) | 8 (26.7%) | 24 (16.3%) | |
| Parity | 0.40 | ||||
| Nulliparous | 152 (83.1%) | 59 (86.8%) | 27 (75.0%) | 125 (85.6%) | |
| Multiparous | 31 (16.9%) | 9 (113.2%) | 9 (25.0%) | 21 (14.4%) | |
| CA-125 | <0.001 | ||||
| <30 IU/L | 121 (65.8%) | 31 (44.3%) | 7 (18.9%) | 73 (48.0%) | |
| ≥30 IU/L | 39 (21.2%) | 9 (12.9%) | 4 (10.8%) | 27 (17.8%) | |
| Not measured | 24 (13.0%) | 30 (42.9%) | 26 (70.3%) | 52 (34.2%) | |
| Carcinoma component | <0.001 | ||||
| Low-grade | 86 (46.7%) | 8 (11.4%) | 8 (21.6%) | 50 (32.9%) | |
| High-grade | 98 (53.3%) | 62 (88.6%) | 29 (78.4%) | 102 (67.1%) | |
| Sarcoma component | 0.48 | ||||
| Homologous | 117 (63.6%) | 42 (60.0%) | 25 (67.6%) | 86 (55.6%) | |
| Heterologous | 67 (36.4%) | 28 (40.0%) | 12 (32.4%) | 66 (43.4%) | |
| Sarcoma dominance | 0.017 | ||||
| No | 113 (61.4%) | 33 (50.0%) | 29 (80.6%) | 83 (55.7%) | |
| Yes | 71 (38.6%) | 33 (50.0%) | 7 (19.4%) | 66 (44.3%) | |
| Tumor size | 0.29 | ||||
| <5 cm | 83 (46.9%) | 25 (36.2%) | 17 (54.8%) | 70 (47.0%) | |
| ≥5 cm | 94 (53.1%) | 44 (63.8%) | 14 (45.2%) | 79 (53.0%) | |
| Myometrial invasion | 0.07 | ||||
| ≤50% | 119 (64.7%) | 48 (68.6%) | 18 (48.6%) | 108 (71.1%) | |
| >50% | 65 (35.3%) | 22 (31.4%) | 19 (51.4%) | 44 (28.9%) | |
| LVSI | 0.02 | ||||
| No | 97 (52.7%) | 32 (45.7%) | 19 (52.8%) | 100 (65.8%) | |
| Yes | 87 (47.3%) | 38 (54.3%) | 17 (47.2%) | 52 (34.2%) | |
| Pelvic lymphadenectomy | <0.001 | ||||
| Performed | 149 (81.0%) | 63 (90.0%) | 24 (64.9%) | 91 (59.9%) | |
| Not performed | 35 (19.0%) | 7 (10.0%) | 13 (35.1%) | 61 (40.1%) | |
| Sampled nodes | 29 (2–81) | 11 (1–39) | 10(2–25) | 18 (1–78) | <0.001 |
| Aortic lymphadenectomy | <0.001 | ||||
| Performed | 97 (52.7%) | 42 (60.0%) | 14 (37.8%) | 38 (25.0%) | |
| Not performed | 87 (47.3%) | 28 (40.0%) | 23 (62.2%) | 114(75.0%) | |
| Sampled nodes | 19 (1–72) | 4(1–16) | 4(1–26) | 4 (1 – 54) | <0.001 |
Mean (± SD), median (range), or number (%) is shown. Percentage is per row for the initial grouping and per column for remaining characteristics. Percentages are per Columns. One-way ANOVA test, Kruskal-Wallis H test, or chi-square test for P-values. Significant P-values are emboldened. Abbreviations: BMI, body mass index; CA-125, cancer antigen 125; and LVSI, lymphovascular space invasion.
3.2. Rationale of recurrence pattern
Median follow-up for the entire cohort was 35.2 (range 0.1–211.2) months: 18.2 months for women who died of uterine carcinosarcoma (n = 86, 19.4%) and 41.2 months for women who were censored at the last visit (n = 357, 80.6%). There were 144 (32.5%) women who had disease recurrence with median time-to-recurrence being 10.1 months. The most common recurrent pattern was distant-recurrence (n = 106, 24.0%) with 1, 2, and 5-year cumulative recurrence rates being 13.0%, 20.7%, and 28.1%, respectively. Local-recurrence was seen in 51 (11.5%) cases with 1, 2, and 5-year cumulative recurrence rates being 9.3%, 12.6%, and 13.3%, respectively. Vaginal cuff and pelvic recurrences were seen in 24 (5.4%) and 33 (7.5%) cases, respectively. When combined, distant-recurrence alone was the most common recurrence pattern (n = 84, 62.2%) followed by local-recurrence alone (n = 29, 21.5%) and both local/distant-recurrence (n = 22, 16.3%). Local-recurrence was associated with shorter time-to-recurrence compared to distant-recurrence (median time to local-recurrence alone 7.1 months, both local-/distant-recurrence 8.8 months, and distant-recurrence alone 12.8 months, P = 0.006).
3.3. Survival outcomes
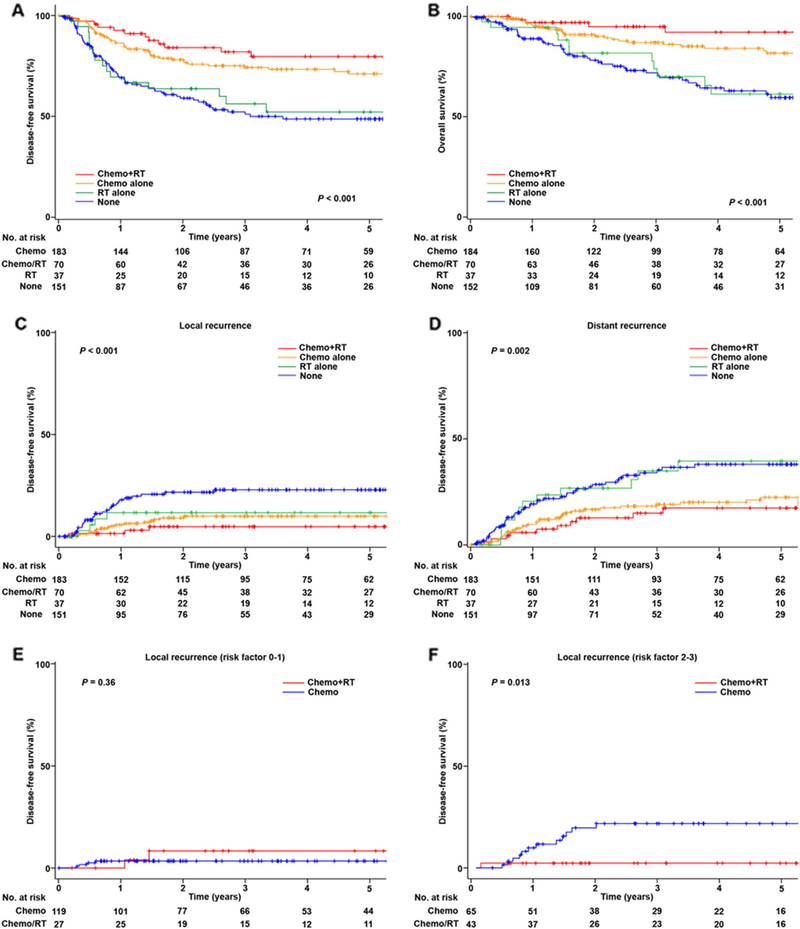
DFS was examined based on adjuvant therapy pattern. When chemotherapy and radiotherapy were analyzed as separate variables (Table S1), only chemotherapy use was associated with improved DFS (5-year rates, 73.1% versus 50.0%, P < 0.001) but not radiotherapy (69.9% versus 61.2%, P = 0.17) on univariate analysis. On multivariate analysis, chemotherapy use was independently associated with improved DFS compared to non-use (adjusted-HR 0.50, 95%CI 0.35–0.71, P < 0.001), and similar finding was observed for OS (adjusted-HR 0.30 95%CI 0.19–0.47, P < 0.001). When combination patterns of chemotherapy and radiotherapy were examined (Table 3), radiotherapy alone was independently associated with decreased DFS compared to chemotherapy alone (5-year rates, 52.9% versus 70.7%, adjusted-HR 2.29, 95%CI 1.26–4.15, P = 0.006) on multivariate analysis. However, combination of chemotherapy/radiotherapy and chemotherapy alone groups had statistically similar DFS (5-year rates, 79.4% versus 70.7%, adjusted-HR 0.71 95%CI 0.39–1.30, P = 0.27; Fig. 2A). Similar findings were also observed for OS (Table 3 and Fig. 2B). Among the chemotherapy/radiotherapy group, sequence of chemotherapy and radiotherapy was not statistically associated with DFS (5-year rates, chemotherapy then radiotherapy versus sandwich therapy, 75.2% versus 84.8%, P = 0.18).
Table 3.
Survival outcome of stage I uterine carcinosarcoma (n = 443).
| Characteristic | No. | Disease-free survival |
Overall survival |
||||||||
| 5-yr (%) | Univariate |
Multivariate |
5-yr (%) | Univariate |
Multivariate |
||||||
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | ||||
| Age | <0.001 | <0.001 | <0.001 | 0.003 | |||||||
| <60 years | 145 | 76.9% | 1 | 1 | 85.7% | 1 | 1 | ||||
| ≥60 years | 298 | 56.2% | 2.47 (1.64–3.72) | 2.13 (1.40–3.25) | 67.8% | 2.77 (1.61–4.77) | 2.39 (1.36–4.20) | ||||
| CA-125 | 0.007 | 0.045 | |||||||||
| <30 IU/L | 232 | 70.2% | 1 | 79.4% | 1 | ||||||
| ≥30 IU/L | 79 | 50.4% | 1.86 (1.23–2.82) | 62.3% | 1.87 (1.10–3.17) | ||||||
| Not tested | 132 | 56.9% | 1.50 (1.02–2.21) | 70.7% | 1.51 (0.92–2.47) | ||||||
| Carcinoma | 0.078 | 0.074 | |||||||||
| Low-grade | 152 | 69.6% | 1 | 81.3% | 1 | ||||||
| High-grade | 291 | 59.7% | 1.38 (0.96–1.99) | 70.2% | 1.54 (0.96–2.48) | ||||||
| Sarcoma | 0.025 | ||||||||||
| Homologous | 270 | 67.8% | 1 | ||||||||
| Heterologous | 173 | 56.5% | 1.46 (1.05–2.02) | ||||||||
| Size | 0.001 | 0.002 | 0.019 | ||||||||
| <5 cm | 195 | 69.9% | 1 | 1 | 79.6% | 1 | |||||
| ≥5 cm | 231 | 57.1% | 1.74 (1.23–2.47) | 1.75 (1.22–2.50) | 68.2% | 1.68 (1.08–2.59) | |||||
| Sarcoma dominance | 0.074 | 0.034 | 0.021 | ||||||||
| No | 258 | 66.4% | 1 | 79.1% | 1 | 1 | |||||
| Yes | 177 | 56.7% | 1.35 (0.97–1.88) | 65.3% | 1.57 (1.03–2.39) | 1.68 (1.08–2.60) | |||||
| Myometrial invasion | <0.001 | 0.008 | 0.011 | 0.005 | |||||||
| ≤50% | 293 | 69.6% | 1 | 1 | 78.5% | 1 | 1 | ||||
| >50% | 150 | 51.2% | 1.87 (1.35–2.60) | 1.60 (1.13–2.28) | 66.4% | 1.72 (1.13–2.62) | 1.85 (1.20–2.85) | ||||
| LVSI | 0.13 | 0.12 | |||||||||
| No | 248 | 66.9% | 1 | 78.3% | 1 | ||||||
| Yes | 194 | 59.4% | 1.29 (0.93–1.79) | 70.4% | 1.40 (0.91–2.14) | ||||||
| Pelvic lymphadenectomy | <0.001 | 0.002 | |||||||||
| Performed | 327 | 68.8% | 1 | 77.6% | 1 | ||||||
| Not performed | 116 | 47.0% | 1.47 (1.24–1.75) | 63.9% | 1.42 (1.14–1.76) | ||||||
| Aortic lymphadenectomy | 0.005 | 0.004 | |||||||||
| Performed | 191 | 70.6% | 1 | 80.2% | 1 | ||||||
| Not performed | 252 | 57.8% | 1.28 (1.07–1.52) | 69.9% | 1.40 (1.11–1.76) | ||||||
| Adjuvant therapy | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| Chemo alone | 184 | 70.7% | 1 | 1 | 81.5% | 1 | 1 | ||||
| Chemo + RT | 70 | 79.4% | 0.76 (0.42–1.39) | 0.71 (0.39–1.30) | 0.27 | 92.6% | 0.51 (0.19–1.32) | 0.47 (0.18–1.24) | 0.13 | ||
| RT alone | 37 | 52.9% | 1.94 (1.10–3.43) | 2.29 (1.26–4.15) | 0.006 | 62.3% | 2.17 (1.07–4.40) | 2.65 (1.29–5.44) | 0.008 | ||
| None | 152 | 49.2% | 2.28 (1.56–3.32) | 2.23 (1.51–3.29) | <0.001 | 59.6% | 2.63 (1.62–4.28) | 2.48 (1.51–4.06) | <0.001 | ||
Log-rank test for univariate analysis and Cox proportional hazard regression model for multivariate analysis. Variables with P < 0.20 in univariate analysis were entered in the initial model, and only variable with P < 0.05 are listed in the final model for conditional backward method. Event-to-variable ratio ≥ 10 indicates absence of overadjustment. Significant P-values are emboldened. Abbreviations: CA-125, cancer antigen 125; postop, postoperative; LVSI, lymphovascular space invasion; 5-yr (%), 5-year survival proportion; HR, hazard ratio; and CI, confidence interval; chemo, chemotherapy; and RT, radiotherapy.
Fig. 2.
Disease-free survival of uterine carcinosarcoma (n = 443). Log-rank test for P-values. Survival curves are shown for A) disease-free survival, B) overall survival, C) cumulative incidence for local recurrence in the pelvis with or without the vaginal cuff, D) cumulative incidence for distant recurrence in outside the pelvis, E) cumulative incidence for local recurrence among cases with 0–1 risk factor, and F) cumulative incidence for local recurrence among cases with 2–3 risk factors. Risk factors: high-grade carcinoma, > 50% myometrial tumor invasion, and sarcoma dominance. Abbreviations: chemo, chemotherapy; and RT, radiotherapy.
3.4. Local-recurrence pattern
The risk of local-recurrence was examined based on adjuvant therapy pattern (Table S2). With absence of adjuvant therapy, 5-year cumulative risk of local-recurrence was 12.1%. Both radiotherapy (5-year cumulative incidence, 7.3% versus 15.3%, P = 0.048) and chemotherapy (8.7% versus 19.8%, P < 0.001) were significantly associated with decreased risk of local-recurrence on univariate analysis. However, on multivariate analysis, only chemotherapy remained an independent predictor for decreased risk of local-recurrence (adjusted-HR 0.46, 95%CI 0.25–0.83, P = 0.01). When combination patterns for chemotherapy and radiotherapy were examined (Table 4 and Fig. 2C), women who received radiotherapy alone had a similar risk of local-recurrence compared to those just receiving chemotherapy alone (5-year cumulative incidence, 11.3% versus 10.1%, adjusted-HR 1.22, 95%CI 0.41–3.69, P = 0.72). Although cumulative incidence is lower, the combination of chemotherapy/radiotherapy had a statistically similar local-recurrence risk compared to chemotherapy alone (5.1% versus 10.1%, adjusted-HR 0.46, 95%CI 0.13–1.58, P = 0.22).
Table 4.
Local and distant recurrence in stage I uterine carcinosarcoma (n = 443).
| No. | Disease-free survival |
Overall survival |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-yr (%) | Univariate |
Multivariate |
5-yr (%) | Univariate |
Multivariate |
||||||
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | ||||
| Age | <0.001 | 0.007 | 0.003 | 0.038 | |||||||
| <60 years | 145 | 5.7% | 1 | 1 | 19.7% | 1 | 1 | ||||
| ≥60 years | 298 | 17.5% | 3.65 (1.64–8.09) | 3.06 (1.36–6.86) | 32.9% | 1.96 (1.25–3.07) | 1.63 (1.03–2.61) | ||||
| CA-125 | 0.042 | 0.024 | |||||||||
| <30 IU/L | 232 | 11.1% | 1 | 23.0% | 1 | ||||||
| ≥30 IU/L | 79 | 22.4% | 2.24 (1.17–4.30) | 41.5% | 1.90 (1.19–3.04) | ||||||
| Not tested | 132 | 12.8% | 1.22 (0.63–2.37) | 31.3% | 1.28 (0.81–2.03) | ||||||
| Carcinoma | 0.17 | ||||||||||
| Low-grade | 152 | 23.9% | 1 | ||||||||
| High-grade | 291 | 30.7% | 1.34 (0.88–2.04) | ||||||||
| Sarcoma | 0.12 | ||||||||||
| Homologous | 270 | 25.3% | 1 | ||||||||
| Heterologous | 173 | 32.9% | 1.35 (0.92–1.99) | ||||||||
| Size | 0.059 | 0.001 | 0.003 | ||||||||
| <5 cm | 195 | 10.2% | 1 | 21.7% | 1 | 1 | |||||
| ≥5 cm | 231 | 17.0% | 1.73 (0.97–3.07) | 33.9% | 1.96 (1.30–2.96) | 1.89 (1.24–2.89) | |||||
| Sarcoma dominance | 0.07 | ||||||||||
| No | 258 | 25.2% | 1 | ||||||||
| Yes | 177 | 34.4% | 1.42 (0.97–2.09) | ||||||||
| Myometrial invasion | 0.018 | 0.011 | <0.001 | 0.01 | |||||||
| ≤50% | 293 | 10.5% | 1 | 1 | 23.0% | 1 | 1 | ||||
| >50% | 150 | 19.4% | 1.91 (1.11–3.29) | 2.08 (1.19–3.63) | 38.5% | 1.93 (1.32–2.83) | 1.71 (1.14–2.56) | ||||
| LVSI | 0.06 | ||||||||||
| No | 248 | 24.0% | 1 | ||||||||
| Yes | 194 | 33.8% | 1.44 (0.98–2.12) | ||||||||
| Pelvic lymphadenectomy | <0.001 | 0.001 | |||||||||
| Performed | 327 | 9.8% | 1 | 24.7% | 1 | ||||||
| Not performed | 116 | 24.7% | 1.68 (1.28–2.21) | 38.9% | 1.38 (1.13–1.69) | ||||||
| Aortic lymphadenectomy | 0.027 | 0.008 | |||||||||
| Performed | 191 | 8.8% | 1 | 21.9% | 1 | ||||||
| Not performed | 252 | 17.0% | 1.40 (1.03–1.88) | 33.0% | 1.31 (1.07–1.61) | ||||||
| Adjuvant type | <0.001 | 0.004 | 0.002 | 0.001 | |||||||
| Chemo alone | 184 | 10.1% | 1 | 1 | 22.6% | 1 | 1 | ||||
| Chemo + RT | 70 | 5.1% | 0.48 (0.14–1.64) | 0.46 (0.13–1.58) | 0.22 | 17.7% | 0.46 (0.89–1.72) | 0.88 (0.45–1.71) | 0.71 | ||
| RT alone | 37 | 11.3% | 1.32 (0.44–3.94) | 1.22 (0.41–3.69) | 0.72 | 38.6% | 1.92 (0.99–3.69) | 2.22 (1.11–4.41) | 0.023 | ||
| None | 152 | 12.1% | 2.62 (1.42–4.84) | 2.44 (1.31–4.56) | 0.005 | 37.9% | 2.04(1.31–3.17) | 2.14 (1.35–3.39) | 0.001 | ||
Log-rank test for univariate analysis and Cox proportional hazard regression model for multivariate analysis. Variables with P < 0.20 in univariate analysis were entered in the initial model, and only variable with P < 0.05 are listed in the final model for conditional backward method. Event-to-variable ratio ≥ 10 indicates absence of overadjustment. Significant P-values are emboldened. Abbreviations: CA-125, cancer antigen 125; postop, postoperative; LVSI, lymphovascular space invasion; 5-yr (%), 5-year cumulative incidence; HR, hazard ratio; and CI, confidence interval. Brachytherapy; and WPRT, whole pelvic radiotherapy.
3.5. Distant-recurrence pattern
The risk of distant-recurrence was examined based on the adjuvant therapy given (Table S2). With absence of adjuvant therapy, 5-year cumulative risk of distant-recurrence was 37.9%. Radiotherapy use was not associated with distant-recurrence on univariate analysis (5-year cumulative incidence, 25.0% versus 29.2%, P = 0.53). Chemotherapy use was independently associated with decreased risk of distant recurrence on multivariate analysis (5-year cumulative rates 21.2% versus 38.0%, adjusted-HR 0.48, 95%CI 0.33–0.71, P < 0.001). When combining chemotherapy and radiotherapy patterns (Table 4 and Fig. 2D), the radiotherapy alone group had a significantly increased risk of distant-recurrence as compared to the chemotherapy alone group (5-year cumulative rates, 38.6% versus 22.6%, adjusted-HR 2.22, 95%CI 1.11–4.41, P = 0.023). The risk of distant-recurrence was similar between the chemotherapy/radiotherapy group and the chemotherapy alone group (5-year cumulative rates 17.7% versus 22.6%, adjusted-HR 0.88, 95%CI 0.45–1.71, P = 0.71).
3.6. Tumor factors and treatment response
Patterns of recurrence were examined based on treatment modality (Table 5). While radiotherapy had no effects on distant recurrence across the tumor factors (all, P > 0.05), radiotherapy significantly reduced the rate of local recurrence when the adenocarcinoma was high-grade (5-year cumulative rates, 7.3% versus 18.3%, P = 0.021), there was deep myometrial tumor invasion (5.2% versus 25.0%, P = 0.017), and there was sarcoma dominance (2.3% versus 20.1%, P = 0.018). The chemotherapy/radiotherapy group had similar local-recurrence risk to the chemotherapy alone group when the tumor had no or a single risk factor of the three aforementioned factors (P = 0.36; Fig. 2E). However, when the tumor had ≥ 2 risk factors, the chemotherapy/radiotherapy group had a significantly decreased local-recurrence risk compared to the chemotherapy alone group (5-year cumulative rates, 2.5% versus 21.8%, HR 0.12, 95%CI 0.02–0.90, P = 0.013; Fig. 2F). Among cases who did not have pelvic lymphadenectomy, radiotherapy decreased local-recurrence but it did not reach statistical significance (5-year cumulative rates 10.3% versus 27.3%, P = 0.18). Chemotherapy significantly decreased local- and distant-recurrences in the majority of tumor factors, but it did not decrease local-recurrence when the tumor exhibited low-grade carcinoma and deep myometrial tumor invasion (both, P > 0.05).
Table 5.
Patterns of recurrence based on treatment modality (n = 443).
| No. | Radiotherapy |
Chemotherapy |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Local-recurrence |
Distant-recurrence |
Local-recurrence |
Distant-recurrence |
||||||
| 5-yr (%) | P-value | 5-yr (%) | P-value | 5-yr (%) | P-value | 5-yr (%) | P-value | ||
| Carcinoma | |||||||||
| Low-grade | 152 | 6.9% vs 11.0% | 0.71 | 0% vs 26.0% | 0.06 | 8.7% vs 13.8% | 0.21 | 21.5% vs 26.6% | 0.16 |
| High-grade | 291 | 7.3%vs 18.3% | 0.021 | 29.1%vs 31.5% | 0.76 | 8.7% vs 23.6% | <0.001 | 21.1% vs 44.0% | <0.001 |
| Sarcoma | |||||||||
| Homologous | 270 | 8.5% vs 13.3% | 0.36 | 26.6% vs 24.9% | 0.49 | 9.0% vs 16.8% | 0.028 | 20.1% vs 33.0% | 0.011 |
| Heterologous | 173 | 5.1% vs 18.3% | 0.057 | 23.7% vs 35.7% | 0.09 | 8.1%vs 25.2% | 0.004 | 23.1% vs 45.4% | 0.005 |
| Myometrial invasion | |||||||||
| ≤50% | 293 | 8.5% vs 10.8% | 0.57 | 18.4% vs 24.1% | 0.29 | 3.5% vs 19.5% | <0.001 | 18.5% vs 28.5% | 0.01 |
| >50% | 150 | 5.2% vs 25.0% | 0.017 | 35.9% vs 39.6% | 0.94 | 18.7% vs 20.2% | 0.36 | 26.4% vs 56.9% | 0.002 |
| LVSI | |||||||||
| No | 248 | 8.2% vs 14.5% | 0.29 | 17.0% vs 25.6% | 0.23 | 9.8% vs 17.3% | 0.034 | 18.4% vs 29.5% | 0.059 |
| Yes | 194 | 5.8% vs 16.3% | 0.09 | 30.5% vs 33.6% | 0.91 | 7.9% vs 24.6% | 0.001 | 23.7% vs 50.1% | 0.001 |
| Sarcoma dominance | |||||||||
| No | 258 | 10.7% vs 12.3% | 0.75 | 25.0% vs 24.8% | 0.83 | 6.3% vs 19.2% | 0.002 | 16.4% vs 35.7% | <0.001 |
| Yes | 177 | 2.3% vs 20.1% | 0.018 | 36.3% vs 28.6% | 0.27 | 12.4% vs 21.1% | 0.038 | 28.6% vs 44.8% | 0.02 |
| Size | |||||||||
| <5 cm | 195 | 7.3% vs 10.9% | 0.57 | 20.3% vs 22.1% | 0.95 | 3.1% vs 18.9% | <0.001 | 13.6% vs 31.8% | 0.005 |
| ≥5 cm | 231 | 7.3% vs 19.7% | 0.053 | 29.2% vs 35.1% | 0.39 | 12.3% vs 22.6% | 0.017 | 26.5% vs 45.6% | 0.001 |
| Pelvic lymphadenectomy | |||||||||
| Performed | 327 | 6.5% vs 10.9% | 0.25 | 24.1% vs 24.9% | 0.92 | 6.7% vs 15.7% | 0.003 | 19.7% vs 34.7% | 0.006 |
| Not performed | 116 | 10.3% vs 27.3% | 0.18 | 28.9% vs 40.5% | 0.40 | 19.7% vs 25.6% | 0.36 | 29.5% vs 34.6% | 0.13 |
| Aortic lymphadenectomy | |||||||||
| Performed | 191 | 6.2% vs 10.0% | 0.39 | 18.9% vs 23.3% | 0.62 | 7.3% vs 13.2% | 0.13 | 20.3% vs 26.6% | 0.30 |
| Not performed | 252 | 8.5% 19.0% | 0.11 | 31.6% vs 33.1% | 0.88 | 10.4% vs 22.1% | 0.007 | 22.5% vs 41.9% | 0.003 |
Log-rank test for P-values. Significant P-values are emboldened. Abbreviations: 5-yr (%), 5-year cumulative incidence; and LVSI, lymphovascular space invasion.
3.7. Stage IA disease
Sub-analysis was performed for stage IA uterine carcinosarcoma cases (n = 293). The most common adjuvant treatment modality was chemotherapy alone (n = 119, 40.6%) followed by chemotherapy/ radiotherapy (n = 48, 16.4%), and radiotherapy alone (n = 18, 6.1%). There were 108 (36.9%) women who did not receive adjuvant therapy. Among the chemotherapy/radiotherapy group, the majority of radiotherapy was WPRT-based (n = 35, 72.9%) followed by ICBT alone (n = 13, 27.1%). The use of chemotherapy was associated with a higher 5-year DFS rate compared to a non-chemotherapy treatment approach: chemotherapy/radiotherapy 84.3%, chemotherapy alone 77.5%, radiotherapy alone 54.3%, and none 57.0% (P < 0.001). Similar findings were observed for 5-year OS rates: chemotherapy/radiotherapy 95.7%, chemotherapy alone 87.1%, radiotherapy alone 56.3%, and none 66.6% (P < 0.001). Among 48 cases who received chemotherapy/radiotherapy, local-recurrence risk was lower in WPRT-based therapy than ICBT alone although it did not demonstrate statistical significance (5-year cumulative rates, 3.5% versus 9.1%, P = 0.48).
4. Discussion
The effectiveness of adjuvant therapy for stage I uterine carcinosarcoma has been relatively understudied in the past, and available previous studies were limited in sample size (27–111 cases) likely due to the rare nature of this tumor [11,12]. Our study not only validated prior findings that chemotherapy is superior to radiotherapy, but also highlights the importance of chemotherapy for this uterine malignancy that has a high risk of distant-recurrence even in stage I disease [12].
Key findings of this investigation are that stage I uterine carcinosarcoma had a disproportionally high risk of distant-recurrence, and systemic chemotherapy after hysterectomy-based surgical treatment reduced the rate of distant-recurrence. Adjuvant chemotherapy is also effective at reducing local-recurrence, and adding radiotherapy to chemotherapy may enhance the local-control effects if the tumors have two or more risk factors.
Deep myometrial tumor invasion was significantly associated with decreased chemotherapy effects for local-recurrence control in this study (Table 5). In contrast, radiotherapy was found to be effective for local-recurrence control when tumor had deep myometrial tumor invasion. This finding can indeed support the fundamental concept of combining systemic chemotherapy and radiotherapy for early-stage uterine carcinosarcoma by making up for the weakness of each treatment effect. That is, when there is evidence of deep myometrial tumor invasion, chemotherapy is effective for distant-recurrence control but insufficient to control local-recurrence whereas radiotherapy has no effect on distant-recurrence control but reduces the local-recurrence risk.
While the carcinoma component rather than the sarcoma component is the driving force for tumor progression and is the main treatment target in uterine carcinosarcoma [7], a possible therapeutic implication of sarcoma dominance that was observed in our study deserves further discussion. That is, use of radiotherapy was significantly effective for prevention of local-recurrence when the dominant component of the tumor consisted of sarcoma (Table 5). Our prior study on uterine carcinosarcoma found that when the sarcoma component metastasizes, the tendency is to spread loco-regionally to the pelvis whereas the carcinoma component tends to spread hematogenously and lymphatically to areas distant from the uterus [19]. These findings suggest that the sarcoma factor may be an important determinant when considering radiotherapy. Indeed, adjuvant radiotherapy is considered an effective modality to reduce local-recurrence in a pooled-analysis of nearly 1500 cases of uterine leiomyosarcoma and endometrial stromal sarcoma, of which both sarcoma types are the majority of homologous element for uterine carcinosarcoma [31]. In addition, radiotherapy had a trend towards effectiveness when the sarcoma element had heterologous type although it did not demonstrate a statistically significant difference (Table 5). Because radiotherapy is an integral component of treatment for genital tract rhabdomyosarcoma which is the most common heterologous element in uterine carcinosarcoma [19,32,33], evaluating adjuvant radiotherapy in tumors containing this sarcoma element may merit further investigation.
Current National Comprehensive Cancer Network (NCCN) management guidelines considers adjuvant chemotherapy as one of the treatment options for stage IA uterine carcinosarcoma [8]. A non-chemotherapy option with tumor-directed radiotherapy is also listed as an alternative approach for adjuvant therapy. In our analysis of stage IA disease, however, non-chemotherapy treatment had increased risk of both local- and distant-recurrences compared to a chemotherapy-based counterpart. Therefore, even in stage IA disease, adjuvant chemotherapy-based treatment is important to optimize outcome. Because ICBT has a comparable effectiveness for vaginal cuff recurrence with reduced radiation-related adverse effects compared to WPRT [34], adding ICBT to chemotherapy may be a reasonable option for adjuvant treatment for this disease as suggested by the NCCN guidelines [8].
In our study, not every woman had lymphadenectomy and only 43% of women underwent complete staging with pelvic and aortic lymphadenectomy. This implies that considerable proportion of women may have had occult or microscopic stage IIIC disease because uterine carcinosarcoma has a high risk of nodal metastasis [7]. Indeed, unstaged women had increased risks of both local- and distant-recurrences compared to staged women (Table 4). When lymphadenectomy was not performed, radiotherapy may reduce the local-recurrence risk although it did not demonstrate statistical significance (10.3% versus 27.3% for no pelvic lymphadenectomy; and 8.5% versus 19.0% for no aortic lymphadenectomy; Table 5). Our study was limited in a sample size, and further study is warranted to examine this association.
Strengths of this study are the evaluation of a large sample size of a relatively rare tumor with comprehensive tumor information. Additionally, we performed a direct comparison of treatment effects between chemotherapy/radiotherapy and chemotherapy alone. Confirmation of the diagnosis of uterine carcinosarcoma by archived histopathology slide review by gynecologic pathologists further enriched the quality of this study. Also, this study was conducted in national and regional cancer centers. Weaknesses of this study are that this is a retrospective study that may have missed possible confounding factors. For example, the exact indication and reason for chemotherapy and radiotherapy were not abstracted from the medical records. The majority of the study population were of Asian ethnicity, thus the findings may not be generalizable to other populations.
Other limitations of this study include that we do not have information regarding the toxicity profile of the adjuvant therapy. Because WPRT and six cycles of systemic chemotherapy may cause substantial adverse effects especially in an elderly population, which is the most common age group affected of this disease, careful assessment of risks and benefits of adjuvant therapy will be warranted. For example, one trial noted that > 20% of patients did not complete combination of adjuvant chemotherapy and radiotherapy for uterine carcinosarcoma due to toxicity or patient decline [35]. In addition, sample size was inadequate to do a sub-analysis for a comparison of WPRT versus ICBT as well as a comparison for taxane/platinum-doublet versus other chemotherapy agents. Lastly, there may be a type II error due to lack of power to detect the statistical difference for survival between chemotherapy/radiotherapy and chemotherapy alone. For instance, the sample size to detect difference in local-recurrence between chemotherapy/radiotherapy and chemotherapy alone groups (5.1% versus 10.1%) was underpowered (<80%) with a-level of 0.05 and β-level of 0.20. Based on our results, 1513 cases for the chemotherapy/radiotherapy group and 3979 cases for the chemotherapy alone group would be needed in a study designed to detect a difference in local-recurrence between the two treatment modalities with 80% power.
There are a few important clinical implications of the current study in terms of postoperative management for women with stage I uterine carcinosarcoma. First, we endorse the importance of adjuvant chemotherapy even in stage IA disease. Second, we introduce the concept of selective radiotherapy to a group of women with certain high risk factors who might benefit the most from its addition. That is, women with multiple risk factors including high-grade carcinoma, sarcoma dominance, and deep myometrial tumor invasion may receive an optimal benefit-to-risk ratio from radiotherapy. To minimize the adverse events from this combination therapy of chemotherapy and radiotherapy in elder women, the utility of this selective radiotherapy approach merits further investigation.
Supplementary Material
HIGHLIGHTS.
Stage I uterine carcinosarcoma (UCS) has a high incidence of distant recurrence.
Adjuvant chemotherapy may be effective to decrease both local/distant recurrences.
Adding radiotherapy to chemotherapy may be effective if tumor has ≥2 risk factors.
Acknowledgments
Financial support
Ensign Endowment for Gynecologic Cancer Research (K.M.).
Footnotes
Disclosure statement
There is no conflict of interest in all authors for this study.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2017.02.001.
References
- [1].Sutton G, Uterine sarcomas 2013, Gynecol. Oncol. 130 (2013) 3–5. [DOI] [PubMed] [Google Scholar]
- [2].Trope CG, Abeler VM, Kristensen GB, Diagnosis and treatment of sarcoma of the uterus. A review, Acta Oncol 51 (2012) 694–705. [DOI] [PubMed] [Google Scholar]
- [3].Kedzia W, Pruski D, Iwaniec K, Przybylski M, Friebe Z, Rajpert-Kedzia H, Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus: clinicoimmunohistochemical and histogenetic characteristics, Folia Histochem. Cytobiol. 50 (2012) 513–518. [DOI] [PubMed] [Google Scholar]
- [4].D'Angelo E, Prat J, Pathology of mixed Mullerian tumours, Best Pract. Res. Clin. Obstet. Gynaecol. 25 (2011) 705–718. [DOI] [PubMed] [Google Scholar]
- [5].Kernochan LE, Garcia RL, Carcinosarcomas (malignant mixed Mullerian tumor) of the uterus: advances in elucidation of biologic and clinical characteristics, J. Natl. Compr. Cancer Netw. 7 (2009) 550–556. [DOI] [PubMed] [Google Scholar]
- [6].Wada H, Enomoto T, Fujita M, Yoshino K, Nakashima R, Kurachi H, Haba T, Wakasa K, Shroyer KR, Tsujimoto M, Hongyo T, Nomura T, Murata Y, Molecular evidence that most but not all carcinosarcomas of the uterus are combination tumors, Cancer Res. 57 (1997) 5379–5385. [PubMed] [Google Scholar]
- [7].Cantrell LA, Blank SV, Duska LR, Uterine carcinosarcoma: a review of the literature, Gynecol. Oncol. 137 (2015) 581–588. [DOI] [PubMed] [Google Scholar]
- [8].Uterine neoplasms. NCCN Clinical Practice Guideline in Oncology http://www.nccn.org (accessed 11/15/2016).
- [9].Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, Yordan E, Brady MF, Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study, Cancer 71 (1993) 1702–1709. [DOI] [PubMed] [Google Scholar]
- [10].Spanos WJ Jr., Peters LJ, Oswald MJ, Patterns of recurrence in malignant mixed Mullerian tumor of the uterus, Cancer 57 (1986) 155–159. [DOI] [PubMed] [Google Scholar]
- [11].Cantrell LA, Havrilesky L, Moore DT, O'Malley D, Liotta M, Secord AA, Nagel CI, Cohn DE, Fader AN, Wallace AH, Rose P, Gehrig PA, A multi-institutional cohort study of adjuvant therapy in stage I—II uterine carcinosarcoma, Gynecol. Oncol. 127 (2012) 22–26. [DOI] [PubMed] [Google Scholar]
- [12].Leath CA 3rd, Numnum TM, Kendrick JEt, Frederick PJ, Rocconi RP, Conner MG, Straughn JM, Patterns of failure for conservatively managed surgical stage I uterine carcinosarcoma: implications for adjuvant therapy, Int. J. Gynecol. Cancer 19 (2009) 888–891. [DOI] [PubMed] [Google Scholar]
- [13].Rauh-Hain JA, Starbuck KD, Meyer LA, Clemmer J, Schorge JO, Lu KH, Del Carmen MG, Patterns of care, predictors and outcomes of chemotherapy for uterine carcinosarcoma: a National Cancer Database analysis, Gynecol. Oncol. 139 (2015) 84–89. [DOI] [PubMed] [Google Scholar]
- [14].Reed NS, Mangioni C, Malmstrom H, Scarfone G, Poveda A, Pecorelli S, Tateo S, Franchi M, Jobsen JJ, Coens C, Teodorovic I, Vergote I, Vermorken JB, Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: a European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874), Eur. J. Cancer 44 (2008) 808–818. [DOI] [PubMed] [Google Scholar]
- [15].Callister M, Ramondetta LM, Jhingran A, Burke TW, Eifel PJ, Malignant mixed Mullerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome, Int. J. Radiat. Oncol. Biol. Phys. 58 (2004) 786–796. [DOI] [PubMed] [Google Scholar]
- [16].Chi DS, Mychalczak B, Saigo PE, Rescigno J, Brown CL, The role of whole-pelvic irradiation in the treatment of early-stage uterine carcinosarcoma, Gynecol. Oncol. 65 (1997) 493–498. [DOI] [PubMed] [Google Scholar]
- [17].Galaal K, van der Heijden E, Godfrey K, Naik R, Kucukmetin A, Bryant A, Das N, Lopes AD, Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma, Cochrane Database Syst. Rev (2013), CD006812.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, Cohn DE, Ioffe OB, A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I—IV carcinosarcoma (CS) of the uterus, Gynecol. Oncol. 107 (2007) 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Matsuo K, Takazawa Y, Ross MS, Elishaev E, Podzielinski I, Yunokawa M, Sheridan TB, Bush SH, Klobocista MM, Blake EA, Takano T, Matsuzaki S, Baba T, Satoh S, Shida M, Nishikawa T, Ikeda Y, Adachi S, Yokoyama T, Takekuma M, Fujiwara K, Hazama Y, Kadogami D, Moffitt MN, Takeuchi S, Nishimura M, Iwasaki K, Ushioda N, Johnson MS, Yoshida M, Hakam A, Li SW, Richmond AM, Machida H, Mhawech-Fauceglia P, Ueda Y, Yoshino K, Yamaguchi K, Oishi T, Kajiwara H, Hasegawa K, Yasuda M, Kawana K, Suda K, Miyake TM, Moriya T, Yuba Y, Morgan T, Fukagawa T, Wakatsuki A, Sugiyama T, Pejovic T, Nagano T, Shimoya K, Andoh M, Shiki Y, Enomoto T, Sasaki T, Mikami M, Shimada M, Konishi I, Kimura T, Post MD, Shahzad MM, Im DD, Yoshida H, Omatsu K, Ueland FR, Kelley JL, Karabakhtsian RG, Roman LD, Significance of histologic pattern of carcinoma and sarcoma components on survival outcomes of uterine carcinosarcoma, Ann. Oncol 27 (2016) 1257–1266. [DOI] [PubMed] [Google Scholar]
- [20].Matsuo K, Ross MS, Bush SH, Yunokawa M, Blake EA, Takano T, Baba T, Satoh S, Shida M, Ikeda Y, Adachi S, Yokoyama T, Takekuma M, Takeuchi S, Nishimura M, Iwasaki K, Yanai S, Klobocista MM, Johnson MS, Machida H, Ueda Y, Hasegawa K, Miyake TM, Nagano T, Pejovic T, Shahzad MM, Im DD, Omatsu K, Ueland FR, Kelley JL, Roman LD, Tumor characteristics and survival outcomes of women with tamoxifen-related uterine carcinosarcoma, Gynecol. Oncol. 144 (2016) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huang GS, Chiu LG, Gebb JS, Gunter MJ, Sukumvanich P, Goldberg GL, Einstein MH, Serum CA125 predicts extrauterine disease and survival in uterine carcinosarcoma, Gynecol. Oncol. 107 (2007) 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferguson SE, Tornos C, Hummer A, Barakat RR, Soslow RA, Prognostic features of surgical stage I uterine carcinosarcoma, Am. J. Surg. Pathol. 31 (2007) 1653–1661. [DOI] [PubMed] [Google Scholar]
- [24].Pecorelli S, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium, Int. J. Gynaecol. Obstet 105 (2009) 103–104. [DOI] [PubMed] [Google Scholar]
- [25].Kaplan EL, Meier P, Nonparametric estimation from incomplete observations, J. Am. Stat. Assoc. 53 (1958) 457–481. [Google Scholar]
- [26].Cox DR, Regression models and life-tables, J. R. Stat. Soc. Ser. B Stat Methodol 34 (1972) 187–220. [Google Scholar]
- [27].Lawless JF, Singhal K, Efficient screening of nonnormal regression models, Biometrics 34 (1978) 318–327. [Google Scholar]
- [28].Mansfield ER, Helms BP, Detecting multicollinearity, Am. Stat. 36 (1982) 158–160. [Google Scholar]
- [29].Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR, A simulation study of the number of events per variable in logistic regression analysis, J. Clin. Epidemiol. 49 (1996) 1373–1379. [DOI] [PubMed] [Google Scholar]
- [30].Schisterman EF, Cole SR, Platt RW, Overadjustment bias and unnecessary adjustment in epidemiologic studies, Epidemiology 20 (2009) 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sampath S, Gaffney DK, Role of radiotherapy treatment of uterine sarcoma, Best Pract. Res. Clin. Obstet. Gynaecol. 25 (2011) 761–772. [DOI] [PubMed] [Google Scholar]
- [32].Arndt CA, Donaldson SS, Anderson JR, Andrassy RJ, Laurie F, Link MP, Raney RB, Maurer HM, Crist WM, What constitutes optimal therapy for patients with rhabdomyosarcoma of the female genital tract? Cancer 91 (2001) 2454–2468. [PubMed] [Google Scholar]
- [33].Terezakis SA, Wharam MD, Radiotherapy for rhabdomyosarcoma: indications and outcome, Clin. Oncol. (R. Coll. Radiol.) 25 (2012) 27–35. [DOI] [PubMed] [Google Scholar]
- [34].Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Mens JW, Slot A, Kroese MC, van Bunningen BN, Ansink AC, van Putten WL, Creutzberg CL, Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial, Lancet 375 (2010) 816–823. [DOI] [PubMed] [Google Scholar]
- [35].Einstein MH, Klobocista M, Hou JY, Lee S, Mutyala S, Mehta K, Reimers LL, Kuo DY, Huang GS, Goldberg GL, Phase II trial of adjuvant pelvic radiation “sandwiched” between ifosfamide or ifosfamide plus cisplatin in women with uterine carcinosarcoma, Gynecol. Oncol. 124 (2011) 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.